Sun, Feb 22, 2026
[Archive]
Volume 21, Issue 5 (May 2023)
IJRM 2023, 21(5): 367-378 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bahreiny S S, Harooni E, Dabbagh M R, Ebrahimi R. Circulating serum preptin levels in women with polycystic ovary syndrome: A systematic review and meta-analysis. IJRM 2023; 21 (5) :367-378
URL: http://ijrm.ir/article-1-2650-en.html
URL: http://ijrm.ir/article-1-2650-en.html
1- Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. , bahreiny.s@ajums.ac.ir
2- Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3- Department of Biology, Faculty of Sciences, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
4- Department of Cell and Molecular Biology, Faculty of Chemistry, University of Kashan, Kashan, Iran.
2- Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3- Department of Biology, Faculty of Sciences, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
4- Department of Cell and Molecular Biology, Faculty of Chemistry, University of Kashan, Kashan, Iran.
Full-Text [PDF 2291 kb]
(1360 Downloads)
| Abstract (HTML) (1803 Views)
Full-Text: (355 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is a prevalent endocrine and metabolic disease in women of gestational age (1). It is characterized by ovulation disorder, hyperandrogenism, and polycystic ovarian morphology (2). Women with PCOS may suffer from various problems, such as infertility, endocrine, and metabolic disorders (3). The risk of depression and its symptoms appears to be higher in women with PCOS, which may be related to factors such as hyperinsulinemia, hyperandrogenism, and increased levels of inflammation (4, 5).
The underlying cause of PCOS is unknown and probably multifactorial, which has implications for proposed treatments. Many studies have suggested that PCOS is caused due to ovarian abnormalities (6-9). One criterion for PCOS is based on the Rotterdam criteria, which demands the presence of 3 features: hyperandrogenism, polycystic ovaries on ultrasound, and menstrual irregularities (10, 11). In addition, PCOS is closely associated with obesity, insulin resistance (IR), impaired glucose tolerance, hypertension, type 2 diabetes mellitus, dyslipidemia, and cardiovascular disease (12). Recent studies on metabolism-regulating proteins and peptides have gained interest as potential biomarkers for PCOS disorders (13-16).
Among the new markers involved in metabolic disorders, preptin is of great importance (17). Preptin is a 34-amino acids hormone produced by the gene encoding insulin-like growth factor 2. This peptide is co-secreted with insulin in response to glucose from the pancreatic beta-cells and stimulates insulin secretion (18, 19). The primary function of preptin involves the regulation of carbohydrate, protein, and lipid metabolism by modulating insulin secretion (20).
Studies show a direct relationship between preptin serum levels and the fasting homeostasis model assessment of insulin resistance (HOMA-IR) (21). Some studies have also showed the association between preptin serum levels and a high HOMA-IR in PCOS participants (18, 22). However, the effect of preptin on the pathogenesis of PCOS is still unclear, and the results seem controversial (23, 24) .
Therefore, this meta-analysis aimed to analyze previous studies on the association between serum preptin levels and PCOS and to investigate whether preptin can be recommended as a new biomarker for PCOS.
2. Materials and Methods
2.1. Protocol and registration
This systematic review and meta-analysis were conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to ensure transparency and rigor in the review process (25, 26) .Our systematic review was registered in PROSPERO (the international database for prospectively registered systematic reviews).
2.2 . Eligibility criteria
All the observational studies published from January 2000 to August 2022 that defined PCOS according to the Rotterdam criteria and reported serum/plasma or follicular fluid preptin levels in PCOS participants compared with non-PCOS controls were included (10). In addition, some studies were excluded for the following reasons: I) incorrect comparison subjects and study design such as interventional studies, II) review articles, studies not conducted in humans, and conference articles, III) studies with non-extractable data.
2.3. Literature search
A literature search was performed for human studies published in PubMed, Web of Science, Scopus, Cochrane, EMBASE, and Google Scholar search engines from January 2000 to August 2022. The following keywords were used: “Preptin”, “ProIGF-II”, “Insulin-Like Growth Factor II”, “polycystic ovary syndrome”, “PCOS”, “polycystic ovary” using coordinating conjunctions “AND” and “OR”. Also, references to related articles were searched manually. Search terms were supplemented by searching for available gray literature. In addition, all records were entered into the EndNote 9 software, and duplicate studies were removed after the screening.
2.4. Study selection
2 independent reviewers thoroughly scanned the available literature to assess the eligibility criteria for the study (S.B. and M.D.). Any disagreements between the reviewers were resolved through collaborative discussion. In addition, a third reviewer was brought in to provide further insights and help reach a consensus (R.E.).
2.5. Data extraction
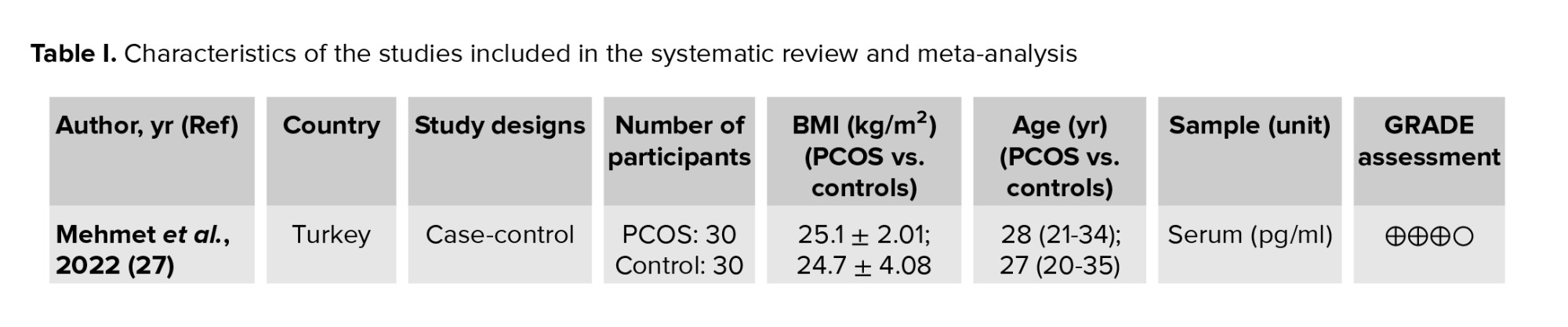
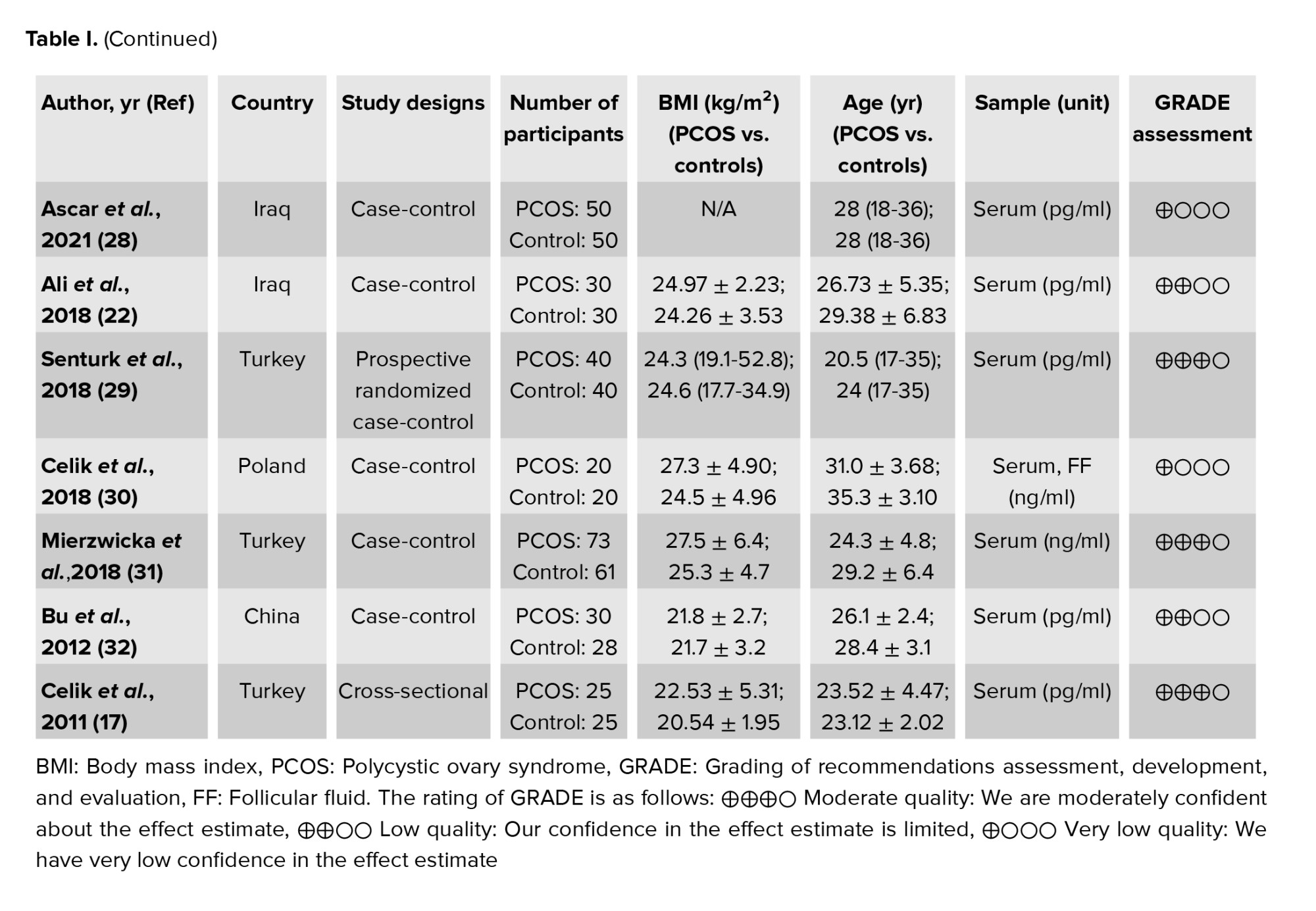
The following data were extracted according to a pre-prepared checklist: origin of study characteristics (name of first author, year of publication, and location of study), study design (cross-sectional, cohort, or case-control), participant characteristics (age, number of participants, and body mass index), measurements, diagnostic criteria, and reported outcomes. In order to ensure the reliability and credibility of the evidence, the grading of recommendations assessment, development, and evaluation approach was applied to assess the quality of the studies included in this meta-analysis. Our evaluation indicated that the studies were between moderate and poor quality, mainly due to the risk of bias, the inconsistency of effect estimators, and imprecision caused by small sample sizes, leading to a high degree of uncertainty. Therefore, while our meta-analysis suggests a positive association between preptin levels and PCOS, caution should be exercised in interpreting the results. Nevertheless, our study adds to the existing body of knowledge on the role of preptin in the pathogenesis of PCOS and highlights the need for more research in this area (Table I).
2.6 . Risk of bias assessment in the included studies
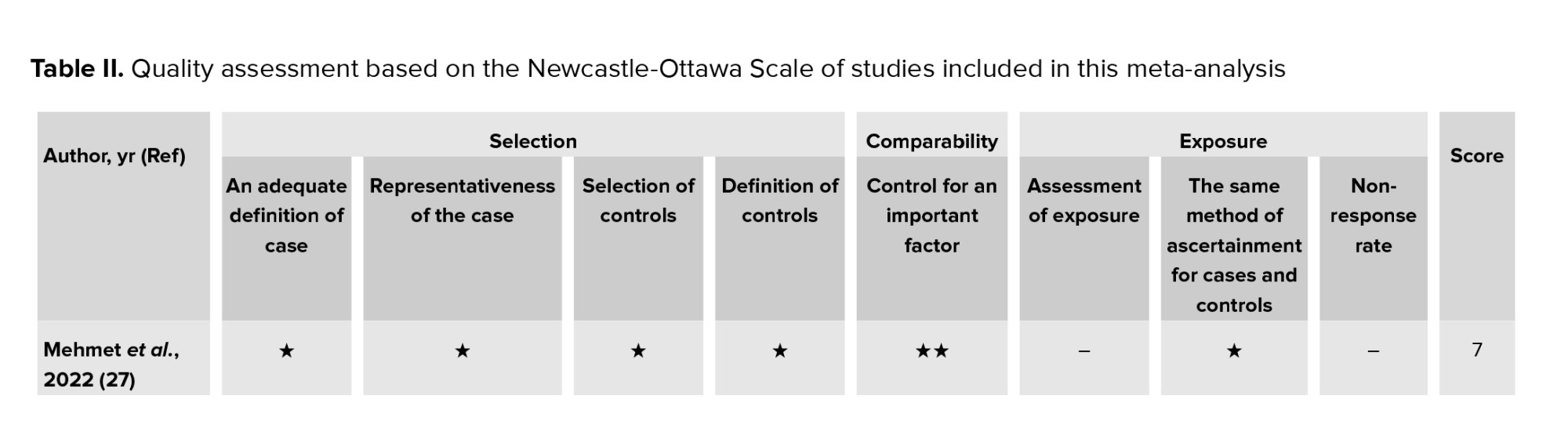
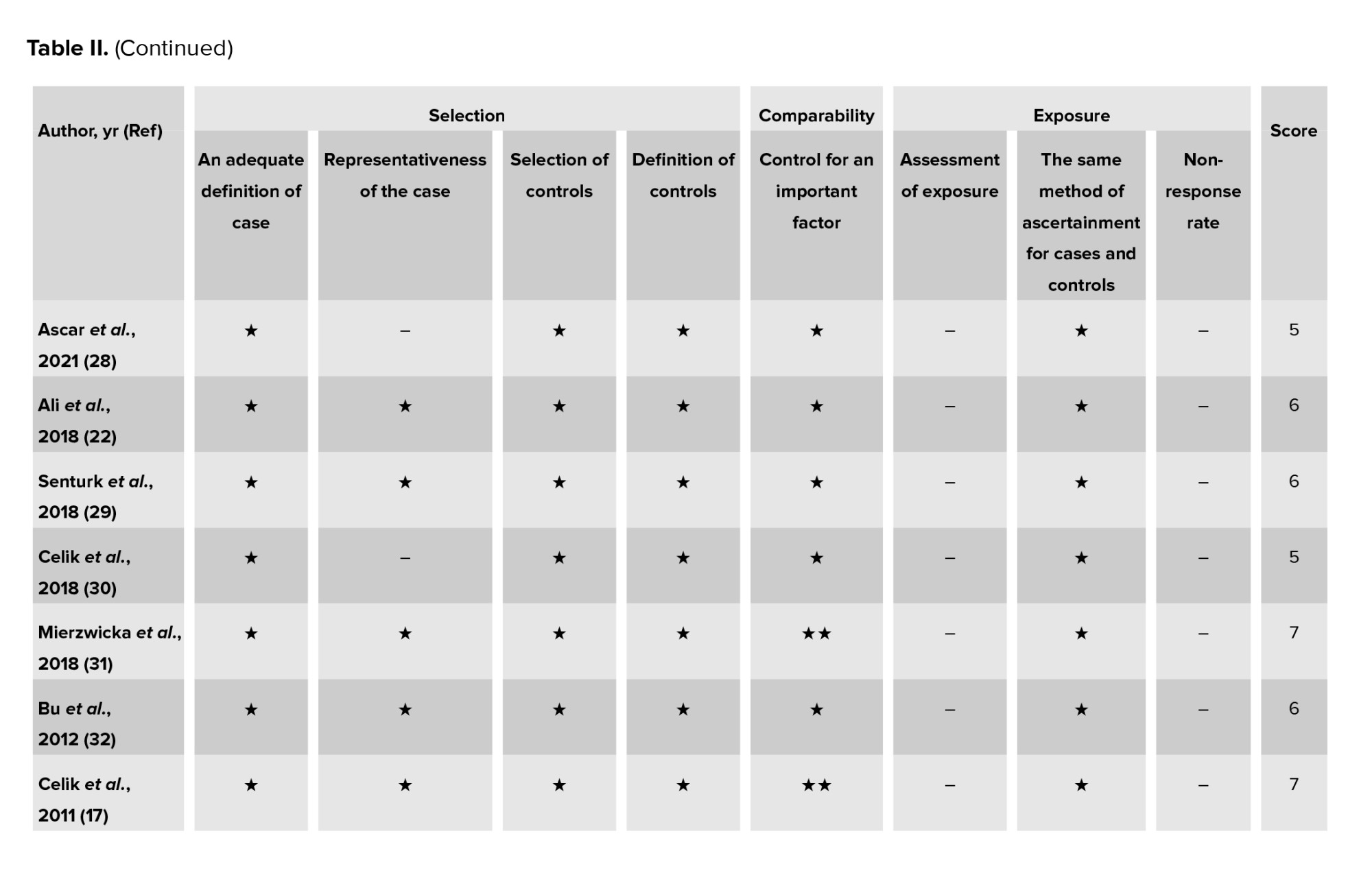
Study quality was assessed separately by 2 researchers (S.B. and M.D.) using the Newcastle-Ottawa scale (NOS) for all observational studies (cross-sectional, case-control, and cohort studies) (33). The scale NOS has 8 stages, which includes 3 domains: selection, comparison, and relating to type of outcome (cohort studies) or exposure (case-control studies). A score of 0 or 1 was assigned for each criterion, depending on whether the benchmark was satisfactorily met. Each study was assigned a point scale of 9 stars, with ratings of 5-9 stars representing high quality, and ratings of 0-4 stars representing low quality. Any discrepancies in the NOS rating of the studies were resolved through discussions among the authors. The quality assessment outcomes for all the included studies are presented in (Table II).
2.7. Statistical analysis
Standardized mean difference (SMD) and 95% confidence interval (CI) were used to estimate circulating preptin levels in PCOS cases compared with controls (34, 35). The
Publication bias was assessed using Egger's and Begg's tests (38, 39). A p-value of 0.05 was used to determine significant publication bias. Sources of heterogeneity were identified by subgroup analysis and meta-regression. Sensitivity analysis was also performed to calculate the impact of each study by removing a single study. Statistical data analysis was performed using comprehensive meta-analysis v3.7z software.
3. Results
3.1. Characteristics of the included studies
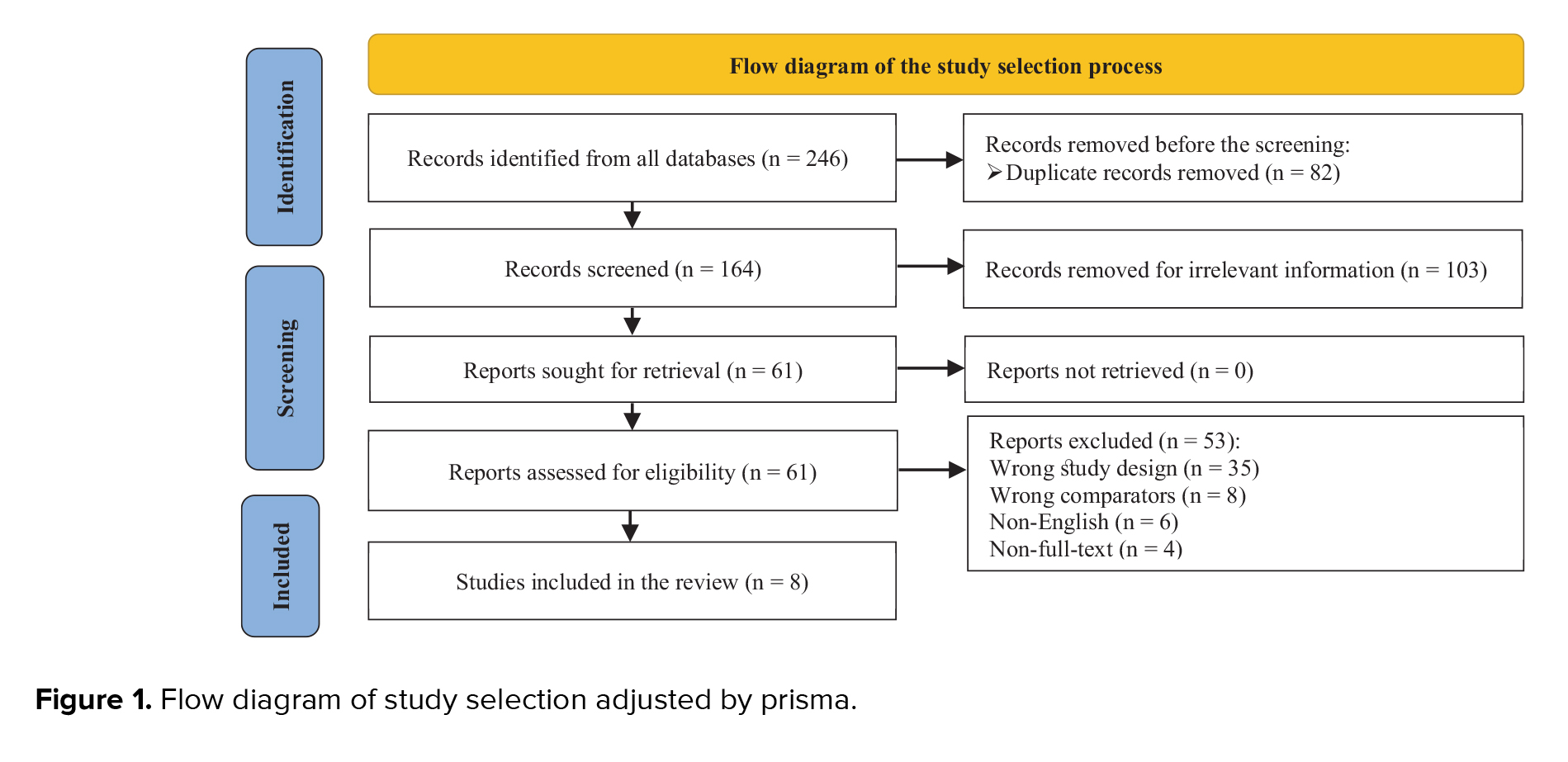
The search strategy identified 246 articles in the database, but 82 duplicate records were removed before the screening. A total of 103 articles were excluded after reviewing the titles and abstracts. Afterward, 61 potentially relevant articles were evaluated by a full-text review. Among these articles, 43 studies were excluded because they had the wrong study design and comparison group, and 10 were excluded for other reasons. Thus, 8 studies (involving 582 participants) met our selection criteria (Figure 1).
3.2 . Association and comparisons details
In 8 studies with a sample size of 582, preptin levels were examined in PCOS participants compared with the control group. Subgroup analysis and meta-regression analysis were also performed to reveal the source of heterogeneity based on body mass index, age, HOMA-IR, geographic region, and year of the studies. This extensive evaluation has led to an improved understanding of the relationship between preptin levels and PCOS and provides valuable insights into the potential factors that contribute to the observed heterogeneity.
3.3. Relationship between serum preptin levels and PCOS
3.3.1. Meta‐analysis
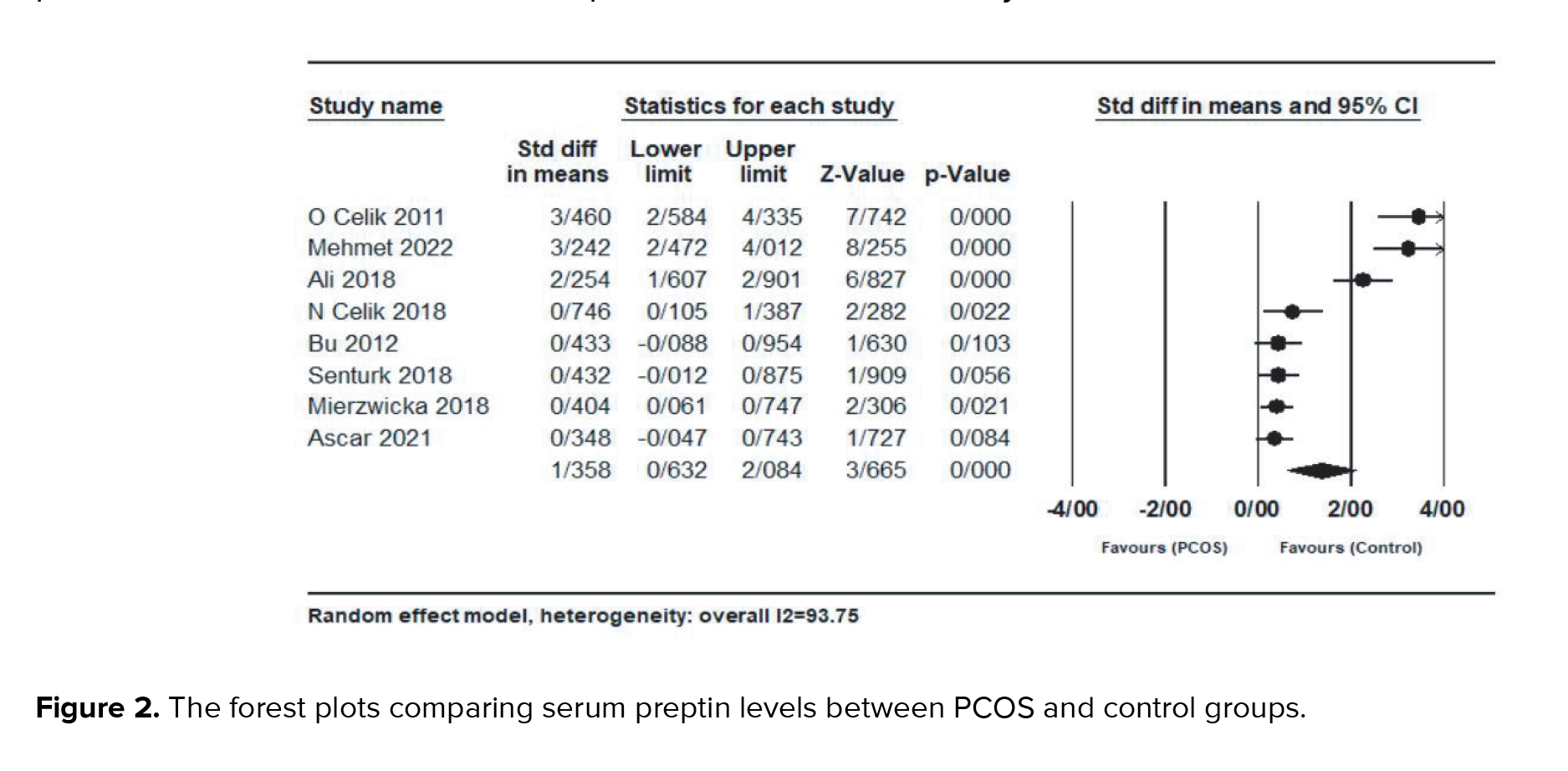
Result showed a significantly higher preptin level in the PCOS group compared with control group (SMD = 1.35; 95% CI: 0.63-2.08; p < 0.001) and there was significant heterogeneity between studies (
3.3.2. Subgroup analysis
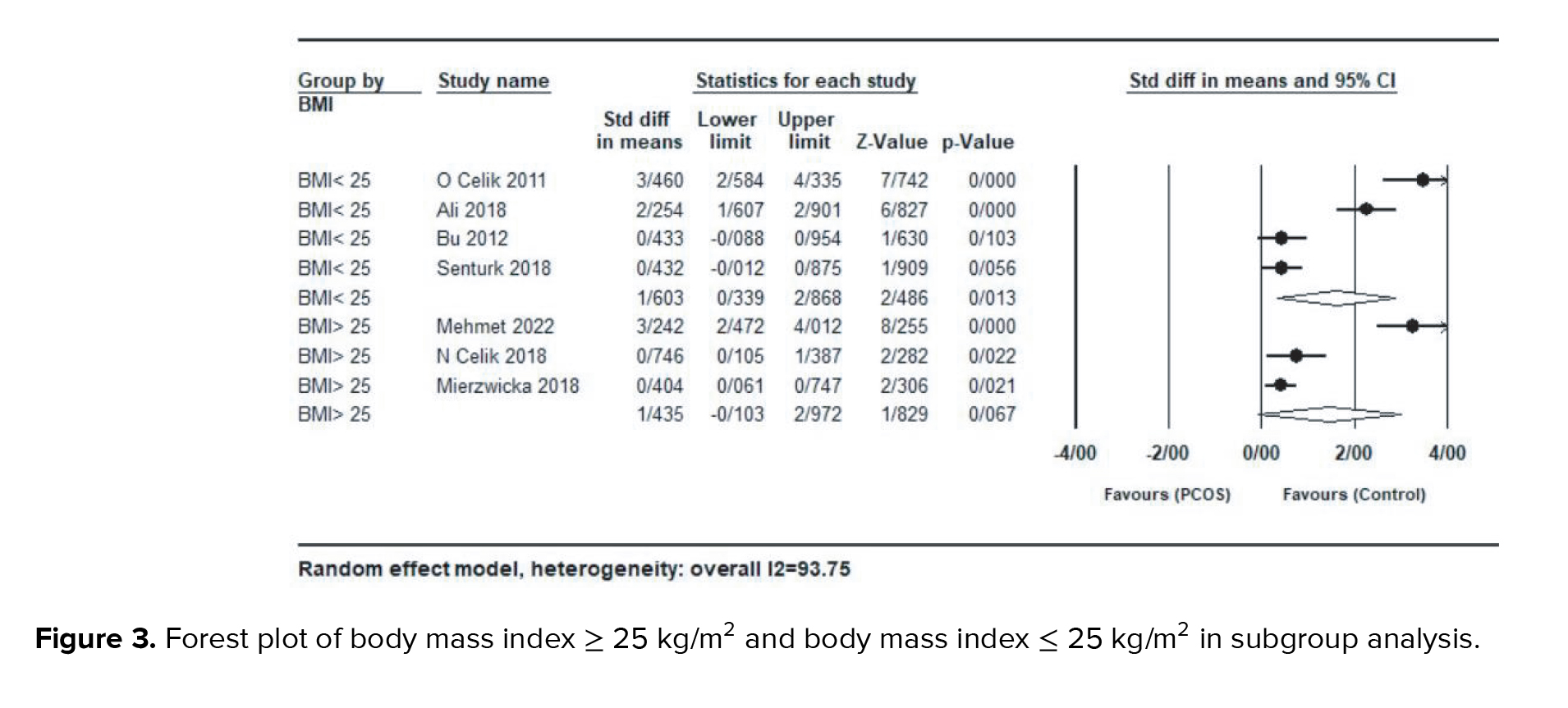
Subgroup analysis based on BMI, age, and HOMA-IR for each study were also performed. In a subgroup analysis based on studies with a mean BMI ≥ 25 kg/m2 or ≤ 25 kg/m2, women with BMI > 25 kg/m2 (SMD = 1.43; 95% CI: -0.10-2.97; p = 0.06), had lower serum preptin levels compared with the group with BMI < 25 kg/m2 (SMD = 1.60; 95% CI: 0.33-2.86; p = 0.01) (Figure 3).
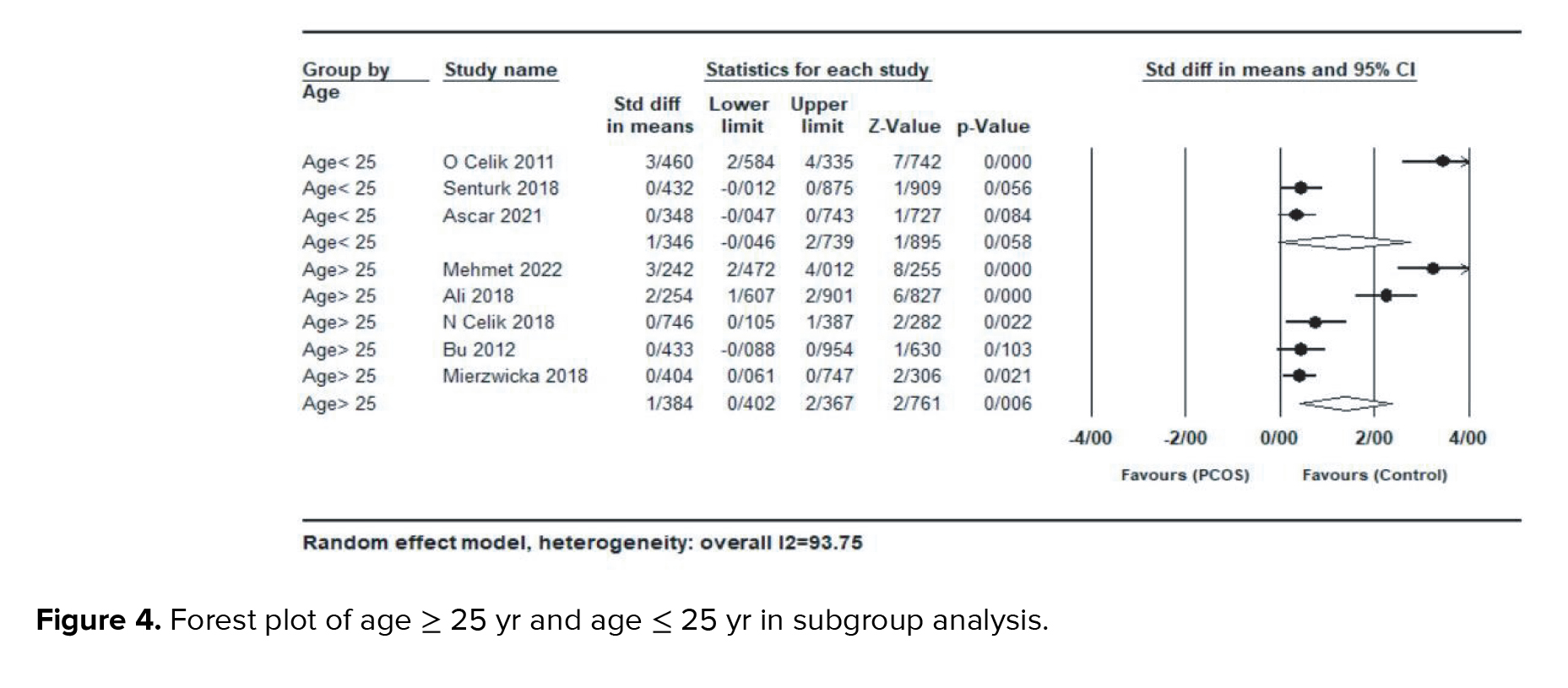
In a subgroup analysis by studies with mean age ≥ 25 yr or ≤ 25 yr, no significant difference was observed between women with age > 25 yr (SMD = 1.38; 95% CI: 0.40-2.36; p < 0.001) and women with age < 25 yr (SMD = 1.34; 95% CI: -0.04-2.73; p = 0.058) (Figure 4).
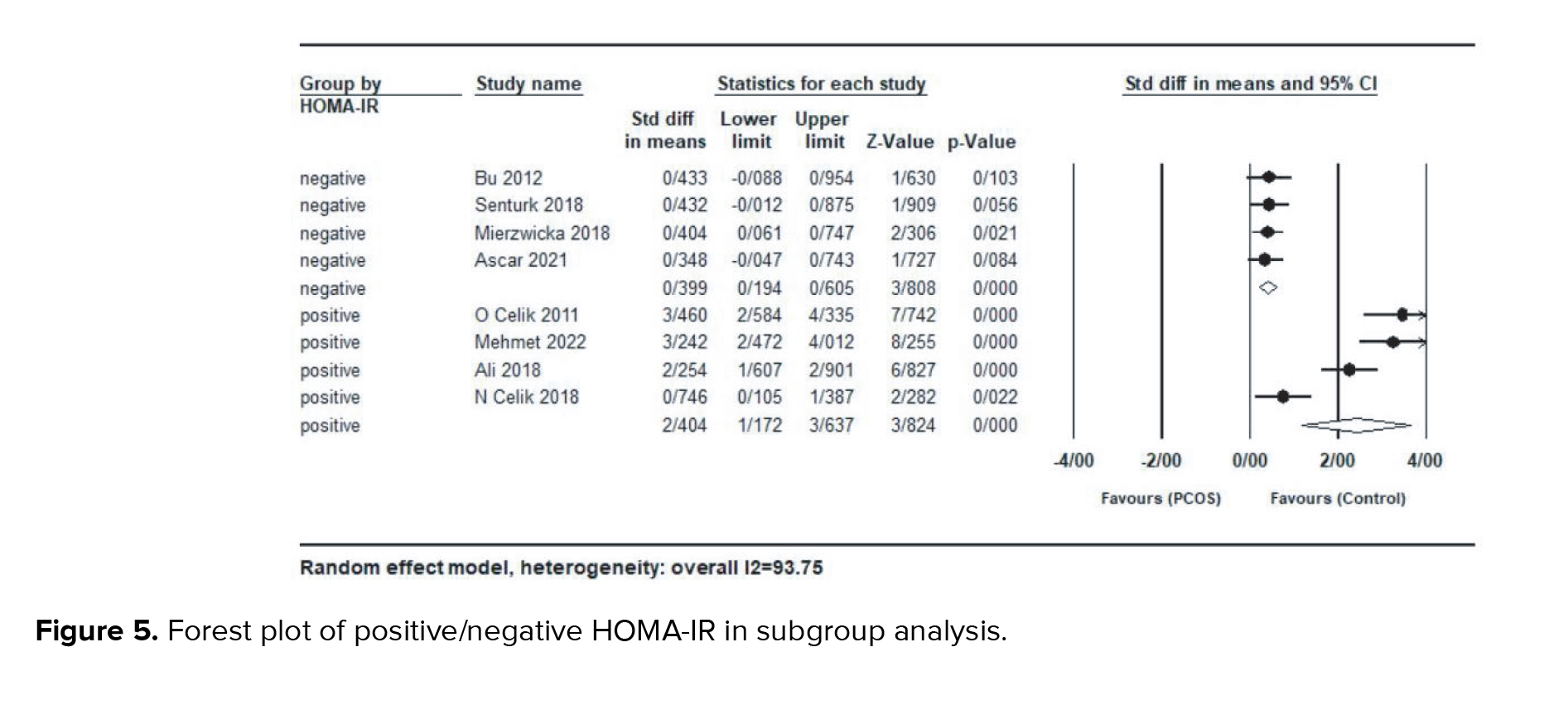
In a subgroup analysis based on studies with a mean HOMA-IR ≥ 3 (positive for IR) or ≤ 3 (negative for IR), women with HOMA-IR > 3 (SMD = 2.40; 95% CI: 1.17-3.63; p < 0.001), had significantly higher serum preptin levels compared to the group with HOMA-IR < 3 (SMD = 0.39; 95% CI: 0.19-0.60; p < 0.001) (Figure 5).
3.3.3. Meta-regression analysis
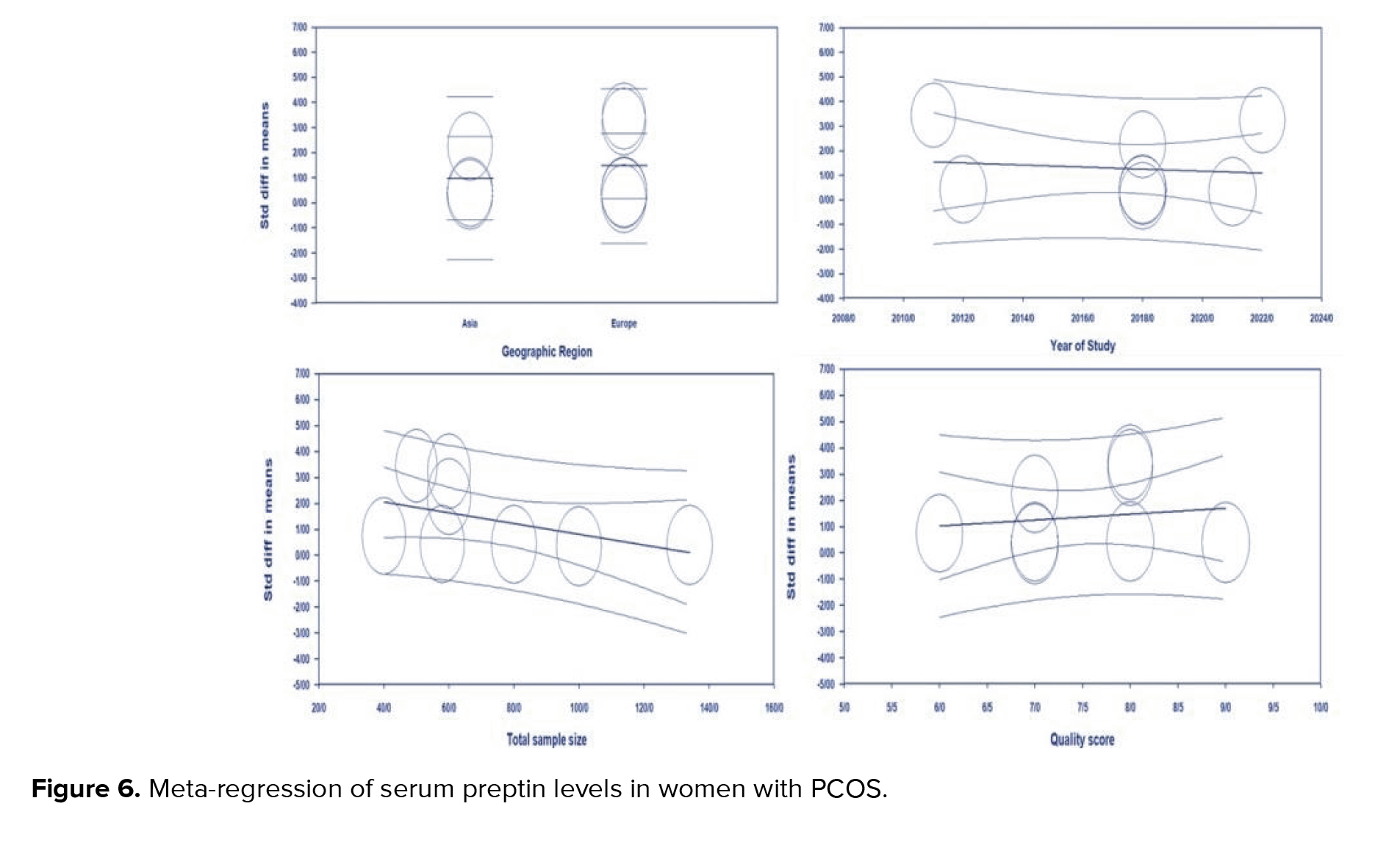
The meta-regression for SMD of preptin level based on geographic region (meta-regression coefficient: 0.489; 95% CI: -1.19 to 2.17; p = 0.56), year of studies (meta-regression coefficient: -0.04; 95% CI: -0.26 to 0.17, p = 0.71), sample size (meta-regression coefficient: -0.020; 95% CI: -0.04 to 0.00, p = 0.97), quality score (meta-regression coefficient: 0.224; 95% CI: -0.71 to 1.16, p = 0.64) was not significant (Figure 6).
3.4. Publication bias and sensitivity analysis
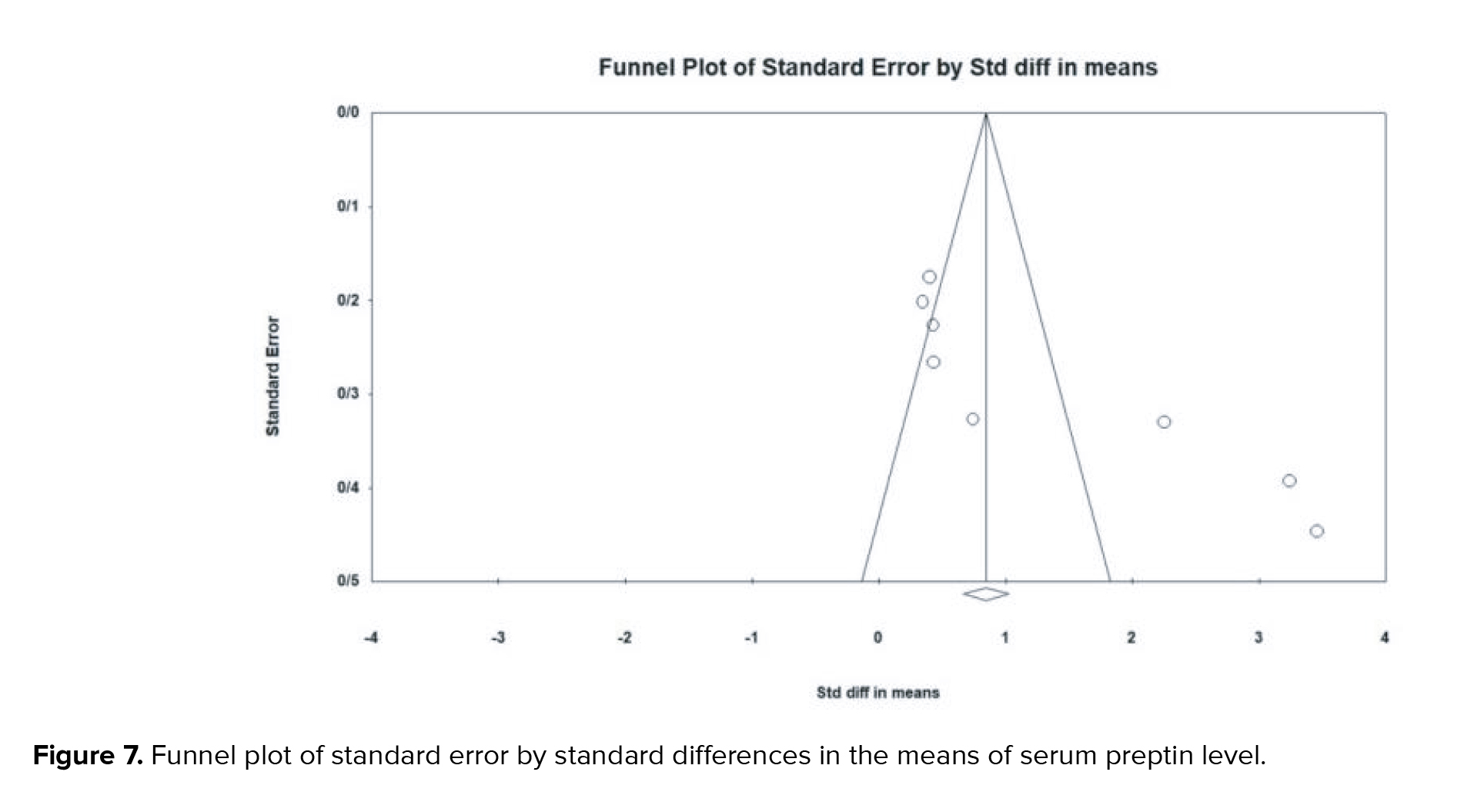
There is substantial evidence of publication bias with respect to the SMD of preptin levels. This bias was identified through both Begg's test (p < 0.001) and Egger's test (p < 0.001) (Figure 7).
However, it is essential to note that the Cochrane handbook for systematic reviews of interventions states that publication bias may be unreliable if fewer than 10 studies were conducted (40). We also performed a sensitivity analysis to confirm the stability and reliability of our outcome. The overall effect size did not change significantly when the individual studies were removed, indicating the reliability of the analysis.
4. Discussion
PCOS is a current endocrinal disorder. This syndrome results in infertility, IR, obesity, cardiovascular problems, and other health problems (41). Preptin is an oligopeptide secreted by pancreatic β-cells that has emerged as a potential new biomarker for diagnosing of PCOS because of its role in promoting insulin secretion (42, 43).
Through a meta-analysis of 8 studies conducted between 2011 and 2022, with a total sample size of 582 participants, our findings suggest a significant positive association between high preptin levels and PCOS. These findings provide compelling evidence for the potential role of preptin in the pathogenesis of PCOS, suggesting that preptin may be a key biomarker for identifying and monitoring the progression of this common endocrine disorder . In a previous study, it has shown an increase in serum preptin levels in participants with PCOS compared to the control group (17). Also, in other studies conducted, a significant correlation was suggested between preptin levels in women with PCOS (22). These findings contradict the results of prior reports (32), in which no significant relationship was found between preptin levels and PCOS status.
In this meta-analysis, the value of I2 = 93.75% indicates a large heterogeneity between studies; therefore, subgroup analysis and meta-regression were done to determine the cause of heterogeneity. Subgroup analysis was performed based on age, BMI, and HOMA-IR ratio. No significant association was observed between preptin levels and BMI in healthy women compared to PCOS participants, which agrees with previous findings (29, 31). Also, the meta-analysis results based on age showed no significant difference between preptin levels in the PCOS and control groups. The HOMA-IR ratio was considered as a factor for the heterogeneity of the effect size in the analysis of subgroups; according to the results of a subgroup analysis based on the HOMA-IR ratio, a positive correlation was observed between increased preptin levels in women with PCOS and an increased HOMA-IR ratio. In addition, meta-regression analysis was performed to investigate potential factors that may contribute to heterogeneity. The results indicated that the SMD of serum preptin levels were not significantly associated with geographic region, publication year, sample size, or quality score. These findings suggest that the effect of preptin on PCOS is consistent across different regions and study periods and is independent of study size and quality. However, other factors such as age, BMI, and insulin resistance may contribute to the observed heterogeneity, and further investigation is warranted to elucidate these factors.
Although previous studies have presented inconsistent findings regarding the link between PCOS and serum preptin levels, our study is the inaugural meta-analysis to elucidate the association between circulating preptin levels and PCOS. It is imperative to acknowledge and consider the inherent limitations of this meta-analysis during the interpretation of its results. These include the reality that the number of studies is insufficient due to the novelty of the topic. However, further studies with a larger statistical population could support our findings. In addition, the data collected for this research were based on English-language articles, and it was not possible to access non-English-language articles, abstracts, and dissertations. Finally, it should be noted that the results of this study require additional research and should be evaluated with caution.
5. Conclusion
Our meta-analysis revealed an association between serum preptin levels and PCOS participants, suggesting that preptin could be used as a novel biomarker for PCOS. Moreover, this association was influenced by IR.
Acknowledgments
Although this study did not receive any financial support, the authors would like to express their appreciation for the researchers whose articles were used in the present research.
Conflict of Interest
The authors declare no conflict of interest.
Polycystic ovary syndrome (PCOS) is a prevalent endocrine and metabolic disease in women of gestational age (1). It is characterized by ovulation disorder, hyperandrogenism, and polycystic ovarian morphology (2). Women with PCOS may suffer from various problems, such as infertility, endocrine, and metabolic disorders (3). The risk of depression and its symptoms appears to be higher in women with PCOS, which may be related to factors such as hyperinsulinemia, hyperandrogenism, and increased levels of inflammation (4, 5).
The underlying cause of PCOS is unknown and probably multifactorial, which has implications for proposed treatments. Many studies have suggested that PCOS is caused due to ovarian abnormalities (6-9). One criterion for PCOS is based on the Rotterdam criteria, which demands the presence of 3 features: hyperandrogenism, polycystic ovaries on ultrasound, and menstrual irregularities (10, 11). In addition, PCOS is closely associated with obesity, insulin resistance (IR), impaired glucose tolerance, hypertension, type 2 diabetes mellitus, dyslipidemia, and cardiovascular disease (12). Recent studies on metabolism-regulating proteins and peptides have gained interest as potential biomarkers for PCOS disorders (13-16).
Among the new markers involved in metabolic disorders, preptin is of great importance (17). Preptin is a 34-amino acids hormone produced by the gene encoding insulin-like growth factor 2. This peptide is co-secreted with insulin in response to glucose from the pancreatic beta-cells and stimulates insulin secretion (18, 19). The primary function of preptin involves the regulation of carbohydrate, protein, and lipid metabolism by modulating insulin secretion (20).
Studies show a direct relationship between preptin serum levels and the fasting homeostasis model assessment of insulin resistance (HOMA-IR) (21). Some studies have also showed the association between preptin serum levels and a high HOMA-IR in PCOS participants (18, 22). However, the effect of preptin on the pathogenesis of PCOS is still unclear, and the results seem controversial (23, 24) .
Therefore, this meta-analysis aimed to analyze previous studies on the association between serum preptin levels and PCOS and to investigate whether preptin can be recommended as a new biomarker for PCOS.
2. Materials and Methods
2.1. Protocol and registration
This systematic review and meta-analysis were conducted according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines to ensure transparency and rigor in the review process (25, 26) .Our systematic review was registered in PROSPERO (the international database for prospectively registered systematic reviews).
2.2 . Eligibility criteria
All the observational studies published from January 2000 to August 2022 that defined PCOS according to the Rotterdam criteria and reported serum/plasma or follicular fluid preptin levels in PCOS participants compared with non-PCOS controls were included (10). In addition, some studies were excluded for the following reasons: I) incorrect comparison subjects and study design such as interventional studies, II) review articles, studies not conducted in humans, and conference articles, III) studies with non-extractable data.
2.3. Literature search
A literature search was performed for human studies published in PubMed, Web of Science, Scopus, Cochrane, EMBASE, and Google Scholar search engines from January 2000 to August 2022. The following keywords were used: “Preptin”, “ProIGF-II”, “Insulin-Like Growth Factor II”, “polycystic ovary syndrome”, “PCOS”, “polycystic ovary” using coordinating conjunctions “AND” and “OR”. Also, references to related articles were searched manually. Search terms were supplemented by searching for available gray literature. In addition, all records were entered into the EndNote 9 software, and duplicate studies were removed after the screening.
2.4. Study selection
2 independent reviewers thoroughly scanned the available literature to assess the eligibility criteria for the study (S.B. and M.D.). Any disagreements between the reviewers were resolved through collaborative discussion. In addition, a third reviewer was brought in to provide further insights and help reach a consensus (R.E.).
2.5. Data extraction
The following data were extracted according to a pre-prepared checklist: origin of study characteristics (name of first author, year of publication, and location of study), study design (cross-sectional, cohort, or case-control), participant characteristics (age, number of participants, and body mass index), measurements, diagnostic criteria, and reported outcomes. In order to ensure the reliability and credibility of the evidence, the grading of recommendations assessment, development, and evaluation approach was applied to assess the quality of the studies included in this meta-analysis. Our evaluation indicated that the studies were between moderate and poor quality, mainly due to the risk of bias, the inconsistency of effect estimators, and imprecision caused by small sample sizes, leading to a high degree of uncertainty. Therefore, while our meta-analysis suggests a positive association between preptin levels and PCOS, caution should be exercised in interpreting the results. Nevertheless, our study adds to the existing body of knowledge on the role of preptin in the pathogenesis of PCOS and highlights the need for more research in this area (Table I).


2.6 . Risk of bias assessment in the included studies
Study quality was assessed separately by 2 researchers (S.B. and M.D.) using the Newcastle-Ottawa scale (NOS) for all observational studies (cross-sectional, case-control, and cohort studies) (33). The scale NOS has 8 stages, which includes 3 domains: selection, comparison, and relating to type of outcome (cohort studies) or exposure (case-control studies). A score of 0 or 1 was assigned for each criterion, depending on whether the benchmark was satisfactorily met. Each study was assigned a point scale of 9 stars, with ratings of 5-9 stars representing high quality, and ratings of 0-4 stars representing low quality. Any discrepancies in the NOS rating of the studies were resolved through discussions among the authors. The quality assessment outcomes for all the included studies are presented in (Table II).


2.7. Statistical analysis
Standardized mean difference (SMD) and 95% confidence interval (CI) were used to estimate circulating preptin levels in PCOS cases compared with controls (34, 35). The
Publication bias was assessed using Egger's and Begg's tests (38, 39). A p-value of 0.05 was used to determine significant publication bias. Sources of heterogeneity were identified by subgroup analysis and meta-regression. Sensitivity analysis was also performed to calculate the impact of each study by removing a single study. Statistical data analysis was performed using comprehensive meta-analysis v3.7z software.
3. Results
3.1. Characteristics of the included studies
The search strategy identified 246 articles in the database, but 82 duplicate records were removed before the screening. A total of 103 articles were excluded after reviewing the titles and abstracts. Afterward, 61 potentially relevant articles were evaluated by a full-text review. Among these articles, 43 studies were excluded because they had the wrong study design and comparison group, and 10 were excluded for other reasons. Thus, 8 studies (involving 582 participants) met our selection criteria (Figure 1).

3.2 . Association and comparisons details
In 8 studies with a sample size of 582, preptin levels were examined in PCOS participants compared with the control group. Subgroup analysis and meta-regression analysis were also performed to reveal the source of heterogeneity based on body mass index, age, HOMA-IR, geographic region, and year of the studies. This extensive evaluation has led to an improved understanding of the relationship between preptin levels and PCOS and provides valuable insights into the potential factors that contribute to the observed heterogeneity.
3.3. Relationship between serum preptin levels and PCOS
3.3.1. Meta‐analysis
Result showed a significantly higher preptin level in the PCOS group compared with control group (SMD = 1.35; 95% CI: 0.63-2.08; p < 0.001) and there was significant heterogeneity between studies (
3.3.2. Subgroup analysis
Subgroup analysis based on BMI, age, and HOMA-IR for each study were also performed. In a subgroup analysis based on studies with a mean BMI ≥ 25 kg/m2 or ≤ 25 kg/m2, women with BMI > 25 kg/m2 (SMD = 1.43; 95% CI: -0.10-2.97; p = 0.06), had lower serum preptin levels compared with the group with BMI < 25 kg/m2 (SMD = 1.60; 95% CI: 0.33-2.86; p = 0.01) (Figure 3).
In a subgroup analysis by studies with mean age ≥ 25 yr or ≤ 25 yr, no significant difference was observed between women with age > 25 yr (SMD = 1.38; 95% CI: 0.40-2.36; p < 0.001) and women with age < 25 yr (SMD = 1.34; 95% CI: -0.04-2.73; p = 0.058) (Figure 4).
In a subgroup analysis based on studies with a mean HOMA-IR ≥ 3 (positive for IR) or ≤ 3 (negative for IR), women with HOMA-IR > 3 (SMD = 2.40; 95% CI: 1.17-3.63; p < 0.001), had significantly higher serum preptin levels compared to the group with HOMA-IR < 3 (SMD = 0.39; 95% CI: 0.19-0.60; p < 0.001) (Figure 5).
3.3.3. Meta-regression analysis
The meta-regression for SMD of preptin level based on geographic region (meta-regression coefficient: 0.489; 95% CI: -1.19 to 2.17; p = 0.56), year of studies (meta-regression coefficient: -0.04; 95% CI: -0.26 to 0.17, p = 0.71), sample size (meta-regression coefficient: -0.020; 95% CI: -0.04 to 0.00, p = 0.97), quality score (meta-regression coefficient: 0.224; 95% CI: -0.71 to 1.16, p = 0.64) was not significant (Figure 6).
3.4. Publication bias and sensitivity analysis
There is substantial evidence of publication bias with respect to the SMD of preptin levels. This bias was identified through both Begg's test (p < 0.001) and Egger's test (p < 0.001) (Figure 7).
However, it is essential to note that the Cochrane handbook for systematic reviews of interventions states that publication bias may be unreliable if fewer than 10 studies were conducted (40). We also performed a sensitivity analysis to confirm the stability and reliability of our outcome. The overall effect size did not change significantly when the individual studies were removed, indicating the reliability of the analysis.






4. Discussion
PCOS is a current endocrinal disorder. This syndrome results in infertility, IR, obesity, cardiovascular problems, and other health problems (41). Preptin is an oligopeptide secreted by pancreatic β-cells that has emerged as a potential new biomarker for diagnosing of PCOS because of its role in promoting insulin secretion (42, 43).
Through a meta-analysis of 8 studies conducted between 2011 and 2022, with a total sample size of 582 participants, our findings suggest a significant positive association between high preptin levels and PCOS. These findings provide compelling evidence for the potential role of preptin in the pathogenesis of PCOS, suggesting that preptin may be a key biomarker for identifying and monitoring the progression of this common endocrine disorder . In a previous study, it has shown an increase in serum preptin levels in participants with PCOS compared to the control group (17). Also, in other studies conducted, a significant correlation was suggested between preptin levels in women with PCOS (22). These findings contradict the results of prior reports (32), in which no significant relationship was found between preptin levels and PCOS status.
In this meta-analysis, the value of I2 = 93.75% indicates a large heterogeneity between studies; therefore, subgroup analysis and meta-regression were done to determine the cause of heterogeneity. Subgroup analysis was performed based on age, BMI, and HOMA-IR ratio. No significant association was observed between preptin levels and BMI in healthy women compared to PCOS participants, which agrees with previous findings (29, 31). Also, the meta-analysis results based on age showed no significant difference between preptin levels in the PCOS and control groups. The HOMA-IR ratio was considered as a factor for the heterogeneity of the effect size in the analysis of subgroups; according to the results of a subgroup analysis based on the HOMA-IR ratio, a positive correlation was observed between increased preptin levels in women with PCOS and an increased HOMA-IR ratio. In addition, meta-regression analysis was performed to investigate potential factors that may contribute to heterogeneity. The results indicated that the SMD of serum preptin levels were not significantly associated with geographic region, publication year, sample size, or quality score. These findings suggest that the effect of preptin on PCOS is consistent across different regions and study periods and is independent of study size and quality. However, other factors such as age, BMI, and insulin resistance may contribute to the observed heterogeneity, and further investigation is warranted to elucidate these factors.
Although previous studies have presented inconsistent findings regarding the link between PCOS and serum preptin levels, our study is the inaugural meta-analysis to elucidate the association between circulating preptin levels and PCOS. It is imperative to acknowledge and consider the inherent limitations of this meta-analysis during the interpretation of its results. These include the reality that the number of studies is insufficient due to the novelty of the topic. However, further studies with a larger statistical population could support our findings. In addition, the data collected for this research were based on English-language articles, and it was not possible to access non-English-language articles, abstracts, and dissertations. Finally, it should be noted that the results of this study require additional research and should be evaluated with caution.
5. Conclusion
Our meta-analysis revealed an association between serum preptin levels and PCOS participants, suggesting that preptin could be used as a novel biomarker for PCOS. Moreover, this association was influenced by IR.
Acknowledgments
Although this study did not receive any financial support, the authors would like to express their appreciation for the researchers whose articles were used in the present research.
Conflict of Interest
The authors declare no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Pattology
References
1. Zeng X, Xie Y-J, Liu Y-T, Long S-L, Mo Z-C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta 2020; 502: 214-221. [DOI:10.1016/j.cca.2019.11.003] [PMID]
2. Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril 2012; 97: 28-38.e25. [DOI:10.1016/j.fertnstert.2011.09.024] [PMID]
3. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev 2015; 36: 487-525. [DOI:10.1210/er.2015-1018] [PMID] [PMCID]
4. Banting LK, Gibson-Helm M, Polman R, Teede HJ, Stepto NK. Physical activity and mental health in women with polycystic ovary syndrome. BMC Womens Health 2014; 14: 51. [DOI:10.1186/1472-6874-14-51] [PMID] [PMCID]
5. Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: A systematic review and meta-analysis. Endocrine 2018; 62: 318-325. [DOI:10.1007/s12020-018-1692-3] [PMID]
6. Witchel SF, Teede HJ, Peña AS. Curtailing PCOS. Pediatr Res 2020; 87: 353-361. [DOI:10.1038/s41390-019-0615-1] [PMID]
7. Aversa A, La Vignera S, Rago R, Gambineri A, Nappi RE, Calogero AE, et al. Fundamental concepts and novel aspects of polycystic ovarian syndrome: Expert consensus resolutions. Front Endocrinol 2020; 11: 516. [DOI:10.3389/fendo.2020.00516] [PMID] [PMCID]
8. Escobar-Morreale HF. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018; 14: 270-284. [DOI:10.1038/nrendo.2018.24] [PMID]
9. Bachelot A. Polycystic ovarian syndrome: Clinical and biological diagnosis. Ann Biol Clin 2016; 74: 661-667. [DOI:10.1684/abc.2016.1184] [PMID]
10. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81: 19-25. [DOI:10.1016/j.fertnstert.2003.10.004]
11. Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2013; 98: 4565-4592. [DOI:10.1210/jc.2013-2350] [PMID] [PMCID]
12. Baldani DP, Skrgatic L, Ougouag R. Polycystic ovary syndrome: Important underrecognised cardiometabolic risk factor in reproductive-age women. Int J Endocrinol 2015; 2015: 786362. [DOI:10.1155/2015/786362] [PMID] [PMCID]
13. Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): An update. Arch Gynecol Obstet 2021; 303: 631-643. [DOI:10.1007/s00404-020-05951-2] [PMID]
14. Bannigida DM, Nayak BS, Vijayaraghavan R. Insulin resistance and oxidative marker in women with PCOS. Arch Physiol Biochem 2020; 126: 183-186. [DOI:10.1080/13813455.2018.1499120] [PMID]
15. Deveer R, Engin-Üstün Y, Uysal S, Su FA, Sarıaslan S, Gülerman C, et al. Serum brain natriuretic peptide and C-reactive protein levels in adolescent with polycystic ovary syndrome. Gynecol Endocrinol 2012; 28: 602-605. [DOI:10.3109/09513590.2011.650758] [PMID]
16. Kruszewska J, Laudy-Wiaderny H, Kunicki M. Review of novel potential insulin resistance biomarkers in PCOS patients-the debate is still open. Int J Environ Res Public Health 2022; 19: 2099. [DOI:10.3390/ijerph19042099] [PMID] [PMCID]
17. Celik O, Celik N, Hascalik S, Sahin I, Aydin S, Ozerol E. An appraisal of serum preptin levels in PCOS. Fertil Steril 2011; 95: 314-316. [DOI:10.1016/j.fertnstert.2010.08.058] [PMID]
18. Buchanan CM, Phillips AR, Cooper GJ. Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet beta-cells and enhances insulin secretion. Biochem J 2001; 360: 431-439. [DOI:10.1042/bj3600431] [PMID] [PMCID]
19. van Doorn J. Insulin-like growth factor-II and bioactive proteins containing a part of the E-domain of pro-insulin-like growth factor-II. Biofactors 2020; 46: 563-578. [DOI:10.1002/biof.1623] [PMID] [PMCID]
20. Aydin S. Three new players in energy regulation: Preptin, adropin and irisin. Peptides 2014; 56: 94-110. [DOI:10.1016/j.peptides.2014.03.021] [PMID]
21. Yang G, Li L, Chen W, Liu H, Boden G, Li K. Circulating preptin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Ann Med 2009; 41: 52-56. [DOI:10.1080/07853890802244142] [PMID]
22. Ali HA, Abbas HJ, Naser AN. Preptin and adropin levels as new predictor in women with polycystic ovary syndrome. J Pharm Sci Res 2018; 10: 3005-3008.
23. Mierzwicka A, Bolanowski M. New peptides players in metabolic disorders. Postepy Hig Med Dosw 2016; 70: 881-886. [DOI:10.5604/17322693.1216271] [PMID]
24. Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: What's new? Adv Clin Exp Med 2017; 26: 359-367. [DOI:10.17219/acem/59380] [PMID]
25. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev Esp Cardiol 2021; 74: 790-799. [DOI:10.1016/j.recesp.2021.06.016] [PMID]
26. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011; 39: 91-92. [DOI:10.1016/j.jcms.2010.11.001] [PMID]
27. Mehmet Ak, Bertan Demir M. An assesment of relationship between serum preptin levels and anti-mullerian hormone in infertile womens with polycystic ovary syndrome. Ann Clin Anal Med 2022; 13: 779-782. [DOI:10.4328/ACAM.21117]
28. Ascar IF, Hameed AS. Serum prolactin, Preptin, CCL 18 and genetic polymorphisms in Iraqi women with polycystic ovary syndrome. Baghdad Sci J 2021; 18: 1552-1556. [DOI:10.21123/bsj.2021.18.4(Suppl.).1552]
29. Şentürk Ş, Hatirnaz S, Kanat-Pektaş M. Serum preptin and amylin levels with respect to body mass index in polycystic ovary syndrome patients. Med Sci Monit 2018; 24: 7517-7523. [DOI:10.12659/MSM.912957] [PMID] [PMCID]
30. Celik N, Aydin S, Ugur K, Yardim M, Acet M, Yavuzkir S, et al. Patatin-like phospholipase domain containing 3-gene (adiponutrin), preptin, kisspeptin and amylin regulates oocyte developmental capacity in PCOS. Cell Mol Biol 2018; 64: 7-12. [DOI:10.14715/cmb/2017.64.15.2] [PMID]
31. Mierzwicka A, Kuliczkowska-Plaksej J, Kolačkov K, Bolanowski M. Preptin in women with polycystic ovary syndrome. Gynecol Endocrinol 2018; 34: 470-475. [DOI:10.1080/09513590.2017.1409715] [PMID]
32. Bu Z, Kuok K, Meng J, Wang R, Xu B, Zhang H. The relationship between polycystic ovary syndrome, glucose tolerance status and serum preptin level. Reprod Biol Endocrinol 2012; 10: 10. [DOI:10.1186/1477-7827-10-10] [PMID] [PMCID]
33. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603-605. [DOI:10.1007/s10654-010-9491-z] [PMID]
34. Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: As simple as it gets. J Clin Psychiatry 2020; 81: 20f13681. [DOI:10.4088/JCP.20f13681]
35. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2016; 27: 1785-1805. [DOI:10.1177/0962280216669183] [PMID]
36. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539-1558. [DOI:10.1002/sim.1186] [PMID]
37. Ades AE, Lu G, Higgins JPT. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005; 25: 646-654. [DOI:10.1177/0272989X05282643] [PMID]
38. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088-1101. [DOI:10.2307/2533446]
39. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629-634. [DOI:10.1136/bmj.315.7109.629] [PMID] [PMCID]
40. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd Ed. UK: John Wiley & Sons; 2019. [DOI:10.1002/9781119536604]
41. Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol 2018; 182: 27-36. [DOI:10.1016/j.jsbmb.2018.04.008] [PMID]
42. Xiao C, Li W, Lu T, Wang J, Han J. Preptin promotes proliferation and osteogenesis of MC3T3-E1 cells by upregulating β-catenin expression. IUBMB Life 2019; 71: 854-862. [DOI:10.1002/iub.2016] [PMID]
43. Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest 2017; 40: 1-8. [DOI:10.1007/s40618-016-0523-8] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







