Tue, Jul 1, 2025
[Archive]
Volume 22, Issue 9 (September 2024)
IJRM 2024, 22(9): 701-708 |
Back to browse issues page
Ethics code: IR.SSU.MEDICINE.REC.1400.316
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahmadnia Z, Montazeri F, Dashti S, Sheikhha M H, Lotfi M. Expression of steroidogenesis pathway genes in cumulus cells from women with diminished ovarian reserve after gonadotropin administration: A case-control study. IJRM 2024; 22 (9) :701-708
URL: http://ijrm.ir/article-1-3209-en.html
URL: http://ijrm.ir/article-1-3209-en.html
1- Department of Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,marzeih.lotfi@gmail.com
2- Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,
Full-Text [PDF 375 kb]
(759 Downloads)
| Abstract (HTML) (597 Views)
1. Introduction
Diminished ovarian reserve (DOR) is one of the causes of infertility in women and refers to cases where the antral follicle counts (AFC) < 5 and anti-Mullerian hormone (AMH) level < 1.2 ng/ml (1). The term “ovarian reserve” describes the ovaries capacity for reproduction (2). The hereditary form of DOR is rare and accounts for 4-31% of all cases (3). In 90% of cases, the cause is unknown, and the disease known as idiopathic (3, 4).
Ovarian reserve determines the number and quality of ovarian follicles. To maximize the success rate of infertility treatment, it is essential to evaluate the effective signaling pathways on ovarian reserve (5, 6). Women with DOR, have lower sex hormone levels such as estrogen and inhibin, and higher gonadotropin levels such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (7). Gonadotropins play an important role in the ovulation and folliculogenesis processes. An imbalance in the secretion of hormones can disturb folliculogenesis. Estradiol synthesis is influenced by the appropriate interaction of growth hormones, gonadotropins, LH, and FSH (8, 9).
Steroidogenesis is an effective process in the growth of ovarian follicles through the production of estrogen. It starts in theca cells, where cholesterol is metabolized into androgens. Granulosa cells, on the other hand, are responsible for changing androgen into estrogen (10, 11). The main signaling pathways related to steroidogenesis are cAMP/ Protein kinase A (PKA) and Wnt/β-catenin. PKA is activated by trophic hormones to control steroid hormone production in steroidogenic cells (12). Glycogen synthase kinase 3 beta (GSK3B) plays a key role in the regulation of the Wnt/β-catenin signaling pathway (13).
LH regulates theca cell steroidogenesis by activating the cAMP signaling pathway through a G protein-coupled receptor. FSH controls the granulosa cell’s ability to produce estrogen. FSH binding initiates the cAMP pathway, which in turn causes the synthesis of cytochrome P450 aromatase (CYP19A1) and 17-hydroxysteroid dehydrogenase in relation to LH signaling. CYP19A1 converts thecal androstenedione irreversibly into estrone, and HSD17B1 then changes estrone into the more potent estradiol (14, 15).
One of the crucial elements in the management of assisted reproduction technology is controlled ovarian hyperstimulation. Also, ovarian response varies from person to person; for example, poor responders often have a low in vitro fertilization (IVF) success rate and often need a prolonged ovarian stimulation cycle. Poor IVF outcomes can lead to psychological and financial stress (16). Therefore, it is crucial to find good predictive tools for IVF outcomes, which can identify key genes in the signaling pathways involved in folliculogenesis. Inhibition of follicle-stimulating hormone receptors using siRNA has been reported to influence miR-1261, miR-130a-3p, miR-329-3p, miR-185-5p, miR-144-5p and miR-4463 expressions. Genes that are regulated by the abovementioned miRNAs have been identified using bioinformatic tools, and it has been found that overexpression of miR-4463 plays a key role in poor ovarian response in KGN cells. It has been suggested that miR-4463 is involved in poor response to gonadotrophin injection in women (17).
In the current study, the expression of miR-4463 regulated genes involved in the steroidogenesis pathway, in cumulus cells (CC), following gonadotropin administration in women with DOR and control group were evaluated.
2. Materials and Methods
Participants in this case-control study were women aged between 20 and 34 yr who referred to Yazd Reproductive Biology Research Institute, Yazd, Iran from November 2021 toDecember 2022.
The inclusion criteria in the case group were: AMH < 1.2 ng/ml and AFC < 5 (n = 20), and in control group were AMH > 1.2 ng/ml and AFC < 5 (n = 20) that were candidates for IVF/intracytoplasmic sperm injection cycles because of male problems. Participants with absence of uterine anomaly, endometriosis, history of thyroid disease, chromosomal problems that lead to reduced ovarian reserve, and polycystic ovaries were excluded from this study.
In these women, ovulation stimulation was performed using the antagonist protocol. In such a way that 300 IU of human menopausal gonadotropin (Merional, IBSA, Lugano, Switzerland) is given to patients from the second day of the menstrual cycle to the 6th day. Ultrasound was performed on the 7th day of the patients menstrual cycle. This process was repeated for 5-6 days until follicles with a diameter of 12-15 mm were observed. After observing follicles with the appropriate diameter, 0.25 mg/day of gonadotropin releasing hormone antagonist (Cetrotide; Sereno International S.A., Geneva, Switzerland) was injected. Ovulation stimulation was continued until follicles with a diameter of 16-18 mm were observed. After which ovulation is done.
Then, human chorionic gonadotropin (Pregnyl; Organon, Oss, the Netherlands) was injected. After 36 hr, ovulation was performed and CC were isolated from ovarian follicles by a reproductive biologist.
Patients with low ovarian reserves were divided into 2 groups, depending on the response to ovulation stimulation drugs, the number of oocytes less than 3 and more than 3.
2.1. Total RNA extraction and cDNA synthesis
Total RNA was extracted from CCs. RNA extraction was performed using a TRIZOL reagent. RNA concentration, integrity, and purity were analyzed using a Nanodrop spectrophotometer (DeNovix, USA) and agarose gel electrophoresis. Complementary DNA (cDNA) was synthesized using Pars Toos Company kit, Iran according to the manufacturer’s instructions.
2.2. Quantitative reverse transcription polymerase chain reaction (RT-PCR)
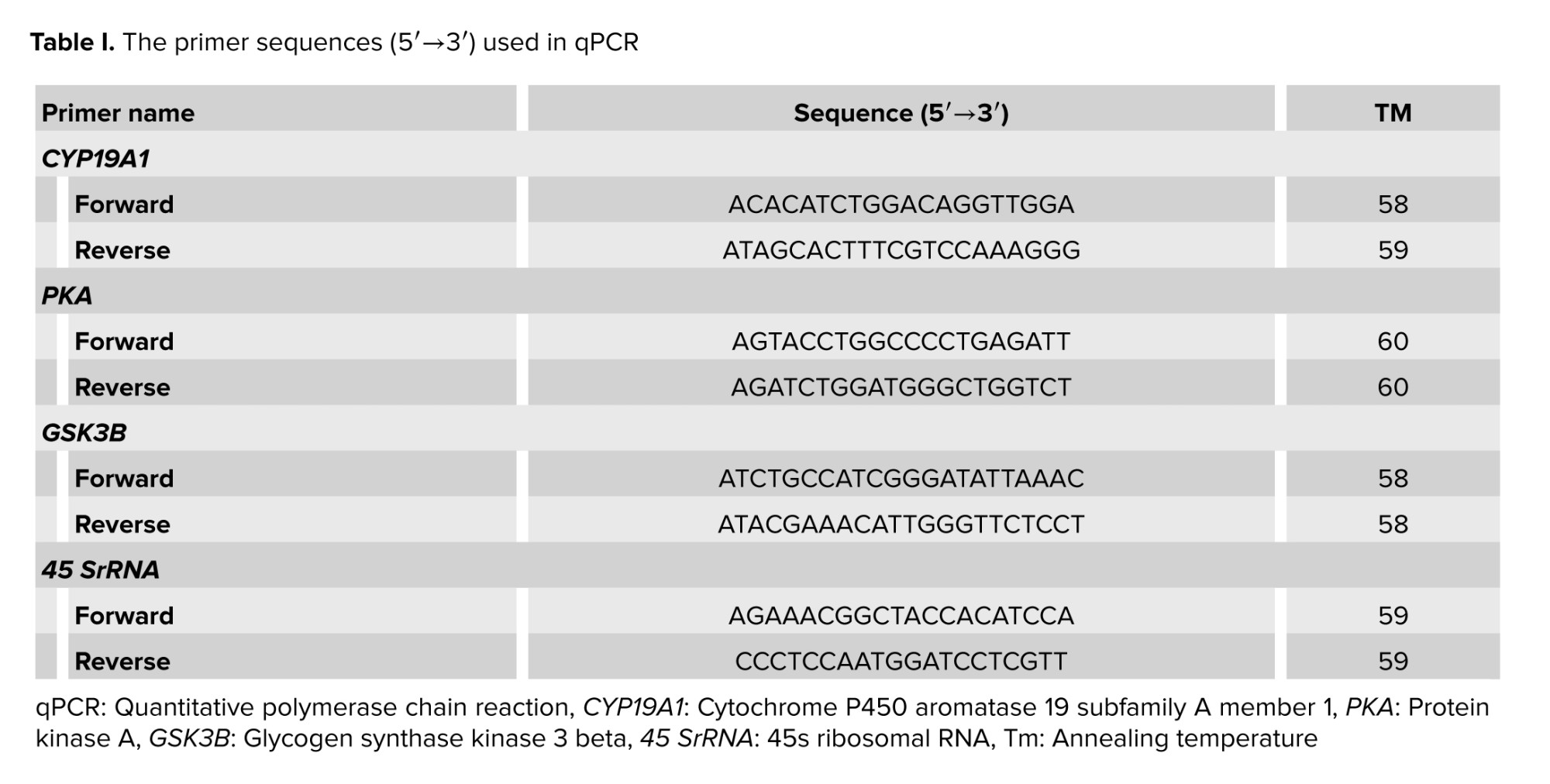
SYBR Green ROX qPCR Master Mix kit (Ampliqon, Denmark/Eppendorf) was utilized to conduct real-time PCR experiments. The PCR amplification settings included a denaturation stage at 95°C for 2 min, then 40 cycles of annealing and extension at 59°C for 25 sec. Comparative Ct (Ct) analysis was used to examine these results. The expression of the reference gene 45s rRNA and the genes CYP19A1, PKA, and GSK3B were examined. These tests were carried out in triplicate and at least 3 times independently (Table I).
2.3. Ethical Considerations
The Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran approved this experimental project (Code: IR.SSU.MEDICINE.REC.1400.316). The Declaration of Helsinki was followed when conducting the study. All individuals who took part in the study provided a written informed consent.
2.4. Statistical Analysis
Data analysis was done by GraphPad Prism (version 8.4.3) and IBM SPSS Statistics (version 26, SPSS Inc., Chicago, USA). One-way ANOVA and multiple t tests were done to check the statistical differences between study groups. The significance was demonstrated by p < 0.05.
3. Results
3.1. Demographic and clinical characteristics
40 women (20 in the DOR group and 20 in the control group) were included in this study. No statistically significant difference was observed between groups; however, the mean BMI of the DOR women was higher than control group (table II).
3.2. CYP19A1, PKA, GSK3B genes expression in CCs
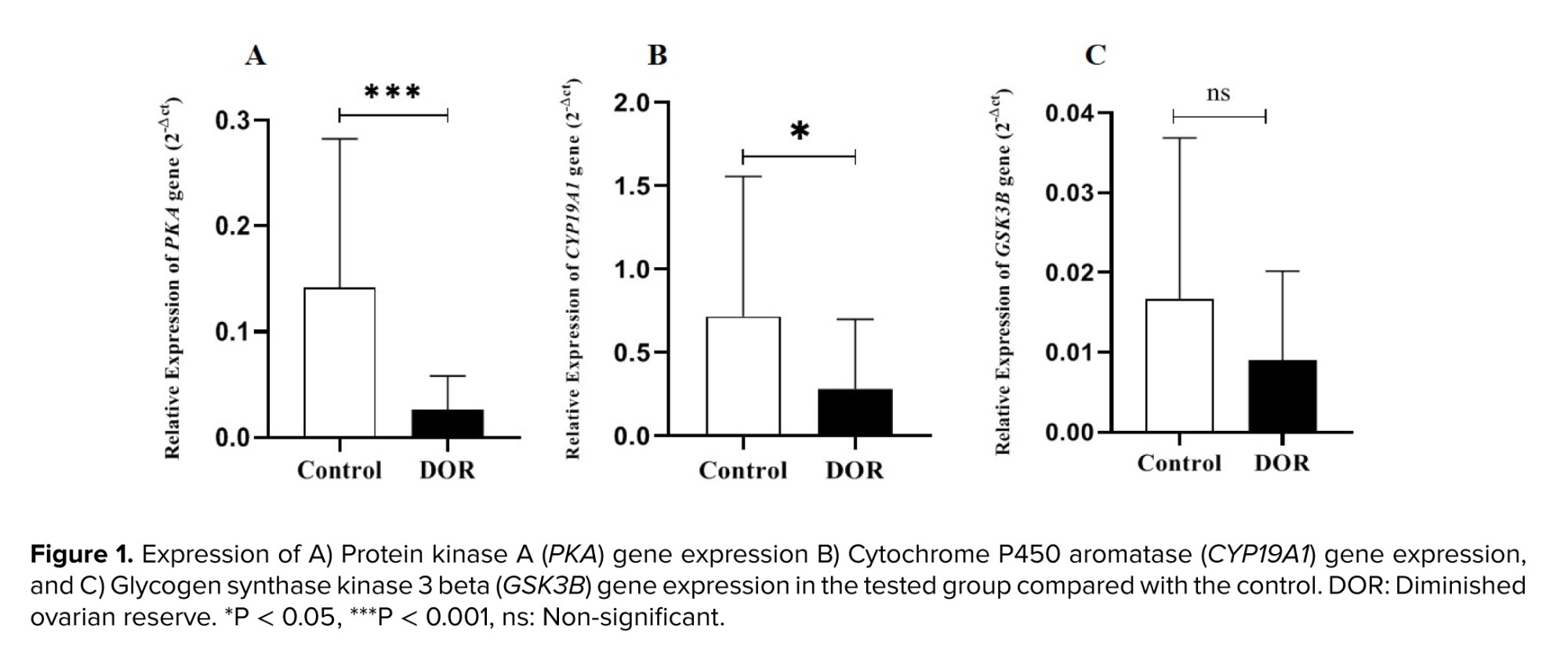
CYP19A1 gene expression in DOR group was significantly lower than in the control group (p = 0.04). A significant difference was observed in the PKA gene expression in the DOR group compared to the control group (p < 0.001). Also, GSK3B gene expression was lower in the study group, although this difference was not statistically significant (p = 0.07). It was also demonstrated that the PKA and GSK3B gene expressions were lower in the study group, although these differences were not statistically significant. Our study's findings also demonstrated that the expression of the PKA gene had decreased in the study group (p = 0.0009) compared to control group. Also, expression of the GSK3B gene was lower in the study group, although this difference was not statistically significant (Figure 1).
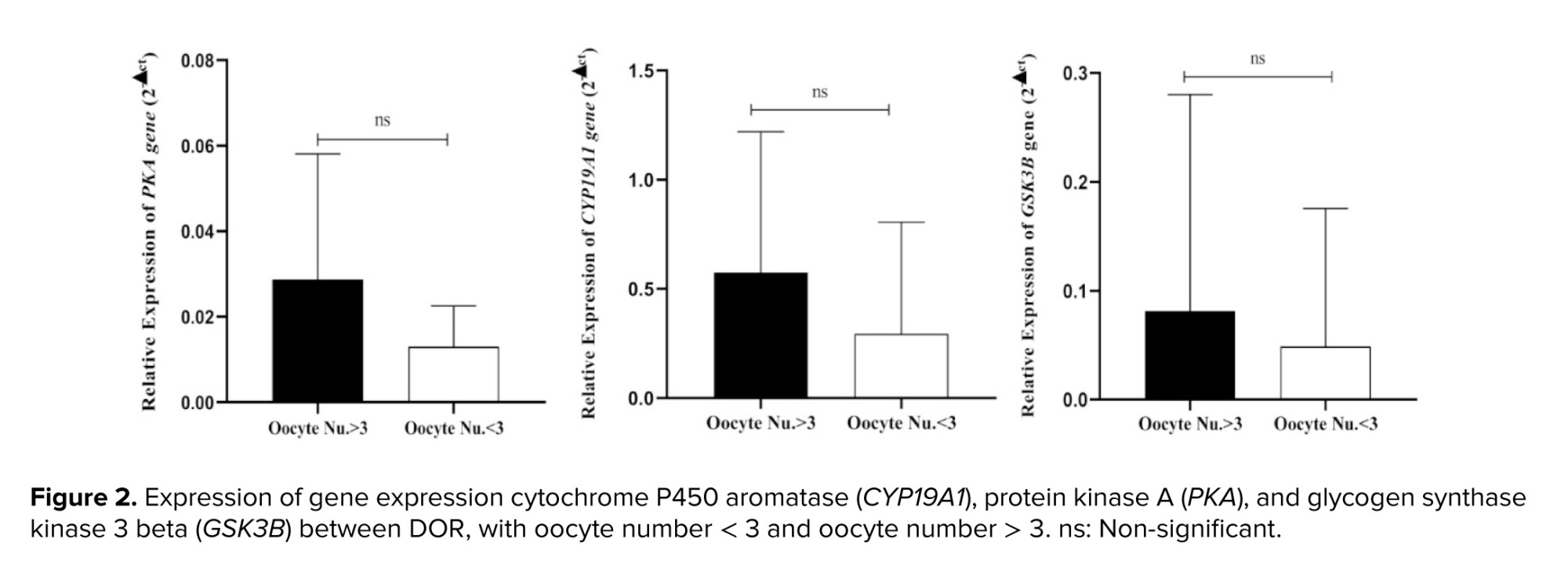
It was also found that the expression of the CYP19A1, PKA, and GSK3B genes was lower in women with < 3 oocytes than in the other group. However, these differences were not statistically significant (Figure 2).

4. Discussion
The results of our study show a decrease in the expression of the CYP19A1 gene in a study group with poor ovarian response compared to the normal group. The essential steroidogenesis enzyme aromatase (P450 arom), which catalyzes the last stages of estrogen production from androgens, is encoded by the CYP19A1 gene. In gonads and extragonadal tissue (brain and adipose tissue), it converts androstenedione to estrone and testosterone to estradiol and estrone (18). In granulosa cells, the aromatase enzyme is involved in the manufacture of estrogen. The quality of oocyte production, which in turn determines the quality of the embryo, is determined by the granulosa cells capacity to perform their roles. According to the study, women with polycystic ovary syndrome had lower average mRNA CYP19A1 expression for aromatase in granulosa cells than women without polycystic ovary syndrome (19).
Many studies demonstrate that DOR in young women can be attributed to changes in the expression of specific genes, such as CYP19A1 and FSHR, in cumulus and granulosa cells (20-22). CYP19-deficient mice have shown that this gene is crucial for the growth of ovarian follicles (23). Our findings also confirmed that the expression of the PKA gene had decreased in the DOR group as compared to the control group.
Numerous studies show the importance of the CAMP/PKA and Wnt signaling pathways in the production of estrogen, the growth of follicles, and ovulation (11-13). The expression of the CYP19A1 gene rises as a result of PKA's activation and effect on the beta-catenin signaling pathways of the Wnt signaling system. A study in 2016 found that ovulation is impacted by any nonfunctional PKA gene mutation (24).
Additionally, the Wnt signaling pathway is essential for the growth and division of follicles as well as ovulation, and its disruption affects both the process of ovulation and folliculogenesis (25). PKA/cAMP and PI3K-AKT signaling pathways have been linked to FSH-related signaling in GC (17, 26, 27). Our study's findings also demonstrated that the expression of the GSK3B gene decreased as compared to control group, although the expression difference was not statistically significant. In this article, we did not observe significant decreases in gene expression in women with fewer than 3 oocytes compared to women with more than 3 oocytes.
5. Conclusion
According to the findings, a defect in the expression of the CYP19A1, GSK3B, and PKA genes, as well as a disruption of the steroidogenesis process, are thought to be the causes of DOR and the variable responses of women to gonadotropin-releasing hormone inducing medications. Therefore, our data may offer new suggestions for molecular indicators to be used in studies of poor responders.
Data Availability
Upon a reasonable request, the corresponding author will provide the data supporting the study’s conclusions.
Author Contributions
MH. Sheikhha and M. Lotfi designed the study and conducted the research. Z. Ahmadnia, F. Montazeri, and M. Lotfi monitored, evaluated, and analyzed the results of the study. Further, Z. Ahmadnia, S. Dashti, MH. Sheikhha, and M. Lotfi reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
This work was supported by Shahid Sadoughi University of Medical Sciences, Yazd, Iran (grant number: 12044). The authors would like to thank Dr. Fatemeh Anbari for technical assistance. We did not apply artificial intelligence for this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (81 Views)
1. Introduction
Diminished ovarian reserve (DOR) is one of the causes of infertility in women and refers to cases where the antral follicle counts (AFC) < 5 and anti-Mullerian hormone (AMH) level < 1.2 ng/ml (1). The term “ovarian reserve” describes the ovaries capacity for reproduction (2). The hereditary form of DOR is rare and accounts for 4-31% of all cases (3). In 90% of cases, the cause is unknown, and the disease known as idiopathic (3, 4).
Ovarian reserve determines the number and quality of ovarian follicles. To maximize the success rate of infertility treatment, it is essential to evaluate the effective signaling pathways on ovarian reserve (5, 6). Women with DOR, have lower sex hormone levels such as estrogen and inhibin, and higher gonadotropin levels such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (7). Gonadotropins play an important role in the ovulation and folliculogenesis processes. An imbalance in the secretion of hormones can disturb folliculogenesis. Estradiol synthesis is influenced by the appropriate interaction of growth hormones, gonadotropins, LH, and FSH (8, 9).
Steroidogenesis is an effective process in the growth of ovarian follicles through the production of estrogen. It starts in theca cells, where cholesterol is metabolized into androgens. Granulosa cells, on the other hand, are responsible for changing androgen into estrogen (10, 11). The main signaling pathways related to steroidogenesis are cAMP/ Protein kinase A (PKA) and Wnt/β-catenin. PKA is activated by trophic hormones to control steroid hormone production in steroidogenic cells (12). Glycogen synthase kinase 3 beta (GSK3B) plays a key role in the regulation of the Wnt/β-catenin signaling pathway (13).
LH regulates theca cell steroidogenesis by activating the cAMP signaling pathway through a G protein-coupled receptor. FSH controls the granulosa cell’s ability to produce estrogen. FSH binding initiates the cAMP pathway, which in turn causes the synthesis of cytochrome P450 aromatase (CYP19A1) and 17-hydroxysteroid dehydrogenase in relation to LH signaling. CYP19A1 converts thecal androstenedione irreversibly into estrone, and HSD17B1 then changes estrone into the more potent estradiol (14, 15).
One of the crucial elements in the management of assisted reproduction technology is controlled ovarian hyperstimulation. Also, ovarian response varies from person to person; for example, poor responders often have a low in vitro fertilization (IVF) success rate and often need a prolonged ovarian stimulation cycle. Poor IVF outcomes can lead to psychological and financial stress (16). Therefore, it is crucial to find good predictive tools for IVF outcomes, which can identify key genes in the signaling pathways involved in folliculogenesis. Inhibition of follicle-stimulating hormone receptors using siRNA has been reported to influence miR-1261, miR-130a-3p, miR-329-3p, miR-185-5p, miR-144-5p and miR-4463 expressions. Genes that are regulated by the abovementioned miRNAs have been identified using bioinformatic tools, and it has been found that overexpression of miR-4463 plays a key role in poor ovarian response in KGN cells. It has been suggested that miR-4463 is involved in poor response to gonadotrophin injection in women (17).
In the current study, the expression of miR-4463 regulated genes involved in the steroidogenesis pathway, in cumulus cells (CC), following gonadotropin administration in women with DOR and control group were evaluated.
2. Materials and Methods
Participants in this case-control study were women aged between 20 and 34 yr who referred to Yazd Reproductive Biology Research Institute, Yazd, Iran from November 2021 toDecember 2022.
The inclusion criteria in the case group were: AMH < 1.2 ng/ml and AFC < 5 (n = 20), and in control group were AMH > 1.2 ng/ml and AFC < 5 (n = 20) that were candidates for IVF/intracytoplasmic sperm injection cycles because of male problems. Participants with absence of uterine anomaly, endometriosis, history of thyroid disease, chromosomal problems that lead to reduced ovarian reserve, and polycystic ovaries were excluded from this study.
In these women, ovulation stimulation was performed using the antagonist protocol. In such a way that 300 IU of human menopausal gonadotropin (Merional, IBSA, Lugano, Switzerland) is given to patients from the second day of the menstrual cycle to the 6th day. Ultrasound was performed on the 7th day of the patients menstrual cycle. This process was repeated for 5-6 days until follicles with a diameter of 12-15 mm were observed. After observing follicles with the appropriate diameter, 0.25 mg/day of gonadotropin releasing hormone antagonist (Cetrotide; Sereno International S.A., Geneva, Switzerland) was injected. Ovulation stimulation was continued until follicles with a diameter of 16-18 mm were observed. After which ovulation is done.
Then, human chorionic gonadotropin (Pregnyl; Organon, Oss, the Netherlands) was injected. After 36 hr, ovulation was performed and CC were isolated from ovarian follicles by a reproductive biologist.
Patients with low ovarian reserves were divided into 2 groups, depending on the response to ovulation stimulation drugs, the number of oocytes less than 3 and more than 3.
2.1. Total RNA extraction and cDNA synthesis
Total RNA was extracted from CCs. RNA extraction was performed using a TRIZOL reagent. RNA concentration, integrity, and purity were analyzed using a Nanodrop spectrophotometer (DeNovix, USA) and agarose gel electrophoresis. Complementary DNA (cDNA) was synthesized using Pars Toos Company kit, Iran according to the manufacturer’s instructions.
2.2. Quantitative reverse transcription polymerase chain reaction (RT-PCR)
SYBR Green ROX qPCR Master Mix kit (Ampliqon, Denmark/Eppendorf) was utilized to conduct real-time PCR experiments. The PCR amplification settings included a denaturation stage at 95°C for 2 min, then 40 cycles of annealing and extension at 59°C for 25 sec. Comparative Ct (Ct) analysis was used to examine these results. The expression of the reference gene 45s rRNA and the genes CYP19A1, PKA, and GSK3B were examined. These tests were carried out in triplicate and at least 3 times independently (Table I).

2.3. Ethical Considerations
The Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran approved this experimental project (Code: IR.SSU.MEDICINE.REC.1400.316). The Declaration of Helsinki was followed when conducting the study. All individuals who took part in the study provided a written informed consent.
2.4. Statistical Analysis
Data analysis was done by GraphPad Prism (version 8.4.3) and IBM SPSS Statistics (version 26, SPSS Inc., Chicago, USA). One-way ANOVA and multiple t tests were done to check the statistical differences between study groups. The significance was demonstrated by p < 0.05.
3. Results
3.1. Demographic and clinical characteristics
40 women (20 in the DOR group and 20 in the control group) were included in this study. No statistically significant difference was observed between groups; however, the mean BMI of the DOR women was higher than control group (table II).
3.2. CYP19A1, PKA, GSK3B genes expression in CCs
CYP19A1 gene expression in DOR group was significantly lower than in the control group (p = 0.04). A significant difference was observed in the PKA gene expression in the DOR group compared to the control group (p < 0.001). Also, GSK3B gene expression was lower in the study group, although this difference was not statistically significant (p = 0.07). It was also demonstrated that the PKA and GSK3B gene expressions were lower in the study group, although these differences were not statistically significant. Our study's findings also demonstrated that the expression of the PKA gene had decreased in the study group (p = 0.0009) compared to control group. Also, expression of the GSK3B gene was lower in the study group, although this difference was not statistically significant (Figure 1).
It was also found that the expression of the CYP19A1, PKA, and GSK3B genes was lower in women with < 3 oocytes than in the other group. However, these differences were not statistically significant (Figure 2).



4. Discussion
The results of our study show a decrease in the expression of the CYP19A1 gene in a study group with poor ovarian response compared to the normal group. The essential steroidogenesis enzyme aromatase (P450 arom), which catalyzes the last stages of estrogen production from androgens, is encoded by the CYP19A1 gene. In gonads and extragonadal tissue (brain and adipose tissue), it converts androstenedione to estrone and testosterone to estradiol and estrone (18). In granulosa cells, the aromatase enzyme is involved in the manufacture of estrogen. The quality of oocyte production, which in turn determines the quality of the embryo, is determined by the granulosa cells capacity to perform their roles. According to the study, women with polycystic ovary syndrome had lower average mRNA CYP19A1 expression for aromatase in granulosa cells than women without polycystic ovary syndrome (19).
Many studies demonstrate that DOR in young women can be attributed to changes in the expression of specific genes, such as CYP19A1 and FSHR, in cumulus and granulosa cells (20-22). CYP19-deficient mice have shown that this gene is crucial for the growth of ovarian follicles (23). Our findings also confirmed that the expression of the PKA gene had decreased in the DOR group as compared to the control group.
Numerous studies show the importance of the CAMP/PKA and Wnt signaling pathways in the production of estrogen, the growth of follicles, and ovulation (11-13). The expression of the CYP19A1 gene rises as a result of PKA's activation and effect on the beta-catenin signaling pathways of the Wnt signaling system. A study in 2016 found that ovulation is impacted by any nonfunctional PKA gene mutation (24).
Additionally, the Wnt signaling pathway is essential for the growth and division of follicles as well as ovulation, and its disruption affects both the process of ovulation and folliculogenesis (25). PKA/cAMP and PI3K-AKT signaling pathways have been linked to FSH-related signaling in GC (17, 26, 27). Our study's findings also demonstrated that the expression of the GSK3B gene decreased as compared to control group, although the expression difference was not statistically significant. In this article, we did not observe significant decreases in gene expression in women with fewer than 3 oocytes compared to women with more than 3 oocytes.
5. Conclusion
According to the findings, a defect in the expression of the CYP19A1, GSK3B, and PKA genes, as well as a disruption of the steroidogenesis process, are thought to be the causes of DOR and the variable responses of women to gonadotropin-releasing hormone inducing medications. Therefore, our data may offer new suggestions for molecular indicators to be used in studies of poor responders.
Data Availability
Upon a reasonable request, the corresponding author will provide the data supporting the study’s conclusions.
Author Contributions
MH. Sheikhha and M. Lotfi designed the study and conducted the research. Z. Ahmadnia, F. Montazeri, and M. Lotfi monitored, evaluated, and analyzed the results of the study. Further, Z. Ahmadnia, S. Dashti, MH. Sheikhha, and M. Lotfi reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
This work was supported by Shahid Sadoughi University of Medical Sciences, Yazd, Iran (grant number: 12044). The authors would like to thank Dr. Fatemeh Anbari for technical assistance. We did not apply artificial intelligence for this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Genetics
References
1. Yin J, Chang H-M, Li R, Leung PCK. Recent progress in the treatment of women with diminished ovarian reserve. Gynecol Obstet Clin Med 2021; 1: 186-189. [DOI:10.1016/j.gocm.2021.10.004]
2. Zhang Q-L, Lei Y-L, Deng Y, Ma R-L, Ding X-S, Xue W, et al. Treatment progress in diminished ovarian reserve: Western and Chinese medicine. Chin J Integr Med 2023; 29: 361-367. [DOI:10.1007/s11655-021-3353-2] [PMID]
3. Zhang Y, Zhang Ch, Shu J, Guo J, Chang H-M, Leung PCK, et al. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: A systematic review and network meta-analysis. Hum Reprod Update 2020; 26: 247-263. [DOI:10.1093/humupd/dmz046] [PMID]
4. Ata B, Seyhan A, Seli E. Diminished ovarian reserve versus ovarian aging: Overlaps and differences. Curr Opin Obstet Gynecol 2019; 31: 139-147. [DOI:10.1097/GCO.0000000000000536] [PMID]
5. Tal R, Seifer DB. Ovarian reserve testing: A user's guide. Am J Obstet Gynecol 2017; 217: 129-140. [DOI:10.1016/j.ajog.2017.02.027] [PMID]
6. Olsen KW, Castillo-Fernandez J, Chan AC, la Cour Freiesleben N, Zedeler A, Bungum M, et al. Identification of a unique epigenetic profile in women with diminished ovarian reserve. Fertil Steril 2021; 115: 732-741. [DOI:10.1016/j.fertnstert.2020.09.009] [PMID]
7. Zhu Q, Li Y, Ma J, Ma H, Liang X. Potential factors result in diminished ovarian reserve: A comprehensive review. J Ovarian Res 2023; 16: 208. [DOI:10.1186/s13048-023-01296-x] [PMID] [PMCID]
8. Lunenfeld B, Bilger W, Longobardi S, Alam V, D'Hooghe T, Sunkara SK. The development of gonadotropins for clinical use in the treatment of infertility. Front Endocrinol 2019; 10: 429. [DOI:10.3389/fendo.2019.00429] [PMID] [PMCID]
9. Fa S, Pogrmic-Majkic K, Samardzija D, Glisic B, Kaisarevic S, Kovacevic R, et al. Involvement of ERK1/2 signaling pathway in atrazine action on FSH-stimulated LHR and CYP19A1 expression in rat granulosa cells. Toxicol Appl Pharmacol 2013; 270: 1-8. [DOI:10.1016/j.taap.2013.03.031] [PMID]
10. Damdimopoulou P, Chiang C, Flaws J. Retinoic acid signaling in ovarian folliculogenesis and steroidogenesis. Reprod Toxicol 2019; 87: 32-41. [DOI:10.1016/j.reprotox.2019.04.007] [PMID] [PMCID]
11. Xiao Ch, Wang J, Zhang Ch. Synthesis, regulatory factors, and signaling pathways of estrogen in the ovary. Reprod Sci 2023; 30: 350-360. [DOI:10.1007/s43032-022-00932-z] [PMID]
12. Gifford JAH. The role of WNT signaling in adult ovarian folliculogenesis. Reproduction 2015; 150: R137-R148. [DOI:10.1530/REP-14-0685] [PMID] [PMCID]
13. Li L, Shi X, Shi Y, Wang Z. The signaling pathways involved in ovarian follicle development. Front Physiol 2021; 12: 730196. [DOI:10.3389/fphys.2021.730196] [PMID] [PMCID]
14. Candelaria JI, Rabaglino MB, Denicol AC. Ovarian preantral follicles are responsive to FSH as early as the primary stage of development. J Endocrinol 2020; 247: 153-168. [DOI:10.1530/JOE-20-0126] [PMID]
15. Casarini L, Crépieux P. Molecular mechanisms of action of FSH. Front Endocrinol 2019; 10: 305. [DOI:10.3389/fendo.2019.00305] [PMID] [PMCID]
16. Cakiroglu Y, Yuceturk A, Karaosmanoglu O, Kopuk SY, Korun ZEU, Herlihy N, et al. Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich plasma (PRP). Aging 2022; 14: 2513-2523. [DOI:10.18632/aging.203972] [PMID] [PMCID]
17. Lee S-Y, Kang Y-J, Kwon J, Nishi Y, Yanase T, Lee K-A, et al. miR-4463 regulates aromatase expression and activity for 17β-estradiol synthesis in response to follicle-stimulating hormone. Clin Exp Reprod Med 2020; 47: 194. [DOI:10.5653/cerm.2019.03412] [PMID] [PMCID]
18. Praveen VP, Ladjouze A, Sauter K-S, Pulickal A, Katharopoulos E, Trippel M, et al. Novel CYP19A1 mutations extend the genotype-phenotype correlation and reveal the impact on ovarian function. J Endocr Soc 2020; 4: bvaa030. [DOI:10.1210/jendso/bvaa030] [PMID] [PMCID]
19. Panghiyangani R, Soeharso P, Suryandari DA, Wiweko B, Kurniati M, Pujianto DA. CYP19A1 gene expression in patients with polycystic ovarian syndrome. J Hum Reprod Sci 2020; 13: 100-103. [DOI:10.4103/jhrs.JHRS_142_18] [PMID] [PMCID]
20. Jirge PR. Poor ovarian reserve. J Hum Reprod Sci 2016; 9: 63-69. [DOI:10.4103/0974-1208.183514] [PMID] [PMCID]
21. Abu-Musa A, Haahr T, Humaidan P. Novel physiology and definition of poor ovarian response; clinical recommendations. Int J Mol Sci 2020; 21: 2110. [DOI:10.3390/ijms21062110] [PMID] [PMCID]
22. Wu Y-G, Barad DH, Kushnir VA, Wang Q, Zhang L, Darmon SK, et al. With low ovarian reserve, hghly individualized egg retrieval (HIER) improves IVF results by avoiding premature luteinization. J Ovarian Res 2018; 11: 23. [DOI:10.1186/s13048-018-0398-8] [PMID] [PMCID]
23. Song D, Huang X-L, Hong L, Yu J-M, Zhang Z-F, Zhang H-Q, et al. Sequence variants in FSHR and CYP19A1 genes and the ovarian response to controlled ovarian stimulation. Fertil Steril 2019; 112: 749-757. [DOI:10.1016/j.fertnstert.2019.05.017] [PMID]
24. Puri P, Little-Ihrig L, Chandran U, Law NC, Hunzicker-Dunn M, Zeleznik AJ. Protein kinase A: A master kinase of granulosa cell differentiation. Sci Rep 2016; 6: 28132. [DOI:10.1038/srep28132] [PMID] [PMCID]
25. Wang W, Wu K, Jia M, Sun S, Kang L, Zhang Q, et al. Dynamic changes in the global microRNAome and transcriptome identify key nodes associated with ovarian development in chickens. Front Genet 2018; 9: 491. [DOI:10.3389/fgene.2018.00491] [PMID] [PMCID]
26. Wang Y, Cheng T, Lu M, Mu Y, Li B, Li X, et al. TMT-based quantitative proteomics revealed follicle-stimulating hormone (FSH)-related molecular characterizations for potentially prognostic assessment and personalized treatment of FSH-positive non-functional pituitary adenomas. EPMA J 2019; 10: 395-414. [DOI:10.1007/s13167-019-00187-w] [PMID] [PMCID]
27. Zareifard A. Le rôle de Janus Kinase 3 (JAK3) dans le développement folliculaire [M.Sc. thesis]. Montreal University; 2022.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







