Sat, Feb 21, 2026
[Archive]
Volume 21, Issue 10 (October 2023)
IJRM 2023, 21(10): 801-808 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Golkar S, Chekini Z, Amjadi F S, Afsharian P, Najafian A, Ghaffari F et al . Expressions of vascular endothelial growth factor A, mucin-1, colony-stimulating factor-1, heparin-binding epidermal growth factor-like growth factor, and fibroblast growth factor 2 genes in the female reproductive tracts of women with ectopic pregnancy: A case-control study. IJRM 2023; 21 (10) :801-808
URL: http://ijrm.ir/article-1-2633-en.html
URL: http://ijrm.ir/article-1-2633-en.html
Sima Golkar1 

 , Zahra Chekini2
, Zahra Chekini2 

 , Fatemeh sadat Amjadi3
, Fatemeh sadat Amjadi3 

 , Parvaneh Afsharian4
, Parvaneh Afsharian4 

 , Aida Najafian5
, Aida Najafian5 

 , Firouzeh Ghaffari *6
, Firouzeh Ghaffari *6 

 , Reza Aflatoonian2
, Reza Aflatoonian2 




 , Zahra Chekini2
, Zahra Chekini2 

 , Fatemeh sadat Amjadi3
, Fatemeh sadat Amjadi3 

 , Parvaneh Afsharian4
, Parvaneh Afsharian4 

 , Aida Najafian5
, Aida Najafian5 

 , Firouzeh Ghaffari *6
, Firouzeh Ghaffari *6 

 , Reza Aflatoonian2
, Reza Aflatoonian2 


1- Department of Genetics Science, Faculty of Basic Sciences and Advanced Technologies in Biology, University of Science and Culture, ACECR, Tehran, Iran. Department of Endocrinology and Female Infertility, Reproductive Biomedicine Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
2- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
3- Department of Anatomy, School of Medicine, Iran University of Medical Science, Tehran, Iran.
4- Department of Genetics, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
5- Department of Infertility, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
6- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran. ,ghafaryf@yahoo.com
2- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
3- Department of Anatomy, School of Medicine, Iran University of Medical Science, Tehran, Iran.
4- Department of Genetics, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran.
5- Department of Infertility, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
6- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran. ,
Keywords: Ectopic pregnancy, Gene expression, Vascular endothelial growth factor A, Mucin-1, Colony-stimulating factor-1, Heparin-binding epidermal growth factor-like growth factor, Fibroblast growth factor 2.
Full-Text [PDF 428 kb]
(953 Downloads)
| Abstract (HTML) (1303 Views)
1. Introduction
Ectopic pregnancy (EP) is the implantation of an embryo somewhere other than the uterine cavity. More than 98% of EPs occur in the fallopian tube (1, 2). EP has become the main cause of maternal morbidity and mortality in the first trimester of pregnancy (3, 4). There is no clear understanding of the molecular mechanisms responsible for EP (1). However, several researchers have suggested the embryo’s arrest within the fallopian tube due to impaired tubal transport as a result of tubal microenvironment alterations leading to inappropriate implantation (1, 5). Several genes have been identified that could affect the tubal environment as well as play a role in endometrial receptivity. These genes include vascular endothelial growth factor A (VEGF-A), Mucin-1 (MUC1), colony-stimulating factor-1 (CSF1), heparin-binding epidermal growth factor-like growth factor (HBEGF), and fibroblast growth factor 2 (FGF2) (6-10).
Angiogenesis is one of the most important aspects of successful implantation (11). The results of previous studies have shown that VEGF-A, as a potent angiogenic factor, has a significantly lower expression in the fallopian tubes of the EP cases (2, 12). MUC1 acts as an epithelial apical surface glycoprotein, which should be removed for successful interaction between blastocyst and endometrium during implantation (13, 14). High levels of MUC1 inhibit cell-cell adhesion, controversy its lower expression leading to EP compared to pseudopregnant women (6, 13, 14). CSF1 is a regulator of macrophage growth and differentiation that plays a significant role in reproduction and pregnancy (15, 16).
HBEGF is a molecular mediator which signals between the endometrium and trophoblast cells. The study results have demonstrated that HBEGF might play role in the attachment and penetration steps of embryo implantation and could be a helpful marker for the implantation window (10). FGF2 is one member of the FGF family, which are multifunctional regulators of different cellular processes including differentiation, migration, and growth (9). Previous investigations have shown that endometrial expression of FGF2 did not change between days 1 and 12 of pregnancy; however, the expression was higher in the proliferative phase rather than the secretory phase (8).
Here, we evaluated the expression of the VEGF-A, MUC1, CSF1, HBEGF, and FGF2 genes in the fallopian tubes and endometria of women with EP in order to investigate the role of genes in the decreasing the endometrial receptivity and increasing the ectopic embryo implantation in the fallopian tube.
2. Materials and Methods
2.1. Subjects
In this case-control study, 5 women who underwent salpingectomy due to EP comprised the case group. These participants referred to the Royan Institute, and Shariati, and Arash hospital, Tehran, Iran during July 2019-August 2021. The ampulla region of the fallopian tube and endometrium were obtained from each case for further investigation. For the control group, as our team previously described, we obtained tissues from 5 cases that underwent hysterectomies due to benign gynecological situations without affect the tubes. The control group were fertile women with regular menstrual cycles, and no evidence of any pathological tubal disorders. To induce normal pregnancy hormonal conditions, we created a state of pseudo-pregnancy according to the previous study (2). This procedure is harmless for these women, as previously stated by other studies (17, 18). All EP cases included the following criteria, age 20-37 yr, regular menstruation, and normal body mass index. The exclusion criteria were as follows, receiving hormonal drugs for 3 months before surgery, salpingitis, smoking, diagnosis of heterotopic pregnancy with vaginal ultrasound, receiving methotrexate, history of using intrauterine devices, history of previous EP, uterine diseases such as (endometriosis, polycystic ovarian syndrome, uterine anomalies, myoma), history of abdominal surgery, and thyroid disease. We removed at least 1 cm of tissue from the site where the embryo was implanted because to prevent the collection of any embryonic or trophoblastic tissues.
2.2. RNA extraction using TRIzol and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA extraction was performed using TRIzol (TRI Reagent, Sigma, Pool, UK) based on the instructions provided in our previous studies (2, 19). After total RNA extraction, we quantified the RNA concentrations by spectrophotometric analysis to assess the quality of the extracted RNA.
The process of creating the first strand of cDNA involved using oligo (dT) primers and the superscript II reverse transcriptase system (Fermentas, Sankt Leon-rot, Germany). To synthesize the cDNA, a master mix was prepared containing 1 µg RNA and 1 µl oligo dT primer with DEPC water in a final volume. Next, we added reaction buffer, dNTP, RNase inhibitor, and reverse transcriptase to the master mix. Reverse transcription was done as follows, incubation for 60 min at 42oC and termination by heating at 70oC for 5 min.
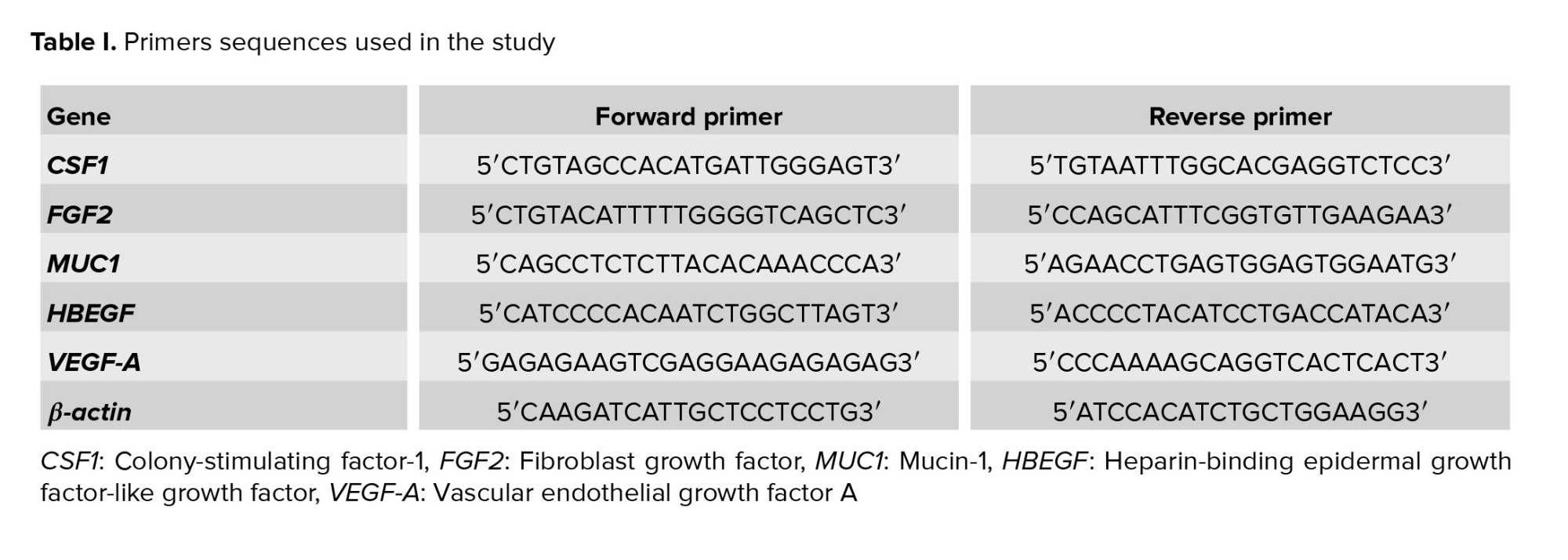
The cDNA was amplified using reverse transcription polymerase chain reaction, and forward and reverse primers of human VEGF-A, MUC1, CSF1, HBEGF, FGF2, and β-actin (Metabion, Martinsried, Germany), and platinum blue PCR super mix (Invitrogen, Paisley, UK). Table I lists the primer sequences. The β-actin was used as the housekeeping gene. The PCR protocol was as follows: 5 min at 95oC for initial denaturation, 40 cycles for 45 sec at 95oC, 45 sec at 60oC, and 45 sec at 72oC. Non-template water was used as a negative control in the experiment to ensure that any amplification observed in the PCR products was due to the presence of the target gene and not to contamination or other factors. The PCR products were separated by electrophoresis on a 1.7% agarose gel (Sigma, UK).
2.3. Real-time quantitative PCR (RT-qPCR)
Real-time PCR was used to assess the relative expressions of the study genes in the case and control groups. RT-qPCR to compare the levels of mRNA expression of certain genes in the case and control groups. The synthesized cDNA, Power SYBR Green Master Mix (Applied Biosystems, UK), and the primers are listed in table I. To perform real-time PCR, for each reaction, we added 5 μl SYBR green reagents, 11 μl molecular grade water, 1 μl of each of the forward and reverse primers, and 2 μl cDNA. The amplification conditions, such as the annealing temperature and the number of cycles, were likely optimized including: 10 min at 95oC, 40 cycles at 95oC for 15 sec, and 60oC for 60 sec. The RT-qPCR was performed under standard conditions, which included the use of triplicate samples.
The quantities of relative VEGF-A, MUC1, CSF1, HBEGF, and FGF2 expressions were compared between the case and control groups. Real-time PCR data were analyzed using the relative cycle threshold (CT) method. The ΔΔCT value was calculated by determining the difference between the case and control groups, which allows for the normalization of the data to a reference gene.
2.4. Ethical considerations
This study was performed following the Declaration of Helsinki and its latest updates and was approved by the Reproductive Biomedicine Research Center Ethics Committee at Royan Institute, Tehran, Iran (Code: IR.ACECR.ROYAN.REC.1398.072). All participants signed the informed consent forms before participating in the study.
2.5. Statistical analysis
Statistical analysis was performed using SPSS software (version 22; Inc, Chicago, IL, USA). All data were shown as mean ± Standard error (SEM). Normal distribution of the data was evaluated by t2-sample Kolmogorov-Smirnov Test. Data were analyzed using the Mann-Whitney Test for comparing values between case and control groups which were not distributed normally. A p-value < 0.05 was considered statistically significant.
3. Results
We examined relative expressions of VEGF-A, MUC1, FGF2, CSF1, and HBEGF genes in 10 cases (n = 5 per group).
3.1. Real-time PCR assessment of relative gene expressions
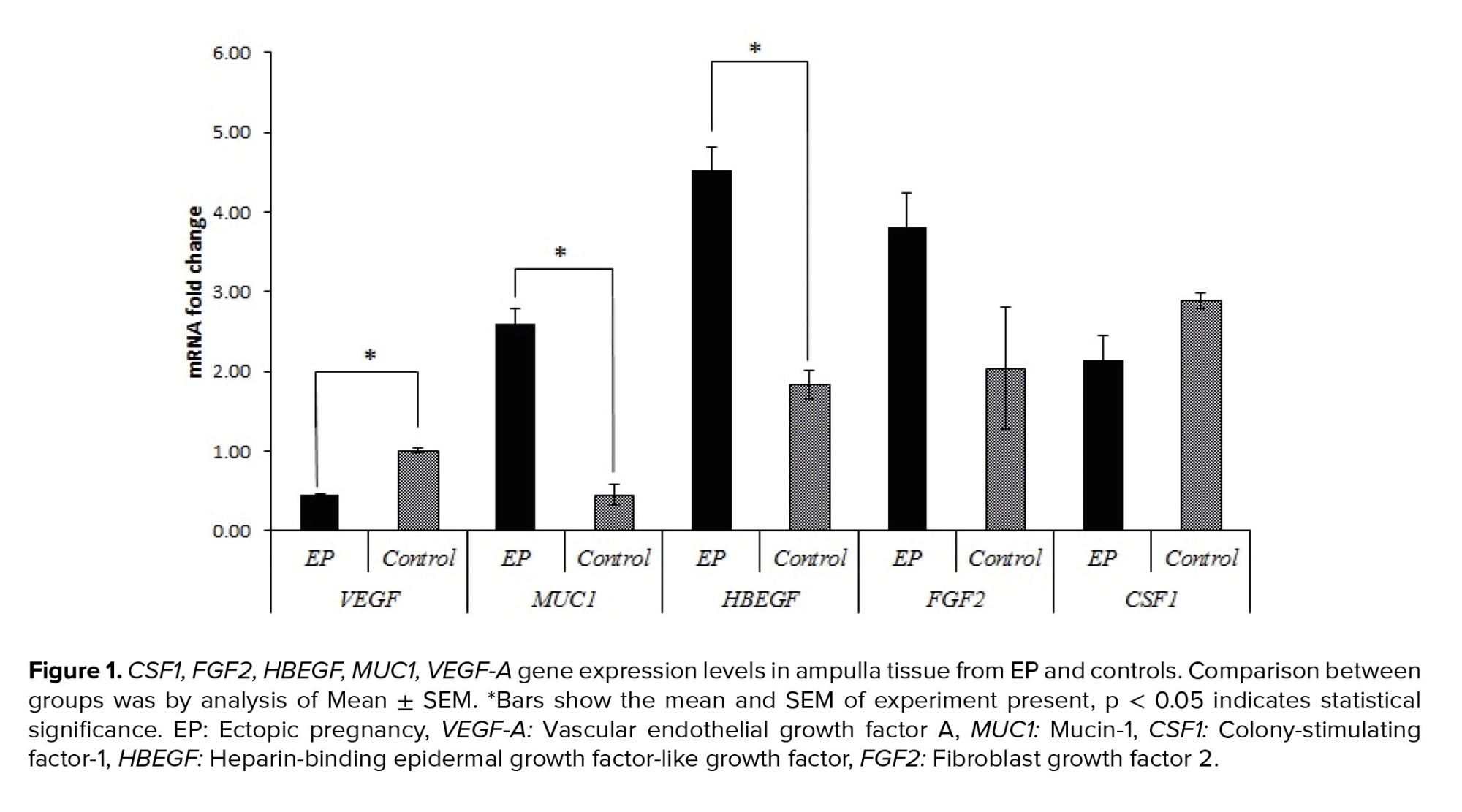
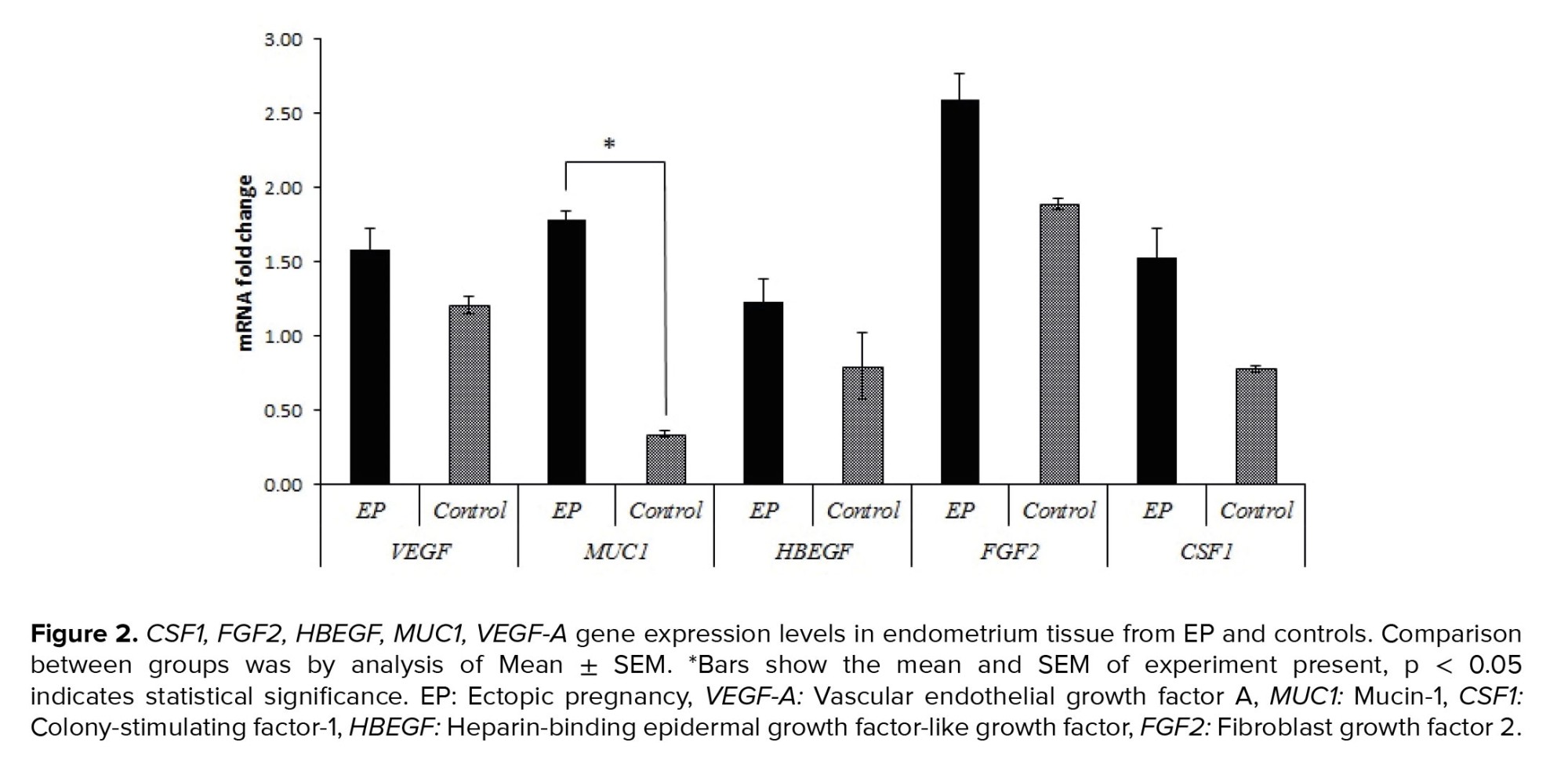
Real-time PCR was used to evaluate the relative gene expressions in the case group compared to the control group. Table II and figures 1 and 2, show the relative expressions of these genes. The results indicated that VEGF-A gene expression in the ampulla region was significantly higher in the control group compared to the case group (p < 0.001). No significant difference was observed in endometrial tissue between the 2 groups (p = 0.082). In terms of the CSF1 and FGF2 gene expressions, no significant differences were observed between the 2 groups in both the ampulla and endometrium (p > 0.05). However, expression of FGF2 in endometrium showed tendency to significant difference (p = 0.057). Furthermore, HBEGF gene expression showed a significantly higher expression in the ampulla region of the case group compared to the control group (p < 0.001), but no significant differences existed between these 2 groups in the endometrium (p = 0.193). The only gene that had significantly different expression in both the ampulla and endometrium between 2 groups in both regions was MUC1, which indicated higher expressions in the case group for both ampulla and endometrium (p < 0.001).

4. Discussion
Based on our results, we have assumed that genes involved in implantation including VEGF-A, MUC1, FGF2, CSF1, and HBEGF might be changed in the endometrium and fallopian tubes during EP.
Our previous investigation showed that VEGF-A has higher expression in all regions of the fallopian tubes in pseudopregnant women compared to EP cases. The current study results confirmed this finding. We noted significantly increased VEGF-A expression in the fallopian tube of the control group compared with the case group. The decreased expression of VEGF-A in EP cases could be due to the effect of hCG on the expressions of VEGF and its receptors (2). We could not find any data that evaluated the expression of VEGF-A in the endometrial tissue of women with EP. Our data showed no significant difference in VEGF-A expression between the 2 groups; however, a slightly higher expression was observed in the EP group in comparison with the control group.
Next, we evaluated the MUC1 expression, which had a significantly lower expression in the fallopian tubes of women with EP compared to normal fallopian tubes (19, 20). Another study on EP cases and pseudopregnant women indicated that MUC1 was localized at the apical surface of the tubal epithelium in both the EP and pseudopregnant groups. These authors also reported significantly lower MUC1 expression in the EP group (6). A later study confirmed significantly decreased MUC1 expression in the fallopian tubes of women with EP (14). Interestingly, the results of another study indicated that MUC1 had higher expression in EP cases (6). These findings could be due to the larger sample size in their study. MUC1 has an important role in endometrial receptivity. There is decreased MUC1 expression in women with recurrent implantation failure compared with normal women (14). Our data showed significantly higher MUC1 expression in both the ampulla and endometria of EP cases compared with controls.
The role of CSF1 in EP has been investigated. Inflammation is a potential cause of EP, and it was found that macrophages might contribute to the regulation of tubal motility. Decreased expression of CSF1, a cytokine that stimulates macrophage production, could cause disruptions in tubal motility, and be involved in EP pathology (15, 21). In the current study, we observed decreased expression of CFS1 in the ampulla region of the fallopian tubes of EP cases when compared with the controls. In contrast, expression was higher in the endometrial tissue of EP cases compared to controls. However, none of these differences were significant, which might be due to the sample size. Statistical analyses indicated that in larger populations, the difference would become significant.
HBEGF is one of the earliest identified molecular mediators of blastocyst implantation and it might play a role in the attachment and penetration steps of embryo implantation (5, 10). We assumed that the expression of this gene could be directly related to a proper implantation process. The results showed a significant increase in HBEGF expression in the fallopian tubes of EP cases compared to the controls; however, no difference was observed in the endometria. These findings strongly suggested that the expression pattern of HBEGF has a direct impact on implantation.
No significant difference was observed between case and control groups in terms of FGF2 expression; however, the expressions in both regions from EP cases were higher than the controls. It has been reported that FGF2 affects endometrium receptivity and proper implantation (8, 9).
5. Conclusion
Our results have shown new evidence about the role of VEGF-A, MUC1, FGF2, CSF1, and HBEGF genes in EP. This is the first study that compares expressions of these genes in both fallopian tube tissue and endometrial tissue. The results strongly suggested that these genes play important roles in proper embryo implantation, and disruptions in their expression patterns could lead to EP. However, more studies should be conducted to confirm the current findings, especially for CSF1. Also, an evaluation of the expression patterns in larger populations is recommended to confirm these results.
Acknowledgments
We thank Mr Khashayar Aflatoonian for expert assistance throughout the study and his support for manuscript preparation. We are grateful to the surgeon and staff of the operating room of Arash hospital for sample collection. This study was supported by a grant (No. 97000007) from Royan Institute, Reproductive Biomedicine Research Center, Tehran, Iran.
Conflict of Interest
All authors declare that there is no conflict of interest.
Full-Text: (228 Views)
1. Introduction
Ectopic pregnancy (EP) is the implantation of an embryo somewhere other than the uterine cavity. More than 98% of EPs occur in the fallopian tube (1, 2). EP has become the main cause of maternal morbidity and mortality in the first trimester of pregnancy (3, 4). There is no clear understanding of the molecular mechanisms responsible for EP (1). However, several researchers have suggested the embryo’s arrest within the fallopian tube due to impaired tubal transport as a result of tubal microenvironment alterations leading to inappropriate implantation (1, 5). Several genes have been identified that could affect the tubal environment as well as play a role in endometrial receptivity. These genes include vascular endothelial growth factor A (VEGF-A), Mucin-1 (MUC1), colony-stimulating factor-1 (CSF1), heparin-binding epidermal growth factor-like growth factor (HBEGF), and fibroblast growth factor 2 (FGF2) (6-10).
Angiogenesis is one of the most important aspects of successful implantation (11). The results of previous studies have shown that VEGF-A, as a potent angiogenic factor, has a significantly lower expression in the fallopian tubes of the EP cases (2, 12). MUC1 acts as an epithelial apical surface glycoprotein, which should be removed for successful interaction between blastocyst and endometrium during implantation (13, 14). High levels of MUC1 inhibit cell-cell adhesion, controversy its lower expression leading to EP compared to pseudopregnant women (6, 13, 14). CSF1 is a regulator of macrophage growth and differentiation that plays a significant role in reproduction and pregnancy (15, 16).
HBEGF is a molecular mediator which signals between the endometrium and trophoblast cells. The study results have demonstrated that HBEGF might play role in the attachment and penetration steps of embryo implantation and could be a helpful marker for the implantation window (10). FGF2 is one member of the FGF family, which are multifunctional regulators of different cellular processes including differentiation, migration, and growth (9). Previous investigations have shown that endometrial expression of FGF2 did not change between days 1 and 12 of pregnancy; however, the expression was higher in the proliferative phase rather than the secretory phase (8).
Here, we evaluated the expression of the VEGF-A, MUC1, CSF1, HBEGF, and FGF2 genes in the fallopian tubes and endometria of women with EP in order to investigate the role of genes in the decreasing the endometrial receptivity and increasing the ectopic embryo implantation in the fallopian tube.
2. Materials and Methods
2.1. Subjects
In this case-control study, 5 women who underwent salpingectomy due to EP comprised the case group. These participants referred to the Royan Institute, and Shariati, and Arash hospital, Tehran, Iran during July 2019-August 2021. The ampulla region of the fallopian tube and endometrium were obtained from each case for further investigation. For the control group, as our team previously described, we obtained tissues from 5 cases that underwent hysterectomies due to benign gynecological situations without affect the tubes. The control group were fertile women with regular menstrual cycles, and no evidence of any pathological tubal disorders. To induce normal pregnancy hormonal conditions, we created a state of pseudo-pregnancy according to the previous study (2). This procedure is harmless for these women, as previously stated by other studies (17, 18). All EP cases included the following criteria, age 20-37 yr, regular menstruation, and normal body mass index. The exclusion criteria were as follows, receiving hormonal drugs for 3 months before surgery, salpingitis, smoking, diagnosis of heterotopic pregnancy with vaginal ultrasound, receiving methotrexate, history of using intrauterine devices, history of previous EP, uterine diseases such as (endometriosis, polycystic ovarian syndrome, uterine anomalies, myoma), history of abdominal surgery, and thyroid disease. We removed at least 1 cm of tissue from the site where the embryo was implanted because to prevent the collection of any embryonic or trophoblastic tissues.
2.2. RNA extraction using TRIzol and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA extraction was performed using TRIzol (TRI Reagent, Sigma, Pool, UK) based on the instructions provided in our previous studies (2, 19). After total RNA extraction, we quantified the RNA concentrations by spectrophotometric analysis to assess the quality of the extracted RNA.
The process of creating the first strand of cDNA involved using oligo (dT) primers and the superscript II reverse transcriptase system (Fermentas, Sankt Leon-rot, Germany). To synthesize the cDNA, a master mix was prepared containing 1 µg RNA and 1 µl oligo dT primer with DEPC water in a final volume. Next, we added reaction buffer, dNTP, RNase inhibitor, and reverse transcriptase to the master mix. Reverse transcription was done as follows, incubation for 60 min at 42oC and termination by heating at 70oC for 5 min.
The cDNA was amplified using reverse transcription polymerase chain reaction, and forward and reverse primers of human VEGF-A, MUC1, CSF1, HBEGF, FGF2, and β-actin (Metabion, Martinsried, Germany), and platinum blue PCR super mix (Invitrogen, Paisley, UK). Table I lists the primer sequences. The β-actin was used as the housekeeping gene. The PCR protocol was as follows: 5 min at 95oC for initial denaturation, 40 cycles for 45 sec at 95oC, 45 sec at 60oC, and 45 sec at 72oC. Non-template water was used as a negative control in the experiment to ensure that any amplification observed in the PCR products was due to the presence of the target gene and not to contamination or other factors. The PCR products were separated by electrophoresis on a 1.7% agarose gel (Sigma, UK).
2.3. Real-time quantitative PCR (RT-qPCR)
Real-time PCR was used to assess the relative expressions of the study genes in the case and control groups. RT-qPCR to compare the levels of mRNA expression of certain genes in the case and control groups. The synthesized cDNA, Power SYBR Green Master Mix (Applied Biosystems, UK), and the primers are listed in table I. To perform real-time PCR, for each reaction, we added 5 μl SYBR green reagents, 11 μl molecular grade water, 1 μl of each of the forward and reverse primers, and 2 μl cDNA. The amplification conditions, such as the annealing temperature and the number of cycles, were likely optimized including: 10 min at 95oC, 40 cycles at 95oC for 15 sec, and 60oC for 60 sec. The RT-qPCR was performed under standard conditions, which included the use of triplicate samples.
The quantities of relative VEGF-A, MUC1, CSF1, HBEGF, and FGF2 expressions were compared between the case and control groups. Real-time PCR data were analyzed using the relative cycle threshold (CT) method. The ΔΔCT value was calculated by determining the difference between the case and control groups, which allows for the normalization of the data to a reference gene.

2.4. Ethical considerations
This study was performed following the Declaration of Helsinki and its latest updates and was approved by the Reproductive Biomedicine Research Center Ethics Committee at Royan Institute, Tehran, Iran (Code: IR.ACECR.ROYAN.REC.1398.072). All participants signed the informed consent forms before participating in the study.
2.5. Statistical analysis
Statistical analysis was performed using SPSS software (version 22; Inc, Chicago, IL, USA). All data were shown as mean ± Standard error (SEM). Normal distribution of the data was evaluated by t2-sample Kolmogorov-Smirnov Test. Data were analyzed using the Mann-Whitney Test for comparing values between case and control groups which were not distributed normally. A p-value < 0.05 was considered statistically significant.
3. Results
We examined relative expressions of VEGF-A, MUC1, FGF2, CSF1, and HBEGF genes in 10 cases (n = 5 per group).
3.1. Real-time PCR assessment of relative gene expressions
Real-time PCR was used to evaluate the relative gene expressions in the case group compared to the control group. Table II and figures 1 and 2, show the relative expressions of these genes. The results indicated that VEGF-A gene expression in the ampulla region was significantly higher in the control group compared to the case group (p < 0.001). No significant difference was observed in endometrial tissue between the 2 groups (p = 0.082). In terms of the CSF1 and FGF2 gene expressions, no significant differences were observed between the 2 groups in both the ampulla and endometrium (p > 0.05). However, expression of FGF2 in endometrium showed tendency to significant difference (p = 0.057). Furthermore, HBEGF gene expression showed a significantly higher expression in the ampulla region of the case group compared to the control group (p < 0.001), but no significant differences existed between these 2 groups in the endometrium (p = 0.193). The only gene that had significantly different expression in both the ampulla and endometrium between 2 groups in both regions was MUC1, which indicated higher expressions in the case group for both ampulla and endometrium (p < 0.001).



4. Discussion
Based on our results, we have assumed that genes involved in implantation including VEGF-A, MUC1, FGF2, CSF1, and HBEGF might be changed in the endometrium and fallopian tubes during EP.
Our previous investigation showed that VEGF-A has higher expression in all regions of the fallopian tubes in pseudopregnant women compared to EP cases. The current study results confirmed this finding. We noted significantly increased VEGF-A expression in the fallopian tube of the control group compared with the case group. The decreased expression of VEGF-A in EP cases could be due to the effect of hCG on the expressions of VEGF and its receptors (2). We could not find any data that evaluated the expression of VEGF-A in the endometrial tissue of women with EP. Our data showed no significant difference in VEGF-A expression between the 2 groups; however, a slightly higher expression was observed in the EP group in comparison with the control group.
Next, we evaluated the MUC1 expression, which had a significantly lower expression in the fallopian tubes of women with EP compared to normal fallopian tubes (19, 20). Another study on EP cases and pseudopregnant women indicated that MUC1 was localized at the apical surface of the tubal epithelium in both the EP and pseudopregnant groups. These authors also reported significantly lower MUC1 expression in the EP group (6). A later study confirmed significantly decreased MUC1 expression in the fallopian tubes of women with EP (14). Interestingly, the results of another study indicated that MUC1 had higher expression in EP cases (6). These findings could be due to the larger sample size in their study. MUC1 has an important role in endometrial receptivity. There is decreased MUC1 expression in women with recurrent implantation failure compared with normal women (14). Our data showed significantly higher MUC1 expression in both the ampulla and endometria of EP cases compared with controls.
The role of CSF1 in EP has been investigated. Inflammation is a potential cause of EP, and it was found that macrophages might contribute to the regulation of tubal motility. Decreased expression of CSF1, a cytokine that stimulates macrophage production, could cause disruptions in tubal motility, and be involved in EP pathology (15, 21). In the current study, we observed decreased expression of CFS1 in the ampulla region of the fallopian tubes of EP cases when compared with the controls. In contrast, expression was higher in the endometrial tissue of EP cases compared to controls. However, none of these differences were significant, which might be due to the sample size. Statistical analyses indicated that in larger populations, the difference would become significant.
HBEGF is one of the earliest identified molecular mediators of blastocyst implantation and it might play a role in the attachment and penetration steps of embryo implantation (5, 10). We assumed that the expression of this gene could be directly related to a proper implantation process. The results showed a significant increase in HBEGF expression in the fallopian tubes of EP cases compared to the controls; however, no difference was observed in the endometria. These findings strongly suggested that the expression pattern of HBEGF has a direct impact on implantation.
No significant difference was observed between case and control groups in terms of FGF2 expression; however, the expressions in both regions from EP cases were higher than the controls. It has been reported that FGF2 affects endometrium receptivity and proper implantation (8, 9).
5. Conclusion
Our results have shown new evidence about the role of VEGF-A, MUC1, FGF2, CSF1, and HBEGF genes in EP. This is the first study that compares expressions of these genes in both fallopian tube tissue and endometrial tissue. The results strongly suggested that these genes play important roles in proper embryo implantation, and disruptions in their expression patterns could lead to EP. However, more studies should be conducted to confirm the current findings, especially for CSF1. Also, an evaluation of the expression patterns in larger populations is recommended to confirm these results.
Acknowledgments
We thank Mr Khashayar Aflatoonian for expert assistance throughout the study and his support for manuscript preparation. We are grateful to the surgeon and staff of the operating room of Arash hospital for sample collection. This study was supported by a grant (No. 97000007) from Royan Institute, Reproductive Biomedicine Research Center, Tehran, Iran.
Conflict of Interest
All authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Genetics
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




