Wed, Nov 12, 2025
[Archive]
Volume 22, Issue 7 (July 2024)
IJRM 2024, 22(7): 593-604 |
Back to browse issues page
Ethics code: IR.AJUMS.MEDICINE.REC.1400.053
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Najafi Chamgordani S, Esmaeil N, Hashemi M, Amari A, Seyedtabib M, Ghafourian M. Evaluation of the natural killer cell subsets and their relationship with serum interferon gamma and vitamin D levels in women with stages III and IV endometriosis: A case-control study. IJRM 2024; 22 (7) :593-604
URL: http://ijrm.ir/article-1-2944-en.html
URL: http://ijrm.ir/article-1-2944-en.html
Samira Najafi Chamgordani1 

 , Nafiseh Esmaeil2
, Nafiseh Esmaeil2 

 , Maryam Hashemi3
, Maryam Hashemi3 

 , Afshin Amari4
, Afshin Amari4 

 , Maryam Seyedtabib5
, Maryam Seyedtabib5 

 , Mehri Ghafourian *6
, Mehri Ghafourian *6 




 , Nafiseh Esmaeil2
, Nafiseh Esmaeil2 

 , Maryam Hashemi3
, Maryam Hashemi3 

 , Afshin Amari4
, Afshin Amari4 

 , Maryam Seyedtabib5
, Maryam Seyedtabib5 

 , Mehri Ghafourian *6
, Mehri Ghafourian *6 


1- Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2- Department of Immunology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. & Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
5- Department of Biostatistics and Epidemiology, School of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
6- Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. & Fertility, Infertility and Perinatology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. ,ghafourianbm@gmail.com
2- Department of Immunology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Department of Obstetrics and Gynecology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. & Cellular and Molecular Research Center, Medical Basic Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
5- Department of Biostatistics and Epidemiology, School of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
6- Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. & Fertility, Infertility and Perinatology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. ,
Full-Text [PDF 1544 kb]
(661 Downloads)
| Abstract (HTML) (907 Views)
Full-Text: (138 Views)
- Introduction
Almost 10% women of reproductive age have endometriosis, one of the most prevalent gynecological and estrogen-dependent disorders. This condition, which is characterized by tissue implants resembling endometrial glands and stroma on the visceral and peritoneal surfaces of the pelvis as well as on the ovaries and outside the uterine cavity (1), is linked to symptoms like dyspareunia, dysmenorrhea, dysuria, pelvic pain, and infertility (2). Physical examination, pelvic ultrasound, transvaginal ultrasound, and pelvic magnetic resonance imaging are some of the diagnostic procedures for endometriosis. However, laparoscopy is the only reliable and conclusive method for diagnosing endometriosis.
Hormone therapy, painkillers, and surgery are categorized as the most available treatments used for endometriosis (3). According to the reported studies, it seems that a dysregulated immune response is closely related to the occurrence of endometriosis. The phenotype and function of natural killer (NK) cells in the peritoneum, blood, and endometrium can influence the pathophysiology of endometriosis (4). Based on CD56 and CD16 expression, NK cells are divided into 2 groups: CD56bright CD16- NK cells and CD56dim CD16+ NK cells. The cytokines, including interleukin (IL) 10, IL-13, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ), are mostly produced by CD56bright cells, which comprise 10% of all NK cells in the peripheral blood (PB) and are primarily found in the decidua and uterine endometrium (5).
On the other hand, 90% or so of all circulating PB NK cells are CD56dim cells with strong cytotoxicity (2). In the past few years, phosphorus and calcium homeostasis regulation and the role of low vitamin D levels in the occurrence of various diseases of the female reproduction system, such as endometriosis, have been assessed (6).
It has been demonstrated that vitamin D is crucial for controlling immunological responses. Due to the immunoregulatory properties of vitamin D, this prohormone is also considered in endometriosis, as its function leads to the suppression of IL-1β and TNF-α expression in this condition (7). Regarding immune system dysfunction in women with endometriosis, cytokine imbalances have been reported. In this regard, the role of IFN-γ has been particularly highlighted in the severity and immunopathogenesis of endometriosis (6). Moreover, the vitamin D receptor is found on the surface of most immune cells, including lymphocytes, and it controls the production of cytokines, including IL-4, IL-5, IL-6, IL-10, IL-13, and IFN-γ (8). It may show that vitamin D is essential for the growth, maturation, differentiation, and cytotoxicity of NK cells, as well as their function (9).
Not much research has been done on how vitamin D affects immune cells in endometriosis-affected women's PB. The current study was designed to compare endometriosis cases to healthy individuals in terms of the percentage of NK cells and their subsets in PB and their relationship to the serum levels of vitamin D and IFN-γ.
Hormone therapy, painkillers, and surgery are categorized as the most available treatments used for endometriosis (3). According to the reported studies, it seems that a dysregulated immune response is closely related to the occurrence of endometriosis. The phenotype and function of natural killer (NK) cells in the peritoneum, blood, and endometrium can influence the pathophysiology of endometriosis (4). Based on CD56 and CD16 expression, NK cells are divided into 2 groups: CD56bright CD16- NK cells and CD56dim CD16+ NK cells. The cytokines, including interleukin (IL) 10, IL-13, tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ), are mostly produced by CD56bright cells, which comprise 10% of all NK cells in the peripheral blood (PB) and are primarily found in the decidua and uterine endometrium (5).
On the other hand, 90% or so of all circulating PB NK cells are CD56dim cells with strong cytotoxicity (2). In the past few years, phosphorus and calcium homeostasis regulation and the role of low vitamin D levels in the occurrence of various diseases of the female reproduction system, such as endometriosis, have been assessed (6).
It has been demonstrated that vitamin D is crucial for controlling immunological responses. Due to the immunoregulatory properties of vitamin D, this prohormone is also considered in endometriosis, as its function leads to the suppression of IL-1β and TNF-α expression in this condition (7). Regarding immune system dysfunction in women with endometriosis, cytokine imbalances have been reported. In this regard, the role of IFN-γ has been particularly highlighted in the severity and immunopathogenesis of endometriosis (6). Moreover, the vitamin D receptor is found on the surface of most immune cells, including lymphocytes, and it controls the production of cytokines, including IL-4, IL-5, IL-6, IL-10, IL-13, and IFN-γ (8). It may show that vitamin D is essential for the growth, maturation, differentiation, and cytotoxicity of NK cells, as well as their function (9).
Not much research has been done on how vitamin D affects immune cells in endometriosis-affected women's PB. The current study was designed to compare endometriosis cases to healthy individuals in terms of the percentage of NK cells and their subsets in PB and their relationship to the serum levels of vitamin D and IFN-γ.
- Materials and Methods
- Study participants
In this case-control study, 59 reproductive age women (18-45 yr) were enrolled. The study was conducted in the Immunology Department of Isfahan University of Medical Sciences, Isfahan, Iran between November 2021 and June 2022.
2 groups were included in the study: 29 women with late endometriosis (stages III and IV) according to the revised American Society for Reproductive Medicine classification (10) as the case group and 30 healthy women with at least one live birth, without clinical or para-clinical evidence of endometriosis, as the control group. A gynecologist chose the case group, among the individuals who referred to the Shahid Beheshti hospital in Isfahan, Iran. The inclusion criteria were those who complained of pelvic pain, including dysmenorrhea, dyspareunia, or dyschezia, and whose endometriosis was diagnosed by transvaginal or transrectal sonography. On an average, 20 severe cases of endometriosis come to this center every month.
The following were the exclusion criteria for both groups: The existence of known autoimmune disorders and other acute or chronic inflammatory disorders, diagnosis of malignant neoplasia and infectious disease, diagnosed systemic disease including liver, kidney, coronary disease, diabetes, and hypertension, hormonal treatment (including oral contraceptives, gonadotropin-releasing hormone analogs, etc.); vitamin D supplementation during the previous 3 months, pregnancy, and menopause. In both the follicular and luteal phases of the menstrual cycle, 5 ml of PB were drawn from each participant and divided into 2 parts: 2 ml of the blood was transferred to an ethylenediamine tetra acetic acid tube for flow cytometry. The remaining 3 ml was collected in a different tube and left to clot at room temperature. The serum was then separated and kept at -70°C until serum vitamin D and IFN-γ were measured.
2 groups were included in the study: 29 women with late endometriosis (stages III and IV) according to the revised American Society for Reproductive Medicine classification (10) as the case group and 30 healthy women with at least one live birth, without clinical or para-clinical evidence of endometriosis, as the control group. A gynecologist chose the case group, among the individuals who referred to the Shahid Beheshti hospital in Isfahan, Iran. The inclusion criteria were those who complained of pelvic pain, including dysmenorrhea, dyspareunia, or dyschezia, and whose endometriosis was diagnosed by transvaginal or transrectal sonography. On an average, 20 severe cases of endometriosis come to this center every month.
The following were the exclusion criteria for both groups: The existence of known autoimmune disorders and other acute or chronic inflammatory disorders, diagnosis of malignant neoplasia and infectious disease, diagnosed systemic disease including liver, kidney, coronary disease, diabetes, and hypertension, hormonal treatment (including oral contraceptives, gonadotropin-releasing hormone analogs, etc.); vitamin D supplementation during the previous 3 months, pregnancy, and menopause. In both the follicular and luteal phases of the menstrual cycle, 5 ml of PB were drawn from each participant and divided into 2 parts: 2 ml of the blood was transferred to an ethylenediamine tetra acetic acid tube for flow cytometry. The remaining 3 ml was collected in a different tube and left to clot at room temperature. The serum was then separated and kept at -70°C until serum vitamin D and IFN-γ were measured.
-
- Sample size
Based on a study with similar parameters (the disparity in the KIR2DL1_NK cells index between the case and control groups) (11), and considering a 95% confidence level and 80% test power, the sample size was estimated at 25 patients per group. Ultimately, we enrolled 30 patients in each group using the following formula.
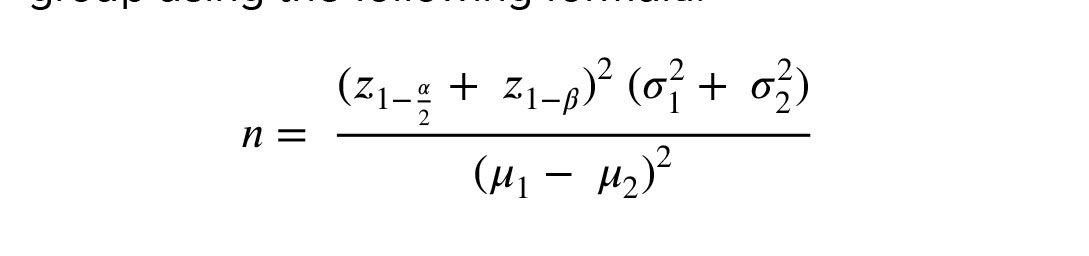
2.3. Isolation of peripheral mononuclear cells (PBMC) and flow cytometry analysis
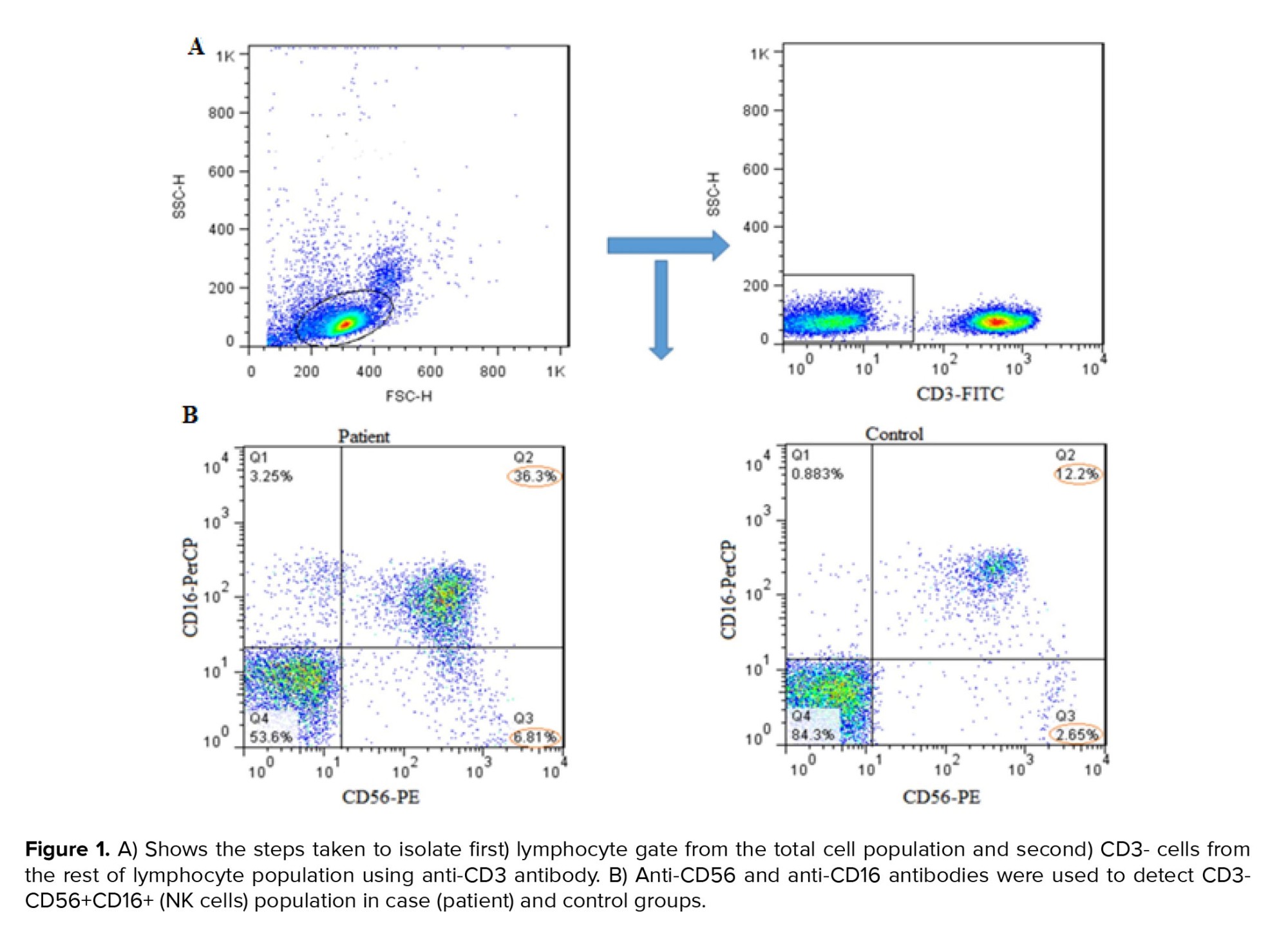
PBMCs were isolated by Ficoll density gradient centrifugation (density, 1.077 ± 0.002) (Sigma, USA) and washed 2 times with phosphate-buffered saline. PBMCs were then stained with FITC-Anti-CD3 (BioLegend, 344804), PerCP-Anti-CD16 (BioLegend, 360719), and PE-Anti-CD56 (BioLegend, 362508), and incubated for 30 min at 4°C in the dark. Finally, stained PBMCs were detected by FACSCalibur flow cytometer (Becton Dickinson, USA), and at least 50,000 events were recorded for each sample, and the data were analyzed using FlowJo software 10.0 (TreeStar, Ashland, U.S.A.). Fluorescence minus one control was utilized for the CD56 and CD16 antibodies. The lymphocyte population was first gated in forward scatter/side scatter plots, further CD3- cells were selected in this population. Finally, CD3-, CD16+, and CD56+ cells were considered as NK cells.
2.4. Enzyme-linked immunosorbent (ELISA) assay
The concentration of IFN-γ in the serum samples was quantified using an IFN-γ ELISA kit (Karmania Pars Gene, Iran), and the 25(OH) vitamin D concentration was measured using an ELISA kit (Euroimmun Co., Germany) according to the manufacturer’s instructions.
2.5. Ethical considerations
All participants in the study gave their informed consent, and the protocol was authorized by the Ahvaz Jundishapur University of Medical Sciences Ethics Committee, Ahvaz, Iran (Code: IR.AJUMS.MEDICINE.REC.1400.053).
2.6. Statistical analysis
The normal or non-normal distribution of data were checked using the Kolmogorov-Smirnov test. The independent t test was used to analyze normal data, whereas the Mann-Whitney test was used for non-normal data. Statistical comparative tests were performed using the Mann-Whitney method, and Spearman's non-parametric method was used to check the correlation. The Chi-square test of Independence was used to evaluate if 2 categorical variables were associated. All analyses were performed at the p-value ≤ 0.05 error level and with the help of GraphPad Prism 9.3.0 software. The results are reported as Mean ± SEM (standard error of mean).
2.3. Isolation of peripheral mononuclear cells (PBMC) and flow cytometry analysis
PBMCs were isolated by Ficoll density gradient centrifugation (density, 1.077 ± 0.002) (Sigma, USA) and washed 2 times with phosphate-buffered saline. PBMCs were then stained with FITC-Anti-CD3 (BioLegend, 344804), PerCP-Anti-CD16 (BioLegend, 360719), and PE-Anti-CD56 (BioLegend, 362508), and incubated for 30 min at 4°C in the dark. Finally, stained PBMCs were detected by FACSCalibur flow cytometer (Becton Dickinson, USA), and at least 50,000 events were recorded for each sample, and the data were analyzed using FlowJo software 10.0 (TreeStar, Ashland, U.S.A.). Fluorescence minus one control was utilized for the CD56 and CD16 antibodies. The lymphocyte population was first gated in forward scatter/side scatter plots, further CD3- cells were selected in this population. Finally, CD3-, CD16+, and CD56+ cells were considered as NK cells.
2.4. Enzyme-linked immunosorbent (ELISA) assay
The concentration of IFN-γ in the serum samples was quantified using an IFN-γ ELISA kit (Karmania Pars Gene, Iran), and the 25(OH) vitamin D concentration was measured using an ELISA kit (Euroimmun Co., Germany) according to the manufacturer’s instructions.
2.5. Ethical considerations
All participants in the study gave their informed consent, and the protocol was authorized by the Ahvaz Jundishapur University of Medical Sciences Ethics Committee, Ahvaz, Iran (Code: IR.AJUMS.MEDICINE.REC.1400.053).
2.6. Statistical analysis
The normal or non-normal distribution of data were checked using the Kolmogorov-Smirnov test. The independent t test was used to analyze normal data, whereas the Mann-Whitney test was used for non-normal data. Statistical comparative tests were performed using the Mann-Whitney method, and Spearman's non-parametric method was used to check the correlation. The Chi-square test of Independence was used to evaluate if 2 categorical variables were associated. All analyses were performed at the p-value ≤ 0.05 error level and with the help of GraphPad Prism 9.3.0 software. The results are reported as Mean ± SEM (standard error of mean).
- Results
3.1. Clinical characteristics of the participants
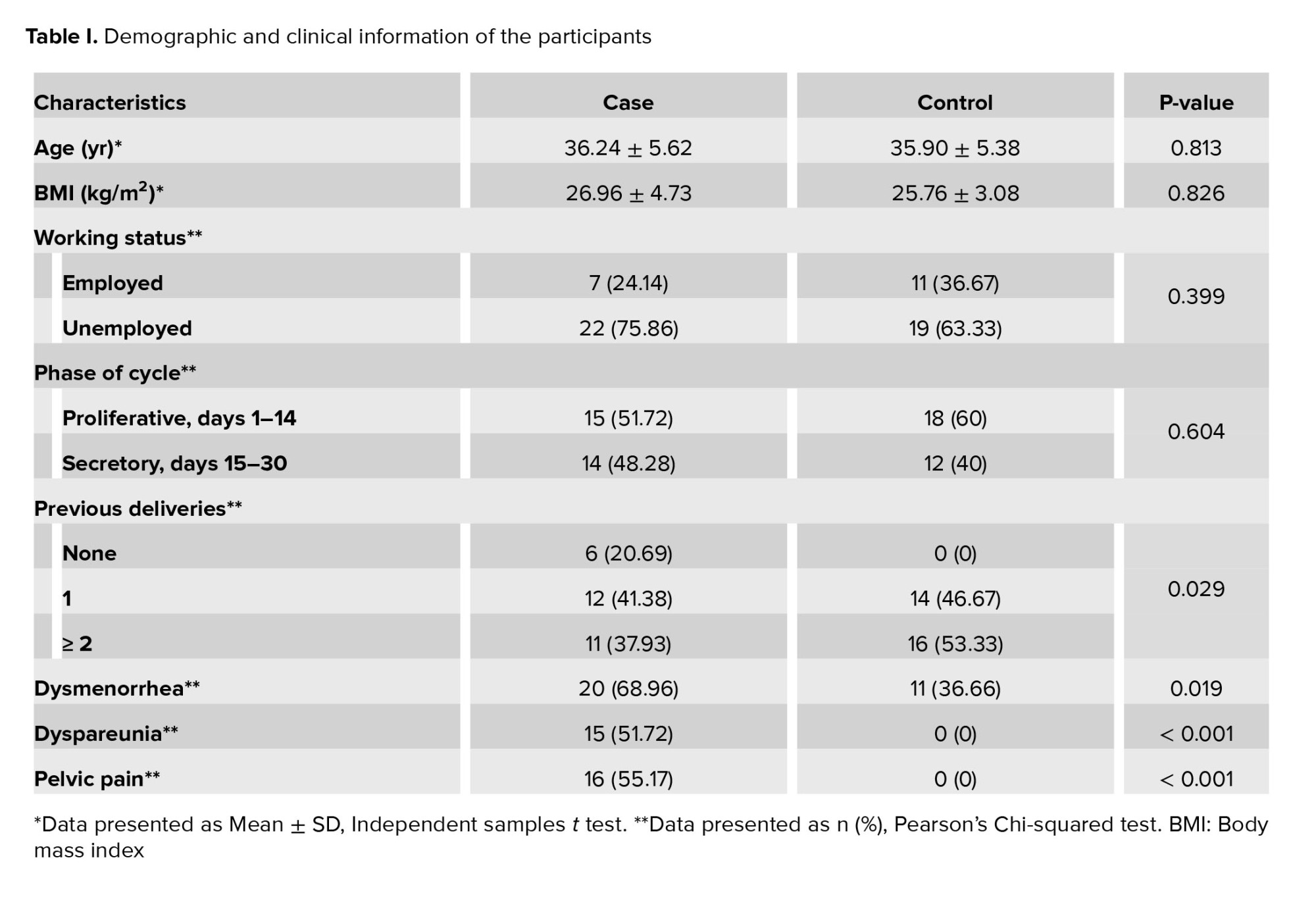
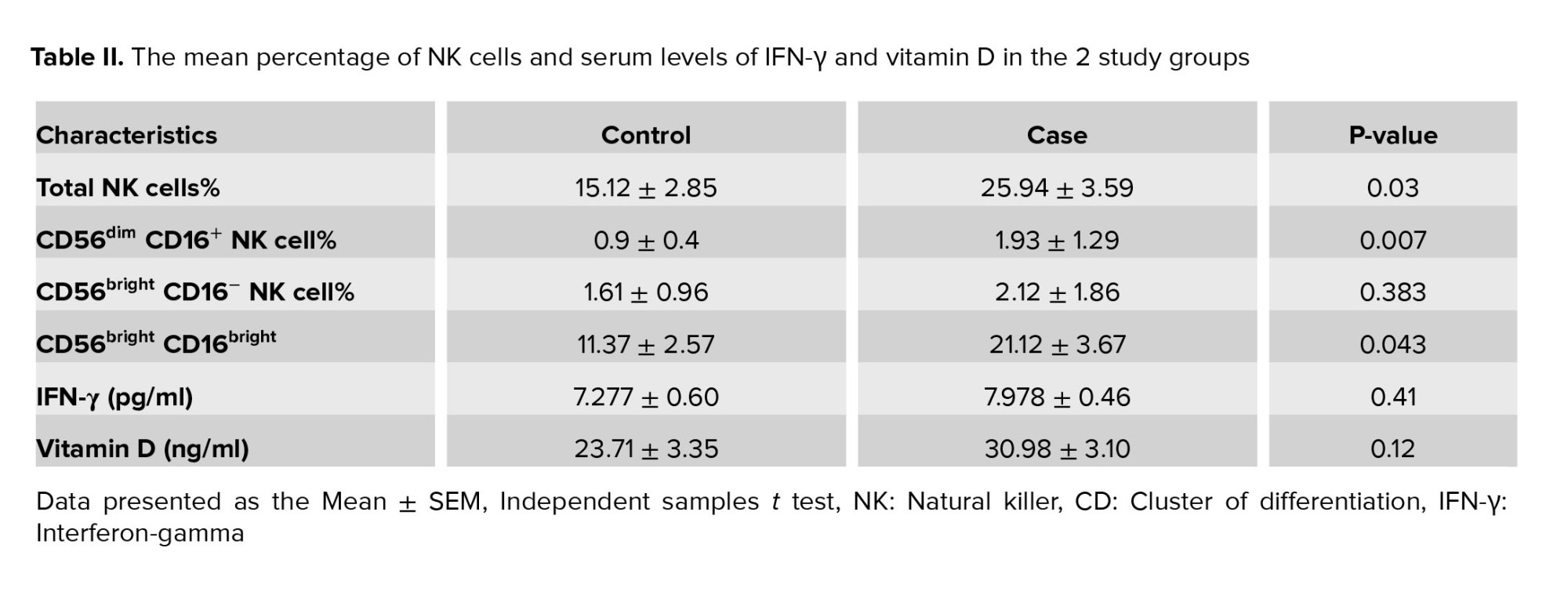
59 women were enrolled in the study, including 29 women with endometriosis and 30 healthy women as the control group. No statistically significant difference was observed in the age and body mass index of the studied women (Table I). According to table I, more women in the case group compared with the control group experienced dysmenorrhea (68.96 vs 36.66). Also, symptoms such as dyspareunia (p < 0.001) and pelvic pain (p < 0.001) were significantly higher in case group than in the control group. Table II shows the mean percentage of NK cells and serum levels of IFN-γ and vitamin D in the 2 study groups.
3.2. Evaluation of NK cells population
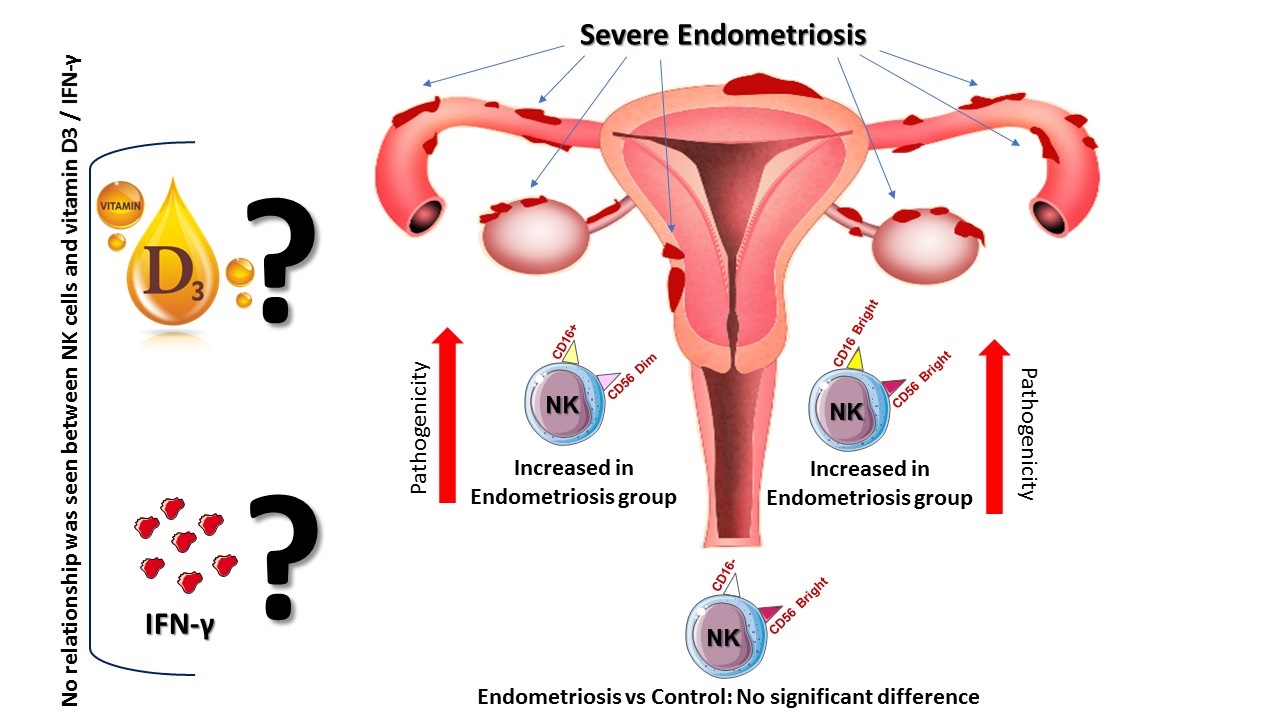
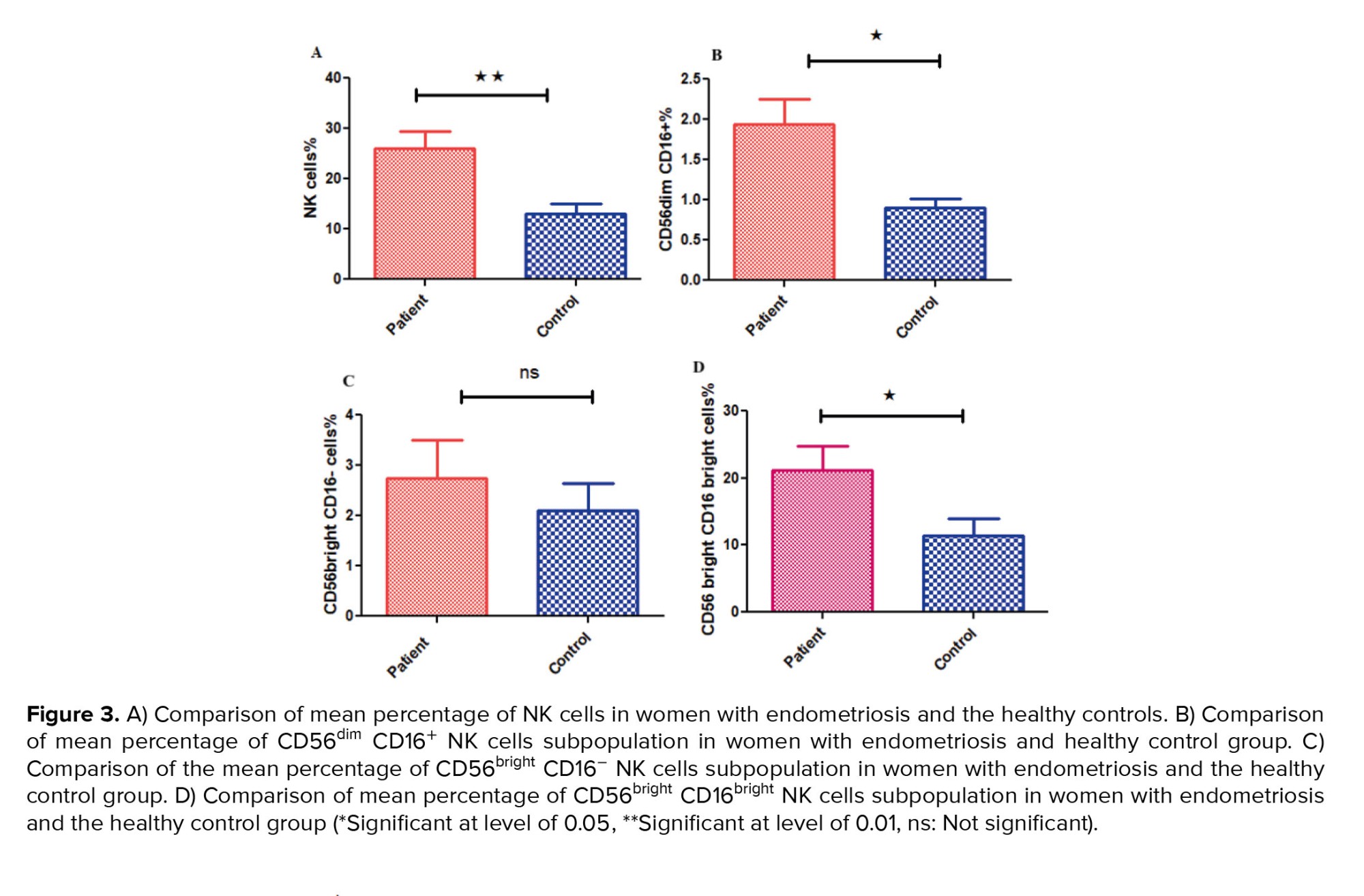
For determining the NK cells population, lymphocyte gates in the total cell population were isolated, and then in the CD3- cells population we selected CD16+ and CD56+ cells as NK cells (Figure 1A, B). The mean percentage of the total NK cells population (in the CD3- lymphocyte population) in the case group showed a significant increase compared to the controls (p = 0.03) (Figure 2A, 3A).
Also, a significant increase was observed in NK cells subpopulation in case group compared to the healthy control group, including CD56dim CD16+ (p = 0.007) (Figure 2B, 3B) and CD56bright CD16bright NK cells subpopulation (in CD3- lymphocyte population) (p = 0.043) (Figure 2D, 3D). However, no significant difference was observed in CD56bright CD16- subpopulation (p = 0.383) (Figure 2C, 3C). Also, no difference was observed in NK cells (p = 0.072) percentage in the endometriosis subjects according to variations related to the phase of the menstrual cycle.
Also, a significant increase was observed in NK cells subpopulation in case group compared to the healthy control group, including CD56dim CD16+ (p = 0.007) (Figure 2B, 3B) and CD56bright CD16bright NK cells subpopulation (in CD3- lymphocyte population) (p = 0.043) (Figure 2D, 3D). However, no significant difference was observed in CD56bright CD16- subpopulation (p = 0.383) (Figure 2C, 3C). Also, no difference was observed in NK cells (p = 0.072) percentage in the endometriosis subjects according to variations related to the phase of the menstrual cycle.
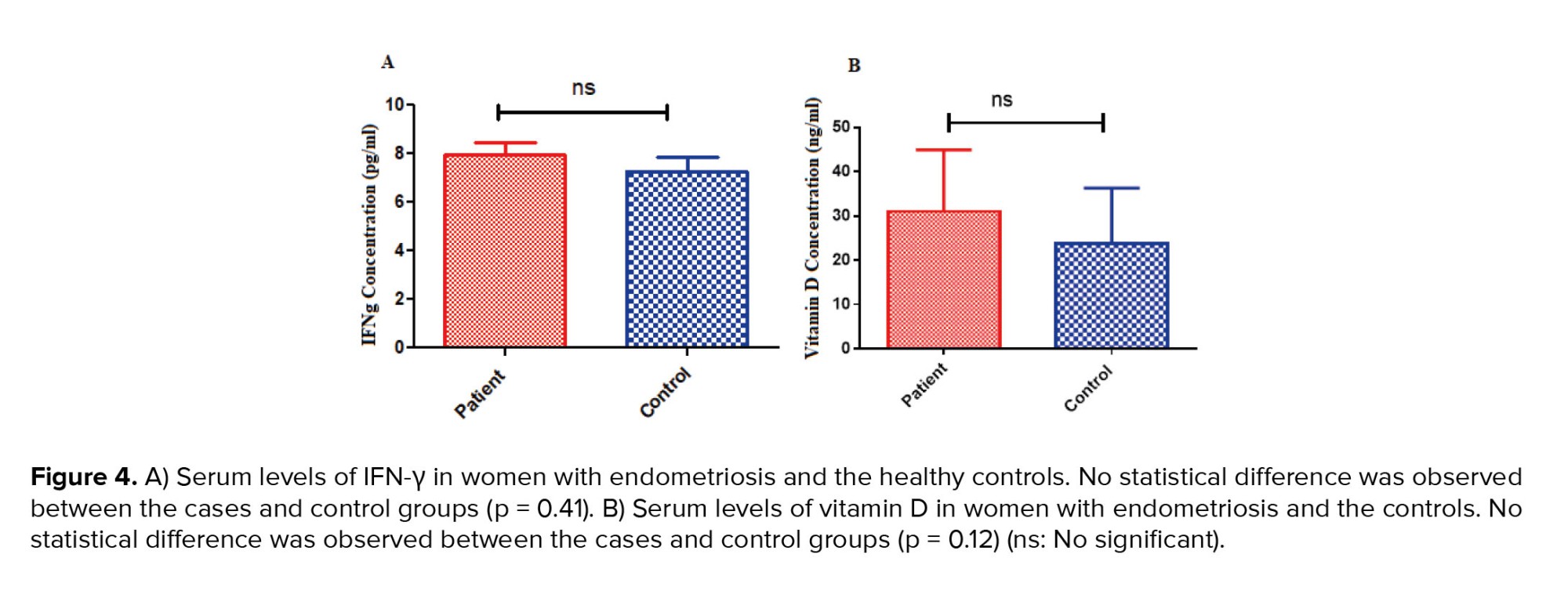
3.3. Comparison of serum IFN-γ and vitamin D
The serum level of IFN-γ increased in women with endometriosis compared to the control group, but no statistically significant difference was seen (p = 0.41) (Figure 4A). Although the serum level of vitamin D in women with endometriosis was increased compared to the control group, it was not statistically significant (p = 0.12) (Figure 4B).
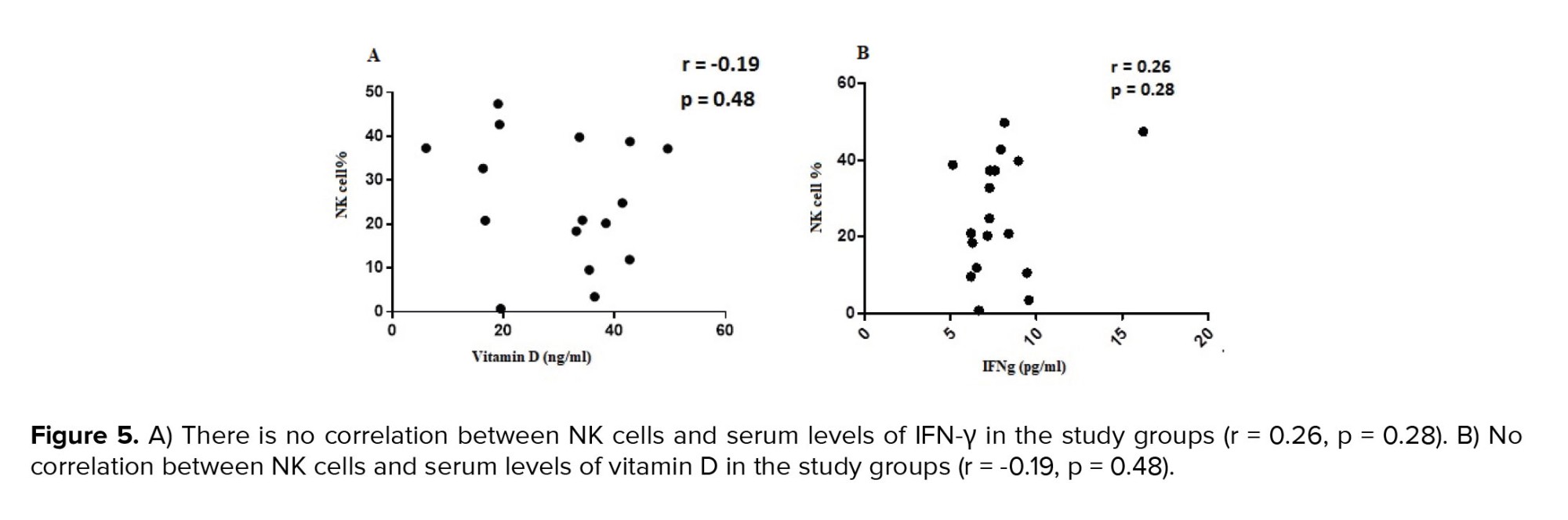
3.4. Correlation between NK cells percentage and serum levels of IFN-γ in the endometriosis group
Spearman's correlation test was performed to investigate the association between NK cells and IFN-γ serum concentration. The result showed that no correlation was observed between the percentage of NK cells and the serum level of IFN-γ (r = 0.26, p = 0.28) (Figure 5A). Also, no correlation was observed between the percentage of NK cells subpopulation CD56dim CD16+ (r = 0.201, p = 0.42) and CD56bright CD16bright (r = -0.43, p = 0.07), and the serum level of IFN-γ in the studied group. However, a significant positive correlation was observed between the percentage of NK cells subpopulation CD56bright CD16-, (r = 0.631, p = 0.007), and the serum levels of IFN-γ in the studied group.
3.5. Correlation between NK cells percentage and serum vitamin D level in the endometriosis group
No correlation was observed between the percentage of NK cells and serum vitamin D level in the studied group (r = -0.19, p = 0.48) (Figure 5B). Also, no correlation was observed between the percentage of CD56dim CD16+ (r = 0.306, p = 0.217), CD56bright CD16- (r = -0.016, p = 0.951), and CD56bright CD16bright (r = 0.265, p = 0.287) NK cells subpopulation and serum vitamin D level in the studied group.

4. Discussion
In this study, we observed that the total NK cells population in the case group was significantly increased compared to the control group. Also, a significant increase in CD56dim CD16+ and CD56bright CD16bright NK cells subpopulation was detected in endometriosis individuals compared to the control group. Consistent with our findings, it was reported that the mean percentage of NK cells in patients with endometriosis is significantly higher than in healthy individuals (12). NK T cells, another important factor in innate immunity, showed a significantly lower percentage in endometriosis cases compared to control group in the study. Different studies have reported contradictory results regarding the frequency of NK cells in PB and peritoneal fluid, with decreases or lack of differences between women with endometriosis and controls (2). It should be noted that in the study of Schmitz et al., they did not observe any significant difference in NK cells subsets of PB or menstrual effluent between endometriosis individuals and control group (13). Also, in our study, no difference was observed in NK cells percentage in the endometriosis subjects according to variations related to the phase of the menstrual cycle, which is in accordance with the other similar study (14). Despite the increase of NK cells in the PB and endometrium of individuals with endometriosis compared to the healthy control group, a decrease in the activity of these cells has been reported, and a huge decrease in the activity of these cells occurs at the advanced stages of endometriosis (15, 16).
NK cell functions are controlled by a variety of killer-activating receptors and killer-inhibitory receptors (KIR). Positive and negative signal transmission in NK cells happen through these receptors, respectively. KIR molecules are a multigenic family of immunoglobulin-like families that realize major histocompatibility complex class I and suppress NK cell activation (17). The increased expression of KIR in peripheral NK cells has been indicated in endometriosis subjects (18). Upregulation of inhibitory receptors on the cell surface of NK cells has been indicated in endometriosis individuals compared to healthy subjects (5). Increased expression of inhibitory receptors in NK cells may be the reason for the decreased activity of these cells. These reports reflect that with less activity from NK cells, the immune system tries to increase the number of these cells to prevent endometriosis. Studies have demonstrated that Th17 cells and IL-17A have essential functions in the onset of endometriosis. These cells promote the survival of endometrial cells and make them resistant to NK cell cytotoxicity. This happens through the activation of ERK1/2 signaling (19).
It was shown that IFN-γ stimulates apoptosis in eutopic endometrial cells but not in ectopic endometrial cells and may be the cause of the uncontrolled growth of endometriotic lesions (20). In addition, it has been shown that IFN-γ increases the expression of intracellular adhesion molecule-1 and may be related to the defective function of NK cells in endometriosis (21). Ectopic endometrial cells probably evade the cytotoxic mechanisms of NK cells by inhibiting.
In the present study, the serum levels of this cytokine increased in women with endometriosis compared to the control group, but it was not statistically significant. Our results regarding the concentration of IFN-γ in PB samples were consistent with another study (22). The author from another study reported that despite observing a significant increase in the levels of IFN-γ in the peritoneal fluid of individuals with endometriosis compared to the control group, they did not observe any difference in the level of cytokine IFN-γ in the PB of the cases compared to the control group (23). However, conflicting reports have been published regarding the concentration of this cytokine between endometriosis and non-endometriosis cases (8, 24). Additionally, it was shown that the expression of NKp46 and its co-expressed activating receptors are altered in endometriosis, leading to a reduction in NK cell cytotoxicity. As a result, endometrial cells in the abdominal cavity may not be eliminated by NK cells, leading to the production of TNF-α and IFN-γ (25).
Vitamin D deficiency is a predisposing factor for various diseases in women, such as uterine fibroids, endometriosis, breast cancer, recurrent pregnancy loss, and in vitro fertilization failure (26, 27). Vitamin D regulates immune responses through its receptor, which is located on the surface of most immune cells, including lymphocytes. It can affect the production of IL-4, IL-5, IL-6, IL-10, IL-13, and IFN-γ cytokines. According to the studies, it has been determined that vitamin D plays a role in the development, maturation, differentiation, and cytotoxicity of NK cells (9).
Irrespective of the presence or absence of cytokines, vitamin D decreases NK cells cytotoxicity while increasing inhibitory receptor expression and decreasing activation receptors. It also prevents the lethal activity of cytotoxic T cells and NK cells by reducing the polarization of perforin on the surface of cells. Accordingly, it has been reported that NK cells' function is greatly influenced by vitamin D (28). We observed a rise in serum vitamin D levels in the PB of women with endometriosis compared to the control group, but this was not statistically significant. Consistent with our findings, other studies did not observe any difference in serum vitamin D levels between cases with endometriosis and healthy subjects. In another study, endometriosis women with treatment showed higher levels of 25(OH) vitamin D in their serum compared with those without (29). Moreover, there are conflicting reports regarding serum vitamin D concentrations between endometriosis and non-endometriosis cases (30). Differences in vitamin D measurement methods, receptor polymorphism, and binding protein may contribute to varying reports of vitamin D's role in endometriosis.
In this study, we focused on measuring the amount of NK cells and different subtypes of NK cells, as well as examining the correlation of these NK cell subsets with vitamin D and IFN-γ. However, the study has limitations. We only measured the amount of NK cells in PB, not the endometrial tissue. Additionally, we measured the level of IFN-γ in serum, not intracellularly. Finally, we did not investigate the vitamin D receptor at the level of immune cells.
5. Conclusion
In conclusion, we found that the percentage of total NK cells, CD56dim CD16+ and CD56bright CD16bright NK cell subsets, were significantly increased in women with endometriosis. In addition, the level of serum vitamin D and IFN-γ increased in women with endometriosis compared to control group. However, no significant difference was observed. According to these findings, the study of the function and percentage of NK cell subsets can be useful in assessing and treating endometriosis disease. However, more comprehensive studies in the future are required to draw definitive conclusions about these observations.
Data availability
Data associated with the paper is available upon reasonable request from the corresponding author.
Author contributions
S.NCh: Conceptualization and methodology and writing original draft. M.Gh: Conceptualization and supervision, and writing-review and editing. N.E: Flow cytometry analysis. A.A: Software and validation. M.S: Formal analysis. M.H: Taking endometrial biopsies. All authors read and approved the final manuscript.
Acknowledgments
Financial support was received from the Fertility, Infertility, and Perinatology Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Grant no: FIRC-0021). The authors express gratitude to all women who participated in the study. We did not use artificial intelligence in any way in preparing the manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
3.5. Correlation between NK cells percentage and serum vitamin D level in the endometriosis group
No correlation was observed between the percentage of NK cells and serum vitamin D level in the studied group (r = -0.19, p = 0.48) (Figure 5B). Also, no correlation was observed between the percentage of CD56dim CD16+ (r = 0.306, p = 0.217), CD56bright CD16- (r = -0.016, p = 0.951), and CD56bright CD16bright (r = 0.265, p = 0.287) NK cells subpopulation and serum vitamin D level in the studied group.







4. Discussion
In this study, we observed that the total NK cells population in the case group was significantly increased compared to the control group. Also, a significant increase in CD56dim CD16+ and CD56bright CD16bright NK cells subpopulation was detected in endometriosis individuals compared to the control group. Consistent with our findings, it was reported that the mean percentage of NK cells in patients with endometriosis is significantly higher than in healthy individuals (12). NK T cells, another important factor in innate immunity, showed a significantly lower percentage in endometriosis cases compared to control group in the study. Different studies have reported contradictory results regarding the frequency of NK cells in PB and peritoneal fluid, with decreases or lack of differences between women with endometriosis and controls (2). It should be noted that in the study of Schmitz et al., they did not observe any significant difference in NK cells subsets of PB or menstrual effluent between endometriosis individuals and control group (13). Also, in our study, no difference was observed in NK cells percentage in the endometriosis subjects according to variations related to the phase of the menstrual cycle, which is in accordance with the other similar study (14). Despite the increase of NK cells in the PB and endometrium of individuals with endometriosis compared to the healthy control group, a decrease in the activity of these cells has been reported, and a huge decrease in the activity of these cells occurs at the advanced stages of endometriosis (15, 16).
NK cell functions are controlled by a variety of killer-activating receptors and killer-inhibitory receptors (KIR). Positive and negative signal transmission in NK cells happen through these receptors, respectively. KIR molecules are a multigenic family of immunoglobulin-like families that realize major histocompatibility complex class I and suppress NK cell activation (17). The increased expression of KIR in peripheral NK cells has been indicated in endometriosis subjects (18). Upregulation of inhibitory receptors on the cell surface of NK cells has been indicated in endometriosis individuals compared to healthy subjects (5). Increased expression of inhibitory receptors in NK cells may be the reason for the decreased activity of these cells. These reports reflect that with less activity from NK cells, the immune system tries to increase the number of these cells to prevent endometriosis. Studies have demonstrated that Th17 cells and IL-17A have essential functions in the onset of endometriosis. These cells promote the survival of endometrial cells and make them resistant to NK cell cytotoxicity. This happens through the activation of ERK1/2 signaling (19).
It was shown that IFN-γ stimulates apoptosis in eutopic endometrial cells but not in ectopic endometrial cells and may be the cause of the uncontrolled growth of endometriotic lesions (20). In addition, it has been shown that IFN-γ increases the expression of intracellular adhesion molecule-1 and may be related to the defective function of NK cells in endometriosis (21). Ectopic endometrial cells probably evade the cytotoxic mechanisms of NK cells by inhibiting.
In the present study, the serum levels of this cytokine increased in women with endometriosis compared to the control group, but it was not statistically significant. Our results regarding the concentration of IFN-γ in PB samples were consistent with another study (22). The author from another study reported that despite observing a significant increase in the levels of IFN-γ in the peritoneal fluid of individuals with endometriosis compared to the control group, they did not observe any difference in the level of cytokine IFN-γ in the PB of the cases compared to the control group (23). However, conflicting reports have been published regarding the concentration of this cytokine between endometriosis and non-endometriosis cases (8, 24). Additionally, it was shown that the expression of NKp46 and its co-expressed activating receptors are altered in endometriosis, leading to a reduction in NK cell cytotoxicity. As a result, endometrial cells in the abdominal cavity may not be eliminated by NK cells, leading to the production of TNF-α and IFN-γ (25).
Vitamin D deficiency is a predisposing factor for various diseases in women, such as uterine fibroids, endometriosis, breast cancer, recurrent pregnancy loss, and in vitro fertilization failure (26, 27). Vitamin D regulates immune responses through its receptor, which is located on the surface of most immune cells, including lymphocytes. It can affect the production of IL-4, IL-5, IL-6, IL-10, IL-13, and IFN-γ cytokines. According to the studies, it has been determined that vitamin D plays a role in the development, maturation, differentiation, and cytotoxicity of NK cells (9).
Irrespective of the presence or absence of cytokines, vitamin D decreases NK cells cytotoxicity while increasing inhibitory receptor expression and decreasing activation receptors. It also prevents the lethal activity of cytotoxic T cells and NK cells by reducing the polarization of perforin on the surface of cells. Accordingly, it has been reported that NK cells' function is greatly influenced by vitamin D (28). We observed a rise in serum vitamin D levels in the PB of women with endometriosis compared to the control group, but this was not statistically significant. Consistent with our findings, other studies did not observe any difference in serum vitamin D levels between cases with endometriosis and healthy subjects. In another study, endometriosis women with treatment showed higher levels of 25(OH) vitamin D in their serum compared with those without (29). Moreover, there are conflicting reports regarding serum vitamin D concentrations between endometriosis and non-endometriosis cases (30). Differences in vitamin D measurement methods, receptor polymorphism, and binding protein may contribute to varying reports of vitamin D's role in endometriosis.
In this study, we focused on measuring the amount of NK cells and different subtypes of NK cells, as well as examining the correlation of these NK cell subsets with vitamin D and IFN-γ. However, the study has limitations. We only measured the amount of NK cells in PB, not the endometrial tissue. Additionally, we measured the level of IFN-γ in serum, not intracellularly. Finally, we did not investigate the vitamin D receptor at the level of immune cells.
5. Conclusion
In conclusion, we found that the percentage of total NK cells, CD56dim CD16+ and CD56bright CD16bright NK cell subsets, were significantly increased in women with endometriosis. In addition, the level of serum vitamin D and IFN-γ increased in women with endometriosis compared to control group. However, no significant difference was observed. According to these findings, the study of the function and percentage of NK cell subsets can be useful in assessing and treating endometriosis disease. However, more comprehensive studies in the future are required to draw definitive conclusions about these observations.
Data availability
Data associated with the paper is available upon reasonable request from the corresponding author.
Author contributions
S.NCh: Conceptualization and methodology and writing original draft. M.Gh: Conceptualization and supervision, and writing-review and editing. N.E: Flow cytometry analysis. A.A: Software and validation. M.S: Formal analysis. M.H: Taking endometrial biopsies. All authors read and approved the final manuscript.
Acknowledgments
Financial support was received from the Fertility, Infertility, and Perinatology Research Center of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (Grant no: FIRC-0021). The authors express gratitude to all women who participated in the study. We did not use artificial intelligence in any way in preparing the manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Horne AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022; 379: e070750. [DOI:10.1136/bmj-2022-070750] [PMID]
2. Fukui A, Mai Ch, Saeki Sh, Yamamoto M, Takeyama R, Kato T, et al. Pelvic endometriosis and natural killer cell immunity. Am J Reprod Immunol 2021; 85: e13342. [DOI:10.1111/aji.13342] [PMID]
3. Weaver J, Chakladar S, Mirchandani K, Liu Z. Surgical and pharmacological treatment patterns in women with endometriosis: A descriptive analysis of insurance claims. J Women's Health 2022; 31: 1003-1011. [DOI:10.1089/jwh.2021.0060] [PMID]
4. Hoogstad-van Evert J, Paap R, Nap A, van der Molen R. The promises of natural killer cell therapy in endometriosis. Int J Mol Sci 2022; 23: 5539. [DOI:10.3390/ijms23105539] [PMID] [PMCID]
5. Reis JL, Rosa NN, Ângelo-Dias M, Martins C, Borrego LM, Lima J. Natural killer cell receptors and endometriosis: A systematic review. Int J Mol Sci 2022; 24: 331. [DOI:10.3390/ijms24010331] [PMID] [PMCID]
6. Almassinokiani F, Emadi S, Khodaverdi S, Salehiniya H. Comparison of serum levels of vitamin D between women with and without endometriosis. J Minim Invasive Surg Sci 2015; 4: e30560. [DOI:10.17795/minsurgery-3056]
7. Altieri B, Muscogiuri G, Barrea L, Mathieu C, Vallone CV, Mascitelli L, et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev Endocr Metab Disord 2017; 18: 335-346. [DOI:10.1007/s11154-016-9405-9] [PMID]
8. Malutan AM, Drugan T, Ciortea R, Bucuri C, Rada MP, Mihu D. Endometriosis-associated changes in serum levels of interferons and chemokines. Turk J Med Sci 2017; 47: 115-122. [DOI:10.3906/sag-1507-185] [PMID]
9. Skrobot A, Demkow U, Wachowska M. Immunomodulatory role of vitamin D: A review. Adv Exp Med Biol 2018; 1108: 13-23. [DOI:10.1007/5584_2018_246] [PMID]
10. The American Fertility Society. Revised american fertility society classification of endometriosis: 1985. Fertil Steril 1985; 43: 351-352. [DOI:10.1016/S0015-0282(16)48430-X] [PMID]
11. Maeda N, Izumiya C, Yamamoto Y, Oguri H, Kusume T, Fukaya T. Increased killer inhibitory receptor KIR2DL1 expression among natural killer cells in women with pelvic endometriosis. Fertil Steril 2002; 77: 297-302.
https://doi.org/10.1016/S0015-0282(01)03249-6 [DOI:10.1016/S0015-0282(01)02964-8]
12. Walankiewicz M, Grywalska E, Krasowska E, Hymos A, Sobstyl M, Kotarski J. The role of NK and NKT-like cells in endometriosis. Eur J Obstet Gynecol Reprod Biol 2016; 206 (Suppl.): e122. [DOI:10.1016/j.ejogrb.2016.07.314]
13. Schmitz T, Hoffmann V, Olliges E, Bobinger A, Popovici R, Nößner E, et al. Reduced frequency of perforin-positive CD8+ T cells in menstrual effluent of endometriosis patients. J Reprod Immunol 2021; 148: 103424. [DOI:10.1016/j.jri.2021.103424] [PMID]
14. Dias Jr JA, Podgaec S, de Oliveira RM, Marin MLC, Baracat EC, Abrão MS. Patients with endometriosis of the rectosigmoid have a higher percentage of natural killer cells in peripheral blood. J Minim Invasive Gynecol 2012; 19: 317-324. [DOI:10.1016/j.jmig.2011.12.021] [PMID]
15. Ścieżyńska A, Komorowski M, Soszyńska M, Malejczyk J. NK cells as potential targets for immunotherapy in endometriosis. J Clin Med 2019; 8: 1468. [DOI:10.3390/jcm8091468] [PMID] [PMCID]
16. Ghafourian M, Mousavi-Salehi A, Moramazi F, Ranjbari N. Immunohistochemical evaluation of NKP46 receptor expression and the number of NK cells in the endometrium of patients with endometriosis. Iran J Immunol 2024; 21: 27-36.
17. Quatrini L, Della Chiesa M, Sivori S, Mingari MC, Pende D, Moretta L. Human NK cells, their receptors and function. Europ J Immunol 2021; 51: 1566-1579. [DOI:10.1002/eji.202049028] [PMID] [PMCID]
18. Thiruchelvam U, Wingfield M, O'Farrelly C. Natural killer cells: Key players in endometriosis. Am J Reprod Immunol 2015; 74: 291-301. [DOI:10.1111/aji.12408] [PMID]
19. Kang Y-J, Cho HJ, Lee Y, Park A, Kim MJ, Jeung IC, et al. IL-17A and Th17 cells contribute to endometrial cell survival by inhibiting apoptosis and NK cell mediated cytotoxicity of endometrial cells via ERK1/2 pathway. Immune Netw 2023; 23: e14. [DOI:10.4110/in.2023.23.e14] [PMID] [PMCID]
20. Nishida M, Nasu K, Ueda T, Fukuda J, Takai N, Miyakawa I. Endometriotic cells are resistant to interferon-γ-induced cell growth inhibition and apoptosis: A possible mechanism involved in the pathogenesis of endometriosis. Mol Hum Reprod 2005; 11: 29-34. [DOI:10.1093/molehr/gah133] [PMID]
21. Wu M-H, Yang B-Ch, Lee Y-Ch, Wu P-L, Hsu Ch-Ch. The differential expression of intercellular adhesion molecule‐1 (ICAM‐1) and regulation by interferon‐gamma during the pathogenesis of endometriosis. Am J Reprod Immunol 2004; 51: 373-380. [DOI:10.1111/j.1600-0897.2004.00171.x] [PMID]
22. Hassa H, Tanir HM, Tekin B, Kirilmaz S, Sahin Mutlu F. Cytokine and immune cell levels in peritoneal fluid and peripheral blood of women with early-and late-staged endometriosis. Arch Gynecol Obstet 2009; 279: 891-895. [DOI:10.1007/s00404-008-0844-8] [PMID]
23. Podgaec S, Abrao M, Dias Jr J, Rizzo LV, De Oliveira R, Baracat EC. Endometriosis: An inflammatory disease with a Th2 immune response component. Hum Reprod 2007; 22: 1373-1379. [DOI:10.1093/humrep/del516] [PMID]
24. Xia T, Zeng K, Peng Q, Wu X, Lei X. Clinical significance of serum Th1/Th2 cytokines in patients with endometriosis. Women Health 2023; 63: 73-82. [DOI:10.1080/03630242.2022.2144986] [PMID]
25. Saeki S, Fukui A, Mai C, Takeyama R, Yamaya A, Shibahara H. Co-expression of activating and inhibitory receptors on peritoneal fluid NK cells in women with endometriosis. J Reprod Immunol 2023; 155: 103765. [DOI:10.1016/j.jri.2022.103765] [PMID]
26. Mousavi Salehi A, Ghafourian M, Amari A, Zargar M. Evaluation of NKT cell percentage and function and its relationship with serum IFN-γ and vitamin D levels in women with recurrent spontaneous abortion and recurrent implantation failure. Iran J Immunol 2022; 19: 369-377.
27. Dabrowski FA, Grzechocinska B, Wielgos M. The role of vitamin D in reproductive health- a Trojan horse or the golden fleece? Nutrients 2015; 7: 4139-4153. [DOI:10.3390/nu7064139] [PMID] [PMCID]
28. Ota K, Dambaeva S, Kim MWI, Han AR, Fukui A, Gilman‐Sachs A, et al. 1, 25‐dihydroxy‐vitamin D3 regulates NK‐cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur J Immunol 2015; 45: 3188-3199. [DOI:10.1002/eji.201545541] [PMID]
29. Matasariu DR, Mandici CE, Ursache A, Bausic AIG, Bujor IE, Cristofor AE, et al. Vitamin D and mitosis evaluation in endometriosis: A step toward discovering the connection? Biomedicines 2023; 11: 2102. [DOI:10.3390/biomedicines11082102] [PMID] [PMCID]
30. Delbandi A-A, Torab M, Abdollahi E, Khodaverdi S, Rokhgireh S, Moradi Z, et al. Vitamin D deficiency as a risk factor for endometriosis in Iranian women. J Reprod Immunol 2021; 143: 103266. [DOI:10.1016/j.jri.2020.103266] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |