Thu, Feb 5, 2026
[Archive]
Volume 22, Issue 1 (January 2024)
IJRM 2024, 22(1): 31-42 |
Back to browse issues page
Ethics code: No: [2020] 211
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dai X, He Y, Li S, Sun Y, Sun W, Tian Z, et al . Modulating the RFamide-related peptide-3/G protein-coupled receptor 147 signaling pathway with nourishing Yin-removing fire herbal mixture to alleviate precocious puberty in female rats: An experimental study. IJRM 2024; 22 (1) :31-42
URL: http://ijrm.ir/article-1-3155-en.html
URL: http://ijrm.ir/article-1-3155-en.html
Xiaoli Dai1 

 , Yuanyuan He1
, Yuanyuan He1 

 , Suhuan Li2
, Suhuan Li2 

 , Yanyan Sun1
, Yanyan Sun1 

 , Wen Sun1
, Wen Sun1 

 , Zhanzhuang Tian3
, Zhanzhuang Tian3 

 , Jian Yu1
, Jian Yu1 

 , Nurgul Yerkinkyzy Ablakimova4
, Nurgul Yerkinkyzy Ablakimova4 

 , Yonghong Wang *5
, Yonghong Wang *5 




 , Yuanyuan He1
, Yuanyuan He1 

 , Suhuan Li2
, Suhuan Li2 

 , Yanyan Sun1
, Yanyan Sun1 

 , Wen Sun1
, Wen Sun1 

 , Zhanzhuang Tian3
, Zhanzhuang Tian3 

 , Jian Yu1
, Jian Yu1 

 , Nurgul Yerkinkyzy Ablakimova4
, Nurgul Yerkinkyzy Ablakimova4 

 , Yonghong Wang *5
, Yonghong Wang *5 


1- Traditional Chinese Medicine Department, Children's Hospital of Fudan University, Shanghai, China.
2- Yantaishan Hospital, Shangdong, China.
3- Department of Integrative Medicine and Neurobiology, Fudan University, Shanghai, China.
4- Department of Pharmacology, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan.
5- Traditional Chinese Medicine Department, Children's Hospital of Fudan University, Shanghai, China. ,wyhekyy@126.com
2- Yantaishan Hospital, Shangdong, China.
3- Department of Integrative Medicine and Neurobiology, Fudan University, Shanghai, China.
4- Department of Pharmacology, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan.
5- Traditional Chinese Medicine Department, Children's Hospital of Fudan University, Shanghai, China. ,
Keywords: Nourishing Yin-removing fire, RFamide-related peptide-3, G protein-coupled receptor 147, Hypo-thalamus, Puberty, Precocious.
Full-Text [PDF 1402 kb]
(949 Downloads)
| Abstract (HTML) (1025 Views)
Full-Text: (175 Views)
1. Introduction
Precocious puberty (PP) is a prevalent endocrine disorder in children with premature onset of secondary sexual characteristics (1). In China, the study estimated a prevalence of 6.19% (11.47% girls, 3.26% boys) based on a survey of 4058 primary school students (2). The onset of puberty is a complex biological process under the control of neuroendocrine pathways with multiple factors involved in regulating the hypothalamic-pituitary-gonadal (HPG) axis (3). Puberty initiation relies on crucial mechanisms such as activating the gonadotropin-releasing hormone (GnRH) pulse generator and the emergence of GnRH peaks, which is the key player in puberty onset, meanwhile, the disruption of GnRH release leads to gonadotropin-dependent PP (4).
Kisspeptin, a neuropeptide encoded by the Kiss1 gene, plays a vital role in activating the hypothalamic-HPG axis. By stimulating the hypothalamus, Kiss1 acts upstream of GnRH and is recognized as a critical regulator of GnRH secretion, which may help control the timing of puberty (5). GnRH neuron regulation involves excitatory and inhibitory networks, with gonadotropin-inhibitory hormone (GnIH) playing a significant inhibitory role (6). As an inhibitor of HPG axis, GnIH was the first to be identified in the Japanese quail, marking its initial discovery (7). GnIH could directly inhibit GnRH release in the quail brain, which could open a new window in the knowledge of HPG axis function (8). The RFamide-related peptide (RFRP) precursor, the mammalian equivalent of GnIH, produces 2 neuropeptides: RFRP1 and RFRP3 (9).
The G protein-coupled receptor 147 (GPR147) receptor, a novel GPR, has been identified in the Japanese quail. GPR147 binds to RFRP3 on some GnRH and kisspeptin neurons. Additionally, RFRP3 may influence GnRH and kisspeptin neurons (10). Meanwhile, they conducted intracerebroventricular injections of RFRP3 in female rats, observing downregulation of GnRH and Kiss1 mRNA expression in the hypothalamus and significant delays in puberty onset (11). These findings suggest that RFRP3/GPRl47 signal pathway can function as an inhibitory regulator of reproductive capability, by regulating gonadotrophin synthesis and release through its effects on the HPG axis.
The nourishing Yin-removing fire (NYRF) herbal mixture (Shanghai pharmacists system number Z05170908, consists of Rehmannia glutinosa [Sheng-Di-Huang], Scrophularia buergeriana [Xuan-Shen], and Anemarrhena asphodeloides [Zhi-Mu], etc.), an experienced prescription originating from the famous TCM experts, has been used to treat PP for decades (12). We have observed that it could mitigate the progression of sexual characteristics in PP girls with kidney-yin deficiency and fire hyperactivity (13). In previous experimental studies, we have found that NYRF mixture could suppress the expression of kisspeptin and GnRH in the hypothalamus of female PP rats (14).
Considering the multi-target therapeutic effects of the Chinese herbal mixtures, we investigated the modulation of NYRF herbal mixture to the RFRP3/GPR147 signal pathway in the hypothalamus of PP female rats, in order to provide additional insights into the therapeutic mechanism of the NYRF herbal mixture in the context of PP.
2. Materials and Methods
2.1. Animal procurement and housing
Newborn Sprague-Dawley rats, along with their mother, were procured from the Laboratory Animal Business Department, of Shanghai Institute of Family Planning (license number SCXK [Shanghai] 2018-0006) to conduct an experimental study. The rats were housed under standard conditions at the Department of Integrative Medicine and Neurobiology, Fudan University, Shanghai, China which included a temperature of 22 ± 2oC, humidity of 30-40%, and a 12-hr light/dark cycle. Adequate food and water were provided to the rats ad libitum.
2.2. Preparation of the herbal mixture
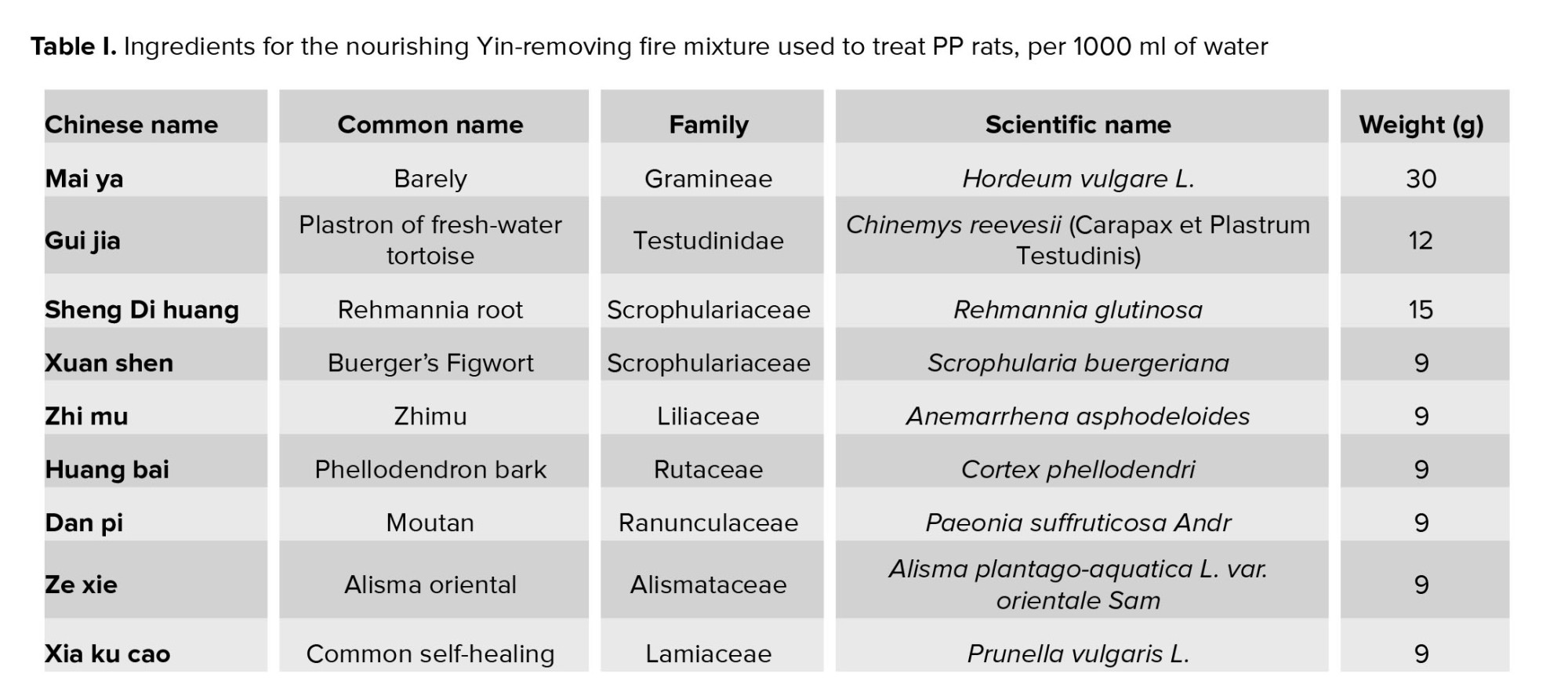
The initial formulation of the NYRF herbal mixture, formulated by the Department of Integrative Medicine at Children's hospital of Fudan University (Shanghai Medicine: Z05170908 consisted of ingredients such as Rehmannia glutinosa, Scrophulariae Radix [dried root of Scrophularia ningpoensis], and Phellodendri Amurensis Cortex [dried bark of Phellodendron chinense], etc.) (Table I). The reparation method employed in this study follows the protocol outlined in our prior research (15).
To obtain the NYRF herbal mixture extract, the traditional method of water extraction followed by alcohol precipitation was chosen (16). The crude drugs were decocted using a thermostat electric set (Zhengzhou Great Wall Scientific Industrial & Trade Co. Ltd., Zhengzhou, China) at 100oC for 40 min. The set was refilled with water, and the decoction continued for another 40 min. The extractive liquid was then accumulated and concentrated on a rotary evaporator (Buchi, Switzerland) for 15 min at 4oC at 1000 rpm/min. Absolute ethanol was slowly added to the extractive liquid until the ethanol concentration reached 60%. The mixture was then incubated at 4oC for 72 hr to remove the impurities that might dissolve in ethanol and precipitate to the bottom. The supernatant was extracted and the ethanol was subsequently removed from it using a rotary evaporator for 15 min at 4oC at 1000 rpm/min. Following this method, the final drug was obtained at a concentration of 2.7 gr per/ml (15).
2.3. Study design, animal grouping, and drug administration
In an experimental study, 24 rats were allocated randomly into 4 groups (n = 6): the normal group, the model group, the normal saline group, and the NYRF group. Except for the rats in the normal group, all the other groups were administered a single subcutaneous injection of 300 µg/25 µL danazol (dissolved in ethylene glycol: ethanol, 1:1) on postnatal day (PD)5 to induce the PP model (17). From PD15 to PD30, the rats in the NYRF group received an oral administration of the NYRF extract twice daily (1 mL/100 gr), while the rats in the normal saline group were given an equal volume of saline. No additional criteria were employed for participant inclusion or exclusion.
To minimize potential confounders, each participant's order of treatments and measurements were randomized. Animal cages were randomly assigned and periodically rotated to mitigate any potential bias associated with cage location. These measures were taken to minimize extraneous factors and enhance the study’s internal validity. At the stage of group allocation, the researchers responsible for assigning the rats to their respective groups were aware of the group assignments. However, during the experiment, the outcome assessment, the subsequent data analysis, and the individuals involved were blinded to the group allocation to minimize potential biases and ensure objective evaluation and analysis of the results.
2.4. Sex character observation, samples management
Daily monitoring of vaginal opening commenced from PD15, and the timing of vaginal opening was documented. After the day of vaginal opening, vaginal smears were taken regularly every morning to observe estrous cycle phases until PD30. There are 4 phases in the entire estrous cycle: proestrus, estrus, metestrus, and diestrus, which last 4-5 days. The vaginal secretions comprise 3 types of cells: leucocytes, cornified epithelial cells, and nucleated epithelial cells (18). During the estrus phase, there is a high presence of enucleated cornified epithelial cells. The cytoplasm is granular, and the cells have an irregular shape. At PD30, all rats (n = 6 in each group) were anesthetized through intraperitoneal injection of pentobarbital sodium (50 mg/kg) (19). Serum samples were collected and stored at -80°C for subsequent hormone level analysis. The hypothalamus was also collected and stored at -80°C, whereas the uterus and ovaries were excised and weighed.
2.5. Organ coefficients, hormone level detection
The uterine and ovarian coefficients were computed (n = 6 in each group) utilizing the organ index formula: ([organ wet weight (g)/body weight (g)] ×104). To determine the levels of estradiol (E2), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) in the serum samples, enzyme-linked immunosorbent assay kits (eBioscience, Affymetrix, USA) were employed.
2.6. Quantitative real-time polymerase chain reaction (qRT-PCR)
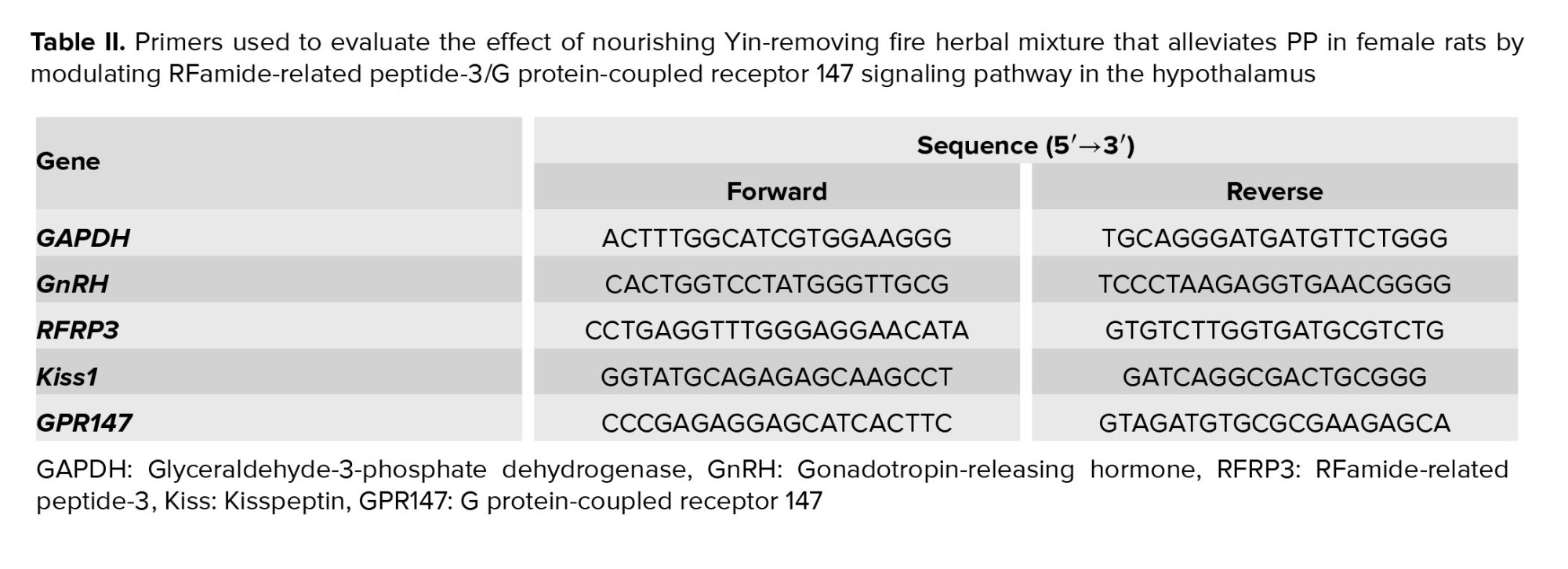
Total RNA was extracted using the Trizol method and the concentration and purity were determined using the ultraviolet spectrophotometer (OD260/OD280, 1.8~2.0). Then, the Real-Time PCR Master Mix Code (Toyobo, Japan) was used to reverse transcribe the RNA into cDNA. The primer sequences, as listed in table II, were designed and synthesized by Shanghai Jierui. The qRT-PCR system was conducted following the ReverTra Ace qRT-PCR kit protocol (Toyobo, Japan), which involved an initial step at 95oC for 180 sec, followed by 40 cycles of 95oC for 5 sec and 60oC for 30 sec. The relative expression of mRNA in the hypothalamus of rats (n = 6/each) was determined using the 2-△△Ct method.
2.7. Protein analysis by western blotting
Protein extraction from the hypothalamus of rats (n = 6/each) was conducted using radioimmunoprecipitation assay (RIPA) buffer, and the protein concentration was determined using a bicinchoninic acid kit (Biosharp, Hefei, China). Gels were prepared and concentrated accordingly to separate the proteins based on their molecular weight. The protein samples were then subjected to electrophoresis and transferred onto polyvinylidene fluoride membranes. These membranes were blocked with 5% bovine serum albumin (BSA) in tris-buffered saline containing 0.05% tween 20 (TBST) and subsequently incubated overnight at 4oC with primary antibodies, including anti-kisspeptin antibody (Abcam, US), anti-RFRP3 antibody (Santa Cruz, US), and anti-GPR147 antibody (Biorbyt, UK). After 3 washes with TBST for 10 min each, the membranes were exposed to suitable secondary antibodies for 1 hr. Chemiluminescence detection was performed using an electrochemiluminescence reagent, and the intensity of the protein bands was quantified using ImageJ software.
2.8. Ethical considerations
The study was approved by the Ethical Committee of the Children’s hospital of Fudan University, Shanghai, China (No: [2020] 211). The animals involved in the study were treated and handled in accordance with the Ethical Guidelines of Fudan University, Shanghai, China.
2.9. Statistical analysis
The study outcomes encompassed several parameters, including the timing of vaginal opening, ovarian coefficient, uterine coefficient, LH, FSH, LH/FSH ratio, E2, GnRH mRNA expression, Kiss1 mRNA expression, kisspeptin protein expression, RFRP3 mRNA expression, RFRP3 protein expression, GPR147 mRNA expression, and GPR147 protein expression. The comparisons between the groups were conducted using one-way ANOVA and post hoc Tukey test. Pearson correlation analysis was utilized to evaluate correlations. A significance level of p < 0.05 was considered statistically significant. The calculation was based on the protein expression data obtained after the study. Statistical analyses were carried out using SPSS for Windows program, version 22 (SPSS Inc., Chicago, Illinois, USA). Group means and standard errors were reported in both text and graphs using GraphPad Prism version 9.0 for Windows (GraphPad Software Inc., San Diego, CA, USA).
The sample size calculation for this study was conducted using G*Power 3.9.11.2 software, considering a desired statistical power of 0.8, an alpha error level of 0.05, and employing a One-way ANOVA test for 4 groups.
3. Results
3.1. Assessment of vaginal opening time, sex organ development, estrous cycle phases, body weight in different groups
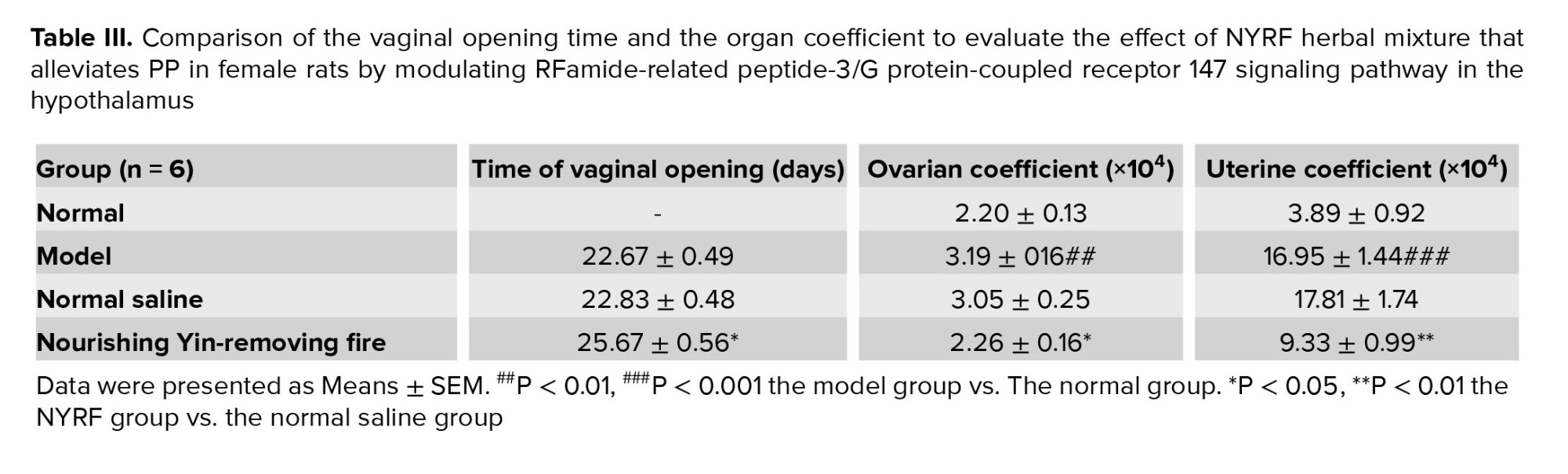
The timing of vaginal opening was carefully documented throughout the experimental period. No instances of vaginal opening were observed in the normal group until PD30. In contrast, the model group exhibited significantly early vaginal opening compared to the normal group. Notably, the NYRF group showed a considerably delayed time of vaginal opening in comparison to both the model group (p = 0.01, Table III) and the normal saline group (p = 0.01).
The organ coefficients of the uterus and ovaries were analyzed to evaluate reproductive organ development. Remarkably, the ovarian and uterine coefficient of rats in the model group exhibited a notable increase compared to those in the normal group (p = 0.004; p = 0.000). In contrast, the NYRF group displayed lower ovarian and uterine coefficients than the model group (p = 0.008; p = 0.003) and the normal saline group (p = 0.026; p = 0.001).
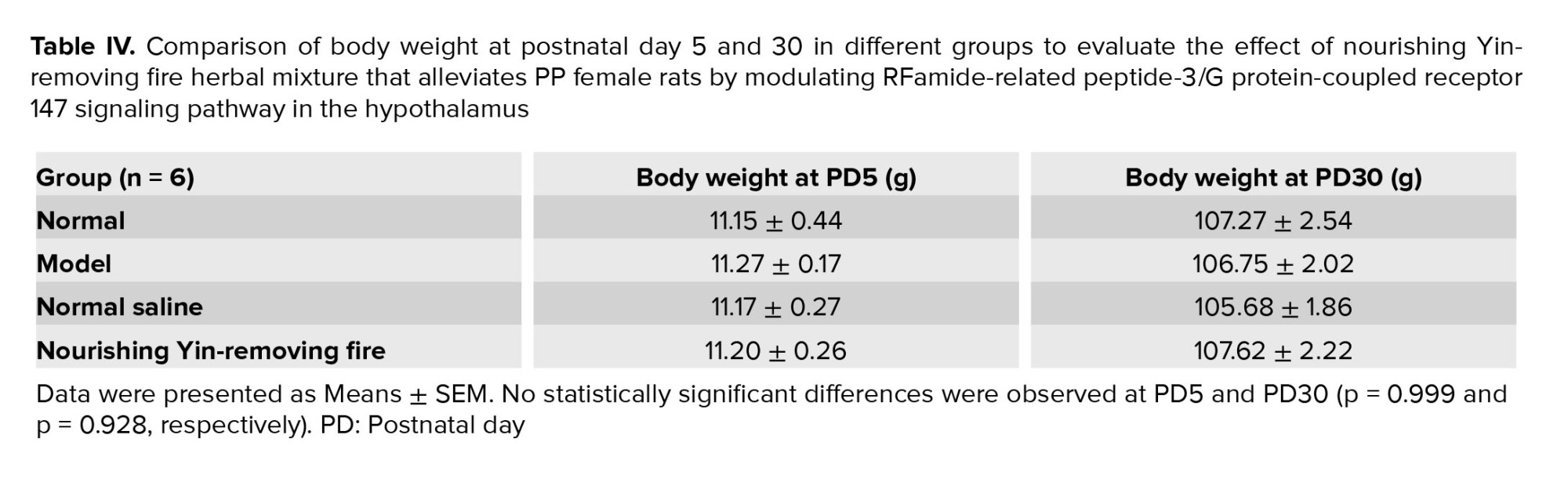
After the day of vaginal opening, the daily vaginal smears were examined to observe estrous cycle phases until PD30. The time of the first estrus phase in the model group and the normal saline group was (29.67 ± 0.21) days and (29.50 ± 0.22) days, respectively. No statistically significant differences were observed in the 2 groups (p = 0.599). However, until PD30 the first regular estrous cycle was still not observed in 4 groups. Considering the potential influence of body fat on Kiss1 expression, the weights of the animals in the 4 experimental groups were recorded at PD5 and PD30, when the samples were obtained. No statistically significant differences were observed in different rat groups at PD5 and PD30 (p = 0.993 and p = 0.928, respectively) (Table IV).
3.2. Analysis of hormone levels in rats from various groups
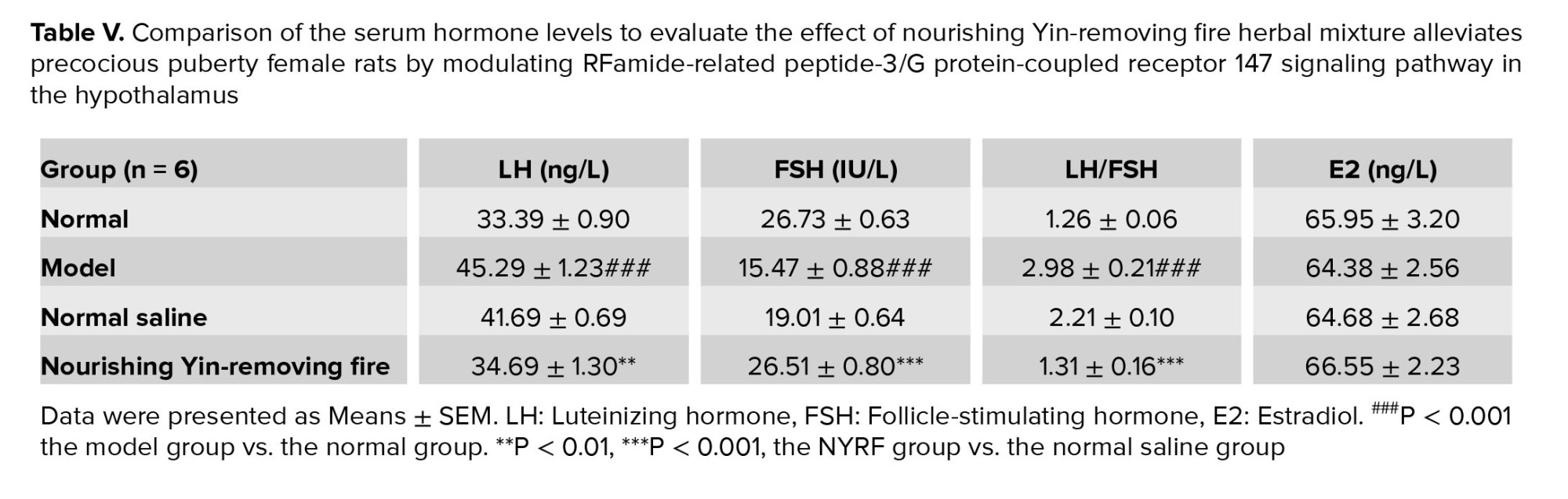
Compared to the normal group, rats in the model group displayed elevated levels of LH and LH/FSH, while exhibiting reduced levels of FSH (all p = 0.000; Table V). Notably, rats in the NYRF group exhibited lower levels of LH and LH/FSH than the model and normal saline groups (all p = 0.000). Interestingly, no significant differences were observed in E2 levels between the groups (p = 0.975, the normal group vs. the model group; p = 0.939, the NYRF group vs. the model group; p = 0.960, the NYRF group vs. the normal saline group).
3.3. Analysis of GnRH and kisspeptin expression in the hypothalamus of rats from different groups
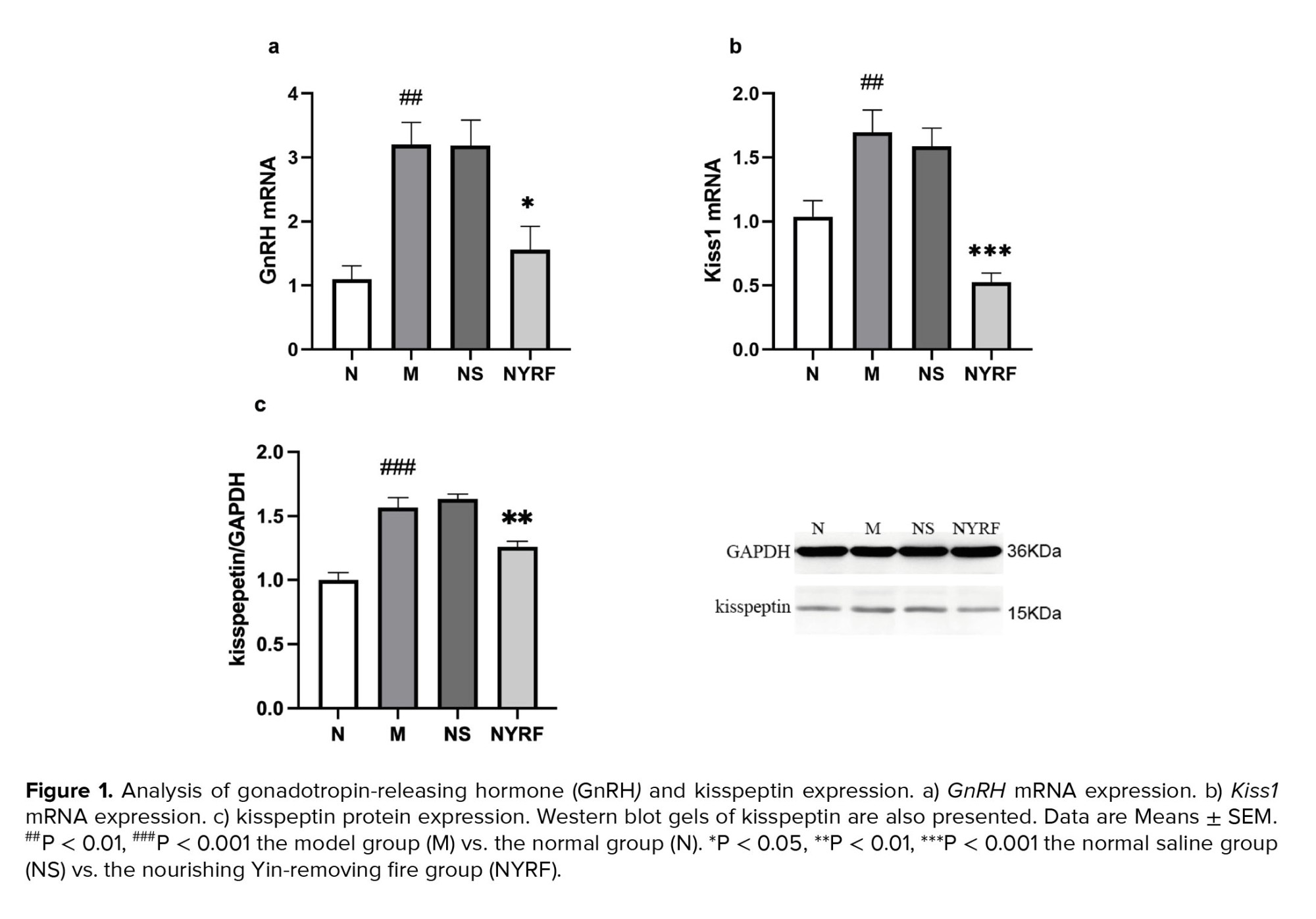
Compared to the normal group, the model group exhibited a significant increase in the expression of GnRH and Kiss1 mRNA in the hypothalamus (p = 0.001 and p = 0.012, respectively; Figure 1). The expression of GnRH mRNA in the NYRF group was lower than that in the model group, and the normal saline group (p = 0.013 and p = 0.014, respectively), and a similar trend was observed for Kiss1 mRNA expression (p = 0.000 and p = 0.000, respectively). To further investigate the kisspeptin expression, we analyzed the protein levels using Western blot analysis. The protein expression of kisspeptin showed a consistent pattern with the mRNA results. The model group displayed higher levels of kisspeptin protein than the normal group (p = 0.000). In contrast, the NYRF group exhibited lower levels of kisspeptin protein compared to both the model group (p = 0.005) and the normal saline group (p = 0.001).
3.4. Analysis of RFRP3 and GPR147 expression in the hypothalamus of rats from different groups
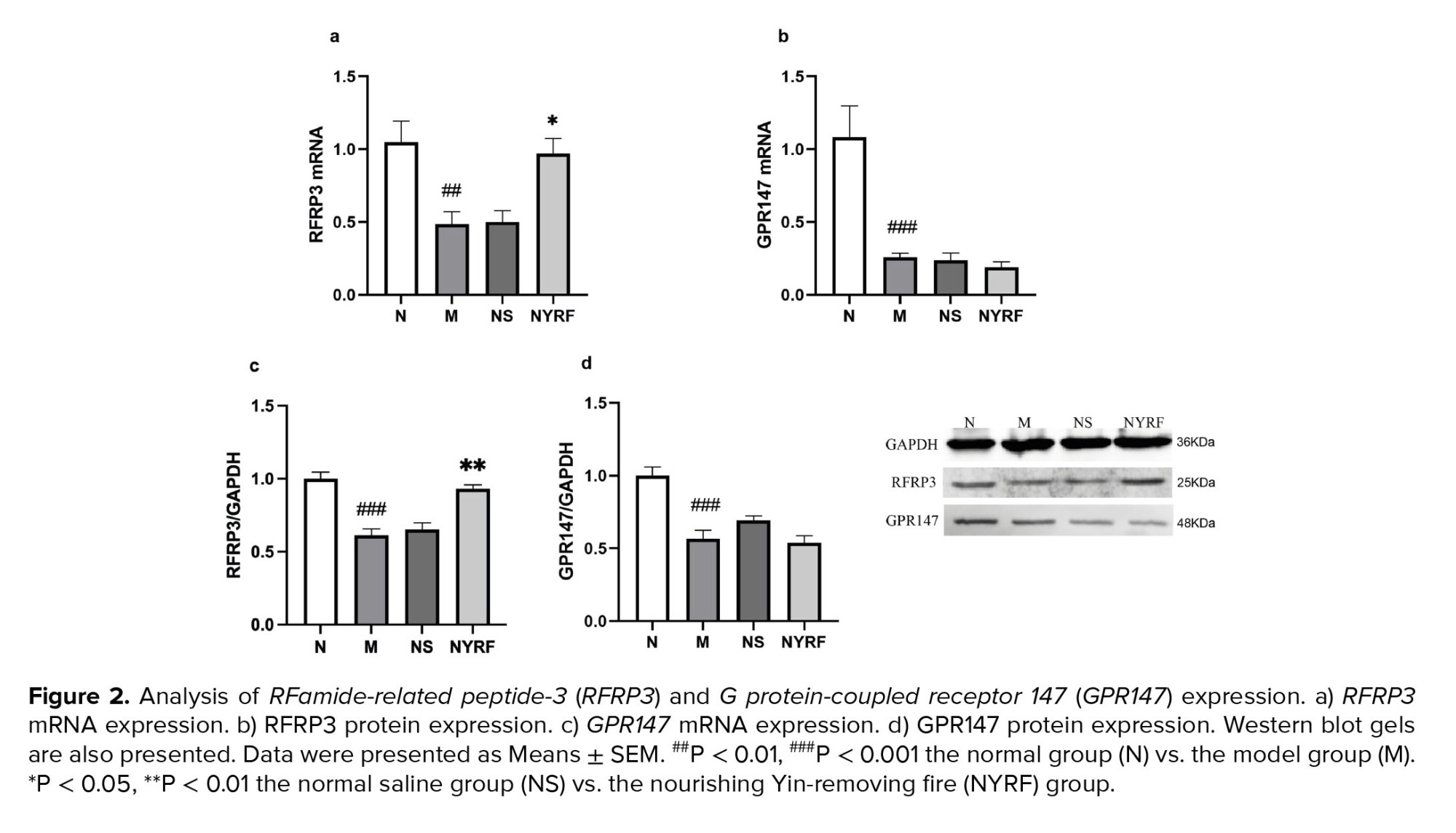
As per our expectations, compared to the normal group, rats in the model group exhibited a significant decrease in both RFRP3 mRNA and protein levels at PD30 (p = 0.006 and p = 0.000, respectively; Figure 2). However, after treatment with the NYRF herbal mixture, the mRNA levels of RFRP3 increased in the NYRF group compared to the model and normal saline groups (p = 0.19 and p = 0.023, respectively), as did the protein levels of RFRP3 in the NYRF group compared to the model and normal saline groups (p = 0.000 and p = 0.645, respectively). Furthermore, we observed considerably lower levels of GPR147 mRNA and protein expression in the model group compared to the normal group (p = 0.000 for both). However, no significant differences were found between the NYRF group, the model group, and the normal saline group in terms of GPR147 mRNA expression (p = 0.974, the NYRF group vs. the model group; p = 0.991, the NYRF group vs. the normal saline group) and protein expression (p = 0.977, the NYRF group vs. the model group; p = 0.171, the NYRF group vs. the normal saline group).
3.5. Correlation analysis of RFRP3 with GnRH, Kiss1 mRNA, and LH levels
We evaluated the correlation between various measured parameters. Notably, RFRP3 exhibited a significant inverse correlation with Kiss1 mRNA (r = -0.55; p = 0.005), GnRH mRNA (r = -0.56; p = 0.005), and LH levels (r = -0.63; p = 0.000).
RFRP3, also known as GnIH, inhibits gonadotropin release and is produced in specific brain regions, exerting an inhibitory effect on gonadal function (22). As one of the inhibitory regulators, it regulates the synthesis and secretion of GnRH. These signaling molecules play important roles in the development of PP (23). The RFRP neurons are primarily distributed in the dorsomedial nucleus of the hypothalamus in rats, and their nerve fibers project to GnRH and kisspeptin neurons, which also express GPR147. These morphological connections provide evidence for the regulation of GnRH and kisspeptin neurons (24). In the PP model induced by danazol, a decline in RFRP3 and GPR147 expression has been observed (25). Similarly, in this study, we found a significant downregulation of RFRP3 and GPR147 expression in the hypothalamus of the model group rats, suggesting that the reduced inhibitory effect of RFRP3/GPR147 on GnRH and kisspeptin neurons contributed to the promotion of PP female rats. Those studies have highlighted the involvement of the RFRP3/GPR147 signaling pathway in the regulation of reproduction and PP pathological process. Previous clinical studies have shown that NYRF herbal mixture delays the onset of advanced puberty, increases final height in adulthood, and delays the age of menarche in girls (14). In experimental studies, we also found that NYRF herbal mixture could affect the hypothalamic kisspeptin signaling pathway-meanwhile, downregulating the increased GnRH expression for treating PP with the NYRF herbal mixture.
It has not been previously reported whether the mechanism of treatment of PP by the NYRF herbal mixture is through the RFRP3/GPR147 signaling pathway. In this study, after the treatment with the NYRF herbal mixture, we found that both the mRNA and protein levels of RFRP3 were increased in the NYRF group compared with the model and normal saline groups at PD30. However, the lower expression levels of kisspeptin and GnRH in the hypothalamus, as well as decreased LH levels, reproductive organ coefficients, and delayed vaginal opening, suggested that the NYRF herbal mixture may delay the onset of puberty in female rats by upregulating hypothalamic RFRP3 expression.
The induction of PP in female rats through subcutaneous injection of danazol has been well-documented. This experimental model is characterized by early vaginal opening, elevated LH levels, increased GnRH and kisspeptin expression in the hypothalamus, an augmented reproductive organ coefficient, and the early onset of the estrous cycle (26). In our experiment, a similar trend in results was observed, a significantly increased expression of GnRH mRNA, Kiss1 mRNA, and kisspeptin in the model group than in the normal group. The NYRF herbal mixture could effectively delay the advanced puberty by downregulating GnRH and kisspeptin expression, as well as the regulation of LH levels. Surprisingly, no significant differences were observed in E2 levels between groups at PD30. Previous studies have indicated that sex hormones could regulate the expression of Kiss1 mRNA in different forebrain nuclei, with diametrically opposite trends in the arcuate nucleus and anterior ventricle. High levels of E2 and progesterone can inhibit GnRH release by suppressing Kiss1 expression in the arcuate nucleus. Interestingly, in the AVPV, elevated levels of E2 stimulate Kiss1 expression, which releases GnRH (27).
According to this background, in our study, the secretion of E2 levels in rats in 4 groups should be under complex regulation. During the onset of puberty, while E2 levels initially rise gradually, a sharp reduction weakens its inhibitory effect.
Consequently, kisspeptin and GnRH levels increase, leading to elevated E2 secretion. Once E2 reaches its peak, it decreases and fluctuates randomly throughout the estrus cycle (28). Due to the early onset of puberty in rats with PP, they exhibit an earlier peak in E2 levels. In contrast, normal rats are still in the ascending phase before puberty onset. This may explain the lack of significant differences in E2 levels between the model group and the normal group at PD30.
As body weight affects the onset of puberty and vice versa, the effect of the NYRF herbal mixture on body weight was assessed in our study (29). In our study, no difference in body weight was observed in the 4 groups on the day of PD5 and PD30 because the NYRF herbal mixture had no negative effect on body weight, which was consistent with the study by Huang et al. in a rat model of PP (14).
There are some limitations in our study. We only investigated the effects of NYRF on the RFRP3/GPR147 signal pathway in PP rats induced by danazol. It is still unclear whether these effects would be observed in other animal models or in humans, particularly considering the effects of nutrients and environmental pollution. In future studies, we will apply other different models of PP for in-depth exploration of the deeper mechanisms of the NYRF herbal mixture on RFRP3/GPR147 signaling pathway.
5. Conclusion
Overall, our study highlights the role of the RFRP3/GPR147 signaling pathway in regulating puberty onset. Upregulation of RFRP3 may contribute to delaying PP, potentially through the modulation of the hypothalamic RFRP3/GPR147 signaling pathway. PP rats treated with the NYRF herbal mixture showed lower expression levels of RFRP3. This suggests that the NYRF herbal mixture has the potential to delay the progression of sexual development in rats with PP by the upregulation of RFRP3 and downregulation of GnRH and kisspeptin in the hypothalamus. These findings provided a deeper understanding of the therapeutic mechanism of the NYRF herbal mixture.
Data availability
Research data is not shared.
Author contributions
Xiaoli Dai and Yuanyuan He designed the study, conducted the research and wrote the paper. Suhuan Li, Yanyan Sun, and Wen Sun monitored, evaluated, and analyzed the result of the study. Yonghong Wang concepted, designed and reviewed the article. Further, Zhanzhuang Tian, Jian Yu, and Nurgul Yerkinkyzy Ablakimova reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (grant No. 81273804; No. 82104932).
Conflict of Interest
The authors declare that there is no conflict of interest.
Precocious puberty (PP) is a prevalent endocrine disorder in children with premature onset of secondary sexual characteristics (1). In China, the study estimated a prevalence of 6.19% (11.47% girls, 3.26% boys) based on a survey of 4058 primary school students (2). The onset of puberty is a complex biological process under the control of neuroendocrine pathways with multiple factors involved in regulating the hypothalamic-pituitary-gonadal (HPG) axis (3). Puberty initiation relies on crucial mechanisms such as activating the gonadotropin-releasing hormone (GnRH) pulse generator and the emergence of GnRH peaks, which is the key player in puberty onset, meanwhile, the disruption of GnRH release leads to gonadotropin-dependent PP (4).
Kisspeptin, a neuropeptide encoded by the Kiss1 gene, plays a vital role in activating the hypothalamic-HPG axis. By stimulating the hypothalamus, Kiss1 acts upstream of GnRH and is recognized as a critical regulator of GnRH secretion, which may help control the timing of puberty (5). GnRH neuron regulation involves excitatory and inhibitory networks, with gonadotropin-inhibitory hormone (GnIH) playing a significant inhibitory role (6). As an inhibitor of HPG axis, GnIH was the first to be identified in the Japanese quail, marking its initial discovery (7). GnIH could directly inhibit GnRH release in the quail brain, which could open a new window in the knowledge of HPG axis function (8). The RFamide-related peptide (RFRP) precursor, the mammalian equivalent of GnIH, produces 2 neuropeptides: RFRP1 and RFRP3 (9).
The G protein-coupled receptor 147 (GPR147) receptor, a novel GPR, has been identified in the Japanese quail. GPR147 binds to RFRP3 on some GnRH and kisspeptin neurons. Additionally, RFRP3 may influence GnRH and kisspeptin neurons (10). Meanwhile, they conducted intracerebroventricular injections of RFRP3 in female rats, observing downregulation of GnRH and Kiss1 mRNA expression in the hypothalamus and significant delays in puberty onset (11). These findings suggest that RFRP3/GPRl47 signal pathway can function as an inhibitory regulator of reproductive capability, by regulating gonadotrophin synthesis and release through its effects on the HPG axis.
The nourishing Yin-removing fire (NYRF) herbal mixture (Shanghai pharmacists system number Z05170908, consists of Rehmannia glutinosa [Sheng-Di-Huang], Scrophularia buergeriana [Xuan-Shen], and Anemarrhena asphodeloides [Zhi-Mu], etc.), an experienced prescription originating from the famous TCM experts, has been used to treat PP for decades (12). We have observed that it could mitigate the progression of sexual characteristics in PP girls with kidney-yin deficiency and fire hyperactivity (13). In previous experimental studies, we have found that NYRF mixture could suppress the expression of kisspeptin and GnRH in the hypothalamus of female PP rats (14).
Considering the multi-target therapeutic effects of the Chinese herbal mixtures, we investigated the modulation of NYRF herbal mixture to the RFRP3/GPR147 signal pathway in the hypothalamus of PP female rats, in order to provide additional insights into the therapeutic mechanism of the NYRF herbal mixture in the context of PP.
2. Materials and Methods
2.1. Animal procurement and housing
Newborn Sprague-Dawley rats, along with their mother, were procured from the Laboratory Animal Business Department, of Shanghai Institute of Family Planning (license number SCXK [Shanghai] 2018-0006) to conduct an experimental study. The rats were housed under standard conditions at the Department of Integrative Medicine and Neurobiology, Fudan University, Shanghai, China which included a temperature of 22 ± 2oC, humidity of 30-40%, and a 12-hr light/dark cycle. Adequate food and water were provided to the rats ad libitum.
2.2. Preparation of the herbal mixture
The initial formulation of the NYRF herbal mixture, formulated by the Department of Integrative Medicine at Children's hospital of Fudan University (Shanghai Medicine: Z05170908 consisted of ingredients such as Rehmannia glutinosa, Scrophulariae Radix [dried root of Scrophularia ningpoensis], and Phellodendri Amurensis Cortex [dried bark of Phellodendron chinense], etc.) (Table I). The reparation method employed in this study follows the protocol outlined in our prior research (15).
To obtain the NYRF herbal mixture extract, the traditional method of water extraction followed by alcohol precipitation was chosen (16). The crude drugs were decocted using a thermostat electric set (Zhengzhou Great Wall Scientific Industrial & Trade Co. Ltd., Zhengzhou, China) at 100oC for 40 min. The set was refilled with water, and the decoction continued for another 40 min. The extractive liquid was then accumulated and concentrated on a rotary evaporator (Buchi, Switzerland) for 15 min at 4oC at 1000 rpm/min. Absolute ethanol was slowly added to the extractive liquid until the ethanol concentration reached 60%. The mixture was then incubated at 4oC for 72 hr to remove the impurities that might dissolve in ethanol and precipitate to the bottom. The supernatant was extracted and the ethanol was subsequently removed from it using a rotary evaporator for 15 min at 4oC at 1000 rpm/min. Following this method, the final drug was obtained at a concentration of 2.7 gr per/ml (15).
2.3. Study design, animal grouping, and drug administration
In an experimental study, 24 rats were allocated randomly into 4 groups (n = 6): the normal group, the model group, the normal saline group, and the NYRF group. Except for the rats in the normal group, all the other groups were administered a single subcutaneous injection of 300 µg/25 µL danazol (dissolved in ethylene glycol: ethanol, 1:1) on postnatal day (PD)5 to induce the PP model (17). From PD15 to PD30, the rats in the NYRF group received an oral administration of the NYRF extract twice daily (1 mL/100 gr), while the rats in the normal saline group were given an equal volume of saline. No additional criteria were employed for participant inclusion or exclusion.
To minimize potential confounders, each participant's order of treatments and measurements were randomized. Animal cages were randomly assigned and periodically rotated to mitigate any potential bias associated with cage location. These measures were taken to minimize extraneous factors and enhance the study’s internal validity. At the stage of group allocation, the researchers responsible for assigning the rats to their respective groups were aware of the group assignments. However, during the experiment, the outcome assessment, the subsequent data analysis, and the individuals involved were blinded to the group allocation to minimize potential biases and ensure objective evaluation and analysis of the results.
2.4. Sex character observation, samples management
Daily monitoring of vaginal opening commenced from PD15, and the timing of vaginal opening was documented. After the day of vaginal opening, vaginal smears were taken regularly every morning to observe estrous cycle phases until PD30. There are 4 phases in the entire estrous cycle: proestrus, estrus, metestrus, and diestrus, which last 4-5 days. The vaginal secretions comprise 3 types of cells: leucocytes, cornified epithelial cells, and nucleated epithelial cells (18). During the estrus phase, there is a high presence of enucleated cornified epithelial cells. The cytoplasm is granular, and the cells have an irregular shape. At PD30, all rats (n = 6 in each group) were anesthetized through intraperitoneal injection of pentobarbital sodium (50 mg/kg) (19). Serum samples were collected and stored at -80°C for subsequent hormone level analysis. The hypothalamus was also collected and stored at -80°C, whereas the uterus and ovaries were excised and weighed.
2.5. Organ coefficients, hormone level detection
The uterine and ovarian coefficients were computed (n = 6 in each group) utilizing the organ index formula: ([organ wet weight (g)/body weight (g)] ×104). To determine the levels of estradiol (E2), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) in the serum samples, enzyme-linked immunosorbent assay kits (eBioscience, Affymetrix, USA) were employed.
2.6. Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using the Trizol method and the concentration and purity were determined using the ultraviolet spectrophotometer (OD260/OD280, 1.8~2.0). Then, the Real-Time PCR Master Mix Code (Toyobo, Japan) was used to reverse transcribe the RNA into cDNA. The primer sequences, as listed in table II, were designed and synthesized by Shanghai Jierui. The qRT-PCR system was conducted following the ReverTra Ace qRT-PCR kit protocol (Toyobo, Japan), which involved an initial step at 95oC for 180 sec, followed by 40 cycles of 95oC for 5 sec and 60oC for 30 sec. The relative expression of mRNA in the hypothalamus of rats (n = 6/each) was determined using the 2-△△Ct method.
2.7. Protein analysis by western blotting
Protein extraction from the hypothalamus of rats (n = 6/each) was conducted using radioimmunoprecipitation assay (RIPA) buffer, and the protein concentration was determined using a bicinchoninic acid kit (Biosharp, Hefei, China). Gels were prepared and concentrated accordingly to separate the proteins based on their molecular weight. The protein samples were then subjected to electrophoresis and transferred onto polyvinylidene fluoride membranes. These membranes were blocked with 5% bovine serum albumin (BSA) in tris-buffered saline containing 0.05% tween 20 (TBST) and subsequently incubated overnight at 4oC with primary antibodies, including anti-kisspeptin antibody (Abcam, US), anti-RFRP3 antibody (Santa Cruz, US), and anti-GPR147 antibody (Biorbyt, UK). After 3 washes with TBST for 10 min each, the membranes were exposed to suitable secondary antibodies for 1 hr. Chemiluminescence detection was performed using an electrochemiluminescence reagent, and the intensity of the protein bands was quantified using ImageJ software.


2.8. Ethical considerations
The study was approved by the Ethical Committee of the Children’s hospital of Fudan University, Shanghai, China (No: [2020] 211). The animals involved in the study were treated and handled in accordance with the Ethical Guidelines of Fudan University, Shanghai, China.
2.9. Statistical analysis
The study outcomes encompassed several parameters, including the timing of vaginal opening, ovarian coefficient, uterine coefficient, LH, FSH, LH/FSH ratio, E2, GnRH mRNA expression, Kiss1 mRNA expression, kisspeptin protein expression, RFRP3 mRNA expression, RFRP3 protein expression, GPR147 mRNA expression, and GPR147 protein expression. The comparisons between the groups were conducted using one-way ANOVA and post hoc Tukey test. Pearson correlation analysis was utilized to evaluate correlations. A significance level of p < 0.05 was considered statistically significant. The calculation was based on the protein expression data obtained after the study. Statistical analyses were carried out using SPSS for Windows program, version 22 (SPSS Inc., Chicago, Illinois, USA). Group means and standard errors were reported in both text and graphs using GraphPad Prism version 9.0 for Windows (GraphPad Software Inc., San Diego, CA, USA).
The sample size calculation for this study was conducted using G*Power 3.9.11.2 software, considering a desired statistical power of 0.8, an alpha error level of 0.05, and employing a One-way ANOVA test for 4 groups.
3. Results
3.1. Assessment of vaginal opening time, sex organ development, estrous cycle phases, body weight in different groups
The timing of vaginal opening was carefully documented throughout the experimental period. No instances of vaginal opening were observed in the normal group until PD30. In contrast, the model group exhibited significantly early vaginal opening compared to the normal group. Notably, the NYRF group showed a considerably delayed time of vaginal opening in comparison to both the model group (p = 0.01, Table III) and the normal saline group (p = 0.01).
The organ coefficients of the uterus and ovaries were analyzed to evaluate reproductive organ development. Remarkably, the ovarian and uterine coefficient of rats in the model group exhibited a notable increase compared to those in the normal group (p = 0.004; p = 0.000). In contrast, the NYRF group displayed lower ovarian and uterine coefficients than the model group (p = 0.008; p = 0.003) and the normal saline group (p = 0.026; p = 0.001).
After the day of vaginal opening, the daily vaginal smears were examined to observe estrous cycle phases until PD30. The time of the first estrus phase in the model group and the normal saline group was (29.67 ± 0.21) days and (29.50 ± 0.22) days, respectively. No statistically significant differences were observed in the 2 groups (p = 0.599). However, until PD30 the first regular estrous cycle was still not observed in 4 groups. Considering the potential influence of body fat on Kiss1 expression, the weights of the animals in the 4 experimental groups were recorded at PD5 and PD30, when the samples were obtained. No statistically significant differences were observed in different rat groups at PD5 and PD30 (p = 0.993 and p = 0.928, respectively) (Table IV).
3.2. Analysis of hormone levels in rats from various groups
Compared to the normal group, rats in the model group displayed elevated levels of LH and LH/FSH, while exhibiting reduced levels of FSH (all p = 0.000; Table V). Notably, rats in the NYRF group exhibited lower levels of LH and LH/FSH than the model and normal saline groups (all p = 0.000). Interestingly, no significant differences were observed in E2 levels between the groups (p = 0.975, the normal group vs. the model group; p = 0.939, the NYRF group vs. the model group; p = 0.960, the NYRF group vs. the normal saline group).
3.3. Analysis of GnRH and kisspeptin expression in the hypothalamus of rats from different groups
Compared to the normal group, the model group exhibited a significant increase in the expression of GnRH and Kiss1 mRNA in the hypothalamus (p = 0.001 and p = 0.012, respectively; Figure 1). The expression of GnRH mRNA in the NYRF group was lower than that in the model group, and the normal saline group (p = 0.013 and p = 0.014, respectively), and a similar trend was observed for Kiss1 mRNA expression (p = 0.000 and p = 0.000, respectively). To further investigate the kisspeptin expression, we analyzed the protein levels using Western blot analysis. The protein expression of kisspeptin showed a consistent pattern with the mRNA results. The model group displayed higher levels of kisspeptin protein than the normal group (p = 0.000). In contrast, the NYRF group exhibited lower levels of kisspeptin protein compared to both the model group (p = 0.005) and the normal saline group (p = 0.001).
3.4. Analysis of RFRP3 and GPR147 expression in the hypothalamus of rats from different groups
As per our expectations, compared to the normal group, rats in the model group exhibited a significant decrease in both RFRP3 mRNA and protein levels at PD30 (p = 0.006 and p = 0.000, respectively; Figure 2). However, after treatment with the NYRF herbal mixture, the mRNA levels of RFRP3 increased in the NYRF group compared to the model and normal saline groups (p = 0.19 and p = 0.023, respectively), as did the protein levels of RFRP3 in the NYRF group compared to the model and normal saline groups (p = 0.000 and p = 0.645, respectively). Furthermore, we observed considerably lower levels of GPR147 mRNA and protein expression in the model group compared to the normal group (p = 0.000 for both). However, no significant differences were found between the NYRF group, the model group, and the normal saline group in terms of GPR147 mRNA expression (p = 0.974, the NYRF group vs. the model group; p = 0.991, the NYRF group vs. the normal saline group) and protein expression (p = 0.977, the NYRF group vs. the model group; p = 0.171, the NYRF group vs. the normal saline group).
3.5. Correlation analysis of RFRP3 with GnRH, Kiss1 mRNA, and LH levels
We evaluated the correlation between various measured parameters. Notably, RFRP3 exhibited a significant inverse correlation with Kiss1 mRNA (r = -0.55; p = 0.005), GnRH mRNA (r = -0.56; p = 0.005), and LH levels (r = -0.63; p = 0.000).





- Discussion
RFRP3, also known as GnIH, inhibits gonadotropin release and is produced in specific brain regions, exerting an inhibitory effect on gonadal function (22). As one of the inhibitory regulators, it regulates the synthesis and secretion of GnRH. These signaling molecules play important roles in the development of PP (23). The RFRP neurons are primarily distributed in the dorsomedial nucleus of the hypothalamus in rats, and their nerve fibers project to GnRH and kisspeptin neurons, which also express GPR147. These morphological connections provide evidence for the regulation of GnRH and kisspeptin neurons (24). In the PP model induced by danazol, a decline in RFRP3 and GPR147 expression has been observed (25). Similarly, in this study, we found a significant downregulation of RFRP3 and GPR147 expression in the hypothalamus of the model group rats, suggesting that the reduced inhibitory effect of RFRP3/GPR147 on GnRH and kisspeptin neurons contributed to the promotion of PP female rats. Those studies have highlighted the involvement of the RFRP3/GPR147 signaling pathway in the regulation of reproduction and PP pathological process. Previous clinical studies have shown that NYRF herbal mixture delays the onset of advanced puberty, increases final height in adulthood, and delays the age of menarche in girls (14). In experimental studies, we also found that NYRF herbal mixture could affect the hypothalamic kisspeptin signaling pathway-meanwhile, downregulating the increased GnRH expression for treating PP with the NYRF herbal mixture.
It has not been previously reported whether the mechanism of treatment of PP by the NYRF herbal mixture is through the RFRP3/GPR147 signaling pathway. In this study, after the treatment with the NYRF herbal mixture, we found that both the mRNA and protein levels of RFRP3 were increased in the NYRF group compared with the model and normal saline groups at PD30. However, the lower expression levels of kisspeptin and GnRH in the hypothalamus, as well as decreased LH levels, reproductive organ coefficients, and delayed vaginal opening, suggested that the NYRF herbal mixture may delay the onset of puberty in female rats by upregulating hypothalamic RFRP3 expression.
The induction of PP in female rats through subcutaneous injection of danazol has been well-documented. This experimental model is characterized by early vaginal opening, elevated LH levels, increased GnRH and kisspeptin expression in the hypothalamus, an augmented reproductive organ coefficient, and the early onset of the estrous cycle (26). In our experiment, a similar trend in results was observed, a significantly increased expression of GnRH mRNA, Kiss1 mRNA, and kisspeptin in the model group than in the normal group. The NYRF herbal mixture could effectively delay the advanced puberty by downregulating GnRH and kisspeptin expression, as well as the regulation of LH levels. Surprisingly, no significant differences were observed in E2 levels between groups at PD30. Previous studies have indicated that sex hormones could regulate the expression of Kiss1 mRNA in different forebrain nuclei, with diametrically opposite trends in the arcuate nucleus and anterior ventricle. High levels of E2 and progesterone can inhibit GnRH release by suppressing Kiss1 expression in the arcuate nucleus. Interestingly, in the AVPV, elevated levels of E2 stimulate Kiss1 expression, which releases GnRH (27).
According to this background, in our study, the secretion of E2 levels in rats in 4 groups should be under complex regulation. During the onset of puberty, while E2 levels initially rise gradually, a sharp reduction weakens its inhibitory effect.
Consequently, kisspeptin and GnRH levels increase, leading to elevated E2 secretion. Once E2 reaches its peak, it decreases and fluctuates randomly throughout the estrus cycle (28). Due to the early onset of puberty in rats with PP, they exhibit an earlier peak in E2 levels. In contrast, normal rats are still in the ascending phase before puberty onset. This may explain the lack of significant differences in E2 levels between the model group and the normal group at PD30.
As body weight affects the onset of puberty and vice versa, the effect of the NYRF herbal mixture on body weight was assessed in our study (29). In our study, no difference in body weight was observed in the 4 groups on the day of PD5 and PD30 because the NYRF herbal mixture had no negative effect on body weight, which was consistent with the study by Huang et al. in a rat model of PP (14).
There are some limitations in our study. We only investigated the effects of NYRF on the RFRP3/GPR147 signal pathway in PP rats induced by danazol. It is still unclear whether these effects would be observed in other animal models or in humans, particularly considering the effects of nutrients and environmental pollution. In future studies, we will apply other different models of PP for in-depth exploration of the deeper mechanisms of the NYRF herbal mixture on RFRP3/GPR147 signaling pathway.
5. Conclusion
Overall, our study highlights the role of the RFRP3/GPR147 signaling pathway in regulating puberty onset. Upregulation of RFRP3 may contribute to delaying PP, potentially through the modulation of the hypothalamic RFRP3/GPR147 signaling pathway. PP rats treated with the NYRF herbal mixture showed lower expression levels of RFRP3. This suggests that the NYRF herbal mixture has the potential to delay the progression of sexual development in rats with PP by the upregulation of RFRP3 and downregulation of GnRH and kisspeptin in the hypothalamus. These findings provided a deeper understanding of the therapeutic mechanism of the NYRF herbal mixture.
Data availability
Research data is not shared.
Author contributions
Xiaoli Dai and Yuanyuan He designed the study, conducted the research and wrote the paper. Suhuan Li, Yanyan Sun, and Wen Sun monitored, evaluated, and analyzed the result of the study. Yonghong Wang concepted, designed and reviewed the article. Further, Zhanzhuang Tian, Jian Yu, and Nurgul Yerkinkyzy Ablakimova reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (grant No. 81273804; No. 82104932).
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Cellular and Molecular Biology of Reproduction
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




