BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijrm.ir/article-1-32-en.html
Infertility affects approximately 15% of all couples trying to conceive. Male factor infertility is the sole or contributing factor in roughly half of these cases, and no identifiable cause can be found in over 25% of infertile males (1). Excessive production of free radicals or reactive oxygen species (ROS) can damage sperm, and ROS have been extensively studied as one of the mechanisms of infertility. Superoxide anion, hydroxyl radical and hydrogen peroxide are some of the major ROS present in seminal plasma. Cells living under aerobic conditions constantly face the oxygen (O2) paradox: O2 is required to support life, but its metabolites such as ROS can modify cell functions, endanger cell survival, or both (2). Hence, any excess ROS must be continuously inactivated in order to maintain normal cell function. This function is taken up by the antioxidants present in the seminal plasma. When there is an excessive production of ROS or impaired antioxidant defense mechanisms, oxidative stress (OS) occurs, which is harmful to spermatozoa.
Physiologic role of ROS
ROS can have beneficial or detrimental effects on sperm functions depending on the nature and the concentration of the ROS as well as the location and length of exposure to ROS (3). During epididymal transit, sperm acquire the ability to move progressively. However, they acquire the ability to fertilize, in the female tract through a series of physiological changes called "capacitation" (4). Under physiological conditions, spermatozoa produce small amounts of ROS, which are needed for capacitation and acrosomal reaction. Superoxide anion appears to play a role in this process (2). Studies have indicated that male germ cells at various stages of differentiation have the potential to generate ROS.Consequences of excessive generation of ROS
Spermatozoa are vulnerable to ROS because their plasma membrane and cytoplasm contain large amounts of polyunsaturated fatty acids (5) Excessive generation of ROS in semen by leukocytes as well as by abnormal spermatozoa could be a cause of infertility (6). Hydrogen peroxide is the major ROS producer in human spermatozoa. Moderately elevated concentrations of hydrogen peroxide do not affect sperm viability but cause sperm immobilization, mostly via depletion of intracellular ATP and the subsequent decrease in the phosphorylation of axonemal proteins (7, 8). High concentrations of hydrogen peroxide induce lipid peroxidation and result in cell death.Gomez et al. (1998) demonstrated that levels of ROS produced by spermatozoa were negatively correlated with the quality of sperm in the original semen (9). A study from our group reported that levels of antioxidants in seminal plasma from infertile men were significantly lower than levels in fertile controls (10). However, pathological levels of ROS detected in semen of infertile men are more likely a result of increased ROS production rather than reduced antioxidant capacity of the seminal plasma (11).
Virtually every human ejaculate is contaminated with potential sources of ROS such as leukocytes and abnormal spermatozoa. It follows that some spermatozoa will incur oxidative damage and a concomitant loss of function. Thus, the impact of ROS on male fertility is a question of degree rather than the presence or absence of the pathology (2).
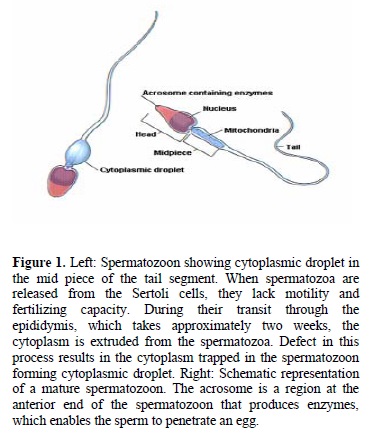
Poor sperm quality is linked to increased ROS generation as a consequence of the presence of excess residual cytoplasm. Spermatozoa undergo a remarkable transformation during the final stage of sperm differentiation and lose their cytoplasm to become mature spermatids. Following spermiation, any residual cytoplasm that is associated with spermatozoa is retained in the mid-piece region as an irregular cytoplasmic mass (Figure 1) (12). If this residual cytoplasm occupies more than one-third of the sperm head, it is termed a cytoplasmic droplet. Under these circumstances, the spermatozoa that are released after spermiation are thought to be immature and functionally defective. They are capable of producing increased amounts of ROS (13). To detect seminal OS, our group created the ROS-total antioxidant capacity (ROS-TAC) score using principal component analysis, which is derived from the ROS and TAC levels. A low ROS-TAC score indicates high seminal OS.
Creatine kinase and its association with infertility
Huszar and Vigue (1994) have found that the morphological irregularities of sperm are significantly correlated with high creatine kinase (CK) activity, an enzyme that is involved in the synthesis and utilization of energy in the sperm (14). CK levels indicate sperm maturity with higher levels being found in immature spermatozoa, which retain their cytoplasmic droplets. High CK levels correlate inversely with the fertilizing potential of the spermatozoa and indicate the degree of cellular immaturity (2).
CK activity is a measure of cellular maturity and fertilizing potential in human spermatozoa. Similarly, recent studies have found an inverse relationship between CK levels and sperm morphological forms and have suggested that CK levels can be used as a reliable marker for sperm quality and fertilizing potential in subfertile men (15). A positive relationship was found between CK activity and the rate of lipid peroxidation, as measured by malondialdehyde (MDA) formation in sperm fractions (14).
Mitochondria: source and target of ROS
Spermatozoa may generate ROS in two ways: 1. the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system at the level of the sperm plasma membrane and 2. nicotinamide adenine dinucleotide (NADH)-dependent oxido-reductase (diphorase) at the level of mitochondria (16). Spermatozoa are rich in mitochondria because they need a constant supply of energy for their motility. Production of ROS is significantly increased in dysfunctional mitochondria, which in turn affect mitochondrial function in spermatozoa (17). Such a relationship could be due to two mutually interconnected phenomena: ROS causing damage to the mitochondrial membrane, and the damaged mitochondrial membrane causing an increase in ROS production.One method to determine mitochondrial function is the assessment of the electrochemical gradient established during the process of oxidative phosphorylation when the protons are pumped from inside the mitochondria to the outside. This inner mitochondrial membrane potential (MMP) has been used by some authors to determine sperm function. Wang, et al. (2003) showed that mitochondrial function as a measure of MMP, is decreased in spermatozoa of infertile men with elevated levels of ROS production and is positively correlated with the sperm concentration (18).
DNA damage and apoptosis induced by ROS
Oxidative damage can cause base degradation, DNA fragmentation and cross-linking of proteins (19). Spermatozoa with damaged DNA lose their ability to fertilize the oocyte. A study by Sun et al. (1997) found a negative correlation between the percentage of spermatozoa with damaged DNA and the fertilization rate (20).Apoptosis, described as programmed cell death, is a physiological phenomenon characterized by cellular morphological and biochemical alterations that cause a cell to die. Apoptosis appears to be strictly regulated by extrinsic and intrinsic factors and can be triggered by a wide variety of stimuli. Examples of extrinsic stimuli that are potentially important in testicular cell apoptosis are irradiation, chemotherapy, and toxin exposure.
Mitochondria play a key role in the mechanism of apoptosis. The integrity of mitochondria is established by the presence of cytochrome C in the inner membrane space. High levels of ROS disrupt the inner and outer mitochondrial membranes. This results in the release from the mitochondria of cytochrome C protein, which activates the caspases and induces apoptosis (Figure 2) (21). Studies in infertile men showed that high levels of cytochrome C in seminal plasma indicate significant mitochondrial damage by ROS. Considerable evidence exists that disruption of mitochondrial functions (e.g., loss of transmembrane potential, permeability transition, and release of cytochrome C leading to impaired electron transport) are important events in many apoptotic cell deaths (22).

A positive relationship between increased sperm damage by ROS and higher levels of the proapoptotic molecules cytochrome C and caspases 9 and 3 was also reported in patients compared to healthy donors indicating mitochondrial injury, increased apoptosis and ultimately DNA damage (Figure 3) (21).

Apoptosis in sperm may also be initiated by ROS-independent pathways involving the cell surface receptor called as Fas or CD 95. Fas is a type I membrane protein that mediates apoptosis. When Fas ligand or anti-Fas antibody binds to Fas, apoptosis occurs (23). Moustafa et al. (2004) determined that infertile patients had high ROS levels in their seminal plasma and higher percentage of apoptosis than normal healthy donors (Figure 2) (24).
Interaction between heavy metals and OS and their impact upon fertility
Lead (Pb) and cadmium (Cd) are highly toxic metals in humans and other mammals. These heavy metals can induce OS through their capacity to interact with ROS, increasing their oxidant activity or by affecting membrane integrity (25). The effect of lead on semen quality was investigated in a study of 85 tollgate workers and the same number of age-matched men living in the same area. The tollgate workers were exposed to traffic pollution and in turn increased quantities of lead, and they had poorer semen parameters (26). In an animal study, lead increased ROS production, reduced sperm motility and sperm-oocyte penetration rate and decreased seminal antioxidants. Lead levels in the semen have been found to have a positive correlation with degradation of DNA bases as measured by 8- hydroxydeoxyguanosine (8-OHdG) (27). Seminal plasma lead levels and artificial insemination cycle fecundity are strongly and negatively correlated (28, 29).Smoking and infertility
Tobacco smoke contains approximately 4,000 compounds such as alkaloids, nitrosamines, and inorganic molecules, and many of these substances are reactive oxygen or nitrogen species (30). A significant positive association has been found between active smoking and sperm DNA fragmentation (20) as well as axonemal damage (31). Smoking is also associated with a decreased sperm count (32). Fraga et al. (1996) found that the level of 8-OHdG (a marker of DNA fragmentation) to be 50% higher in smokers compared to nonsmokers (p=0.005) (33). It is believed that smoking affects spermatogenesis by increasing the production of norepinephrine, which increases the conversion of testosterone to estrogen causing decreased testosterone level (34). A study by our group demonstrated that smoking increases ROS levels and decreases seminal antioxidants (35).
Antioxidants and fertility
Since ROS has both physiological and pathological roles, an array of antioxidants maintains a steady state of ROS in the seminal plasma. Antioxidants act as free radical scavengers to protect spermatozoa against ROS. These antioxidants are superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPX). In addition, semen contains a variety of non-enzymatic antioxidant molecules such as vitamin C, vitamin E, pyruvate, glutathione, and carnitine (3). These antioxidants compensate for the loss of sperm cytoplasmic enzymes as the cytoplasm is extruded during spermiogenesis, which in turn, diminishes endogenous repair mechanisms and enzymatic defenses (36). Agarwal et al.(2004) in an exhaustive review of the literature, found a total of 57 studies related to antioxidants and fertility; 10 studies were randomized controlled trials, 16 were controlled studies, and 31 were uncontrolled studies (2, 37).
Non-enzymatic antioxidants
Vitamin E
In vitro studies show that vitamin E is a major chain-breaking antioxidant in the sperm membranes and it appears to have a dose-dependent protective effect (38). Cryopreservation and thawing procedures are associated with a significant reduction in sperm motility induced by OS, and these effects can be avoided by adding vitamin E to cryoprotectants at a dose of 10mmol/L.
In a randomized double-blind controlled trial, asthenospermic patients (general decrease in sperm motility) received oral vitamin E (300mg/day). This treatment significantly decreased the malondialdehyde (a marker for lipid peroxidation) concentration in spermatozoa and improved sperm motility. Eleven of the 52 treated patients (21%) impregnated their wives; nine of the women successfully ended with normal term deliveries, whereas the other two had a spontaneous abortion in the first trimester. No pregnancies were reported in the wives of the placebo-treated patients (39).
Vitamin E and selenium supplementation lead to a significant decrease in MDA concentrations and improved sperm motility (40). Disparate results in sperm quality and quantity were observed in those reports using doses of vitamin E below 400 mg (41). In contrast, patients taking vitamin B showed no change in sperm motility, but a small decrease in the MDA concentration was observed.
Selenium by itself could potentially protect against oxidative DNA damage in human sperm cells. A significant inverse correlation was observed between 8-OHdG and selenium concentration in seminal plasma (r=-0.40, p<0.01), but the experience with this trace element is scarce (42).
Vitamin C
Vitamin C (ascorbate) is another important chain-breaking antioxidant contributing up to 65% of the antioxidant capacity of the seminal plasma. Vitamin C concentration in seminal plasma exceeds 10 times more than that in blood plasma (364 versus 40 micromoles/L) (43). A dose-dependent effect of vitamin C on sperm motility has been demonstrated. At a dose of 1000 microgram/L, vitamin C positively influenced the motility of the spermatozoa. However, above that level, vitamin C reduced the motility of the spermatozoa. On the other hand, doses under 200 mg of vitamin C did not provide any benefit (44). Vitamin C has been shown to improve sperm quality in smokers. Dawson et al. (1992) recruited 75 men (20 to 35 years old) who were randomly divided into one of three supplementation groups: placebo, vitamin C 200 mg, and vitamin C 1,000 mg. The authors observed no improvement in sperm quality in the placebo group, while the groups receiving vitamin C showed improvement in sperm quality, with the highest improvement in the 1,000-mg group (45).
Fraga et al. (1991) studied the oxidative damage to DNA in relation to the seminal fluid vitamin C levels in the semen samples provided by healthy donors (46). This relationship was studied in two groups. In a group of 24 individuals who were between the ages of 20-50 years, high levels of 8-OHdG were correlated with low seminal plasma vitamin C. The second group of individuals was maintained on a controlled diet that varied only in vitamin C content. When dietary vitamin C was decreased from 250 to 5 mg/day, vitamin C in seminal fluid decreased by half and the level of 8-OHdG in sperm DNA increased 91%. Repletion of dietary vitamin C for 28 days (from 5 mg/day to 250 mg/day) caused a doubling in seminal fluid vitamin C and reduced 8-OHdG by 36%. These results indicate that dietary supplementation protects human sperm from endogenous oxidative DNA damage, thereby decreasing the risk of genetic defects, particularly in populations with low vitamin C levels such as smokers.
Glutathione
Glutathione is the most abundant non-thiol protein in mammalian cells (47). A glutathione deficiency can lead to instability of the mid-piece of sperm, resulting in defective motility (48, 49). It protects plasma membrane from lipid peroxidation, scavenges superoxide and prevents O2- formation. In a study consisting of infertile men with unilateral varicocele or genital tract inflammation, glutathione led to significant improvement in the sperm quality (50, 51).
Other non-enzymatic antioxidants
Molecules such as N-acetyl L-cysteine, carotenoids, coenzyme Q 10 and carnitines, provide excellent antioxidant support. N-acetyl L-cysteine is a precursor of glutathione that improves the sperm motility and reduces the ROS induced DNA damage (52, 53). Carotenoids play an important role in protecting the cells and organisms by scavenging the superoxide radicals (54). Coenzyme Q10 protects lipids against peroxidative damage (55). It scavenges superoxide anion as well as peroxides. Carnitine promotes membrane stability and plays an important role in sperm maturation and development (56).
Enzymatic antioxidants
Superoxide dismutase
Superoxide dismutase (SOD) scavenges both extracellular and intracellular superoxide anion and prevents lipid peroxidation of the plasma membrane. In order to act against H2O2, it must be conjugated with catalase or glutathione peroxidase (57). SOD also prevents premature hyperactivation and capacitation induced by superoxide radicals before ejaculation (58).Glutathione peroxidase/ reductase system
This system forms an excellent protection against lipid peroxidation of plasma membrane of spermatozoa. It scavenges lipid peroxides thereby arresting the progressive chain reaction of lipid peroxidation. It also scavenges hydrogen peroxide (H2O2), which is responsible for the initiation of lipid peroxidation. Glutathione reductase (GRD) stimulates the reduction of glutathione disulfide to reduced glutathione. This ensures a steady supply of the reductive substrate (NADPH) to glutathione peroxidase. Glucose-6-phosphate dehydrogenase (G6PD) is required for the conversion of nicotinamide adenine dinucleotide phosphate (NADP+) to its reduced form (NADPH).
Catalase
Catalase detoxifies both intracellular and extracellular H2O2 to water and oxygen (59). In addition, catalase activates NO-induced sperm capacitation, which is a complex mechanism involving H2O2 (60).
Conclusions
The last decade has seen a phenomenal growth in the field of male infertility mainly due to the increased understanding of ROS and OS. This has lead to the development of various antioxidants. This rapid spurt of various antioxidants as a treatment for male infertility is a cause of concern. Current evidences support the use of systemic antioxidants for the management of selective cases of male infertility as well as in vitro supplements during various sperm preparation technique. However, a definitive conclusion cannot be drawn from the available studies as OS is not the only cause of male infertility. The rationale for antioxidant therapy in infertile males should be based on a high OS status. The dosage of the antioxidant is a critical point, particularly because investigators do not know the required level of ROS for physiological purposes. The dose and duration of treatment are of vital importance. Some of the studies were unable to show the effectiveness of antioxidants on fertility or sperm parameters. This could be either due to short duration of the study or inadequate sample size. There is a rationale supporting the use of antioxidants in infertile male patients with elevated ROS levels or low antioxidant defenses. A consensus is still required on type and dosage of antioxidants to be used and outcome parameters studied. Future multicentric studies with larger samples will be of help to gain a better insight to this essential problem.
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




