Sat, Jan 31, 2026
[Archive]
Volume 22, Issue 12 (December 2024)
IJRM 2024, 22(12): 1015-1024 |
Back to browse issues page
Ethics code: IR.KAUMS.REC.1394.58
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zamani-Badi T, Karimian M, Amini Mahabadi J, Rafatmanesh A, Nikzad H. IL-6-174 G/C transversion might decrease male infertility risk: A case-control study. IJRM 2024; 22 (12) :1015-1024
URL: http://ijrm.ir/article-1-3217-en.html
URL: http://ijrm.ir/article-1-3217-en.html
Tayyebeh Zamani-Badi1 

 , Mohammad Karimian2
, Mohammad Karimian2 

 , Javad Amini Mahabadi3
, Javad Amini Mahabadi3 

 , Atieh Rafatmanesh4
, Atieh Rafatmanesh4 

 , Hossein Nikzad *5
, Hossein Nikzad *5 




 , Mohammad Karimian2
, Mohammad Karimian2 

 , Javad Amini Mahabadi3
, Javad Amini Mahabadi3 

 , Atieh Rafatmanesh4
, Atieh Rafatmanesh4 

 , Hossein Nikzad *5
, Hossein Nikzad *5 


1- Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran.
2- Department of Molecular and Cell Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran.
3- Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran. & Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran.
4- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran.
5- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran. ,hnikzad1343@gmail.com
2- Department of Molecular and Cell Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran.
3- Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran. & Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran.
4- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran.
5- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran. ,
Full-Text [PDF 472 kb]
(506 Downloads)
| Abstract (HTML) (843 Views)
Full-Text: (115 Views)
- Introduction
Infertility is a complex issue influenced by various factors, including anatomical, environmental, lifestyle, physiological, and genetic components. Male infertility is a prevalent concern affecting around 5% of men (1).
While there are several causes of male infertility, including post-testicular obstruction, varicocele, anti-sperm antibody production, and hormonal imbalances, genetic factors are regarded as one of the primary contributing factors (2). Studies have demonstrated that genetic factors such as aneuploidies, Y chromosome microdeletions, mitochondrial DNA abnormalities, point mutations, and variations in key genes can heighten the risk of male infertility (3). Our prior research has also established associations between genetic polymorphisms in certain cytokine gene families, such as interleukin (IL)-1α, IL-1β, IL-1RA, and susceptibility to male infertility (4, 5).
Interleukin 6 (IL-6) is a versatile cytokine found in seminal fluid, typically produced by various cell types, including Sertoli and germ cells (6). Consequently, it plays a regulatory role in the growth, differentiation, and function of sperm. IL-6 levels have been found to correlate with the secretory activity of Sertoli cells and the count of leukocytes in seminal fluid. Moreover, IL-6 is a key marker for detecting inflammation in seminal fluid (7, 8).
Several single nucleotide polymorphisms (SNPs) within the IL-6 promoter region such as -174G > C, -572G > C, -597G > A, -634C > G, -1363 G > T, and -2954G > C have been extensively studied in relation to various diseases, such as deep vein thrombosis, type 2 diabetes mellitus, and cardiovascular conditions (9-12). Particularly, the -174G > C polymorphism has shown an association with male infertility (13). This polymorphism is an upstream genetic variation located at the promoter region. The mutated allele in this polymorphism is C, serving as a replacement for the wild-type allele G (14). Based on their position in gene regulatory regions, these polymorphisms may cause changes in gene expression (15).
The objective of our study was to investigate the potential link between the IL-6-174G/C SNP and the risk of male infertility.
While there are several causes of male infertility, including post-testicular obstruction, varicocele, anti-sperm antibody production, and hormonal imbalances, genetic factors are regarded as one of the primary contributing factors (2). Studies have demonstrated that genetic factors such as aneuploidies, Y chromosome microdeletions, mitochondrial DNA abnormalities, point mutations, and variations in key genes can heighten the risk of male infertility (3). Our prior research has also established associations between genetic polymorphisms in certain cytokine gene families, such as interleukin (IL)-1α, IL-1β, IL-1RA, and susceptibility to male infertility (4, 5).
Interleukin 6 (IL-6) is a versatile cytokine found in seminal fluid, typically produced by various cell types, including Sertoli and germ cells (6). Consequently, it plays a regulatory role in the growth, differentiation, and function of sperm. IL-6 levels have been found to correlate with the secretory activity of Sertoli cells and the count of leukocytes in seminal fluid. Moreover, IL-6 is a key marker for detecting inflammation in seminal fluid (7, 8).
Several single nucleotide polymorphisms (SNPs) within the IL-6 promoter region such as -174G > C, -572G > C, -597G > A, -634C > G, -1363 G > T, and -2954G > C have been extensively studied in relation to various diseases, such as deep vein thrombosis, type 2 diabetes mellitus, and cardiovascular conditions (9-12). Particularly, the -174G > C polymorphism has shown an association with male infertility (13). This polymorphism is an upstream genetic variation located at the promoter region. The mutated allele in this polymorphism is C, serving as a replacement for the wild-type allele G (14). Based on their position in gene regulatory regions, these polymorphisms may cause changes in gene expression (15).
The objective of our study was to investigate the potential link between the IL-6-174G/C SNP and the risk of male infertility.
- Materials and Methods
- Participants with inclusion and exclusion criteria
A case-control study was carried out involving a total of 314 participants, comprising 163 cases and 151 controls. The sample collection was performed between August 2015 and November 2017. Among a total of 314 participants recruited for this investigation, 163 cases were selected from individuals seeking assistance at the Kashan Infertility Center, specifically Shahid Beheshti hospital in Kashan, Iran.
Infertile men included in this study had no history of systemic or other identified genetic diseases, as determined through interviews. Those with conditions such as orchitis, maldescensus of the testis, varicocele, vas deferens obstruction, immune or infectious abnormalities, drug abuse, diabetes mellitus, abnormal hormone profiles (luteinizing hormone, follicle stimulating hormone, and testosterone), abnormal karyotype, and Y-chromosome microdeletions were excluded. Based on spermiogram results, infertile men were classified into subgroups of oligozoospermia (n = 61), asthenozoospermia (n = 60), and nonobstructive azoospermia (NOA; n = 42). The control group consisted of fertile men with normal sperm parameters, randomly selected, who had at least one offspring without assisted reproductive technologies, and were referred to the same clinic. Additionally, men with any known genetic or familial diseases were excluded from the control group. The demographic and clinical features of cases and controls are summarized in table I. After completing the screening process, a 2 ml sample of whole blood was collected from each participant.
2.2. Sample size
Based on a similar study, and using the online server, for comparing a ratio or outcome between 2 groups, considering a significance level (α) of 0.05, study power of 80%, and the maximum observed mutant allele percentage in patients as 40.4% and in healthy individuals as 20%, the sample size for each group is estimated to be 78 individuals (13, 16). However, due to our access, we were able to collect a larger sample size.
2.3. DNA extraction and genotype analysis
Genomic DNA was extracted from blood samples using a commercially available DNA extraction kit (Bioneer Co., Daejeon, Korea). The genotyping of IL-6 polymorphism was done using the polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) method. For this, specific primers were designed in the vicinity of the polymorphic region using Oligo7 software. The sequences of the forward and reverse primers were as follows:
Forward primer (F): 5′-GAAGAGTGGTTCTGCTTCTTAG-3′ and reverse primer (R): 5′-TTTGATAAATCTTTGTTGGAGG-3′.
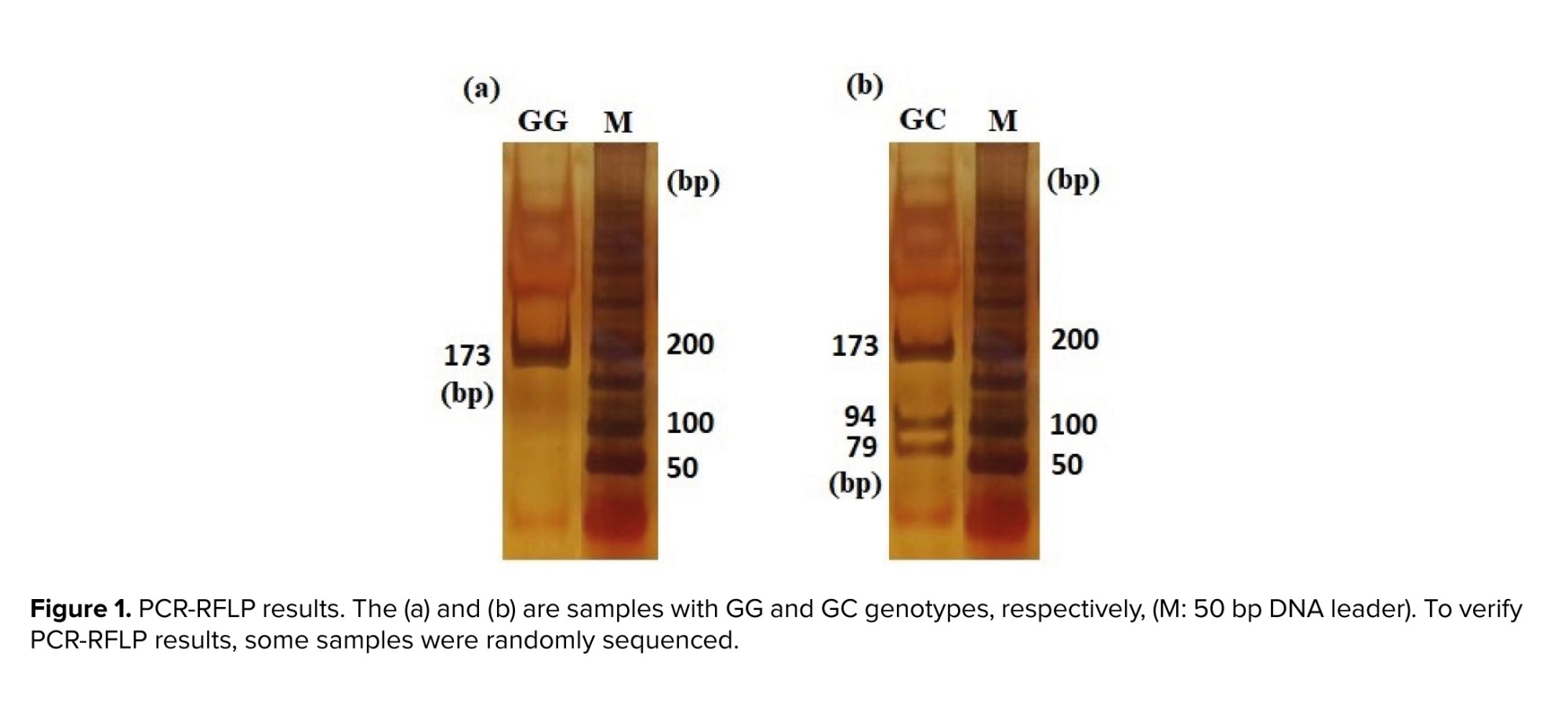
PCR was conducted in a total volume of 20 μl containing 10 μl premix (CinnaGen, Iran), 0.5 μM of forward and reverse primers, and 2 μl (30 ng) template DNA. The PCR was performed in a Peqlab peqSTAR thermal cycler with the following program: 94°C for 5 min, 94°C for 45 sec, 53°C for 45 sec, 72°C for 45 sec, 35 cycles, and final 72°C for 5 min. Subsequently, 5 μl of the PCR products were subjected to digestion using 0.8 units of Hin1II restriction enzyme (#ER1831-Thermo Scientific, Lot: 00471907) with an incubation period of 16 hr at 37°C. The enzyme was subsequently inactivated through incubation at 65°C for 20 min. The digested fragments were separated by electrophoresis on the 8% polyacrylamide gel and visualized using DNA Green Viewer (CinnaGen CO.). Following electrophoresis, 3 distinct genotypes were identified based on their differing sizes: GG (173bp), GC (173, 79, 94), and CC (94, 79) (Figure 1).
2.4. Ethical Considerations
Sample collection was conducted after obtaining informed consent from the participants. This study received ethical approval from Kashan University of Medical Sciences, Kashan, Iran (Code: IR.KAUMS.REC.1394.58).
2.5. Statistical Analysis
Statistical analysis for the case-control study was conducted using Statistical Package for the Social Sciences (SPSS) 22 software for Windows (SPSS Inc., Chicago, USA). To assess the normal distribution of continuous variables, the Kolmogorov-Smirnov test was employed for adjustment. Numerical variables were analyzed with t test. The Hardy-Weinberg equilibrium of observed genotype frequencies were determined using a goodness‐of‐fit Chi‐square test. Associations between categorical variables, including genotype frequencies and odds ratios, were evaluated using the Chi‐square test. A significance level of p < 0.05 was considered statistically significant.
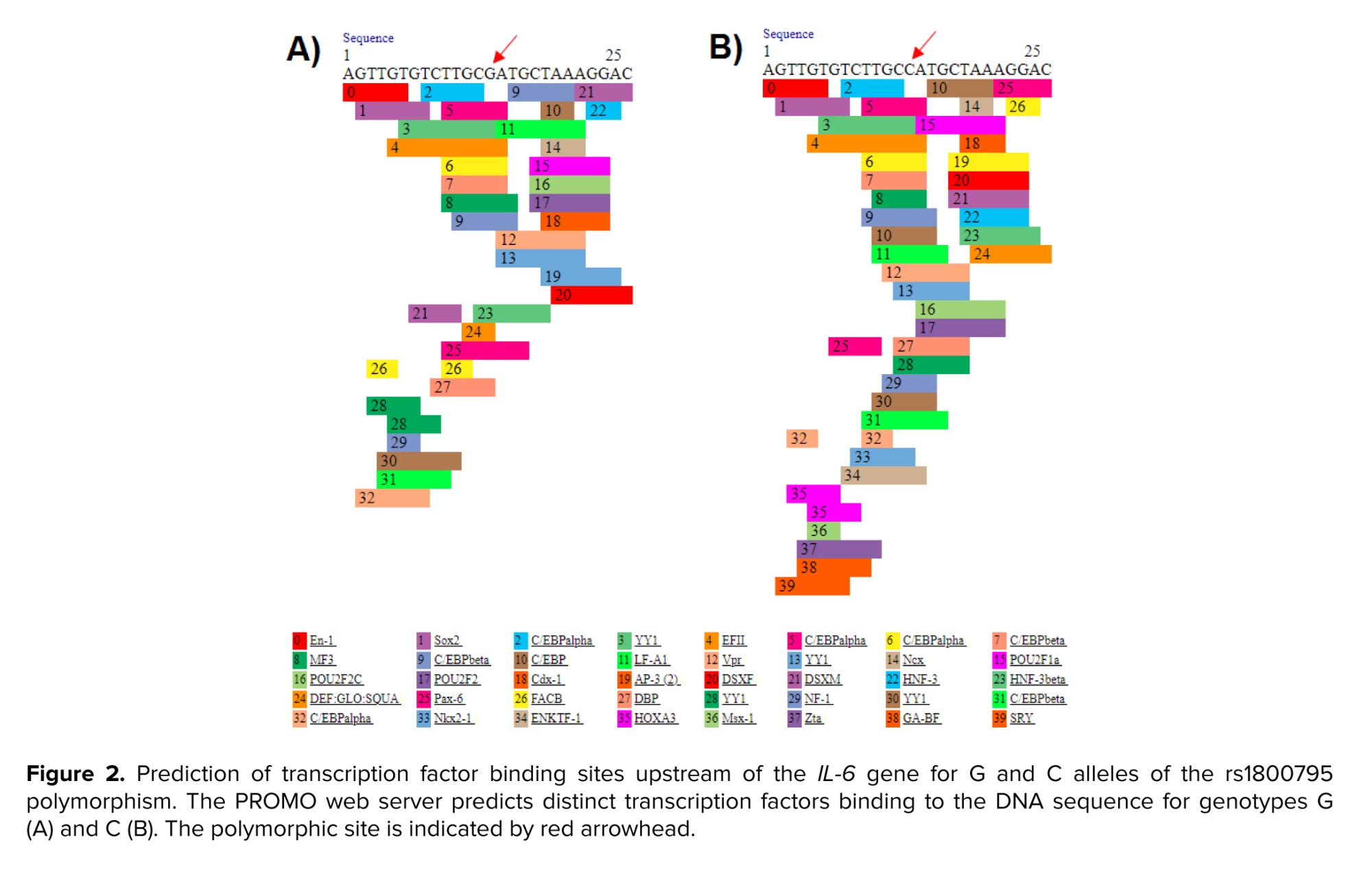
Sometimes, mutant alleles can improve survival, increase biological efficiency, and adapt to the environment (17, 18). There are numerous studies indicating that mutant alleles of SNPs are protective (19, 20). We stated in our article that the C allele is a protective factor against infertility, but this does not mean that the G allele is the cause of infertility. All genetic polymorphisms and mutations are not necessarily risk factors and can have positive effects on the corresponding protein or even RNA. For example, Mobasseri et al. reported that the mutant allele of rs2234693 polymorphism can be a protective factor against male infertility with a positive effect on RNA stability (21). Given that the polymorphism studied is located upstream of the gene, it may affect transcription factor binding sites. The eukaryotic promoter database indicated that the IL-6-174 G/C polymorphism is positioned close to and upstream of the promoter, and the PROMO server showed that the C variant creates additional binding sites for transcription factors for IL-6 (Figure 2), potentially playing an effective role in the gene regulation. Therefore, this may explain the protective role of the IL-6-174 G/C polymorphism against male infertility.
A previous study conducted in the Uttar Pradesh population of North India also demonstrated an association between the IL-6-174 G/C transversion and male infertility. Their subgroup analysis revealed higher levels of apoptosis and necrosis in individuals with oligozoospermia and asthenospermia compared to the control group. Additionally, they found associations with increased levels of reactive oxygen species (ROS), decreased testosterone and luteinizing hormone, and increased prolactin and follicle-stimulating hormone in the infertile group (13).
Male infertility is a complex, multifactorial condition that poses a significant global public health challenge, with a substantial percentage of affected men experiencing conditions such as azoospermia, asthenozoospermia, and oligospermia. An array of factors, including anatomical, environmental, and genetic factors, contribute to male infertility (22).
The process of spermatogenesis, which is vital for producing mature sperm, is influenced by various cytokines. Elevated levels of IL-6 have been associated with disruptions in spermatogenesis, potentially contributing to infertility (23). Notably, there is an inverse relationship between cholesterol levels and IL-6, with cholesterol playing a role in male gonad parameters like Sertoli cell function, germ cell differentiation, and steroidogenesis (24). Any alteration affecting IL-6 integrity can impact reproductive function, directly or indirectly, and is correlated with sperm lipid peroxidation (25).
Among genetic factors, polymorphisms in cytokine genes can alter their structure, function, or expression levels. Polymorphisms in the IL-6 gene have the potential to elevate cytokine levels and induce the generation of ROS, which can have detrimental effects on spermatogenesis (13). Roughly, 30-80% of ROS levels can lead to damage in spermatozoa. Elevated ROS levels can damage sperm membranes, DNA, and proteins, resulting in abnormalities in sperm count, motility, and structural integrity (26). Consequently, these disruptions can manifest as various phenotypes, including azoospermia, oligozoospermia, and asthenozoospermia (27).
In many cases of autosomal recessive traits, individuals who are homozygous for mutated allele experience reduced fitness compared to those who are heterozygous or homozygous for the normal allele. However, there are certain disorders where environmental conditions lead to higher fitness in heterozygotes than in either homozygous genotype. This phenomenon is known as heterozygote advantage. It is significant because even a slight advantage in heterozygotes can lead to an increase in the frequency of the mutated allele within the population, even if the mutation significantly reduces fitness in homozygotes. Instances where natural selection simultaneously works to maintain a harmful allele in the gene pool while also working to eliminate it are called balanced polymorphisms (28). The inheritance pattern of SNPs follows the general principles of Mendelian inheritance, with some subtle differences, and in some accepted models including codominant, dominant-recessive, incomplete dominance, overdominance, and additive. Generally, the inheritance pattern of SNPs can vary depending on the specific SNP and its interaction with other genetic and environmental factors. Additionally, SNPs can exhibit different inheritance patterns in populations or different ethnic groups (29, 30).
For examining only one gene as well as one polymorphism, this preliminary study serves as an initial investigation, providing data that will guide future, more thorough research aimed at drawing clinical conclusions regarding whether this genetic variation can serve as a biomarker indicating susceptibility to male infertility. Considering that investigating the effects of genetic polymorphisms through experimental methods such as in vitro and in vivo approaches is costly and time-consuming, computational methods can be a suitable approach for studying the effects of polymorphisms (15). Given that the polymorphism under study, rs1800795, is an upstream variant, its effects on gene function, particularly on gene expression, are suggested to be explored using bioinformatics tools.
However, it is essential to acknowledge the limitations of our study, particularly regarding gene-gene and gene-environment interactions that were not explored. Considering the results of our study and the absence of the homozygous CC genotype in the entire population under study (both in the case and control groups), we were unable to statistically analyze the behavior of this genotype in male infertility, precisely. With the low frequency of the homozygous CC genotype in the population, obtaining a larger sample size and accessing individuals with the homozygous CC genotype will result in more accurate results, regarding this genotype and its association with male infertility. Additionally, obtaining more precise data would necessitate larger study groups of both infertile and control subjects from diverse ethnic populations, as geographical regions can influence study outcomes. Moreover, exploring additional polymorphisms in IL-6 gene and other inflammatory genes in fertile and infertile men could contribute to obtaining more precise conclusions.
Data Availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author Contributions
H. Nikzad, and M. Karimian designed the study and conducted the research. T. Zamani-Badi, J. Amini Mahabadi, and A. Rafatmanesh evaluated and analyzed the results of the study. Further, M. Karimian, H. Nikzad, T. Zamani-Badi, J. Amini Mahabadi, and A. Rafatmanesh reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data. Due to the multidisciplinary nature of the article and the important role of both corresponding authors in various stages of the research, including design, data analysis, and writing, 2 corresponding authors have been introduced to reflect the equal contribution and importance of each of their expertise.
Acknowledgements
This work is supported by the Vice-Chancellor for Research and Technology of Kashan University of Medical Sciences, Kashan, Iran (grant number: 94058). We would like to express our gratitude to Dr. Hossein Mahmoudi, a urology specialist, for his consultation on this project. We did not use artificial intelligence in the preparation of this article.
Conflict of Interest
The authors declare that there is no conflict of interest.
Infertile men included in this study had no history of systemic or other identified genetic diseases, as determined through interviews. Those with conditions such as orchitis, maldescensus of the testis, varicocele, vas deferens obstruction, immune or infectious abnormalities, drug abuse, diabetes mellitus, abnormal hormone profiles (luteinizing hormone, follicle stimulating hormone, and testosterone), abnormal karyotype, and Y-chromosome microdeletions were excluded. Based on spermiogram results, infertile men were classified into subgroups of oligozoospermia (n = 61), asthenozoospermia (n = 60), and nonobstructive azoospermia (NOA; n = 42). The control group consisted of fertile men with normal sperm parameters, randomly selected, who had at least one offspring without assisted reproductive technologies, and were referred to the same clinic. Additionally, men with any known genetic or familial diseases were excluded from the control group. The demographic and clinical features of cases and controls are summarized in table I. After completing the screening process, a 2 ml sample of whole blood was collected from each participant.
2.2. Sample size
Based on a similar study, and using the online server, for comparing a ratio or outcome between 2 groups, considering a significance level (α) of 0.05, study power of 80%, and the maximum observed mutant allele percentage in patients as 40.4% and in healthy individuals as 20%, the sample size for each group is estimated to be 78 individuals (13, 16). However, due to our access, we were able to collect a larger sample size.
2.3. DNA extraction and genotype analysis
Genomic DNA was extracted from blood samples using a commercially available DNA extraction kit (Bioneer Co., Daejeon, Korea). The genotyping of IL-6 polymorphism was done using the polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) method. For this, specific primers were designed in the vicinity of the polymorphic region using Oligo7 software. The sequences of the forward and reverse primers were as follows:
Forward primer (F): 5′-GAAGAGTGGTTCTGCTTCTTAG-3′ and reverse primer (R): 5′-TTTGATAAATCTTTGTTGGAGG-3′.
PCR was conducted in a total volume of 20 μl containing 10 μl premix (CinnaGen, Iran), 0.5 μM of forward and reverse primers, and 2 μl (30 ng) template DNA. The PCR was performed in a Peqlab peqSTAR thermal cycler with the following program: 94°C for 5 min, 94°C for 45 sec, 53°C for 45 sec, 72°C for 45 sec, 35 cycles, and final 72°C for 5 min. Subsequently, 5 μl of the PCR products were subjected to digestion using 0.8 units of Hin1II restriction enzyme (#ER1831-Thermo Scientific, Lot: 00471907) with an incubation period of 16 hr at 37°C. The enzyme was subsequently inactivated through incubation at 65°C for 20 min. The digested fragments were separated by electrophoresis on the 8% polyacrylamide gel and visualized using DNA Green Viewer (CinnaGen CO.). Following electrophoresis, 3 distinct genotypes were identified based on their differing sizes: GG (173bp), GC (173, 79, 94), and CC (94, 79) (Figure 1).


2.4. Ethical Considerations
Sample collection was conducted after obtaining informed consent from the participants. This study received ethical approval from Kashan University of Medical Sciences, Kashan, Iran (Code: IR.KAUMS.REC.1394.58).
2.5. Statistical Analysis
Statistical analysis for the case-control study was conducted using Statistical Package for the Social Sciences (SPSS) 22 software for Windows (SPSS Inc., Chicago, USA). To assess the normal distribution of continuous variables, the Kolmogorov-Smirnov test was employed for adjustment. Numerical variables were analyzed with t test. The Hardy-Weinberg equilibrium of observed genotype frequencies were determined using a goodness‐of‐fit Chi‐square test. Associations between categorical variables, including genotype frequencies and odds ratios, were evaluated using the Chi‐square test. A significance level of p < 0.05 was considered statistically significant.
3. Results
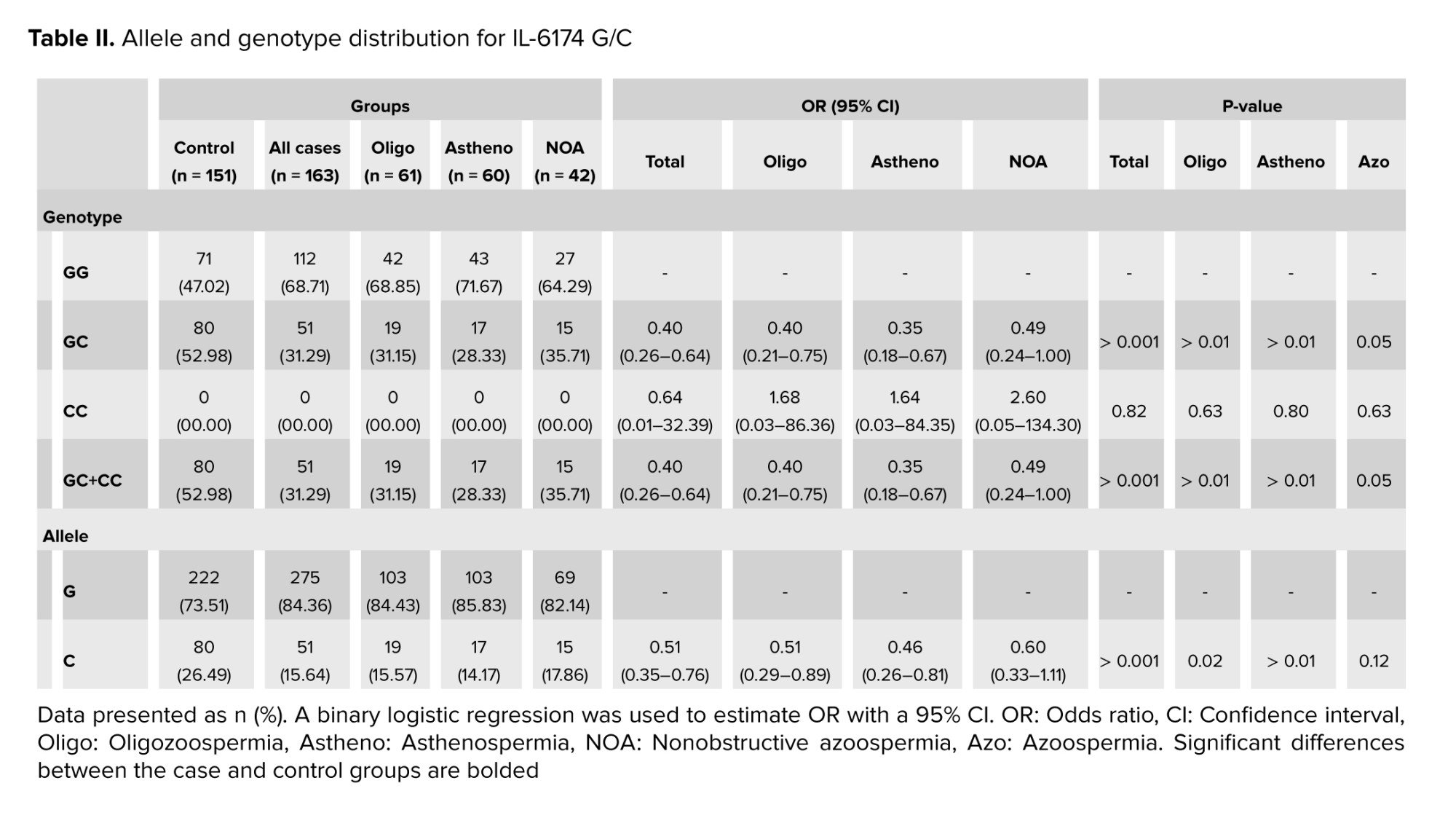
The data demonstrated a significant association between the heterozygous genotype GC and a reduction in the risk of male infertility. Furthermore, the data indicated a meaningful correlation between the GC genotype and a decreased risk of oligozoospermia, and asthenospermia. Additionally, a significant association was observed in a dominant genetic model (GC + CC vs. GG) between the IL-6-174 G/C polymorphism and a decreased risk in male infertility, extending to the subgroups of oligozoospermia and asthenospermia (Table II). The allelic analysis revealed that the C allele was associated with a decreased risk of male infertility. Furthermore, a significant association was identified between the C allele and a reduced risk of oligozoospermia and asthenospermia (Table II). Due to the rarity of the CC genotype in both infertile and healthy individuals (0 frequency), genetic association analysis was not performed, as it requires larger sample sizes for such analyses.
- Discussion
Sometimes, mutant alleles can improve survival, increase biological efficiency, and adapt to the environment (17, 18). There are numerous studies indicating that mutant alleles of SNPs are protective (19, 20). We stated in our article that the C allele is a protective factor against infertility, but this does not mean that the G allele is the cause of infertility. All genetic polymorphisms and mutations are not necessarily risk factors and can have positive effects on the corresponding protein or even RNA. For example, Mobasseri et al. reported that the mutant allele of rs2234693 polymorphism can be a protective factor against male infertility with a positive effect on RNA stability (21). Given that the polymorphism studied is located upstream of the gene, it may affect transcription factor binding sites. The eukaryotic promoter database indicated that the IL-6-174 G/C polymorphism is positioned close to and upstream of the promoter, and the PROMO server showed that the C variant creates additional binding sites for transcription factors for IL-6 (Figure 2), potentially playing an effective role in the gene regulation. Therefore, this may explain the protective role of the IL-6-174 G/C polymorphism against male infertility.
A previous study conducted in the Uttar Pradesh population of North India also demonstrated an association between the IL-6-174 G/C transversion and male infertility. Their subgroup analysis revealed higher levels of apoptosis and necrosis in individuals with oligozoospermia and asthenospermia compared to the control group. Additionally, they found associations with increased levels of reactive oxygen species (ROS), decreased testosterone and luteinizing hormone, and increased prolactin and follicle-stimulating hormone in the infertile group (13).
Male infertility is a complex, multifactorial condition that poses a significant global public health challenge, with a substantial percentage of affected men experiencing conditions such as azoospermia, asthenozoospermia, and oligospermia. An array of factors, including anatomical, environmental, and genetic factors, contribute to male infertility (22).
The process of spermatogenesis, which is vital for producing mature sperm, is influenced by various cytokines. Elevated levels of IL-6 have been associated with disruptions in spermatogenesis, potentially contributing to infertility (23). Notably, there is an inverse relationship between cholesterol levels and IL-6, with cholesterol playing a role in male gonad parameters like Sertoli cell function, germ cell differentiation, and steroidogenesis (24). Any alteration affecting IL-6 integrity can impact reproductive function, directly or indirectly, and is correlated with sperm lipid peroxidation (25).
Among genetic factors, polymorphisms in cytokine genes can alter their structure, function, or expression levels. Polymorphisms in the IL-6 gene have the potential to elevate cytokine levels and induce the generation of ROS, which can have detrimental effects on spermatogenesis (13). Roughly, 30-80% of ROS levels can lead to damage in spermatozoa. Elevated ROS levels can damage sperm membranes, DNA, and proteins, resulting in abnormalities in sperm count, motility, and structural integrity (26). Consequently, these disruptions can manifest as various phenotypes, including azoospermia, oligozoospermia, and asthenozoospermia (27).
In many cases of autosomal recessive traits, individuals who are homozygous for mutated allele experience reduced fitness compared to those who are heterozygous or homozygous for the normal allele. However, there are certain disorders where environmental conditions lead to higher fitness in heterozygotes than in either homozygous genotype. This phenomenon is known as heterozygote advantage. It is significant because even a slight advantage in heterozygotes can lead to an increase in the frequency of the mutated allele within the population, even if the mutation significantly reduces fitness in homozygotes. Instances where natural selection simultaneously works to maintain a harmful allele in the gene pool while also working to eliminate it are called balanced polymorphisms (28). The inheritance pattern of SNPs follows the general principles of Mendelian inheritance, with some subtle differences, and in some accepted models including codominant, dominant-recessive, incomplete dominance, overdominance, and additive. Generally, the inheritance pattern of SNPs can vary depending on the specific SNP and its interaction with other genetic and environmental factors. Additionally, SNPs can exhibit different inheritance patterns in populations or different ethnic groups (29, 30).
For examining only one gene as well as one polymorphism, this preliminary study serves as an initial investigation, providing data that will guide future, more thorough research aimed at drawing clinical conclusions regarding whether this genetic variation can serve as a biomarker indicating susceptibility to male infertility. Considering that investigating the effects of genetic polymorphisms through experimental methods such as in vitro and in vivo approaches is costly and time-consuming, computational methods can be a suitable approach for studying the effects of polymorphisms (15). Given that the polymorphism under study, rs1800795, is an upstream variant, its effects on gene function, particularly on gene expression, are suggested to be explored using bioinformatics tools.
However, it is essential to acknowledge the limitations of our study, particularly regarding gene-gene and gene-environment interactions that were not explored. Considering the results of our study and the absence of the homozygous CC genotype in the entire population under study (both in the case and control groups), we were unable to statistically analyze the behavior of this genotype in male infertility, precisely. With the low frequency of the homozygous CC genotype in the population, obtaining a larger sample size and accessing individuals with the homozygous CC genotype will result in more accurate results, regarding this genotype and its association with male infertility. Additionally, obtaining more precise data would necessitate larger study groups of both infertile and control subjects from diverse ethnic populations, as geographical regions can influence study outcomes. Moreover, exploring additional polymorphisms in IL-6 gene and other inflammatory genes in fertile and infertile men could contribute to obtaining more precise conclusions.

- Conclusion
Data Availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author Contributions
H. Nikzad, and M. Karimian designed the study and conducted the research. T. Zamani-Badi, J. Amini Mahabadi, and A. Rafatmanesh evaluated and analyzed the results of the study. Further, M. Karimian, H. Nikzad, T. Zamani-Badi, J. Amini Mahabadi, and A. Rafatmanesh reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data. Due to the multidisciplinary nature of the article and the important role of both corresponding authors in various stages of the research, including design, data analysis, and writing, 2 corresponding authors have been introduced to reflect the equal contribution and importance of each of their expertise.
Acknowledgements
This work is supported by the Vice-Chancellor for Research and Technology of Kashan University of Medical Sciences, Kashan, Iran (grant number: 94058). We would like to express our gratitude to Dr. Hossein Mahmoudi, a urology specialist, for his consultation on this project. We did not use artificial intelligence in the preparation of this article.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Genetics
References
1. Bhattacharya I, Sharma SS, Majumdar SS. Etiology of male infertility: An update. Reprod Sci 2024; 31: 942-965. [DOI:10.1007/s43032-023-01401-x] [PMID]
2. Karimian M, Parvaresh L, Behjati M. Genetic variations as molecular diagnostic factors for idiopathic male infertility: Current knowledge and future perspectives. Expert Rev Mol Diagn 2021; 21: 1191-1210. [DOI:10.1080/14737159.2021.1985469] [PMID]
3. Karimian M, Babaei F. Large-scale mtDNA deletions as genetic biomarkers for susceptibility to male infertility: A systematic review and meta-analysis. Int J Biol Macromol 2020; 158: 85-93. [DOI:10.1016/j.ijbiomac.2020.04.216] [PMID]
4. Zamani‐Badi T, Nikzad H, Karimian M. IL‐1RA VNTR and IL‐1α 4845G> T polymorphisms and risk of idiopathic male infertility in Iranian men: A case-control study and an in silico analysis. Andrologia 2018; 50: e13081. [DOI:10.1111/and.13081] [PMID]
5. Zamani-Badi T, Karimian M, Azami-Tameh A, Nikzad H. Association of C3953T transition in interleukin 1β gene with idiopathic male infertility in an Iranian population. Hum Fertil 2017; 22: 111-117. [DOI:10.1080/14647273.2017.1384857] [PMID]
6. Alves-Silva T, Freitas GA, Húngaro TGR, Arruda AC, Oyama LM, Avellar MCW, et al. Interleukin-6 deficiency modulates testicular function by increasing the expression of suppressor of cytokine signaling 3 (SOCS3) in mice. Sci Rep 2021; 11: 11456. [DOI:10.1038/s41598-021-90872-6] [PMID] [PMCID]
7. Fraczek M, Kurpisz M. Cytokines in the male reproductive tract and their role in infertility disorders. J Reprod Immunol 2015; 108: 98-104. [DOI:10.1016/j.jri.2015.02.001] [PMID]
8. Li X, Ni M, Xing S, Yu Y, Zhou Y, Yang S, et al. Reactive oxygen species secreted by leukocytes in semen induce self-expression of interleukin-6 and affect sperm quality. Am J Mens Health 2020; 14: 1557988320970053. [DOI:10.1177/1557988320970053] [PMID] [PMCID]
9. Sharma A, Singh K, Biswas A, Ranjan R, Kishor K, Pandey H, et al. Impact of interleukin 6 promoter polymorphisms (-174 G> C,-572 G> C and -597 G> A) on plasma IL-6 levels and their influence on the development of DVT: A study from India. Hematology 2018; 23: 833-838. [DOI:10.1080/10245332.2018.1483546] [PMID]
10. Zhang M, Xu J, Bao X, Niu W, Wang L, Du L, et al. Association between genetic polymorphisms in interleukin genes and recurrent pregnancy loss: A systematic review and meta-analysis. PloS One 2017; 12: e0169891. [DOI:10.1371/journal.pone.0169891] [PMID] [PMCID]
11. Ayelign B, Negash M, Andualem H, Wondemagegn T, Kassa E, Shibabaw T, et al. Association of IL-10 (-1082 A/G) and IL-6 (-174 G/C) gene polymorphism with type 2 diabetes mellitus in Ethiopia population. BMC Endocr Disord 2021; 21: 70. [DOI:10.1186/s12902-021-00738-1] [PMID] [PMCID]
12. González-Castro TB, Hernández-Díaz Y, Pérez-Hernández N, Tovilla-Zárate CA, Juárez-Rojop IE, López-Narvaez ML, et al. Interleukin 6 (rs1800795) gene polymorphism is associated with cardiovascular diseases: A meta-analysis of 74 studies with 86,229 subjects. EXCLI J 2019; 18: 331-355.
13. Shukla KK, Agnihotri S, Gupta A, Mahdi AA, Mohamed EA, Sankhwar SN, et al. Significant association of TNFα and IL-6 gene with male infertility--an explorative study in Indian populations of Uttar Pradesh. Immunol Lett 2013; 156: 30-37. [DOI:10.1016/j.imlet.2013.08.011] [PMID]
14. Sobhan MR, Mahdinezhad-Yazdi M, Dastgheib SA, Ahrar H, Aghili K, Neamatzadeh H. Association of the IL-6 -174G > C (rs1800795) polymorphism with adolescent idiopathic scoliosis: Evidence from a case-control study and meta-analysis. Rev Bras Ortop 2020; 55: 17-26. [DOI:10.1055/s-0039-1700813] [PMID] [PMCID]
15. Jamali S, Karimian M, Nikzad H, Aftabi Y. The c.-190 C>A transversion in promoter region of protamine1 gene as a genetic risk factor for idiopathic oligozoospermia. Mol Biol Rep 2016; 43: 795-802. [DOI:10.1007/s11033-016-4017-8] [PMID]
16. Kumar A, Kapoor R, Mishra S, Joshi A, Tiwari N, Ramamurthy HR. Non-analgesic benefits of combined thoracic epidural analgesia with general anesthesia in on-pump pediatric cardiac surgery: A prospective observational study. Med J Armed Forces India 2024; 80: 80-90. [DOI:10.1016/j.mjafi.2023.01.006] [PMID] [PMCID]
17. Jin Y, Wang J, Bachtiar M, Chong SS, Lee CG. Architecture of polymorphisms in the human genome reveals functionally important and positively selected variants in immune response and drug transporter genes. Hum Genomics 2018; 12: 43. [DOI:10.1186/s40246-018-0175-1] [PMID] [PMCID]
18. Bryk J, Hardouin E, Pugach I, Hughes D, Strotmann R, Stoneking M, et al. Positive selection in East Asians for an EDAR allele that enhances NF-κB activation. PloS One 2008; 3: e2209. [DOI:10.1371/journal.pone.0002209] [PMID] [PMCID]
19. Karimian M, Hosseinzadeh Colagar A. Human MTHFR-G1793A transition may be a protective mutation against male infertility: A genetic association study and in silico analysis. Hum Fertil 2018; 21: 128-136. [DOI:10.1080/14647273.2017.1298161] [PMID]
20. Moradkhani A, Azami M, Assadi S, Ghaderi M, Azarnezhad A, Moradi Y. Association of vitamin D receptor genetic polymorphisms with the risk of infertility: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2024; 24: 398. [DOI:10.1186/s12884-024-06590-0] [PMID] [PMCID]
21. Mobasseri N, Nikzad H, Karimian M. Protective effect of oestrogen receptor α-PvuII transition against idiopathic male infertility: A case-control study and meta-analysis. Reprod Biomed Online 2019; 38: 588-598. [DOI:10.1016/j.rbmo.2019.01.008] [PMID]
22. Mojarrad M, Saburi E, Golshan A, Moghbeli M. Genetics and molecular biology of male infertility among Iranian population: An update. Am J Transl Res 2021; 13: 5767-5785.
23. Dutta S, Sengupta P, Slama P, Roychoudhury S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int J Mol Sci 2021; 22: 10043. [DOI:10.3390/ijms221810043] [PMID] [PMCID]
24. Lubrano V, Gabriele M, Puntoni MR, Longo V, Pucci L. Relationship among IL-6, LDL cholesterol and lipid peroxidation. Cell Mol Biol Lett 2015; 20: 310-322. [DOI:10.1515/cmble-2015-0020] [PMID]
25. Hussain T, Kandeel M, Metwally E, Murtaza G, Kalhoro DH, Yin Y, et al. Unraveling the harmful effect of oxidative stress on male fertility: A mechanistic insight. Front Endocrinol 2023; 14: 1070692. [DOI:10.3389/fendo.2023.1070692] [PMID] [PMCID]
26. Park Y-J, Pang M-G. Mitochondrial functionality in male fertility: From spermatogenesis to fertilization. Antioxidants 2021; 10: 98. [DOI:10.3390/antiox10010098] [PMID] [PMCID]
27. Amorini AM, Listorti I, Bilotta G, Pallisco R, Saab MW, Mangione R, et al. Antioxidant-based therapies in male infertility: Do we have sufficient evidence supporting their effectiveness? Antioxidants 2021; 10: 220. [DOI:10.3390/antiox10020220] [PMID] [PMCID]
28. Rosenberg LE, Rosenberg DD. Human genes and genomes: Science, health, society. 1st Ed. US: Academic Press; 2013.
29. Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature 2009; 461: 747-753. [DOI:10.1038/nature08494] [PMID] [PMCID]
30. Amiteye S. Basic concepts and methodologies of DNA marker systems in plant molecular breeding. Heliyon 2021; 7: e08093. [DOI:10.1016/j.heliyon.2021.e08093] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |