Tue, Jul 15, 2025
[Archive]
Volume 21, Issue 5 (May 2023)
IJRM 2023, 21(5): 415-424 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shoorei H, Jafarabadi M, PourBayranvand S, Salehnia M. Comparison of mouse ovarian follicular development and gene expression in the presence of ovarian tissue extract and sodium selenite: An experimental study. IJRM 2023; 21 (5) :415-424
URL: http://ijrm.ir/article-1-2612-en.html
URL: http://ijrm.ir/article-1-2612-en.html
1- Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. Department of Anatomical Sciences, Faculty of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
2- Vali-E-Asr Reproductive Health Research Center, Family Health Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
3- Vali-E-Asr Reproductive Health Research Center, Family Health Research Institute, Tehran University of Medical Sciences, Tehran, Iran. ,salehnim@modares.ac.ir
2- Vali-E-Asr Reproductive Health Research Center, Family Health Research Institute, Tehran University of Medical Sciences, Tehran, Iran.
3- Vali-E-Asr Reproductive Health Research Center, Family Health Research Institute, Tehran University of Medical Sciences, Tehran, Iran. ,
Keywords: Follicle-stimulating hormone receptor, Ovary, Sodium selenite, Proliferation cell nuclear antigen, Mouse.
Full-Text [PDF 1255 kb]
(1060 Downloads)
| Abstract (HTML) (969 Views)
1. Introduction
Folliculogenesis in mammals is one of the most complex processes that involves direct interaction between somatic cells (such as granulosa and theca cells) and oocytes (1). Different factors such as hormones, growth factors, and antioxidants play critical roles in the growth and maturation of follicles and oocytes (2). Several culture systems, including 2- and 3-dimensional systems, were designed to investigate the effects of these factors during in vitro follicular culture. Culture media usually contain necessary supplementations such as serum, growth factors, hormones, and so on that improves follicular maturation (3).
It has been reported that selenium salts as non-metal chemical elements in large amounts are very toxic, but trace amounts of selenium are necessary for animal cell function (4). The addition of selenium to the culture medium as an antioxidant leads to the maturation of follicles and oocytes (5-10). It was reported that the supplementation of culture media with sodium selenite (SS) could increase the growth and maturation of preantral follicles (5). Also, several studies have demonstrated that supplementation of mouse oocyte maturation media with SS improved not only the oocyte developmental rate but also enhanced its expression of mitochondrial transcription factor A and mitochondrial DNA (6-9). These studies have shown that the reactive oxygen species (ROS) level was decreased in the presence of SS, which acts as an antioxidant (5, 7). It was also shown that selenium is one of the main critical factors in follicular fluid (11).
Ovarian follicular development is also regulated by intra-ovarian factors such as steroid hormones, epidermal growth factor, vascular growth factor, fibroblast growth factor, and growth differentiation factor β that enhance follicular development (11, 12). Some of these factors are produced by the ovarian stromal cells and follicular cells (12). The ovarian microenvironment could improve follicular development by providing these elements. However, the literature shows that supplementation of culture media with follicular fluid could enhance the development and the quality of cumulus-oocyte complex and embryo (12, 13).
Previously, we studied the effects of different concentrations of ovarian tissue extract (OTE) on mouse follicular development. The OTE contains components that mimic the natural composition of ovarian tissues and was obtained from adult mouse ovaries, which contain a lot of follicular fluid that has an impact on follicular development. Our results at the morphological level demonstrated that the OTE increased the development of follicles as its concentration increased within the culture medium (14).
Moreover, this study was designed to bring some additional information and compare the effect of SS and OTE on follicular function and development. In addition, at the mRNA level, the expression of 2 critical genes including follicle-stimulating hormone receptor (FSHR) and proliferation cell nuclear antigen (PCNA) that involve in the maturation and proliferation of were assessed.
2. Materials and Methods
The reagents were prepared by Sigma Aldrich company (Dusseldorf, Germany) except those indicated in the text.
Full-Text: (222 Views)
1. Introduction
Folliculogenesis in mammals is one of the most complex processes that involves direct interaction between somatic cells (such as granulosa and theca cells) and oocytes (1). Different factors such as hormones, growth factors, and antioxidants play critical roles in the growth and maturation of follicles and oocytes (2). Several culture systems, including 2- and 3-dimensional systems, were designed to investigate the effects of these factors during in vitro follicular culture. Culture media usually contain necessary supplementations such as serum, growth factors, hormones, and so on that improves follicular maturation (3).
It has been reported that selenium salts as non-metal chemical elements in large amounts are very toxic, but trace amounts of selenium are necessary for animal cell function (4). The addition of selenium to the culture medium as an antioxidant leads to the maturation of follicles and oocytes (5-10). It was reported that the supplementation of culture media with sodium selenite (SS) could increase the growth and maturation of preantral follicles (5). Also, several studies have demonstrated that supplementation of mouse oocyte maturation media with SS improved not only the oocyte developmental rate but also enhanced its expression of mitochondrial transcription factor A and mitochondrial DNA (6-9). These studies have shown that the reactive oxygen species (ROS) level was decreased in the presence of SS, which acts as an antioxidant (5, 7). It was also shown that selenium is one of the main critical factors in follicular fluid (11).
Ovarian follicular development is also regulated by intra-ovarian factors such as steroid hormones, epidermal growth factor, vascular growth factor, fibroblast growth factor, and growth differentiation factor β that enhance follicular development (11, 12). Some of these factors are produced by the ovarian stromal cells and follicular cells (12). The ovarian microenvironment could improve follicular development by providing these elements. However, the literature shows that supplementation of culture media with follicular fluid could enhance the development and the quality of cumulus-oocyte complex and embryo (12, 13).
Previously, we studied the effects of different concentrations of ovarian tissue extract (OTE) on mouse follicular development. The OTE contains components that mimic the natural composition of ovarian tissues and was obtained from adult mouse ovaries, which contain a lot of follicular fluid that has an impact on follicular development. Our results at the morphological level demonstrated that the OTE increased the development of follicles as its concentration increased within the culture medium (14).
Moreover, this study was designed to bring some additional information and compare the effect of SS and OTE on follicular function and development. In addition, at the mRNA level, the expression of 2 critical genes including follicle-stimulating hormone receptor (FSHR) and proliferation cell nuclear antigen (PCNA) that involve in the maturation and proliferation of were assessed.
2. Materials and Methods
The reagents were prepared by Sigma Aldrich company (Dusseldorf, Germany) except those indicated in the text.
2.1. Study design
In this experimental study from mouse ovaries, the follicles were dissected with 145-155 μm diameter and divided into 3 groups as follows. In the control group the culture media (α-minimum essential medium; αMEM) containing 1% insulin, transferrin, and selenium (Invitrogen, Paisley, UK), 100 mIU/ml recombinant follicle-stimulating hormone (rFSH or Gonal-f; Serono, Switzerland), 100 IU/ml penicillin and 100 mg/ml streptomycin and 10% fetal calf serum (FBS; Gibco, UK). In experimental group I, the culture media was the same as the control, in addition, 10 ng/ml SS was added (5). In experimental group II, the described culture media was used and the FBS was replaced with OTE (14). After 12 days of culture, the survival and developmental rate of follicles and the expression of FSHR and PCNA genes were analyzed and compared. In addition, the levels of 17-β-estradiol and progesterone in the collected media were analyzed.
2.2. Animals and collection of ovarian tissue
For this study, 12-16-day-old female NMRI mice (n = 20) ovaries that contain preantral follicles were used for follicular isolation and 6-8-wk-old adult mice (n = 15) for the preparation of OTE. The mice were maintained in the animal house of the university under standard conditions (12-hr light/12-hr dark cycle, 22-24°C, and 55% humidity). The ovaries were obtained after mice cervical dislocation, then transferred to culture media consisting of 10% FBS, 100 IU/ml penicillin, and 100 mg/ml streptomycin until assessments.
2.3. OTE preparation
The OTE was prepared according to the previous study (14). After dissecting the adult mice ovaries under a stereomicroscope, they (n = 15) were collected and immersed in potassium ammonium chloride solution (pH = 7.2-7.4) for 1 min. Ovaries were then washed 3 times with phosphate-buffered saline (PBS) and put into a glass homogenizer tube. They were homogenized by adding 2 ml of Tris-HCL buffer (pH = 7) and 50 μL of protease inhibitor solution. Then the volume of the solution reached 10 ml after the homogenization of ovarian tissues by Tris-HCL buffer (pH = 7). The obtained solution was sonicated for 2 min under W50 sonication conditions. Finally, the solution was centrifuged at 12000 RPM for 15 min. The supernatant was collected as tissue extract and stored at -80°C until assay.
2.3. OTE preparation
The OTE was prepared according to the previous study (14). After dissecting the adult mice ovaries under a stereomicroscope, they (n = 15) were collected and immersed in potassium ammonium chloride solution (pH = 7.2-7.4) for 1 min. Ovaries were then washed 3 times with phosphate-buffered saline (PBS) and put into a glass homogenizer tube. They were homogenized by adding 2 ml of Tris-HCL buffer (pH = 7) and 50 μL of protease inhibitor solution. Then the volume of the solution reached 10 ml after the homogenization of ovarian tissues by Tris-HCL buffer (pH = 7). The obtained solution was sonicated for 2 min under W50 sonication conditions. Finally, the solution was centrifuged at 12000 RPM for 15 min. The supernatant was collected as tissue extract and stored at -80°C until assay.
2.4. Total protein analysis
500 μL of supernatant of OTE was used to measure the amount of protein. The amount of protein in fetal bovine serum (FBS) was used as a reference to select the concentration of OTE. According to our previous study, the OTE which contains ½ of the protein level in FBS was selected (14).
2.5. 3-dimensional follicle culture
A 3-dimensional culture system based on sodium alginate capsulation was used for follicular culture (14). A mixture of sodium alginate and activated charcoal in distilled water was prepared to eliminate organic pollution. After 24 hr it was diluted with PBS at a concentration of 0.5% (w/v), then each isolated follicle was individually transferred into 7 µL of sodium alginate and a cross-linking solution containing 50 mM CaCl2 and 140 mM NaCl, respectively. After washing the follicles in PBS at least 2 times for 2 min in each, the capsulated follicles (n = 248 in total) were put in the medium under mineral oil. The group without SS and OTE was considered as the control group (n = 90) and in this group, the αMEM culture media was supplemented with 1% insulin, transferrin, and selenium, 100 mIU/ml rFSH, 100 IU/ml penicillin, and 100 mg/ml streptomycin and 10% f FBS. In experimental group I (n = 86 follicles) the FBS was replaced by 10 ng/ml SS (5) and in experimental group II (n = 90 follicles) with OTE as its protein concentration was ½ of FBS protein (14). The half of culture media was renewed by fresh ones at one-day intervals during the culture period and the remaining media was frozen until analysis.
2.6. In vitro ovulation induction
The morphological changes of follicles were studied using inverted microscope, and the follicles that showed a dark appearance were considered dead. By supplementing the culture media with 1.5 IU/ml of human chorionic gonadotropin (hCG; Organon) the oocyte ovulation induction was induced on Day 12 of culture, then 18 hr later the oocyte staging was done. The germinal vesicle oocyte has the prominent nucleus that is arrested in the prophase of meiosis I; the nucleus and polar body were not visible in the germinal vesicle breakdown oocyte, and the presence of the first polar body was demonstrated in the metaphase II (MII) oocyte (13).
2.7. Hormonal assays
At the end of the culture period, steroid hormones, including 17-β-estradiol and progesterone, were analyzed in the collected media (n = 3 in each group). The levels of 17-β-estradiol and progesterone (Biotest AG, Dreieich, Germany) were measured by an enzyme immunoassay, and these experiments were done in triplicates.
2.8. RNA extraction and cDNA synthesis for molecular assessment
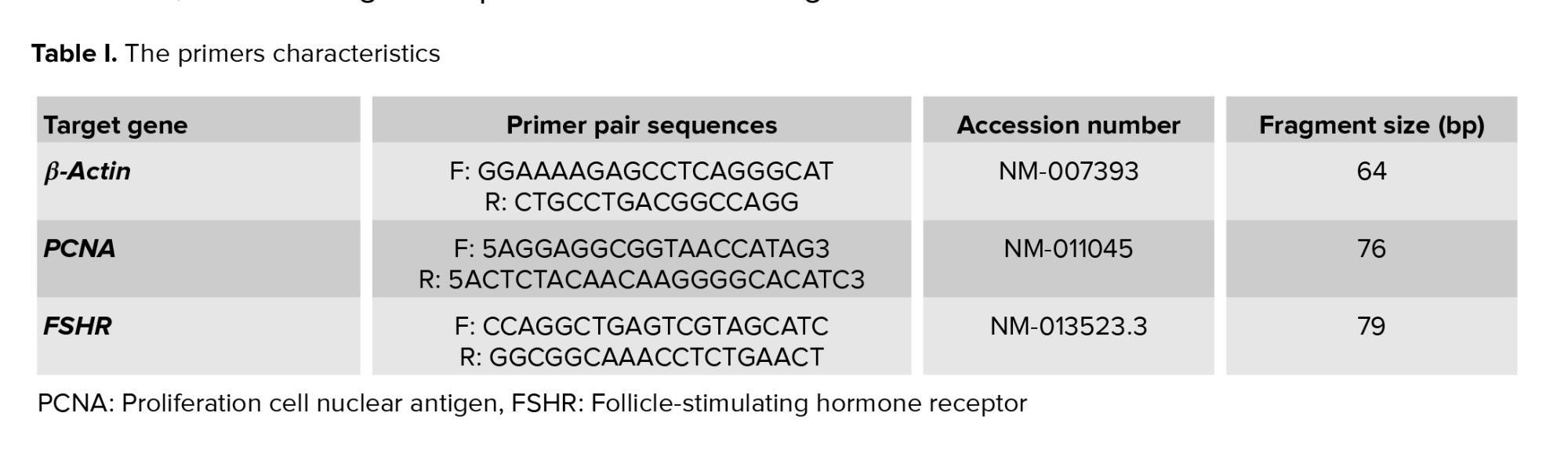
Total RNA was extracted from follicles in each studied group (n = 12 in 3 replicates) using a TRIzol reagent extraction method (Invitrogen, Paisley, UK). By spectrophotometer, the quality of extracted RNA and its level were analyzed. The list of applied primers for PCNA, FSHR, and β-actin was summarized in table I.
2.9. Real-time reverse transcription polymerase chain reaction (RT-PCR)
After extraction of total RNA and cDNA synthesis, according to QuantiTect SYBR Green RT-PCR kit (Applied Biosystems, UK), real-time RT-PCR was performed. Thermal conditions for the process included 3 steps; holding stage (95ºC for 5 min), step 2 was performed at 95ºC for 15 s, 58ºC for 30 s, and 72ºC for 30 s, and the last step (melt curve step) was continued at 95ºC for 15 s, 60ºC for 1 min, and 95ºC for 15 s. Then, the relative quantification of target genes to housekeeping genes was determined.
2.10. Ethical considerations
The animals were used and cared for according to the Ethical Guidelines of Tarbiat Modares University. The Ethics Committee for Animal Research of the Tarbiat Modares University, Tehran, Iran, approved this study (Code: IR.TMU.REC.1394.272).

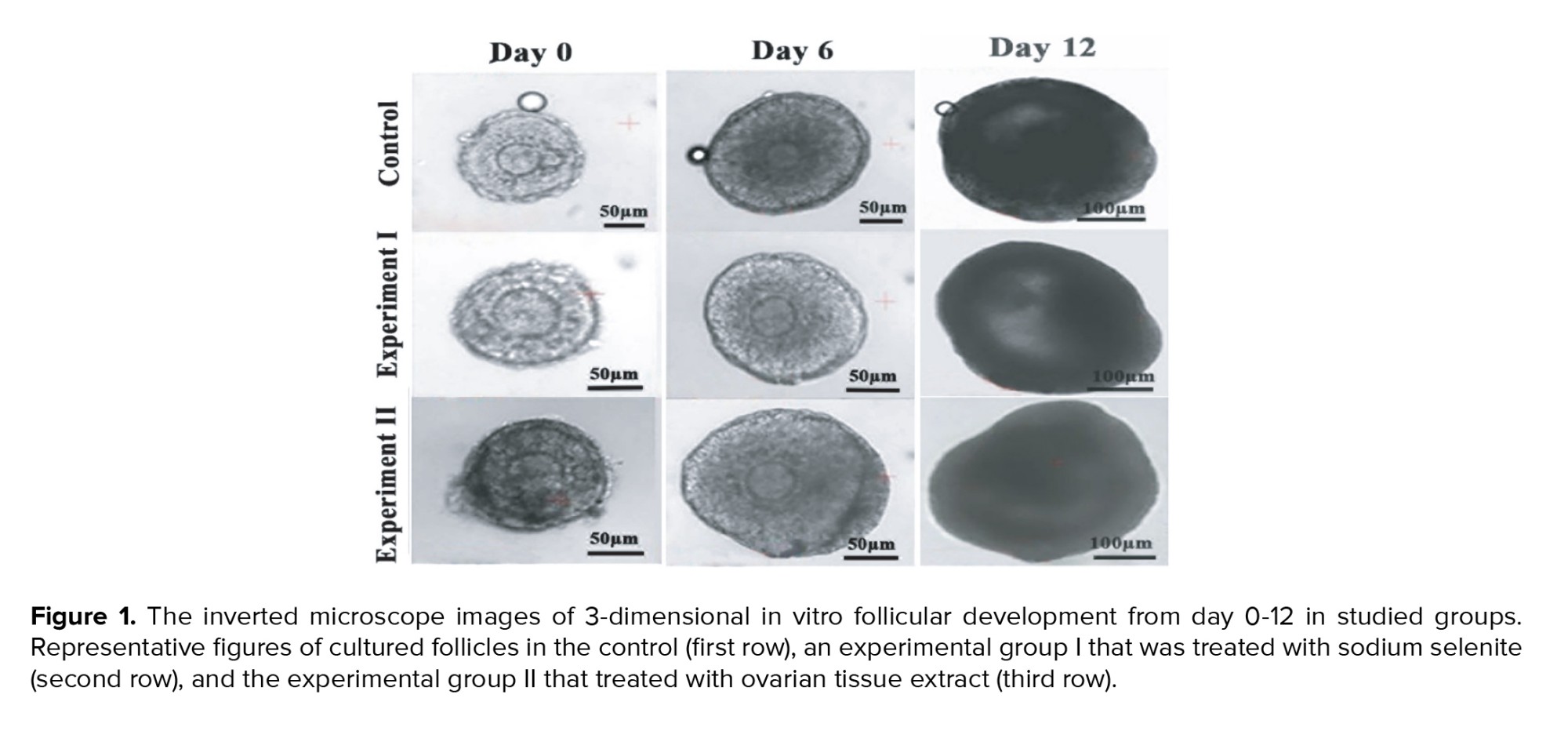
Our results showed the survival, growth, and developmental rates of cultured follicles in 2 experimental groups that were treated with SS or OTE were higher than the control group, and these results revealed that the positive effects of these components on the follicular development.
In agreement with our results, several reports demonstrated the positive effects of SS as an antioxidant, proliferative, and maturation factor that improved follicular development and oocyte maturation (4-10). It was reported that a culture medium supplemented with different concentrations of SS (5, 10, and 15 ng/ml SS) promoted mouse in vitro follicular growth and maturation in a 2-dimensional culture system. Moreover, they demonstrated that 10 ng/ml of SS had better effects on the growth and maturation of the follicle (4).
Another study showed that the activity of glutathione peroxidase of oocytes and the DNA integrity of cumulus cells were increased in the presence of SS during IVM of oocytes (6). Selenium as an integral part of the enzyme glutathione peroxidase improves the survival rate of the cells by the decrease in the level of ROS (5).
In the present study, our results also confirm the similar effects of SS for enhancing follicular development in the 3D culture system. These results may be related to the antioxidant effects of SS by reducing the ROS or by reducing cell apoptosis (5, 7, 15-18).
According to the results of another part of this study, it is suggested that the positive effects of OTE to improve follicular and oocyte development may be related to components within this extract, such as several types of growth factors that could enhance the follicular diameters by increasing the number of granulosa and theca cells (11, 12). These factors are directly and indirectly involved in follicular growth and development. An increase in the number of follicular cells resulted in progress in follicular size that was demonstrated in both experimental groups during the culture period. It is suggested that SS as a proliferative factor and an antioxidant reagent enhances follicular development, while the OTE based on its component that mimics the natural condition and ovarian microenvironment showed the same effect.
However, this effect is more prominent in SS-supplemented group. One explanation for these results is that the presence of some additional components in OTE that are remained or produced during its preparation, interferes with the follicular development. Moreover, in the present study, we did not assay and analyze the content of OTE based on some limitations, thus more additional studies are needed to prove the mentioned suggestions.
The functional potential of granulosa and theca cells can be evaluated by measuring estradiol and progesterone production, and our observations showed that the levels of these hormones were increased in the culture media in both experimental groups in comparison with the control group. However, it was demonstrated that SS by stimulation of estradiol production could support in vitro and in vivo folliculogenesis (19). Also, it is suggested that the similar effect of OTE produces estradiol and progesterone by granulosa and theca cells; however, further study is needed to prove this suggestion.
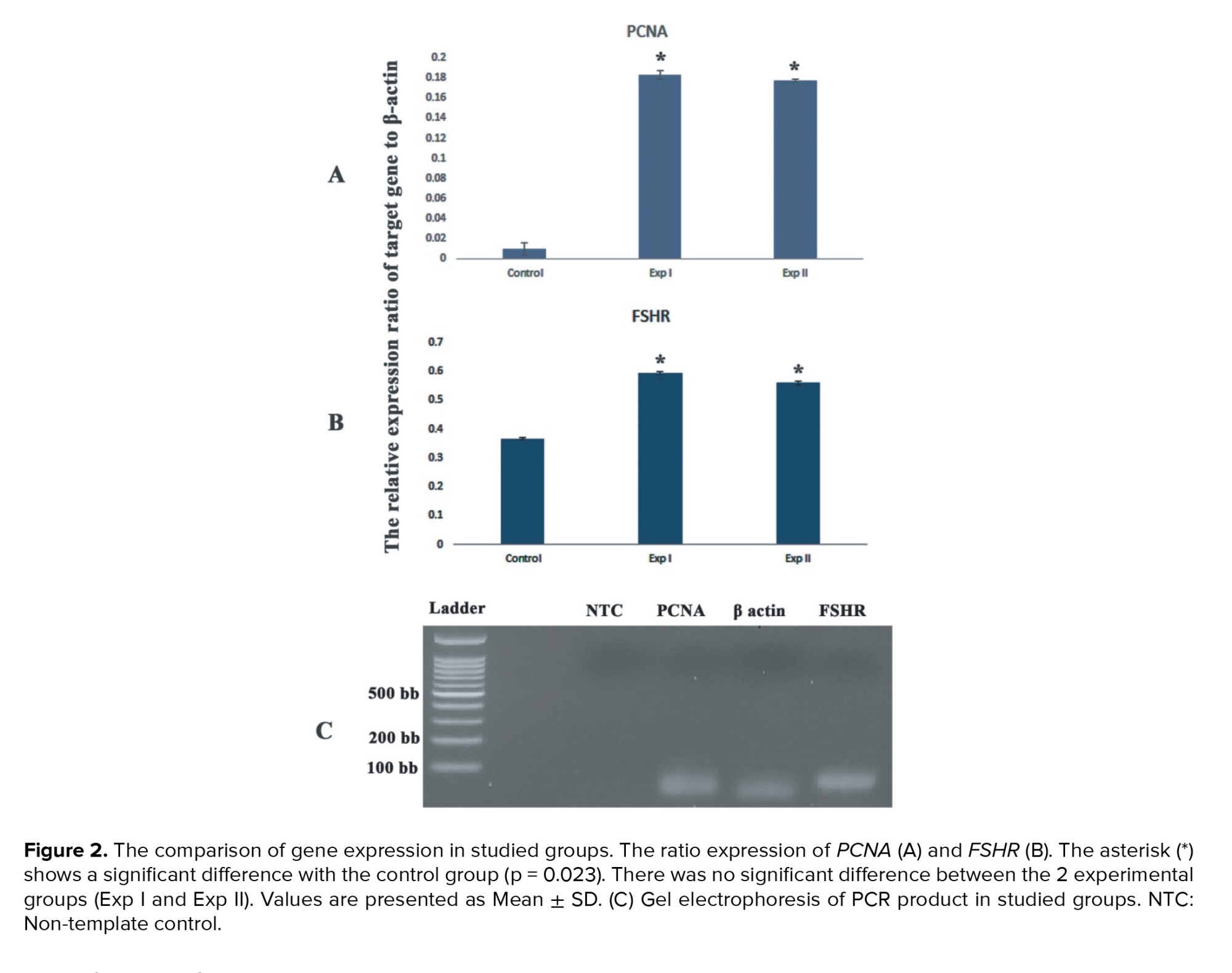
Moreover, the molecular analysis in our study confirmed this suggestion and in this part of the study, we have evaluated the expression of 2 maturation markers in cultured follicles including PCNA and FSHR genes. As the follicular development proceed, the expression and production of these proteins was increased and could be a good indicator of follicular growth (20-22). However, in the present study, our results showed the treatment of follicles in vitro by SS and OTE led to increased expression of PCNA, and FSHR genes. This observation confirmed again the beneficial effects of SS and OTE on the in vitro maturation of follicles.
A similar effect of SS was reported in an in vivo study by investigating the effects of SS on radiation-induced rat ovaries, which showed PCNA expression markedly enhanced in the irradiated rats that were treated with SS in comparison with the non-treated once (23). From the other point of view, FSHR is a G-protein coupled with 7 transmembrane domain receptors and is expressed in the granulosa cells (24). Moreover, the nuclear and cytoplasmic maturation of oocytes could be controlled by the content of OTE via the expression of maturation genes such as FSHR. FSHR is necessary for follicular development, and is also responsible to produce androgens (25). It has been reported that when the transition from the primordial to primary follicle occurred, FSHR expresses progressively (26). The presence of FSHR in the early follicular stages has an indirect effect on follicular development via paracrine factors released by ovarian stromal cells (24, 27).
Acknowledgments
This work was supported by Tarbiat Modares University of Medical Sciences, Tehran, Iran to conduct M.Sc. thesis.
Conflict of Interest
The authors declare that there is no conflict of interest.

2.10. Ethical considerations
The animals were used and cared for according to the Ethical Guidelines of Tarbiat Modares University. The Ethics Committee for Animal Research of the Tarbiat Modares University, Tehran, Iran, approved this study (Code: IR.TMU.REC.1394.272).
2.11. Statistical analysis
Statistical analysis was conducted by SPSS 18 software (SPSS Inc., Chicago, USA). Quantitative variables were expressed as Mean ± SD. The survival rate, developmental rate, production of hormones, and results of real-time RT-PCR, were compared by one-way ANOVA, and Tukey's HSD was used as post hoc test. P < 0.05 was considered to be statistically significant.3. Results
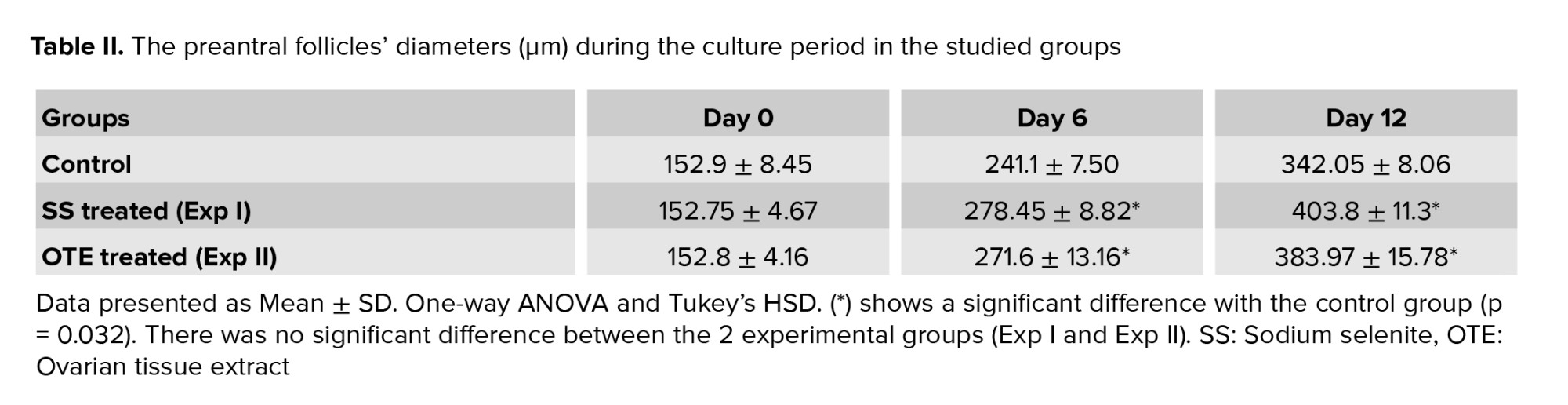
3.1. The diameter of cultured isolated preantral follicles
The morphology of cultured follicles in 3 studied groups under the inverted microscope is demonstrated in figure 1. During the culture period, the follicular size showed a remarkable increase, and their diameter changes during the culture period are summarized and compared in table II. No significant difference was observed in follicular diameters in all studied groups at the beginning of the culture. Moreover, on day 12 of the culture period, these ranges were significantly increased in all groups, and the mean diameter of culture follicles in the 2 experimental groups was significantly higher in comparison with the control group (p = 0.032). However, this mentioned parameter was higher in the SS-treated group (p = 0.019).3.2. Follicular developmental rate
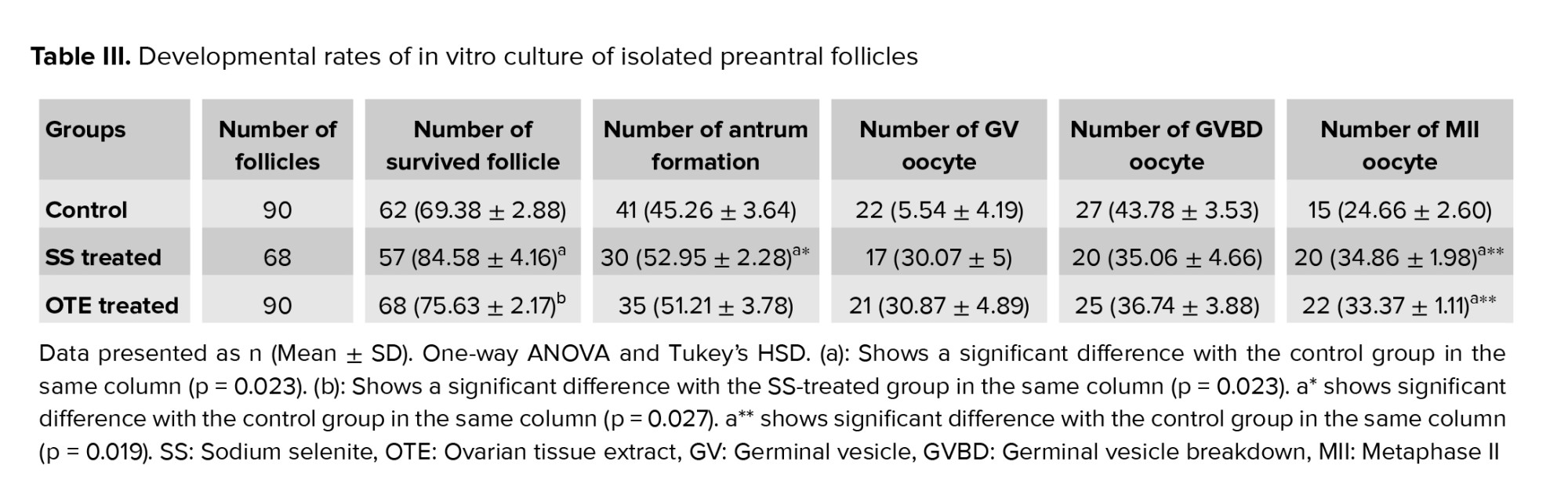
The survival and antrum formation rates in follicles and the rate of released MII oocytes are summarized in table III. At the end of the culture period, amongst the studied follicles in control, SS, and OTE-supplemented groups, the survival rates were found to be higher in SS supplemented group (p = 0.032). In the control group the percentages of antrum formation and the rate of released MII oocytes were significantly lower than in other studied groups (p = 0.027); but there was no statistical difference between SS and OTE groups.3.3. Hormonal assay
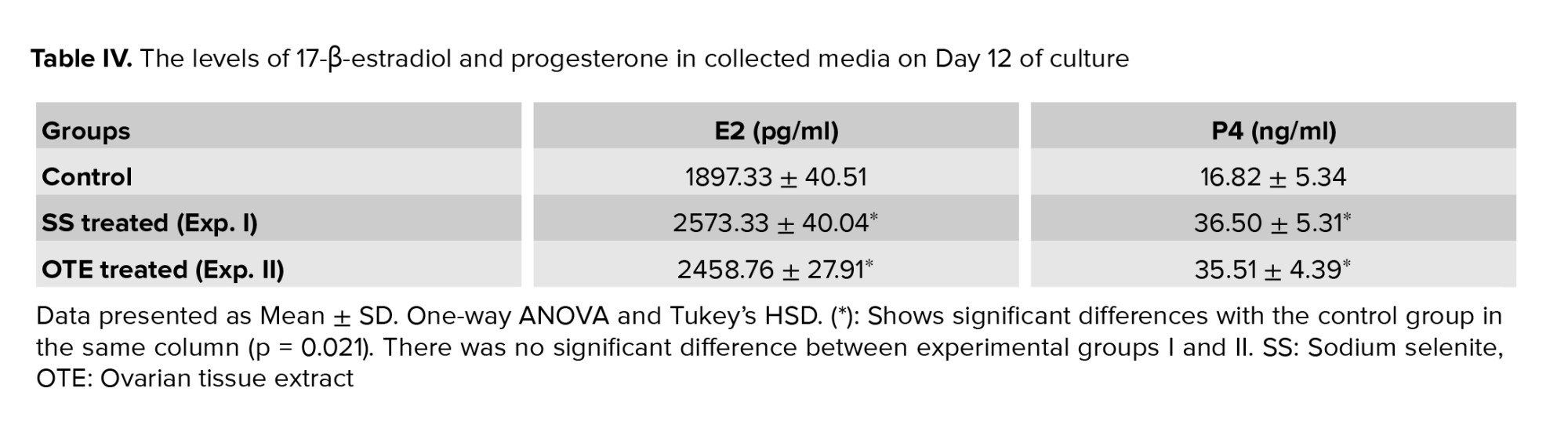
The levels of estradiol and progesterone in the group that was treated with SS and those treated with OTE were increased (p = 0.021) but there was no significant difference between these groups in this regard (Table IV).3.4. The expression of PCNA and FSHR genes
The mRNA levels of PCNA and FSHR genes were evaluated by real-time RT-PCR. The expression ratio of these target genes relative to the housekeeping (β-actin) gene and the representative figure of PCR product electrophoresis are shown in figures 2 A and B. This figure shows the expression of these studied genes was lower in the control group in comparison with both experimental groups (p = 0.023). Also, the representative figure of gel electrophoresis of PCR products in studied groups was demonstrated in figure 2C.





4. Discussion
The present study aimed to evaluate the effect of OTE obtained from the adult mouse on preantral follicular growth, development, and gene expression. For better evaluation of this effect, in another set of experiment, in parallel, the preantral follicles were treated with SS and their development and pattern of gene expression were compared with another experimental and control group.Our results showed the survival, growth, and developmental rates of cultured follicles in 2 experimental groups that were treated with SS or OTE were higher than the control group, and these results revealed that the positive effects of these components on the follicular development.
In agreement with our results, several reports demonstrated the positive effects of SS as an antioxidant, proliferative, and maturation factor that improved follicular development and oocyte maturation (4-10). It was reported that a culture medium supplemented with different concentrations of SS (5, 10, and 15 ng/ml SS) promoted mouse in vitro follicular growth and maturation in a 2-dimensional culture system. Moreover, they demonstrated that 10 ng/ml of SS had better effects on the growth and maturation of the follicle (4).
Another study showed that the activity of glutathione peroxidase of oocytes and the DNA integrity of cumulus cells were increased in the presence of SS during IVM of oocytes (6). Selenium as an integral part of the enzyme glutathione peroxidase improves the survival rate of the cells by the decrease in the level of ROS (5).
In the present study, our results also confirm the similar effects of SS for enhancing follicular development in the 3D culture system. These results may be related to the antioxidant effects of SS by reducing the ROS or by reducing cell apoptosis (5, 7, 15-18).
According to the results of another part of this study, it is suggested that the positive effects of OTE to improve follicular and oocyte development may be related to components within this extract, such as several types of growth factors that could enhance the follicular diameters by increasing the number of granulosa and theca cells (11, 12). These factors are directly and indirectly involved in follicular growth and development. An increase in the number of follicular cells resulted in progress in follicular size that was demonstrated in both experimental groups during the culture period. It is suggested that SS as a proliferative factor and an antioxidant reagent enhances follicular development, while the OTE based on its component that mimics the natural condition and ovarian microenvironment showed the same effect.
However, this effect is more prominent in SS-supplemented group. One explanation for these results is that the presence of some additional components in OTE that are remained or produced during its preparation, interferes with the follicular development. Moreover, in the present study, we did not assay and analyze the content of OTE based on some limitations, thus more additional studies are needed to prove the mentioned suggestions.
The functional potential of granulosa and theca cells can be evaluated by measuring estradiol and progesterone production, and our observations showed that the levels of these hormones were increased in the culture media in both experimental groups in comparison with the control group. However, it was demonstrated that SS by stimulation of estradiol production could support in vitro and in vivo folliculogenesis (19). Also, it is suggested that the similar effect of OTE produces estradiol and progesterone by granulosa and theca cells; however, further study is needed to prove this suggestion.
Moreover, the molecular analysis in our study confirmed this suggestion and in this part of the study, we have evaluated the expression of 2 maturation markers in cultured follicles including PCNA and FSHR genes. As the follicular development proceed, the expression and production of these proteins was increased and could be a good indicator of follicular growth (20-22). However, in the present study, our results showed the treatment of follicles in vitro by SS and OTE led to increased expression of PCNA, and FSHR genes. This observation confirmed again the beneficial effects of SS and OTE on the in vitro maturation of follicles.
A similar effect of SS was reported in an in vivo study by investigating the effects of SS on radiation-induced rat ovaries, which showed PCNA expression markedly enhanced in the irradiated rats that were treated with SS in comparison with the non-treated once (23). From the other point of view, FSHR is a G-protein coupled with 7 transmembrane domain receptors and is expressed in the granulosa cells (24). Moreover, the nuclear and cytoplasmic maturation of oocytes could be controlled by the content of OTE via the expression of maturation genes such as FSHR. FSHR is necessary for follicular development, and is also responsible to produce androgens (25). It has been reported that when the transition from the primordial to primary follicle occurred, FSHR expresses progressively (26). The presence of FSHR in the early follicular stages has an indirect effect on follicular development via paracrine factors released by ovarian stromal cells (24, 27).
5. Conclusion
The OTE and SS have a positive effect on in vitro growth and maturation of mouse preantral follicles via over-expression of FSHR and PCNA genes. Acknowledgments
This work was supported by Tarbiat Modares University of Medical Sciences, Tehran, Iran to conduct M.Sc. thesis.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Vo KCT, Kawamura K. In vitro activation early follicles: From the basic science to the clinical perspectives. Int J Mol Sci 2021; 22: 3785. [DOI:10.3390/ijms22073785] [PMID] [PMCID]
2. Gershon E, Dekel N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int J Mol Sci 2020; 21: 4565. [DOI:10.3390/ijms21124565] [PMID] [PMCID]
3. Yang Q, Zhu L, Jin L. Human follicle in vitro culture including activation, growth, and maturation: A review of research progress. Front Endocrinol (Lausanne) 2020; 11: 548. [DOI:10.3389/fendo.2020.00548] [PMID] [PMCID]
4. Hariharan S, Dharmaraj S. Selenium and selenoproteins: It's role in regulation of inflammation. Inflammopharmacol 2020; 28: 667-695. [DOI:10.1007/s10787-020-00690-x] [PMID] [PMCID]
5. Abedelahi A, Salehnia M, Allameh AA, Davoodi D. Sodium selenite improves the in vitro follicular development by reducing the reactive oxygen species level and increasing the total antioxidant capacity and glutathione peroxide activity. Hum Reprod 2010; 25: 977-985. [DOI:10.1093/humrep/deq002] [PMID]
6. Xiong X, Lan D, Li J, Lin Y, Li M. Selenium supplementation during in vitro maturation enhances meiosis and developmental capacity of yak oocytes. Anim Sci J 2018; 89: 298-306. [DOI:10.1111/asj.12894] [PMID]
7. Ghorbanmehr N, Salehnia M, Amooshahi M. The effects of sodium selenite on mitochondrial DNA copy number and reactive oxygen species levels of in vitro matured mouse oocytes. Cell J 2018; 20: 396-402.
8. Lizarraga RM, Anchordoquy JM, Galarza EM, Farnetano NA, Carranza-Martin A, Furnus CC, et al. Sodium selenite improves in vitro maturation of bos primigenius taurus oocytes. Biol Trace Elem Res 2020; 197: 149-158. [DOI:10.1007/s12011-019-01966-2] [PMID]
9. Malekzadeh Kebria M, Salehnia M, Zavareh S, Moazzeni SS. Effect of sodium selenite on the apoptotic genes expression in in vitro matured mouse metaphase II oocyte. Vet Res Forum 2020; 11: 377-383.
10. Moshaashaee T, Zavareh S, Pourbeiranvand Sh, Salehnia M. The effect of sodium selenite on expression of mitochondrial transcription factor A during in vitro maturation of mouse oocyte. Avicenna J Med Biotechnol 2021; 13: 81-86. [DOI:10.18502/ajmb.v13i2.5526] [PMID] [PMCID]
11. Poormoosavi SM, Behmanesh MA, Najafzadeh Varzi H, Mansouri Sh, Janati S. The effect of follicular fluid selenium concentration on oocyte maturation in women with polycystic ovary syndrome undergoing in vitro fertilization/Intracytoplasmic sperm injection: A cross-sectional study. Int J Reprod BioMed 2021; 19: 689-698. [DOI:10.18502/ijrm.v19i8.9616] [PMID] [PMCID]
12. Devesa J, Caicedo D. The role of growth hormone on ovarian functioning and ovarian angiogenesis. Front Endocrinol (Lausanne) 2019; 10: 450. [DOI:10.3389/fendo.2019.00450] [PMID] [PMCID]
13. Da Broi MG, Giorgi VSI, Wang F, Keefe DL, Albertini D, Navarro PA. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J Assist Reprod Genet 2018; 35: 735-751. [DOI:10.1007/s10815-018-1143-3] [PMID] [PMCID]
14. Shoorei H, Salehnia M, Abdi Sh. [The effects of different concentrations of ovarian tissue extract on preantral mousefollicle development after three-dimensional culture.] Pathobiol Res 2017; 19: 41-53. (in Persian)
15. Barchielli G, Capperucci A, Tanini D. The role of selenium in pathologies: An updated review. Antioxidants (Basel) 2022; 11: 251. [DOI:10.3390/antiox11020251] [PMID] [PMCID]
16. Ren Z, Fan Y, Zhang Z, Chen C, Chen C, Wang X, et al. Sodium selenite inhibits deoxynivalenol-induced injury in GPX1-knockdown porcine splenic lymphocytes in culture. Sci Rep 2018; 8: 17676. [DOI:10.1038/s41598-018-36149-x] [PMID] [PMCID]
17. Colle D, Santos DB, de Souza V, Lopes MW, Leal RB, de Souza Brocardo P, et al. Sodium selenite protects from 3-nitropropionic acid-induced oxidative stress in cultured primary cortical neurons. Mol Biol Rep 2019; 46: 751-762. [DOI:10.1007/s11033-018-4531-y] [PMID]
18. Ma Y-M, Ibeanu G, Wang L-Y, Zhang J-Z, Chang Y, Dong J-D, et al. Selenium suppresses glutamate-induced cell death and prevents mitochondrial morphological dynamic alterations in hippocampal HT22 neuronal cells. BMC Neurosci 2017; 18: 15. [DOI:10.1186/s12868-017-0337-4] [PMID] [PMCID]
19. Mojadadi A, Au A, Salah W, Witting P, Ahmad G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients 2021; 13: 3256. [DOI:10.3390/nu13093256] [PMID] [PMCID]
20. González-Magaña A, Blanco FJ. Human PCNA structure, function, and interactions. Biomolecules 2020; 10: 570. [DOI:10.3390/biom10040570] [PMID] [PMCID]
21. Vatanparast M, Karimi Zarchi M, Nabi A, Khalili MA. Proliferating cell nuclear antigen presentation, as a marker of folliculogenesis, in the transplanted ovarian tissue. J Obstet Gynaecol Res 2021; 47: 4340-4349. [DOI:10.1111/jog.15022] [PMID]
22. Rowe E, Van Horn A, Rockwell LC. CYP17 genotype modifies the impact of anthropometric variation on salivary estradiol in healthy women. Am J Phys Anthropol 2015; 156: 665-670. [DOI:10.1002/ajpa.22676] [PMID]
23. Said RS, Nada AS, El-Demerdash E. Sodium selenite improves folliculogenesis in radiation-induced ovarian failure: A mechanistic approach. PloS One 2012; 7: e50928. [DOI:10.1371/journal.pone.0050928] [PMID] [PMCID]
24. Haldar S, Agrawal H, Saha S, Straughn AR, Roy P, Kakar SS. Overview of follicle stimulating hormone and its receptors in reproduction and in stem cells and cancer stem cells. Int J Biol Sci 2022; 18: 675-692. [DOI:10.7150/ijbs.63721] [PMID] [PMCID]
25. Banerjee AA, Mahale SD. Role of the extracellular and intracellular loops of follicle-stimulating hormone receptor in its function. Front Endocrinol (Lausanne) 2015; 6: 110. [DOI:10.3389/fendo.2015.00110] [PMID] [PMCID]
26. Scarlet D, Walter I, Hlavaty J, Aurich C. Expression and immunolocalisation of follicle-stimulating hormone receptors in gonads of newborn and adult female horses. Reprod Fertil Dev 2015. Online ahead of print. [DOI:10.1071/RD14392] [PMID]
27. Liu Y-X, Zhang Y, Li Y-Y, Liu X-M, Wang X-X, Zhang C-L, et al. Regulation of follicular development and differentiation by intra-ovarian factors and endocrine hormones. Front Biosci (Landmark Ed) 2019; 24: 983-993. [DOI:10.2741/4763] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







