Sat, Feb 21, 2026
[Archive]
Volume 22, Issue 5 (May 2024)
IJRM 2024, 22(5): 383-394 |
Back to browse issues page
Ethics code: IR.IAU.KSH.REC.1402.035
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadi F, Karimi-Dehkordi M, Pouriayevali F. Evaluation of the sperm parameters, oxidative stress, and histopathological effects of vitamin B12 in preventing Helicobacter pylori-induced testicular toxicity: An experimental study. IJRM 2024; 22 (5) :383-394
URL: http://ijrm.ir/article-1-3225-en.html
URL: http://ijrm.ir/article-1-3225-en.html
1- Department of Veterinary, Agriculture Faculty, Kermanshah Branch, Islamic Azad University, Kermanshah, Iran.
2- Department of Clinical Sciences, Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran. ,devsiecnce1374@gmail.com
3- Research Institute of Animal Embryo Technology, Shahrekord University, Shahrekord, Iran.
2- Department of Clinical Sciences, Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran. ,
3- Research Institute of Animal Embryo Technology, Shahrekord University, Shahrekord, Iran.
Full-Text [PDF 2376 kb]
(1457 Downloads)
| Abstract (HTML) (1427 Views)
1. Introduction
Healthy reproduction is the foundation for the species' existence and perpetuation. However, there has recently been an increase in accounts of decreasing male fertility (1). According to estimates, infertility impacts millions of reproductive-aged partners globally (2). Male infertility accounts for approximately half of all infertility instances (3). Male infertility can be caused by a variety of factors, including hereditary abnormalities, steroid hormone disorders, hypogonadism, spermatogenesis failure, heavy alcohol consumption, ejaculation disorders, and reproductive infections.
A wide range of microorganisms, such as bacteria, viruses, and parasites, can invade the reproductive organs of men and initiate a cascade of inflammatory reactions that ultimately lead to a decrease in fertility of men (4).
Helicobacter pylori (H. pylori) is a microaerophilic, gram-negative bacterium with a spiral shape. It thrives in the stomach and triggers a robust cellular and immune system response, leading to chronic gastritis. In combination with bacterial factors, this response contributes to the development of peptic ulcers in 15-20% of infected people and gastric carcinoma in 1-3%, with variations based on the virulence of the infecting organisms and the geographical location (5). There is evidence to suggest that infection with H. pylori, particularly those that produce CagA, may have a detrimental impact on human reproductive capacity (6). The relationship between H. pylori infection and extra-gastrointestinal illnesses is often attributed to oxidative stress as a potential underlying mechanism. However, there are fewer studies on the effective role of antioxidant supplements in reducing oxidative stress caused by H. pylori infection.
Vitamin B12 (-[5, 6-dimethylbenzimidazolyl] cobamidcyanide), also known as cobalamin due to the presence of cobalt in its chemical structure, is one of the 8 recognized B vitamins (7). Vitamin B12 is essential for spermatogenesis (8). It has been proposed that vitamin B12 may affect spermatozoa development. Furthermore, vitamin B12 may function as a direct reactive oxygen species (ROS) absorber. There is a growing body of information that suggests a potentially harmful impact of H. pylori infection on reproductive health. As a result, this study aims to investigate the effect of H. pylori infection on the male reproductive organs and the use of vitamin B12 as a protective factor.
Therefore, the effect of H. pylori infection and the protective effects of vitamin B12 on sperm parameters and testicular tissue are evaluated in this study.
2. Materials and Methods
2.1. Experiment and animals
40 healthy male C57 mice (6 wk old, weight 20-25 gr) were purchased from Pasture Institute, Iran. The mice were kept under controlled environmental conditions, with a consistent room temperature of 25 ± 2°C and a humidity range of 40-70%. The mice were housed in plastic cages containing hardwood chips as bedding material, as well as in polypropylene cages that provided a natural 12-hr light/dark cycle. The mice were provided with a standard laboratory pellet meal and were given unrestricted access to water. Mice were randomly divided into 4 groups (n = 10/each).
The first group was without any injection (control group).
The second group was induced with H. pylori after an overnight fast and orogasterically inoculated 3 times at a 1-day interval with 1×109 CFU H. pylori (H group).
The third group was induced with H. pylori in their stomach and orogasterically inoculated 3 times at a one-day 1 cc phosphate buffered saline (PBS) (HP group).
The fourth group received H. pylori in addition to receiving 50 μg/kg vitamin B12 intraperitoneal injections every week for 3 wk (HB group).
After 1 month, blood samples were taken to confirm the induction of this bacterium in the mice (H. pylori > 20). After confirmation of H. pylori induction, the samples were collected and the study was ended (9).
2.2. H. pylori culture
The H. pylori strain ATCC43504 was cultivated in a microaerophilic environment at a temperature of 37°C on Helicobacter agar for a duration of 5-7 days. The colonies of H. pylori were selected randomly and then submerged in brucella broth that was supplemented with 5-10% fetal bovine serum. This suspension was incubated under microaerophilic circumstances at a temperature of 37°C for 24 hr (10).
2.3. Sample collection
Mice were intraperitoneally anesthetized with ketamine and xylazine, then blood samples were received by heart puncture. Serums were separated from individual blood samples and vitamin B12, testosterone, homocysteine, H. pylori, total antioxidant capacity (TAC), malondialdehyde (MDA) enzyme, superoxide dismutase enzyme, and glutathione peroxidase enzyme were evaluated. Testicles and epididymis were detached and dissected. Cauda sections of epididymis were incubated in a sperm washing medium (VitaSperm, Inoclon, Iran) supplemented with 10% serum for 40 min at 37°C, and sperms were separated and used for assessment of sperm parameters.
2.4. Histopathological evaluation
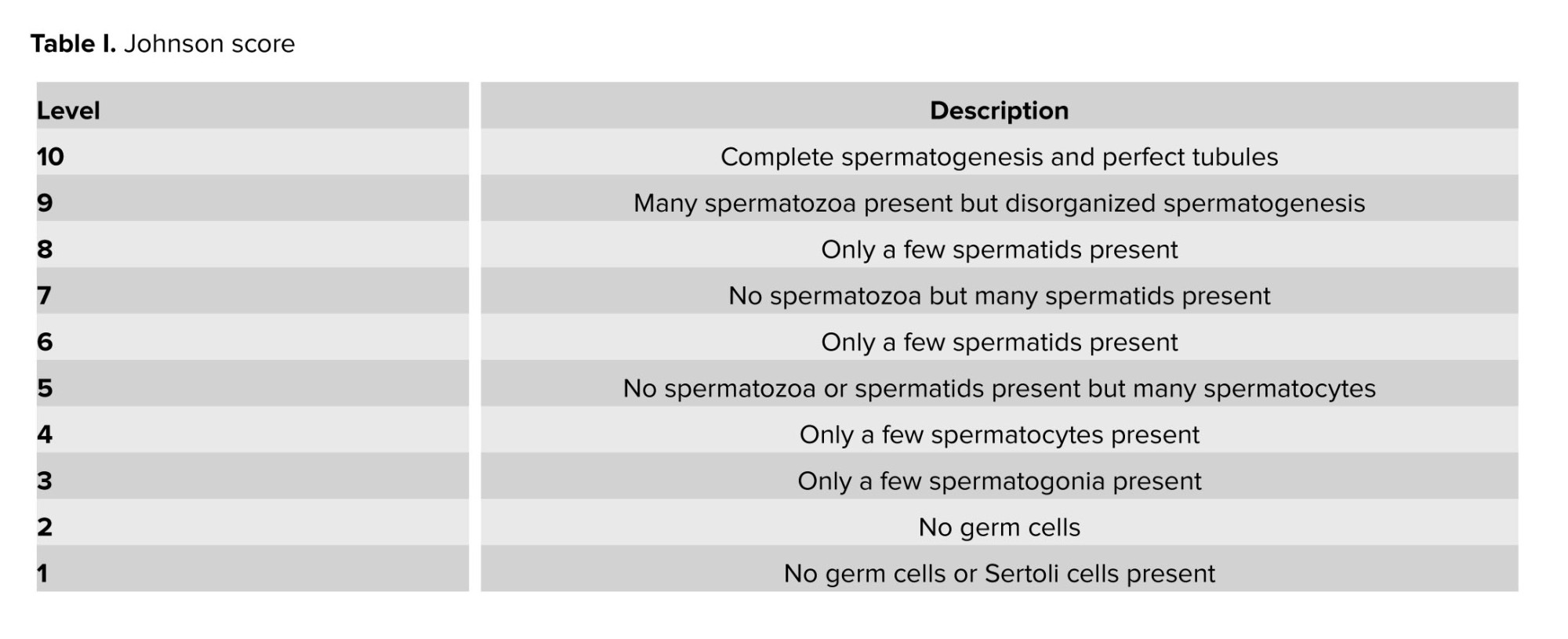
The excised testes were immersed in a 10% formalin solution for 24 hr to facilitate fixation. The paraffin slices underwent detachment and processing, and were subsequently stained using hematoxylin and eosin staining. The testicles were assessed using tubular differentiation index (TDI), spermatogenesis index (SPI) (11), and modified Johnson scores (Table I) (12). To obtain the SPI, the ratio of active spermatogonia cells to inactive spermatogonia cells was calculated. TDI was obtained, and the percentage of spermatogenic seminiferous tubules or differentiated seminiferous tubules were recorded. 100 seminiferous tubules were analyzed for the evaluation of Johnson score, TDI, and SPI (13).
2.5. Assessment of sperm parameters
The measurement of sperm concentration was conducted using a sperm counting chamber (Sperm Processor located, Aurangabad, India). A volume of 20 μl of the sample that was obtained was diluted with an equal volume of water. Subsequently, 10 μl of this diluted mixture was carefully placed into a sperm-counting chamber, allowing for the determination of the number of spermatozoa per milliliter.
The assessment of sperm motility % was conducted using light microscopy at a magnification of ×40. To evaluate sperm movement, a 10 μl aliquot of the material was placed onto a slide that had been preheated, and the proportion of spermatozoa exhibiting progressive motion was documented. The evaluation of sperm morphology was conducted using the eosin/nigrosin staining method. Initially, 20 µl of washed spermatozoa in PBS was mixed with 40 µl of eosin (Merck, Darmstadt, Germany) for 5 min.
Then, 60 µl of nigrosin (Merck, Darmstadt, Germany) was administered. Smears were produced for each sample, and 200 spermatozoa were counted using a CX31 OLYMPUS light microscope (magnification x100). The proportion of spermatozoa with head, neck, and tail abnormalities and total abnormalities were determined (13).
2.6. Serum analyses
The levels of B12 (vitamin B12 II, Roche, Switzerland), testosterone (Testosterone II, Roche, Switzerland), and H. pylori in the serum were assessed using the enzyme-linked immunosorbent assay kits (Elecsys 2010 and Cobas e411 analyzers) according to the manufacturer's instructions. The absorbance was measured spectrophotometrically at a wavelength of 450 nm. The determination of homocysteine serum levels was performed using high-performance liquid chromatography.
2.7. Assessment of serum TAC
The TAC of serum was measured using the ferric-reducing antioxidant power method. The basis of this method is the ability of serum to reduce ferric ions Fe+3 to Fe+2 in the presence of tripyridyltriazine (2, 4, 6-Tripyridyl-s-triazineTPTZ) (number 3682-35-7, Sigma, Germany). In this method, the reaction of Fe+2 with TPTZ reagent creates a blue-colored Fe+2-TPTZ complex with a maximum light absorption property at a wavelength of 593 nm, which can be measured by increasing the concentration of the abovementioned complex by a spectrophotometer (UNICO 2150-UV Spectrophotometer, China) (14).
2.8. Assessment of serum MDA
To measure serum MDA, a working solution containing 0.5 gr of thiobarbituric acid (CAS-number 504-17-6, Merck, Germany) and 80 ml of 20% acetic acid (CAS-number 64-19-7, Merck, Germany) was used. The pH of this solution was adjusted to 3.5 using sodium hydroxide (NaOH) (CAS-number 1310-73-2, Merck, Germany) and its final volume was adjusted to 100 ml by adding 20% acetic acid.
In the next step, 2.5 ml of this solution was poured into a glass test tube along with 100 µl of serum and 100 µl of sodium dodecyl sulfate (1.8%) (CAS-number 151-21-3, Merck, Germany). And the tubes were closed with an aluminum cap. The tubes were placed in a hot water bath for 1 hr and after cooling, they were centrifuged at 4000 rpm (Poland Mpw 260 r). Then, the optical absorption of the supernatant solution was recorded by a spectrophotometer (UNICO 2150-UV Spectrophotometer, China) at a wavelength of 523 nm (15).
2.9. Assessment of serum superoxide dismutase enzyme
To determine the amount of superoxide dismutase enzyme, the Zellbio (ZB-SOD96) kit was used according to the kit protocol. For this purpose, 10 × l of each serum was used and after adding the solutions, its absorbance was measured by a spectrophotometer (UNICO 2150-UV Spectrophotometer, China) at a wavelength of 420 nm.
2.10. Assessment of serum glutathione peroxidase enzyme
To determine the amount of glutathione peroxidase enzyme, both the kit of Zellbio (ZB-GPX96) and the hydrogen peroxide substrate in the kit were used. For this purpose, 10 µl of each serum was used and after adding the solutions, its absorbance was measured by a spectrophotometer (UNICO 2150-UV Spectrophotometer, China) at a wavelength of 412 nm.
2.11. Ethical considerations
All procedures were confirmed according to the ethical guidelines of the Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran, for the Care and Use of Laboratory Animals. This study was approved by the Ethical Committee of Shahrekord Branch, Islamic Azad University, Shahrekord, Iran (Code: IR.IAU.KSH.REC.1402.035).
2.12. Statistical analysis
All the studied data showed normal distribution. Collected data were expressed as the mean ± standard deviation (SD) and p < 0.05 was considered as significant. Where appropriate, ANOVA and independent-sample t test were used to define differences within groups. All the analysis was carried out in SPSS software version 22 (SPSS Science, Chicago, IL, USA).
3. Result
3.1. Histopathology
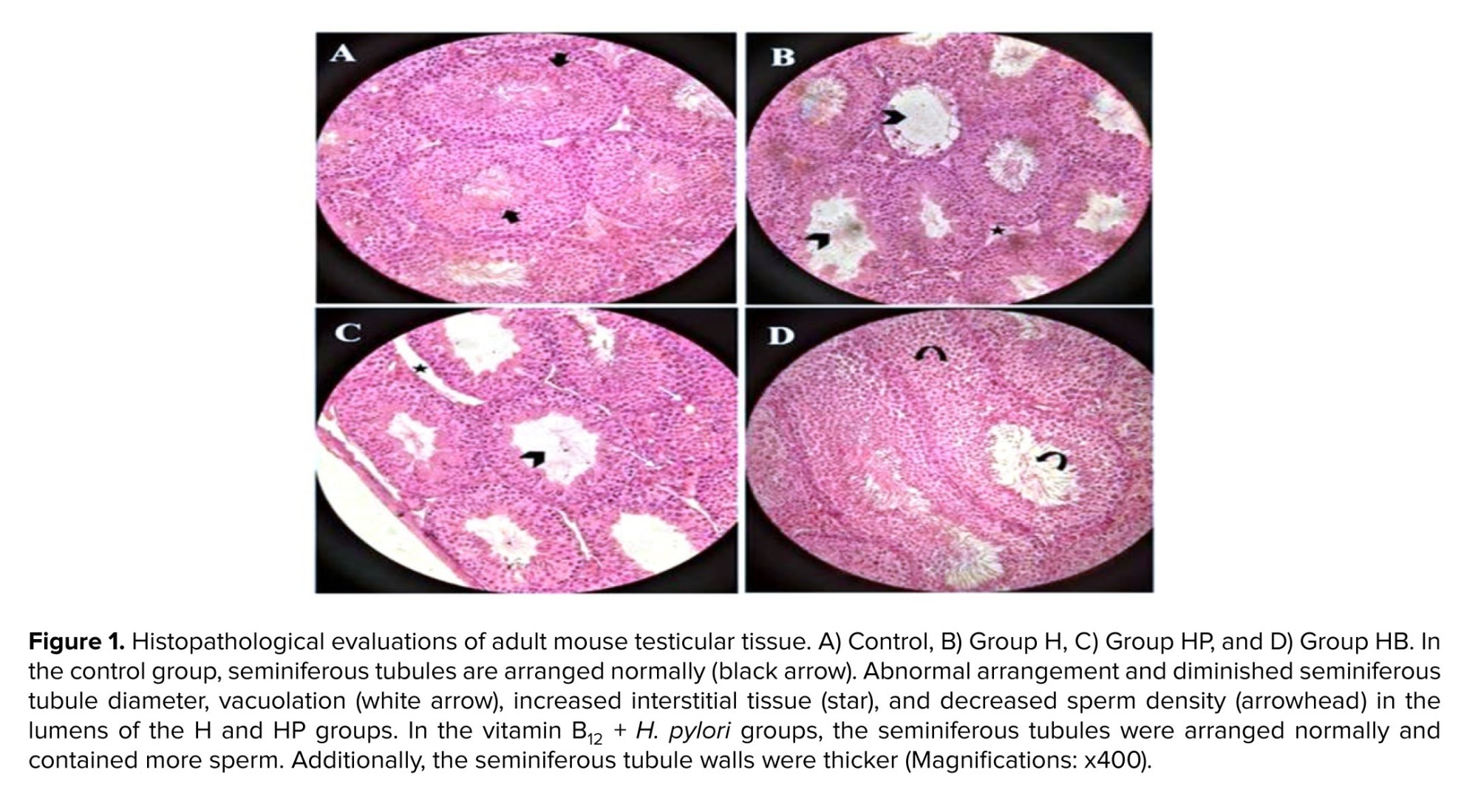
The testicular tissues of the control group were structurally normal and contained an abundance of sperm cells with normal spermatogonia, layered in multiple layers on the seminiferous tubule (Figure 1A). In the control group, the tubular epithelium was mostly unchanged and comprised Sertoli cells besides spermatocytes and spermatogonia on the basement membrane. Our result showed that infection with H. pylori resulted in testicular structural changes characterized by a reduced number of spermatozoa (Figures 1B and C, arrowhead). The seminiferous tubules in the H and HP groups had significant changes compared to the control group, such as seminiferous tubule deterioration and necrosis, reduced interstitial tissue with extensive intracellular spaces, spermatogonia cell loss, and reduction of mature sperm are all indicators of sperm dysfunction.
There was significant progress in the HB group, where there was a partial recovery of germinal cells and spermatozoa (rounded arrow) with abundant spermatogenic cell layers (Figure 1D). Leydig cells and typical, weak sperm have been identified in the seminiferous tubules of the vitamin B12 treatment group.
3.2. Comparison of histological examinations of mice testis
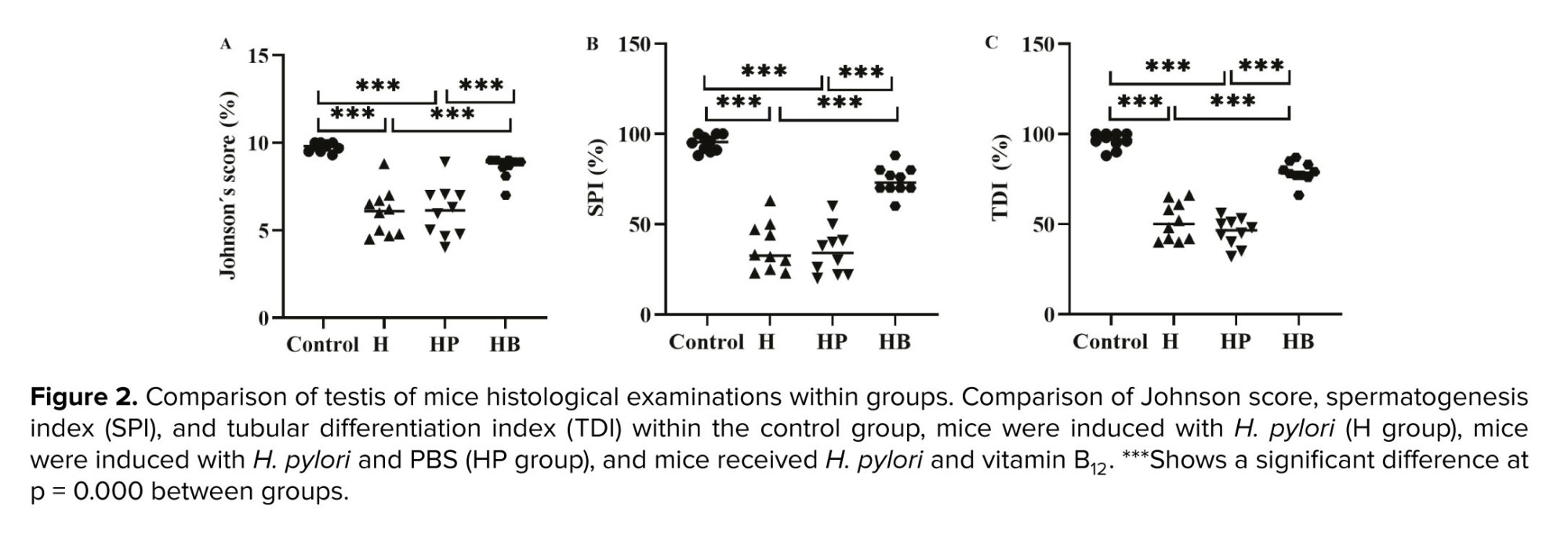
The testicular levels of Johnson scores, TDI, and SPI exhibited a significant decrease in both the H and HP groups when compared to the control and HB groups (p < 0.001). No statistically significant difference was observed between the control and the HB groups in terms of Johnson scores, TDI, and SPI (p = 0.09, Figure 2).
3.3. Sperm analysis
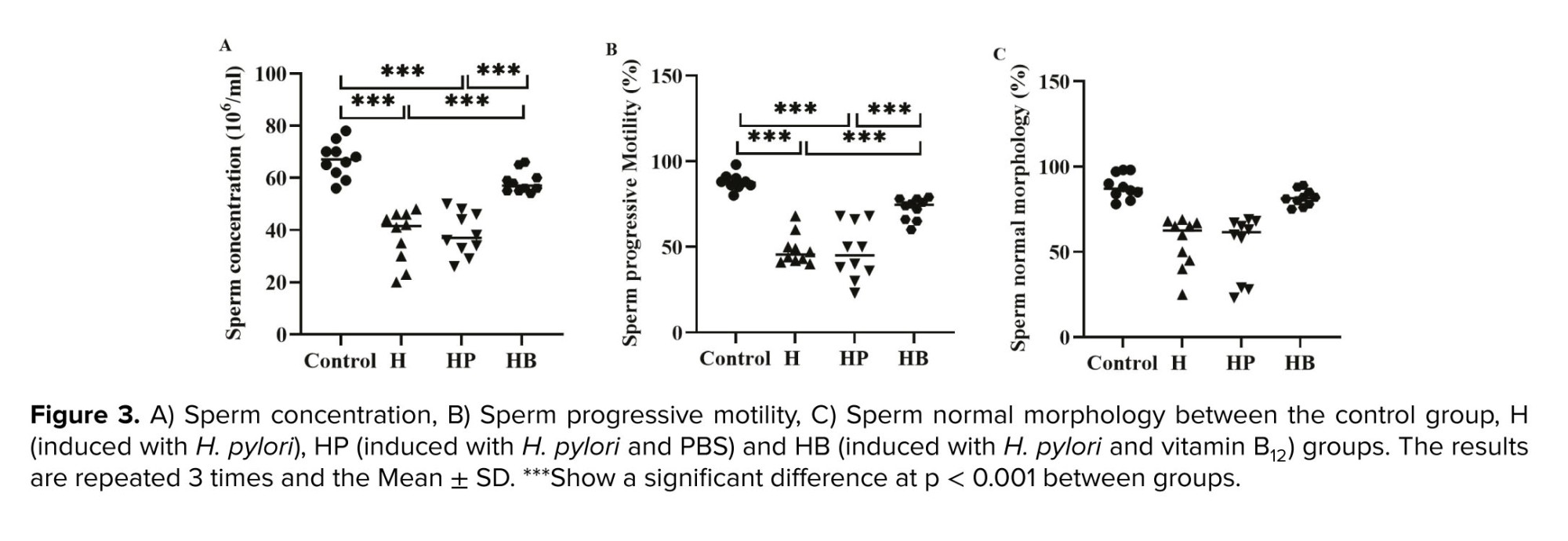
The average values of sperm concentration and progressive sperm motility were found to be considerably lower in the H and HP groups when compared to both the control group and the HB group (p < 0.001). There is no significant variation in the mean value of sperm normal morphology across the various groups (Figure 3).
3.4. Plasma factors
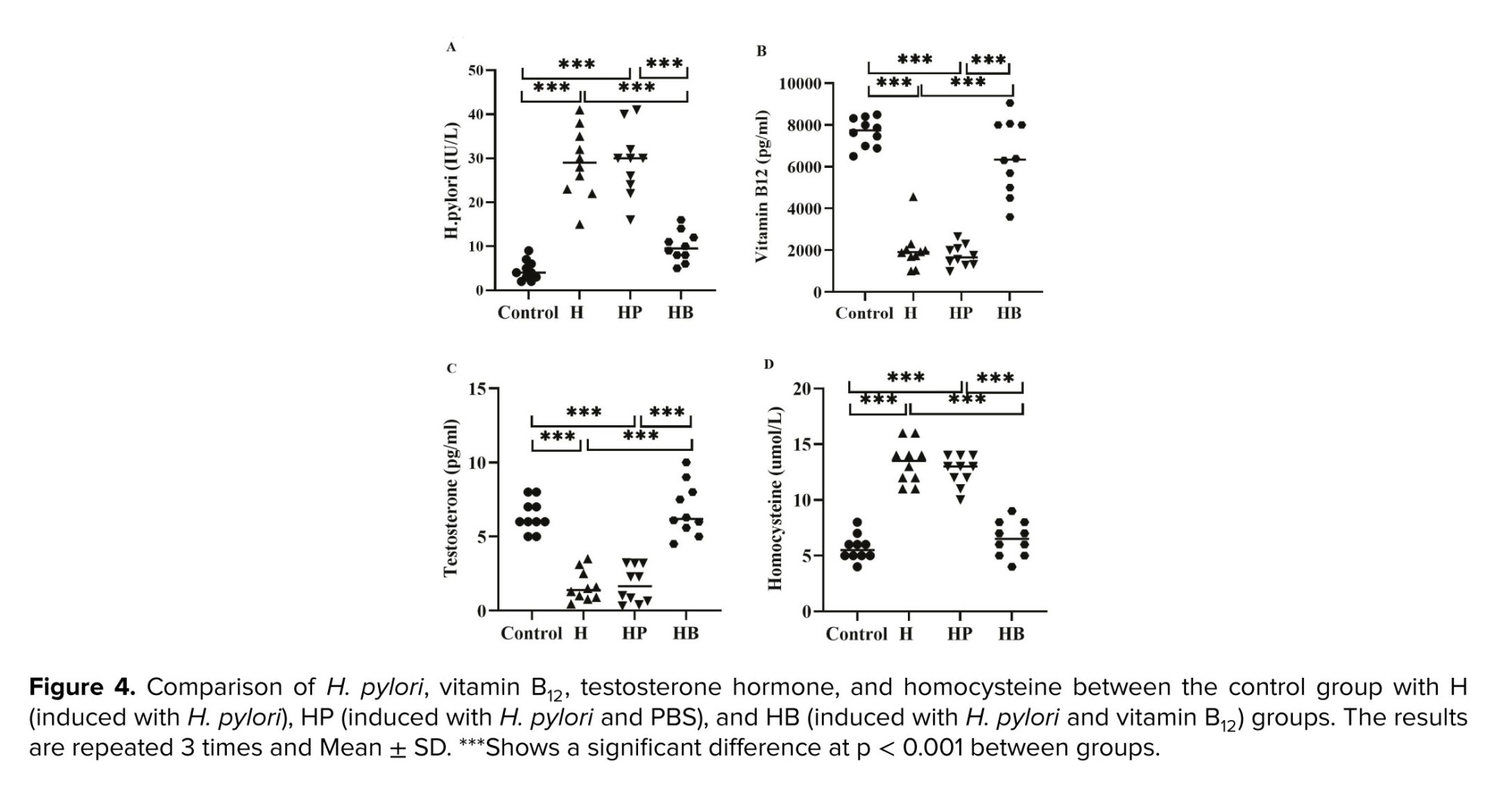
As shown in figure 4, the mean plasma levels of H. pylori, vitamin B12, testosterone, and homocysteine were significantly decreased in the H and HP groups compared to the control and HB groups (p < 0.001). In addition, no significant difference was observed between the control and HB groups with respect to plasma levels of H. pylori (p = 0.178), vitamin B12 (p = 0.092), testosterone (p = 0.903), and homocysteine (p = 0.638).
3.5. The effect of H. pylori and vitamin B12 on MDA level and TAC
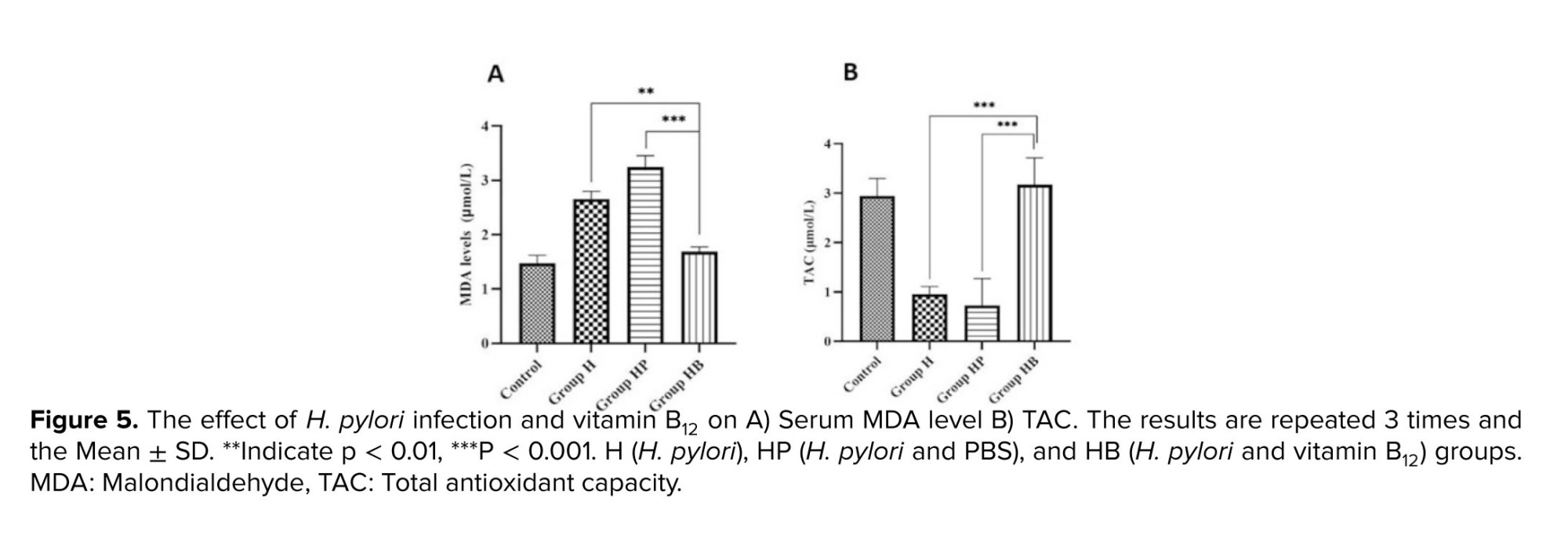
In comparison to control group, the concentration of MDA was significantly higher in the H and HP groups (p < 0.001). In the HB group, the amount of MDA was significantly decreased compared to the HP and H groups (p < 0.001). Moreover, based on our findings, H. pylori infection decreased TAC relative to the control group (p < 0.001). When compared to the H and HP groups, vitamin B12 administration in group 4 substantially averted the decrease in TAC (p < 0.001). Statistical analysis revealed no significant variation between the control and HB groups (Figure 5).
3.6. Effect of H. pylori infection on antioxidant enzyme activity in serum
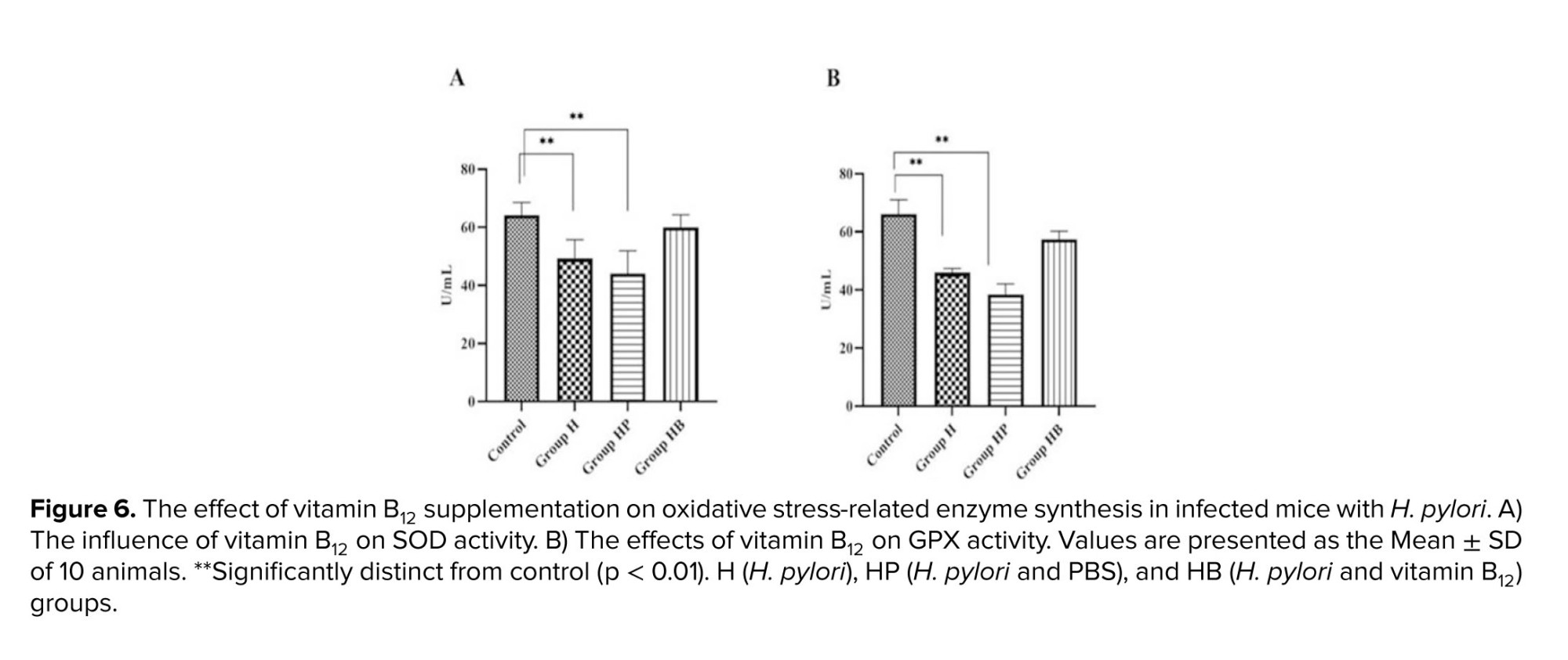
Superoxide dismutase (SOD) is one of the antioxidant enzymes responsible for preventing ROS production in cells. It converts anion superoxide (O2) to low-risk hydrogen peroxide in cells and converts glutathione peroxidase (GPX) and catalase to water. In this study, infection with H. pylori significantly decreased SOD levels compared to the control group (p < 0.01). When compared to the H and HP groups, the results showed that vitamin B12 had a positive effect on SOD and increased its activity. Compared to the control group, the specific activity of GPX was significantly (p < 0.01) reduced in the H and HP groups. Compared to corresponding controls, pre-treatment with vitamin B12 restored this diminished activity (Figure 6).
4. Discussion
Since H. pylori infection has been shown to associate with the histological changes of the seminiferous tubules, the protective effect of vitamin B12 against H. pylori-induced testicular damage was evaluated in the current study after 3 wk of treatment. The spermatogenesis is progressed nearly over the 4-6 wk in rat testes (16). The decreased Johnson score, TDI, and SPI in the H. pylori-infected group confirms the testicular toxicity of H. pylori infection. The data obtained from the serum level of SOD, GPX, MDA, and TAC showed that the testicular damage was associated with increased oxidative stress. While, the higher Johnson score, TDI, and SPI in the H. pylori-infected + vitamin B12 group than in the H. pylori group indicated that vitamin B12 can reduce testicular damage induced by H. pylori and improve the damaged-spermatogenesis. Since the vitamin B12 treatment group showed higher SOD and GPX enzyme activity and TAC as well as lower MDA levels than H and HB groups, the reduced testicular damage in the treatment group can be contributed to increasing antioxidant capacity by vitamin B12.
More than half of the global population is infected with H. pylori. In developing nations, the prevalence of infection persists above 80%, while it has radically decreased in developed nations (17). H. pylori infection may be linked to health problems outside of the gut system, and it may also contribute to the development of inflammatory diseases. H. pylori can induce extra gastric symptoms directly or indirectly via the release of inflammatory mediators and cytokines, molecular mimics, and systemic immune response (18). For many years, research organizations have concentrated on the potential impact of H. pylori infections on sperm quality. H. pylori infection, particularly by isolates producing the CagA protein, has been suggested as a potential concurrent cause of hypofertility and sperm changes because it has been linked to decreased mobility and a rise in unviable sperms (9). It is clear that H. pylori infection reduces sperm motility and viability and has some impact on testicular parameters (19). The exact mechanism by which H. pylori may affect sperm parameters is still unknown completely, although involving antigenic mimicry has been hypothesized. Infected individuals may mount a cellular and humoral immune response to bacterial constituents and products that might cross-react with epithelial cells of various organs such as testicular damage (20).
Several studies have examined the protective effects of potent antioxidants (phytochemicals, vitamins, and enzymes) on the testicles of rodents (12, 21-22).
In this study, vitamin B12 was selected as it has previously shown to protect against testicular damage caused by cimetidine (23). Vitamin B12 has also shown to play a part in the preservation of reproductive processes in the testis. Vitamin B12 promotes DNA production, which aids in cell proliferation (24). Rodents deficient in vitamin B12 have shown testicular pathological conditions characterized by notable diminishment in testis weight, shrinkage of the seminiferous tubules, and aplasia of spermatids and sperm (23, 25). In oligospermically provoked male rats, for example, methylcobalamin at 1000 µg/kg (6 times a week for 5-10 wk) caused a significant rise in the width of the seminiferous tubules as well as sperm concentration (26). In addition, studies revealed that spermatogenesis would be enhanced when a high dose of vitamin B12 is administered to patients with oligospermia in relation to fertility (27). Our results showed that infection with Helicobacter leads to a decrease in serum levels of vitamin B12.
Low amounts of vitamin B12 in the body decrease the enzymatic activity of methionine synthase, which is required to make methionine from homocysteine. This decrease causes a buildup of homocysteine in the bloodstream, also known as hyperhomocysteinemia (> 15 mol/L) (28). It has been discovered that hyperhomocysteinemia is associated with numerous health issues, including reproductive problems (29).
In this study, hyperhomocysteinemia was observed in groups infected with H. pylori. Recent in vitro research uncovered a relationship between sperm parameters such as motility and quantity and thiol concentrations. Such evidence implies a potential homocysteine toxicity to sperm, which may adversely affect sperm parameters due to a depleted vitamin B12 level. Hyperhomocysteinemia inhibits nitric oxide synthase pathways in the body, reducing the amount of nitric oxide generated. The enzyme known as nitric oxide synthase exists in spermatozoa and nitric oxide is essential for proper sperm movement, it is plausible that vitamin B12 deficiency may impair sperm function via hyperhomocysteinemia-induced nitric oxide deficiency (23). Our results in this study showed that there is a significant relationship between infection with H. pylori and the reduction of vitamin B12 and the increase of serum homocysteine.
Increased ROS and decreased antioxidant defense lead to redox imbalance, decreased sperm motility, and DNA damage in sperm (30). Due to the abundance of unsaturated fatty acids in their cell membranes, spermatozoa are extremely susceptible to the detrimental effects of ROS (31). Oxidative stress is associated with an increase in the generation of oxygen-free radicals or an unbalance in the redox defense system.
SOD, GPX, and catalase are the 3 major enzymatic components of this system in sperm (32). Our results showed that the activity of SOD and GPX was decreased in the H. pylori infected. As the quantity of ROS in the tissue rises, plasma membrane lipids become damaged and peroxidized, and internal components are destroyed, with MDA serving as a measure for lipid peroxidation (33). In our study, the oxidative stress and histopathological effects of vitamin B12 pretreatment in the prevention of H. pylori injury in mice were evaluated, and it was discovered that vitamin B12 supplementation significantly decreased plasma MDA levels and increased GPX and SOD activity.
Along with the results obtained from our research, it was reported that infection with H. pylori, especially the CagA strain, can be responsible for infertility in men through a destructive effect on sperm (24).
Furthermore, a study revealed that the presence of H. pylori infection leads to elevated levels of inflammatory cytokines in semen. These elevated levels have been associated with a decrease in sperm motility, potential sperm damage, and ultimately a decline in male fertility (7). Finally, this study's findings reported that vitamin B12 supplementation has a positive impact on preventing and treating complications of H. pylori infection and infertility.
5. Conclusion
The results of this study can help to explain that infection with H. pylori can lead to infertility by inducing harmful changes in testicular tissue and sperm parameters. In addition, H. pylori can cause harm to the reproductive system by destroying the antioxidant balance and decreasing the TAC, and activity of SOD and GPX enzymes. According to the results of the present investigation, vitamin B12 is an effective antioxidant that can decrease oxidative stress, enhance sperm parameters, and reduce the rate of testicular tissue damage. The beneficial effects of vitamin B12 on sperm parameters can be attributed to improve the efficacy of male reproductive organs, reduced homocysteine toxicity, and decreased ROS accumulation. However, additional research, predominantly clinical, is required to confirm these positive effects.
Data availability
Data will be made available on request via email to corresponding authors.
Author contributions
Maryam Karimi-Dehkordi designed the study and conducted the research. Maryam Karimi-Dehkordi and Forogh Mohammadi monitored, evaluated, and analyzed the results of the study. Further, Farnaz Pouriayevali reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
Artificial intelligence has not been used in any way (translation, editing, grammar checking, etc.).
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (328 Views)
1. Introduction
Healthy reproduction is the foundation for the species' existence and perpetuation. However, there has recently been an increase in accounts of decreasing male fertility (1). According to estimates, infertility impacts millions of reproductive-aged partners globally (2). Male infertility accounts for approximately half of all infertility instances (3). Male infertility can be caused by a variety of factors, including hereditary abnormalities, steroid hormone disorders, hypogonadism, spermatogenesis failure, heavy alcohol consumption, ejaculation disorders, and reproductive infections.
A wide range of microorganisms, such as bacteria, viruses, and parasites, can invade the reproductive organs of men and initiate a cascade of inflammatory reactions that ultimately lead to a decrease in fertility of men (4).
Helicobacter pylori (H. pylori) is a microaerophilic, gram-negative bacterium with a spiral shape. It thrives in the stomach and triggers a robust cellular and immune system response, leading to chronic gastritis. In combination with bacterial factors, this response contributes to the development of peptic ulcers in 15-20% of infected people and gastric carcinoma in 1-3%, with variations based on the virulence of the infecting organisms and the geographical location (5). There is evidence to suggest that infection with H. pylori, particularly those that produce CagA, may have a detrimental impact on human reproductive capacity (6). The relationship between H. pylori infection and extra-gastrointestinal illnesses is often attributed to oxidative stress as a potential underlying mechanism. However, there are fewer studies on the effective role of antioxidant supplements in reducing oxidative stress caused by H. pylori infection.
Vitamin B12 (-[5, 6-dimethylbenzimidazolyl] cobamidcyanide), also known as cobalamin due to the presence of cobalt in its chemical structure, is one of the 8 recognized B vitamins (7). Vitamin B12 is essential for spermatogenesis (8). It has been proposed that vitamin B12 may affect spermatozoa development. Furthermore, vitamin B12 may function as a direct reactive oxygen species (ROS) absorber. There is a growing body of information that suggests a potentially harmful impact of H. pylori infection on reproductive health. As a result, this study aims to investigate the effect of H. pylori infection on the male reproductive organs and the use of vitamin B12 as a protective factor.
Therefore, the effect of H. pylori infection and the protective effects of vitamin B12 on sperm parameters and testicular tissue are evaluated in this study.
2. Materials and Methods
2.1. Experiment and animals
40 healthy male C57 mice (6 wk old, weight 20-25 gr) were purchased from Pasture Institute, Iran. The mice were kept under controlled environmental conditions, with a consistent room temperature of 25 ± 2°C and a humidity range of 40-70%. The mice were housed in plastic cages containing hardwood chips as bedding material, as well as in polypropylene cages that provided a natural 12-hr light/dark cycle. The mice were provided with a standard laboratory pellet meal and were given unrestricted access to water. Mice were randomly divided into 4 groups (n = 10/each).
The first group was without any injection (control group).
The second group was induced with H. pylori after an overnight fast and orogasterically inoculated 3 times at a 1-day interval with 1×109 CFU H. pylori (H group).
The third group was induced with H. pylori in their stomach and orogasterically inoculated 3 times at a one-day 1 cc phosphate buffered saline (PBS) (HP group).
The fourth group received H. pylori in addition to receiving 50 μg/kg vitamin B12 intraperitoneal injections every week for 3 wk (HB group).
After 1 month, blood samples were taken to confirm the induction of this bacterium in the mice (H. pylori > 20). After confirmation of H. pylori induction, the samples were collected and the study was ended (9).
2.2. H. pylori culture
The H. pylori strain ATCC43504 was cultivated in a microaerophilic environment at a temperature of 37°C on Helicobacter agar for a duration of 5-7 days. The colonies of H. pylori were selected randomly and then submerged in brucella broth that was supplemented with 5-10% fetal bovine serum. This suspension was incubated under microaerophilic circumstances at a temperature of 37°C for 24 hr (10).
2.3. Sample collection
Mice were intraperitoneally anesthetized with ketamine and xylazine, then blood samples were received by heart puncture. Serums were separated from individual blood samples and vitamin B12, testosterone, homocysteine, H. pylori, total antioxidant capacity (TAC), malondialdehyde (MDA) enzyme, superoxide dismutase enzyme, and glutathione peroxidase enzyme were evaluated. Testicles and epididymis were detached and dissected. Cauda sections of epididymis were incubated in a sperm washing medium (VitaSperm, Inoclon, Iran) supplemented with 10% serum for 40 min at 37°C, and sperms were separated and used for assessment of sperm parameters.
2.4. Histopathological evaluation
The excised testes were immersed in a 10% formalin solution for 24 hr to facilitate fixation. The paraffin slices underwent detachment and processing, and were subsequently stained using hematoxylin and eosin staining. The testicles were assessed using tubular differentiation index (TDI), spermatogenesis index (SPI) (11), and modified Johnson scores (Table I) (12). To obtain the SPI, the ratio of active spermatogonia cells to inactive spermatogonia cells was calculated. TDI was obtained, and the percentage of spermatogenic seminiferous tubules or differentiated seminiferous tubules were recorded. 100 seminiferous tubules were analyzed for the evaluation of Johnson score, TDI, and SPI (13).
2.5. Assessment of sperm parameters
The measurement of sperm concentration was conducted using a sperm counting chamber (Sperm Processor located, Aurangabad, India). A volume of 20 μl of the sample that was obtained was diluted with an equal volume of water. Subsequently, 10 μl of this diluted mixture was carefully placed into a sperm-counting chamber, allowing for the determination of the number of spermatozoa per milliliter.
The assessment of sperm motility % was conducted using light microscopy at a magnification of ×40. To evaluate sperm movement, a 10 μl aliquot of the material was placed onto a slide that had been preheated, and the proportion of spermatozoa exhibiting progressive motion was documented. The evaluation of sperm morphology was conducted using the eosin/nigrosin staining method. Initially, 20 µl of washed spermatozoa in PBS was mixed with 40 µl of eosin (Merck, Darmstadt, Germany) for 5 min.
Then, 60 µl of nigrosin (Merck, Darmstadt, Germany) was administered. Smears were produced for each sample, and 200 spermatozoa were counted using a CX31 OLYMPUS light microscope (magnification x100). The proportion of spermatozoa with head, neck, and tail abnormalities and total abnormalities were determined (13).
2.6. Serum analyses
The levels of B12 (vitamin B12 II, Roche, Switzerland), testosterone (Testosterone II, Roche, Switzerland), and H. pylori in the serum were assessed using the enzyme-linked immunosorbent assay kits (Elecsys 2010 and Cobas e411 analyzers) according to the manufacturer's instructions. The absorbance was measured spectrophotometrically at a wavelength of 450 nm. The determination of homocysteine serum levels was performed using high-performance liquid chromatography.
2.7. Assessment of serum TAC
The TAC of serum was measured using the ferric-reducing antioxidant power method. The basis of this method is the ability of serum to reduce ferric ions Fe+3 to Fe+2 in the presence of tripyridyltriazine (2, 4, 6-Tripyridyl-s-triazineTPTZ) (number 3682-35-7, Sigma, Germany). In this method, the reaction of Fe+2 with TPTZ reagent creates a blue-colored Fe+2-TPTZ complex with a maximum light absorption property at a wavelength of 593 nm, which can be measured by increasing the concentration of the abovementioned complex by a spectrophotometer (UNICO 2150-UV Spectrophotometer, China) (14).
2.8. Assessment of serum MDA
To measure serum MDA, a working solution containing 0.5 gr of thiobarbituric acid (CAS-number 504-17-6, Merck, Germany) and 80 ml of 20% acetic acid (CAS-number 64-19-7, Merck, Germany) was used. The pH of this solution was adjusted to 3.5 using sodium hydroxide (NaOH) (CAS-number 1310-73-2, Merck, Germany) and its final volume was adjusted to 100 ml by adding 20% acetic acid.
In the next step, 2.5 ml of this solution was poured into a glass test tube along with 100 µl of serum and 100 µl of sodium dodecyl sulfate (1.8%) (CAS-number 151-21-3, Merck, Germany). And the tubes were closed with an aluminum cap. The tubes were placed in a hot water bath for 1 hr and after cooling, they were centrifuged at 4000 rpm (Poland Mpw 260 r). Then, the optical absorption of the supernatant solution was recorded by a spectrophotometer (UNICO 2150-UV Spectrophotometer, China) at a wavelength of 523 nm (15).
2.9. Assessment of serum superoxide dismutase enzyme
To determine the amount of superoxide dismutase enzyme, the Zellbio (ZB-SOD96) kit was used according to the kit protocol. For this purpose, 10 × l of each serum was used and after adding the solutions, its absorbance was measured by a spectrophotometer (UNICO 2150-UV Spectrophotometer, China) at a wavelength of 420 nm.
2.10. Assessment of serum glutathione peroxidase enzyme
To determine the amount of glutathione peroxidase enzyme, both the kit of Zellbio (ZB-GPX96) and the hydrogen peroxide substrate in the kit were used. For this purpose, 10 µl of each serum was used and after adding the solutions, its absorbance was measured by a spectrophotometer (UNICO 2150-UV Spectrophotometer, China) at a wavelength of 412 nm.

2.11. Ethical considerations
All procedures were confirmed according to the ethical guidelines of the Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran, for the Care and Use of Laboratory Animals. This study was approved by the Ethical Committee of Shahrekord Branch, Islamic Azad University, Shahrekord, Iran (Code: IR.IAU.KSH.REC.1402.035).
2.12. Statistical analysis
All the studied data showed normal distribution. Collected data were expressed as the mean ± standard deviation (SD) and p < 0.05 was considered as significant. Where appropriate, ANOVA and independent-sample t test were used to define differences within groups. All the analysis was carried out in SPSS software version 22 (SPSS Science, Chicago, IL, USA).
3. Result
3.1. Histopathology
The testicular tissues of the control group were structurally normal and contained an abundance of sperm cells with normal spermatogonia, layered in multiple layers on the seminiferous tubule (Figure 1A). In the control group, the tubular epithelium was mostly unchanged and comprised Sertoli cells besides spermatocytes and spermatogonia on the basement membrane. Our result showed that infection with H. pylori resulted in testicular structural changes characterized by a reduced number of spermatozoa (Figures 1B and C, arrowhead). The seminiferous tubules in the H and HP groups had significant changes compared to the control group, such as seminiferous tubule deterioration and necrosis, reduced interstitial tissue with extensive intracellular spaces, spermatogonia cell loss, and reduction of mature sperm are all indicators of sperm dysfunction.
There was significant progress in the HB group, where there was a partial recovery of germinal cells and spermatozoa (rounded arrow) with abundant spermatogenic cell layers (Figure 1D). Leydig cells and typical, weak sperm have been identified in the seminiferous tubules of the vitamin B12 treatment group.
3.2. Comparison of histological examinations of mice testis
The testicular levels of Johnson scores, TDI, and SPI exhibited a significant decrease in both the H and HP groups when compared to the control and HB groups (p < 0.001). No statistically significant difference was observed between the control and the HB groups in terms of Johnson scores, TDI, and SPI (p = 0.09, Figure 2).
3.3. Sperm analysis
The average values of sperm concentration and progressive sperm motility were found to be considerably lower in the H and HP groups when compared to both the control group and the HB group (p < 0.001). There is no significant variation in the mean value of sperm normal morphology across the various groups (Figure 3).
3.4. Plasma factors
As shown in figure 4, the mean plasma levels of H. pylori, vitamin B12, testosterone, and homocysteine were significantly decreased in the H and HP groups compared to the control and HB groups (p < 0.001). In addition, no significant difference was observed between the control and HB groups with respect to plasma levels of H. pylori (p = 0.178), vitamin B12 (p = 0.092), testosterone (p = 0.903), and homocysteine (p = 0.638).
3.5. The effect of H. pylori and vitamin B12 on MDA level and TAC
In comparison to control group, the concentration of MDA was significantly higher in the H and HP groups (p < 0.001). In the HB group, the amount of MDA was significantly decreased compared to the HP and H groups (p < 0.001). Moreover, based on our findings, H. pylori infection decreased TAC relative to the control group (p < 0.001). When compared to the H and HP groups, vitamin B12 administration in group 4 substantially averted the decrease in TAC (p < 0.001). Statistical analysis revealed no significant variation between the control and HB groups (Figure 5).
3.6. Effect of H. pylori infection on antioxidant enzyme activity in serum
Superoxide dismutase (SOD) is one of the antioxidant enzymes responsible for preventing ROS production in cells. It converts anion superoxide (O2) to low-risk hydrogen peroxide in cells and converts glutathione peroxidase (GPX) and catalase to water. In this study, infection with H. pylori significantly decreased SOD levels compared to the control group (p < 0.01). When compared to the H and HP groups, the results showed that vitamin B12 had a positive effect on SOD and increased its activity. Compared to the control group, the specific activity of GPX was significantly (p < 0.01) reduced in the H and HP groups. Compared to corresponding controls, pre-treatment with vitamin B12 restored this diminished activity (Figure 6).






4. Discussion
Since H. pylori infection has been shown to associate with the histological changes of the seminiferous tubules, the protective effect of vitamin B12 against H. pylori-induced testicular damage was evaluated in the current study after 3 wk of treatment. The spermatogenesis is progressed nearly over the 4-6 wk in rat testes (16). The decreased Johnson score, TDI, and SPI in the H. pylori-infected group confirms the testicular toxicity of H. pylori infection. The data obtained from the serum level of SOD, GPX, MDA, and TAC showed that the testicular damage was associated with increased oxidative stress. While, the higher Johnson score, TDI, and SPI in the H. pylori-infected + vitamin B12 group than in the H. pylori group indicated that vitamin B12 can reduce testicular damage induced by H. pylori and improve the damaged-spermatogenesis. Since the vitamin B12 treatment group showed higher SOD and GPX enzyme activity and TAC as well as lower MDA levels than H and HB groups, the reduced testicular damage in the treatment group can be contributed to increasing antioxidant capacity by vitamin B12.
More than half of the global population is infected with H. pylori. In developing nations, the prevalence of infection persists above 80%, while it has radically decreased in developed nations (17). H. pylori infection may be linked to health problems outside of the gut system, and it may also contribute to the development of inflammatory diseases. H. pylori can induce extra gastric symptoms directly or indirectly via the release of inflammatory mediators and cytokines, molecular mimics, and systemic immune response (18). For many years, research organizations have concentrated on the potential impact of H. pylori infections on sperm quality. H. pylori infection, particularly by isolates producing the CagA protein, has been suggested as a potential concurrent cause of hypofertility and sperm changes because it has been linked to decreased mobility and a rise in unviable sperms (9). It is clear that H. pylori infection reduces sperm motility and viability and has some impact on testicular parameters (19). The exact mechanism by which H. pylori may affect sperm parameters is still unknown completely, although involving antigenic mimicry has been hypothesized. Infected individuals may mount a cellular and humoral immune response to bacterial constituents and products that might cross-react with epithelial cells of various organs such as testicular damage (20).
Several studies have examined the protective effects of potent antioxidants (phytochemicals, vitamins, and enzymes) on the testicles of rodents (12, 21-22).
In this study, vitamin B12 was selected as it has previously shown to protect against testicular damage caused by cimetidine (23). Vitamin B12 has also shown to play a part in the preservation of reproductive processes in the testis. Vitamin B12 promotes DNA production, which aids in cell proliferation (24). Rodents deficient in vitamin B12 have shown testicular pathological conditions characterized by notable diminishment in testis weight, shrinkage of the seminiferous tubules, and aplasia of spermatids and sperm (23, 25). In oligospermically provoked male rats, for example, methylcobalamin at 1000 µg/kg (6 times a week for 5-10 wk) caused a significant rise in the width of the seminiferous tubules as well as sperm concentration (26). In addition, studies revealed that spermatogenesis would be enhanced when a high dose of vitamin B12 is administered to patients with oligospermia in relation to fertility (27). Our results showed that infection with Helicobacter leads to a decrease in serum levels of vitamin B12.
Low amounts of vitamin B12 in the body decrease the enzymatic activity of methionine synthase, which is required to make methionine from homocysteine. This decrease causes a buildup of homocysteine in the bloodstream, also known as hyperhomocysteinemia (> 15 mol/L) (28). It has been discovered that hyperhomocysteinemia is associated with numerous health issues, including reproductive problems (29).
In this study, hyperhomocysteinemia was observed in groups infected with H. pylori. Recent in vitro research uncovered a relationship between sperm parameters such as motility and quantity and thiol concentrations. Such evidence implies a potential homocysteine toxicity to sperm, which may adversely affect sperm parameters due to a depleted vitamin B12 level. Hyperhomocysteinemia inhibits nitric oxide synthase pathways in the body, reducing the amount of nitric oxide generated. The enzyme known as nitric oxide synthase exists in spermatozoa and nitric oxide is essential for proper sperm movement, it is plausible that vitamin B12 deficiency may impair sperm function via hyperhomocysteinemia-induced nitric oxide deficiency (23). Our results in this study showed that there is a significant relationship between infection with H. pylori and the reduction of vitamin B12 and the increase of serum homocysteine.
Increased ROS and decreased antioxidant defense lead to redox imbalance, decreased sperm motility, and DNA damage in sperm (30). Due to the abundance of unsaturated fatty acids in their cell membranes, spermatozoa are extremely susceptible to the detrimental effects of ROS (31). Oxidative stress is associated with an increase in the generation of oxygen-free radicals or an unbalance in the redox defense system.
SOD, GPX, and catalase are the 3 major enzymatic components of this system in sperm (32). Our results showed that the activity of SOD and GPX was decreased in the H. pylori infected. As the quantity of ROS in the tissue rises, plasma membrane lipids become damaged and peroxidized, and internal components are destroyed, with MDA serving as a measure for lipid peroxidation (33). In our study, the oxidative stress and histopathological effects of vitamin B12 pretreatment in the prevention of H. pylori injury in mice were evaluated, and it was discovered that vitamin B12 supplementation significantly decreased plasma MDA levels and increased GPX and SOD activity.
Along with the results obtained from our research, it was reported that infection with H. pylori, especially the CagA strain, can be responsible for infertility in men through a destructive effect on sperm (24).
Furthermore, a study revealed that the presence of H. pylori infection leads to elevated levels of inflammatory cytokines in semen. These elevated levels have been associated with a decrease in sperm motility, potential sperm damage, and ultimately a decline in male fertility (7). Finally, this study's findings reported that vitamin B12 supplementation has a positive impact on preventing and treating complications of H. pylori infection and infertility.
5. Conclusion
The results of this study can help to explain that infection with H. pylori can lead to infertility by inducing harmful changes in testicular tissue and sperm parameters. In addition, H. pylori can cause harm to the reproductive system by destroying the antioxidant balance and decreasing the TAC, and activity of SOD and GPX enzymes. According to the results of the present investigation, vitamin B12 is an effective antioxidant that can decrease oxidative stress, enhance sperm parameters, and reduce the rate of testicular tissue damage. The beneficial effects of vitamin B12 on sperm parameters can be attributed to improve the efficacy of male reproductive organs, reduced homocysteine toxicity, and decreased ROS accumulation. However, additional research, predominantly clinical, is required to confirm these positive effects.
Data availability
Data will be made available on request via email to corresponding authors.
Author contributions
Maryam Karimi-Dehkordi designed the study and conducted the research. Maryam Karimi-Dehkordi and Forogh Mohammadi monitored, evaluated, and analyzed the results of the study. Further, Farnaz Pouriayevali reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
Artificial intelligence has not been used in any way (translation, editing, grammar checking, etc.).
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Epidemiology
References
1. Wang S, Zhang K, Yao Y, Li J, Deng S. Bacterial infections affect male fertility: A focus on the oxidative stress-autophagy axis. Front Cell Dev Biol 2021; 9: 727812. [DOI:10.3389/fcell.2021.727812] [PMID] [PMCID]
2. Oghbaei H, Rastgar Rezaei Y, Nikanfar S, Zarezadeh R, Sadegi M, Latifi Z, et al. Effects of bacteria on male fertility: Spermatogenesis and sperm function. Life Sci 2020; 256: 117891. [DOI:10.1016/j.lfs.2020.117891] [PMID]
3. Biggs SN, Kennedy J, Lewis SL, Hearps S, O'Bryan MK, McLachlan R, et al. Lifestyle and environmental risk factors for unexplained male infertility: Study protocol for Australian Male Infertility Exposure (AMIE), a case-control study. Reprod Health 2023; 20: 32. [DOI:10.1186/s12978-023-01578-z] [PMID] [PMCID]
4. Moridi A, Roozbeh N, Yaghoobi H, Soltani S, Dashti S, Shahrahmani N, et al. Etiology and risk factors associated with infertility. Int J Women's Health Reprod Sci 2019; 7: 346-353. [DOI:10.15296/ijwhr.2019.57]
5. Marchiani S, Baccani I, Tamburrino L, Mattiuz G, Nicolò S, Bonaiuto C, et al. Effects of common gram-negative pathogens causing male genitourinary-tract infections on human sperm functions. Sci Rep 2021; 11: 19177. [DOI:10.1038/s41598-021-98710-5] [PMID] [PMCID]
6. Eini F, Azizi Kutenaei M, Zareei F, Sharifian Dastjerdi Z, Hosseinzadeh Shirzeyli M, Salehi E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with Leukocytospermia. BMC Mol Cell Biol 2021; 22: 42. [DOI:10.1186/s12860-021-00380-8] [PMID] [PMCID]
7. Moretti E, Figura N, Campagna MS, Gonnelli S, Iacoponi F, Collodel G. Sperm parameters and semen levels of inflammatory cytokines in Helicobacter pylori-infected men. Urology 2015; 86: 41-47. [DOI:10.1016/j.urology.2015.02.068] [PMID]
8. Kamarehei F, Taheri M, Mohammadi Y, Shokoohizadeh L, Alikhani MY. Prevalence of Helicobacter pylori virulence genes among Iranian infected patients: A systematic review and meta-analysis. Rev Res Med Microbiol 2020; 31: 191-200. [DOI:10.1097/MRM.0000000000000207]
9. Cho J, Prashar A, Jones NL, Moss SF. Helicobacter pylori infection. Gastroenterol Clin North Am 2021; 50: 261-282. [DOI:10.1016/j.gtc.2021.02.001] [PMID]
10. Taylor NS, Fox JG. Animal models of Helicobacter-induced disease: Methods to successfully infect the mouse. Methods Mol Biol 2012; 921: 131-142. [DOI:10.1007/978-1-62703-005-2_18] [PMID] [PMCID]
11. Shubina OS, Dudenkova NA. The effect of lead on the process of spermatogenesis in sex glands of male albino rats. Vet World 2016; 9: 1129-1134. [DOI:10.14202/vetworld.2016.1129-1134] [PMID] [PMCID]
12. Salehinezhad F, Eshraghi H, Kadivar A, Shirian S, Asghari A, Aali E, et al. Amelioration effects of vitamin E, melatonin, L-carnitine, and atorvastatin, on destructive effects of busulfan in the testes of male rats: A gene expression evaluation. Kafkas Univ Vet Fak Derg 2019; 25: 709-716. [DOI:10.9775/kvfd.2019.21726]
13. Gholami-Ahangaran M, Karimi-Dehkordi M, Akbari Javar A, Haj Salehi M, Ostadpoor M. A systematic review on the effect of Ginger (Zingiber officinale) on improvement of biological and fertility indices of sperm in laboratory animals, poultry and humans. Vet Med Sci 2021; 7: 1959-1969. [DOI:10.1002/vms3.538] [PMID] [PMCID]
14. Ghajari G, Nabiuni M, Amini E. The association between testicular toxicity induced by Li2Co3 and protective effect of Ganoderma lucidum: Alteration of Bax & c-Kit genes expression. Tissue Cell 2021; 72: 101552. [DOI:10.1016/j.tice.2021.101552] [PMID]
15. Nabi A, Entezari F, Miresmaeili SM, Vahidi S, Lorian K, Anbari F, et al. Evaluation of sperm parameters and DNA integrity following different incubation times in PVP medium. Urol J 2022; 19: 232-237.
16. Marchetti F, Aardema M, Beevers C, Yauk CL, Young R, Williams A. Simulation of mouse and rat spermatogenesis to inform genotoxicity testing using OECD test guideline 488. Mutat Res Genet Toxicol Environ Mutagen 2018; 832-833: 19-28. [DOI:10.1016/j.mrgentox.2018.05.020] [PMID]
17. Demir M, Ozdem S. Evaluation of total antioxidant capacity in human seminal plasma. Int J Med Biochem 2022; 5: 54-59. [DOI:10.14744/ijmb.2021.30092]
18. Menevse E, Dursunoglu D, Cetin N, Korucu EN, Erbayram FZ. Evaluation of sialic acid, malondialdehyde and glutathione levels in infertile male. Rev Int Androl 2022; 20: 266-273. [DOI:10.1016/j.androl.2021.05.002] [PMID]
19. Ghajari Gh, Moosavi R. Evaluation of the effects of diazinon toxin on some reproductive parameters in male rats. Pers Med J 2022; 7: 30-35.
20. Faria C, Zakout R, Araújo M. Helicobacter pylori and autoimmune diseases. Biomed Pharmacother 2013; 67: 347-349. [DOI:10.1016/j.biopha.2011.09.015] [PMID]
21. Mohammadi N, Shirian S, Gorji A, Roshanpajouh M, Ahmadi E, Nazari H, et al. The potential protective effect of melatonin and N-acetylcysteine alone and in combination on opioid-induced testicular dysfunction and degeneration in rat. Reprod Toxicol 2023; 120: 108453. [DOI:10.1016/j.reprotox.2023.108453] [PMID]
22. Zangoie R, Eshraghi R, Shirian S, Nazari H, Aali E. Melatonin synergistically enhances protective effect of atorvastatin against busulfan-induced spermatogenesis injuries in a rat model. Comparat Clin Pathol 2020; 29: 161-166. [DOI:10.1007/s00580-019-03040-8]
23. Elsaed WM, Bedeer RF, Eladl MA. Ameliorative effect of vitamin B12 on seminiferous epithelium of cimetidine-treated rats: A histopathological, immunohistochemical and ultrastructural study. Anat Cell Biol 2018; 51: 52-61. [DOI:10.5115/acb.2018.51.1.52] [PMID] [PMCID]
24. El-Kady HM. The plausibility of Helicobacter pylori and CagA strains related infertility among males in Alexandria, Egypt. J High Inst Public Health 2019; 49: 56-63. [DOI:10.21608/jhiph.2019.29467]
25. Kohli A, Kumar R. Role of antioxidants of natural herbs in management of male infertility. J Res Appl Sci Biotechnol 2023; 2: 55-80. [DOI:10.55544/jrasb.2.1.9]
26. Adelakun SA, Ukwenya VO, Akintunde OW. Vitamin B12 ameliorate tramadol-induced oxidative stress, endocrine imbalance, apoptosis and NO/iNOS/NF-κB expression in Sprague Dawley rats through regulatory mechanism in the pituitary-gonadal axis. Tissue Cell 2022; 74: 101697. [DOI:10.1016/j.tice.2021.101697] [PMID]
27. Banihani SA. Vitamin B12 and semen quality. Biomolecules 2017; 7: 42. [DOI:10.3390/biom7020042] [PMID] [PMCID]
28. Beltrame FL, Sasso-Cerri E. Vitamin B12-induced spermatogenesis recovery in cimetidine-treated rats: Effect on the spermatogonia number and sperm concentration. Asian J Androl 2017; 19: 567-572. [DOI:10.4103/1008-682X.182397] [PMID] [PMCID]
29. Froese DS, Fowler B, Baumgartner MR. Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J Inherit Metab Dis 2019; 42: 673-685. [DOI:10.1002/jimd.12009] [PMID]
30. Rehman T, Shabbir MA, Inam‐Ur‐Raheem M, Manzoor MF, Ahmad N, Liu ZW, et al. Cysteine and homocysteine as biomarker of various diseases. Food Sci Nutr 2020; 8: 4696-4707. [DOI:10.1002/fsn3.1818] [PMID] [PMCID]
31. Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J Clin Diagn Res 2017; 11: IE01-IE05. [DOI:10.7860/JCDR/2017/23927.9886] [PMID] [PMCID]
32. Ritchie C, Ko EY. Oxidative stress in the pathophysiology of male infertility. Andrologia 2021; 53: e13581. [DOI:10.1111/and.13581] [PMID]
33. Asghari A, Akbari G, Meghdadi A, Mortazavi P. Protective effect of metformin on testicular ischemia/ reperfusion injury in rats. Acta Cir Bras 2016; 31: 411-416. [DOI:10.1590/S0102-865020160060000008] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







