Tue, Jul 8, 2025
[Archive]
Volume 22, Issue 6 (June 2024)
IJRM 2024, 22(6): 463-472 |
Back to browse issues page
Ethics code: IR.IAU.QOM.REC.1401.072
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Babaei F, Khoshsokhan Muzaffar M, Jannatifar R. The association of sperm BAX and BCL-2 gene expression with reproductive outcome in Oligoasthenoteratozoospermia cases undergoing intracytoplasmic sperm injection: A case-control study. IJRM 2024; 22 (6) :463-472
URL: http://ijrm.ir/article-1-3230-en.html
URL: http://ijrm.ir/article-1-3230-en.html
1- Biology Department, Islamic Azad University, Qom Branch, Qom, Iran.
2- Department of Reproductive Biology, Academic Center for Education, Culture, and Research (ACECR), Qom, Iran. ,Rahiljanati2016@gmail.com
2- Department of Reproductive Biology, Academic Center for Education, Culture, and Research (ACECR), Qom, Iran. ,
Full-Text [PDF 330 kb]
(692 Downloads)
| Abstract (HTML) (656 Views)
Full-Text: (106 Views)
1. Introduction
Apoptosis (programmed cell death) is a regulated process leading to the removal of damaged, surplus, or aged cells during normal development, and its deregulation causes many disorders (1). In the absence of specific cell surface receptors, factors that can directly penetrate the cell and modulate the apoptotic cascade may activate apoptosis (2). Such factors include: heat shock, ultraviolet radiation, reaction oxidative stress, and some drugs. Today, the presence and activity of apoptosis signals in human sperm in response to various stimuli is widely accepted (3).
There are 2 distinct mechanisms for the initiation of apoptosis: extrinsic pathway or receptor apoptosis and endogenous or mitochondrial apoptosis. Specific mechanisms include the cell DNA fragmentation through chromatin degradation during programmed cell death, is a biological mechanism critical for generating appropriate germ cells and maintaining the germ cell-to-Sertoli ratio in the testis (3). In fact, the biochemical feature of apoptosis includes the transfer of phosphatidylserine to the outer plasma membrane, caspase activation, and DNA fragmentation (4). Caspase activity is associated with sperm immaturity, low number, reduced motility, lower fertilization levels (5).
Apoptosis in spermatogenesis is a dynamic process including morphologic differentiation, mitotic divisions of spermatogonia and spermatocytes, and spermatids ending maturation in sperm formation (6). In many types of cells, there are several proteins that play a role in regulating apoptosis. It has been reported that some members of the B-cell lymphoma 2 (BCL-2) family (BCL-w, Mcl-7, BCL-xl, BCL-2) are involved in suppressing apoptosis, and some of them (Bad, Bak, BCL-xs, BAX) in promoting apoptosis (7). BCL-2 protein suppresses apoptosis, while BAX protein promotes apoptosis. BCL-2 helps maintain mitochondrial membrane integrity by binding to mitochondrial outer membrane receptors. When cells are exposed to apoptosis-inducing agents, BAX is translocated from the cytoplasm to the mitochondrial membrane, causing changes in cell membrane permeability (8). Following these changes, cytochrome C is released and induces apoptosis, which ultimately leads to sperm DNA fragmentation.
The primordial germ cells originate from the epiblast and finally migrate to the gonad. Excess cells produced at this time are killed by apoptosis, which mainly depends on coordination between BCL-xL and BAX (9). An abrogated initial wave of apoptosis in transgenic mice overexpressing BCL-2 or BCL-x leads to accumulation of spermatogonia and spermatocytes, resulting in infertile animals (10). BCL-x-knockout mice have severe defects in male germ cells during development.
The balance between proteins that are involved in the induction of apoptosis and those that suppress apoptosis in the cell affects the regulation of apoptosis in the cell. For example, a high BAX/BCL ratio indicates a pro-apoptotic tendency of the cell (11). By increasing the amount of BAX and decreasing BCL-2, a suitable pre-apoptotic environment is prepared for the cell (12). Today, the use of intracytoplasmic sperm injection (ICSI) is suggested for better selection of sperm in patients with severe male factor infertility (13). Poor seminal parameters, including increased sperm DNA fragmentation, decrease blastocyst production rates following in vitro fertilization, suggesting an effect of sperm on preimplantation embryo development in humans (14). Studies have shown that increasing the amount of apoptosis in sperm decreases the quality of sperm parameters and then increases the fragmentation rate in the embryo (15).
This study aims to explore the association of sperm BAX and BCL-2 gene expression with reproductive outcome in oligoasthenoteratozoospermic cases undergoing ICSI.
2. Materials and Methods
2.1. Study design
This study is a case-control method that was randomly sampled among 25 infertile men (oligoasthenoteratozoospermic) undergoing assisted reproductive technology (ART) cycles in the age range of 20-40 yr who were approved by a urologist and referred to the Infertility Research Center, the Academic Center for Education, Culture, and Research (ACECR), Qom, Iran from August 2022-March 2023. Men with total sperm motility < 40% and count < 15 million sperm/ml and normal morphology < 4%, participated as oligoasthenoteratozoospermic according to the analysis by the World Health Organization (WHO) (16). 25 normozoospermic men were included as healthy control in the age range of 20-40 who are healthy according to the analysis of the WHO. Those with smoking and alcohol assumption, varicoceles, systemic diseases, cryptorchidism and external genitalia abnormalities, and hormonal level abnormalities were excluded from this study. Partner’s age of patients who are oligoasthenoteratozoospermic was between 25-35 yr, and there were no female disorder factors such as ovulation disorders, tubal factor, endometrial disease, or endocrine abnormalities. Also, participants with a history of alcohol abuse, drug use, or smoking were excluded from the study.
2.2. Sperm analysis
Following 4-5 days of sexual abstinence, the ejaculates were poured into sterile containers, and more than 1 sample was prepared at an interval of 2 wk from each other. The semen examination was based on the WHO instructions (16).
Computer-assisted sperm analysis system (LABOMED, SDC313B, Germany) was used to assess sperm motility (progressive, non-progressive or quiescent). Papanicolaou staining was also used to evaluate the normal morphology of sperms. To check the number of sperm, after appropriate dilution, 100 sperm per slide were measured by Neubauer chamber through a Nikon Eclipse TE2000 microscope.
2.3. BAX and BCL-2 gene expressions
Total ribonucleic acid (RNA) was extracted from all sperm samples by total RNA extraction as well as first strand cDNA generation using QuantiTect®, and RNeasy Micro kits (Qiagen, Europe). The NanoDrop spectrophotometer was applied to determine RNA concentration, which was adjusted to 1000 ng/μl. The RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific Inc. China) was applied to synthesize cDNA on the same day as guided. The reverse transcription was done in 20 μl reactions (42oC/60 min and then 70oC/5 min) for inactivation of the reverse transcriptase and its product was directly applied in quantitative polymerase chain reaction (PCR) in an independent step to amplify the targets.
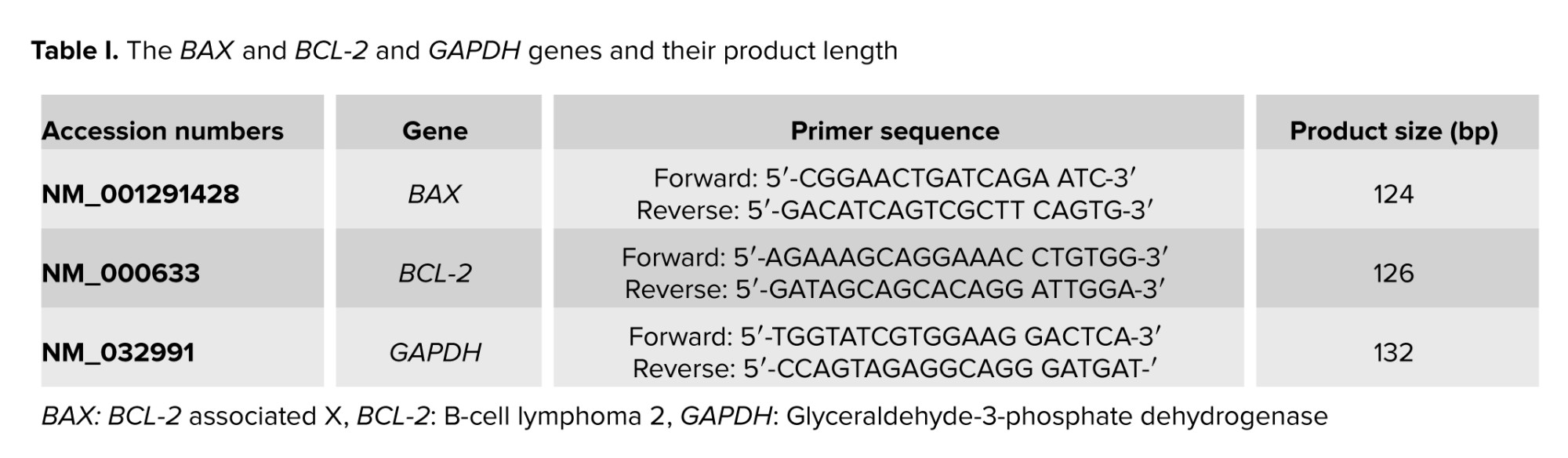
2.4. Real-time PCR
Quantitative real-time PCR was used to evaluate BCL-2 and BAX relative transcript levels using specified primers (Table I). The internal control was the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. The QuantiTect SYBR Green RT-PCR kit (Lot No.: 1201416; Applied Biosystems, UK) was used for PCR run on an ABI 7500 RT-PCR system (Applied Biosystems) considering stage 1 (95oC/5 min) and stage 2 (95oC/20 s, 58°C/30 s, 72°C/20 s) for 40 cycles, followed by a melt curve stage (95°C/15 s, 58°C/1 min, and 95°C/15 s). The specimens were run 2 times to decrease the sampling error, and the duplicates’ mean value was applied for the next calculations. Specimens minus the reverse transcriptase and no template control were run along with the original specimens. The product length, 2% agarose gel, and a melting curve analysis verified amplicon size and specificity. The ratio = (E target) ΔCt target (control sample)/(E ref) ΔCt ref (control-sample) formula was used to calculate the relative transcript levels. The target gene relative transcript levels were calculated using the RT PCR efficiency (E) as well as the threshold cycle difference related to an unfamiliar specimen compared to the control (Ct control-sample). CDNA dilution curves were constructed for each gene to determine the individual real-time PCR efficiencies (E = 10 [- 1/slope]).
2.5. Sperm DNA fragmentation
The terminal deoxynucleotidyl transferase dUTP nick end labeling assay evaluated sperm DNA fragmentation by the in-situ cell death detection kit (Roche, Germany) (17). A fluorescence microscope (BX51, Olympus, Japan) was used to assess 500 sperms for each slide at ×100 magnification. Sperm characterized by redheads had intact DNA, whereas sperm with green heads had fragmented DNA.
2.6. ICSI process, and embryo quality
A long method of ovulation induction was performed in all the cases' wives. 250 gr of recombinant human chorionic gonadotropin (OVIDREL, hCG, Merck-Serono) was injected to trigger follicular development, followed by the extraction of oocytes (18). Which were obtained from follicular fluid quickly after follicle aspiration. Oocytes with the first polar body morphology were used for ICSI. Then, 24 hr after oocyte retrieval, an injection of normal sperm was done to inseminate the oocyte. Then 16-18 hr after ICSI, 2 pronuclei observed by an Olympus inverted microscope using a Hoffmann modulation contrast system (×400) proved fertilization. Embryo quality and cleavage rate were assessed 3 days following ICSI, according to a scoring system (19): grade I, no fragmentation and symmetric blastomeres; grade II, uneven blastomeres and less than 30% fragmentation; and grade III, uneven blastomeres and more than 30% fragmentation. The resulting embryos were cryopreserved. 2 freeze-thaw embryo transfer ICSI cycles were performed in all cases.
2.7. Ethical considerations
The Ethics Committee for Human Subjects Research of Qom Azad University, Qom, Iran accepted this study (Code: IR.IAU.QOM.REC.1401.072). Patients enrolled in the study, and the proband's family gave their written approval.
2.8. Statistical analysis
We used the Statistical Package for Social Sciences (SPSS) version 16 to analyze the data. In addition, variables with a continuous normal distribution were examined using the independent sample t test, while the Mann-Whitney U test was used for data that were not normally distributed. P < 0.05 was considered statistically significant. Findings are shown as mean ± SEM.
3. Results
3.1. Clinical and demographic characteristics
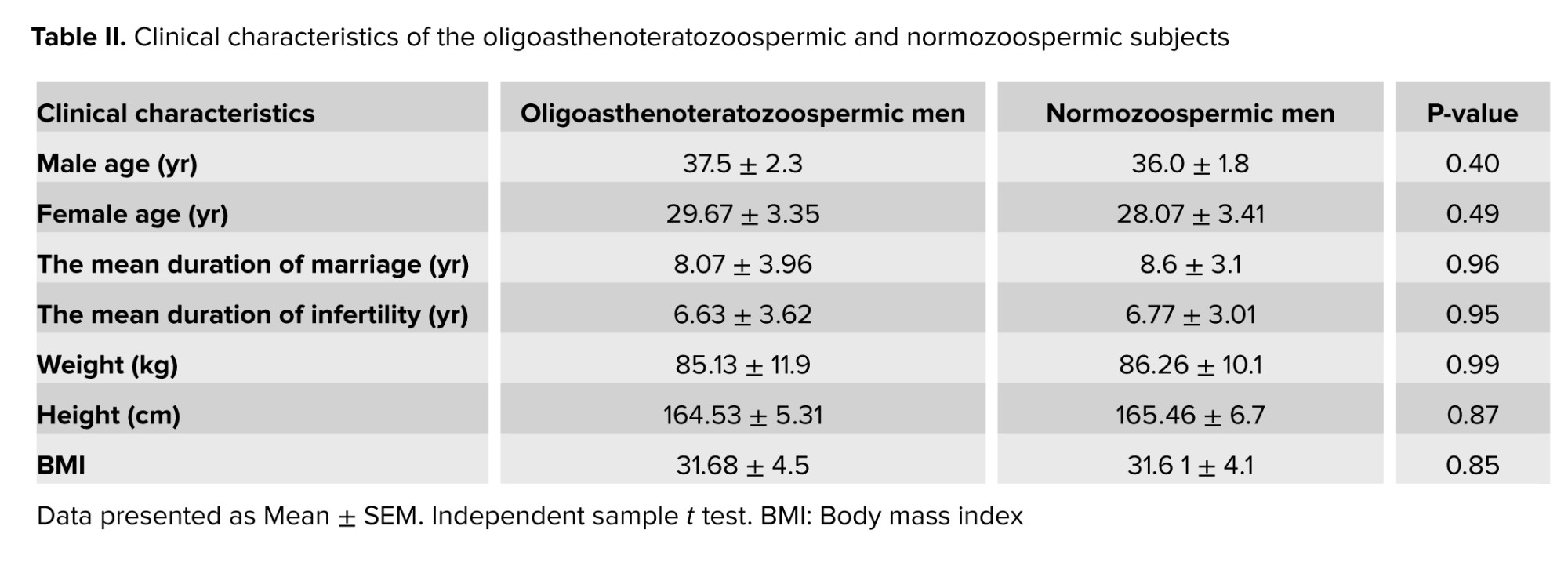
No significant differences were observed in the male age (p = 0.40), female age) p = 0.49) duration of marriage (p = 0.96), or duration of infertility (p = 0.95). Also, no significant differences were observed in weight, height, or BMI between the 2 groups as presented in table II.
3.2. Sperm quality
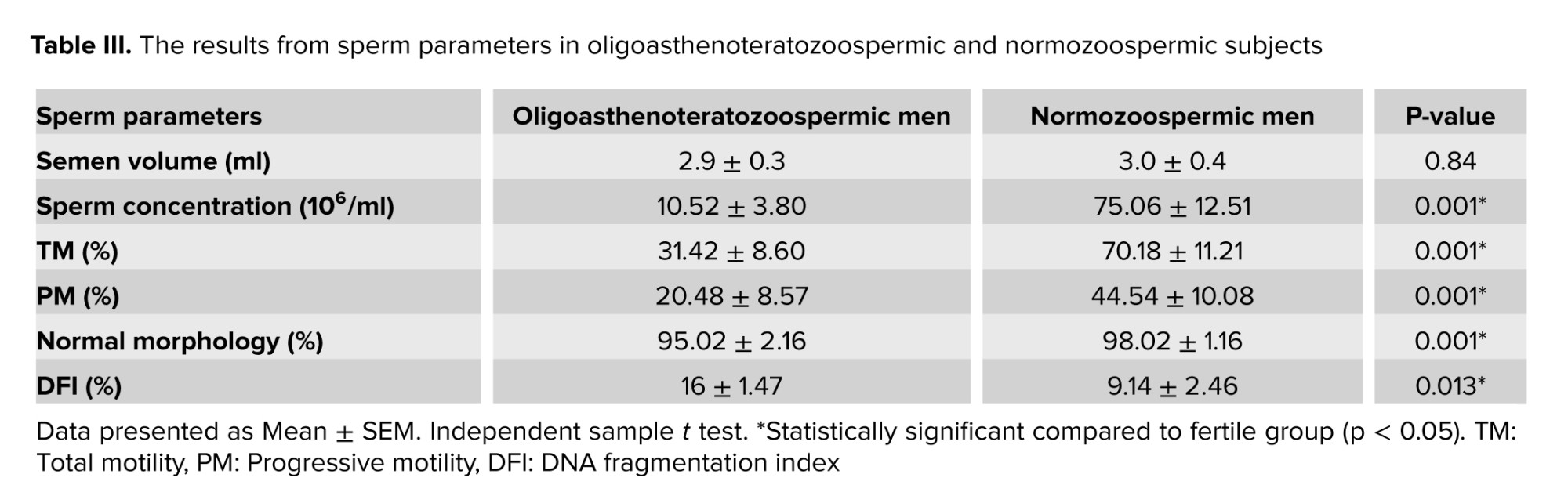
Table III indicate that sperm concentration (p = 0.001), total motility (p = 0.001), progressive motility (p = 0.004), and normal morphology (p = 0.001), between oligoasthenoteratozoospermia, and normozoospermic fertile subjects were significantly different. Also, data were significantly different for DNA fragmentation of sperm between oligoasthenoteratozoospermic, and normozoospermic subjects (p = 0.013). No significant differences were observed in the semen volume between the 2 groups.
3.3. BAX and BCL-2 gene expression
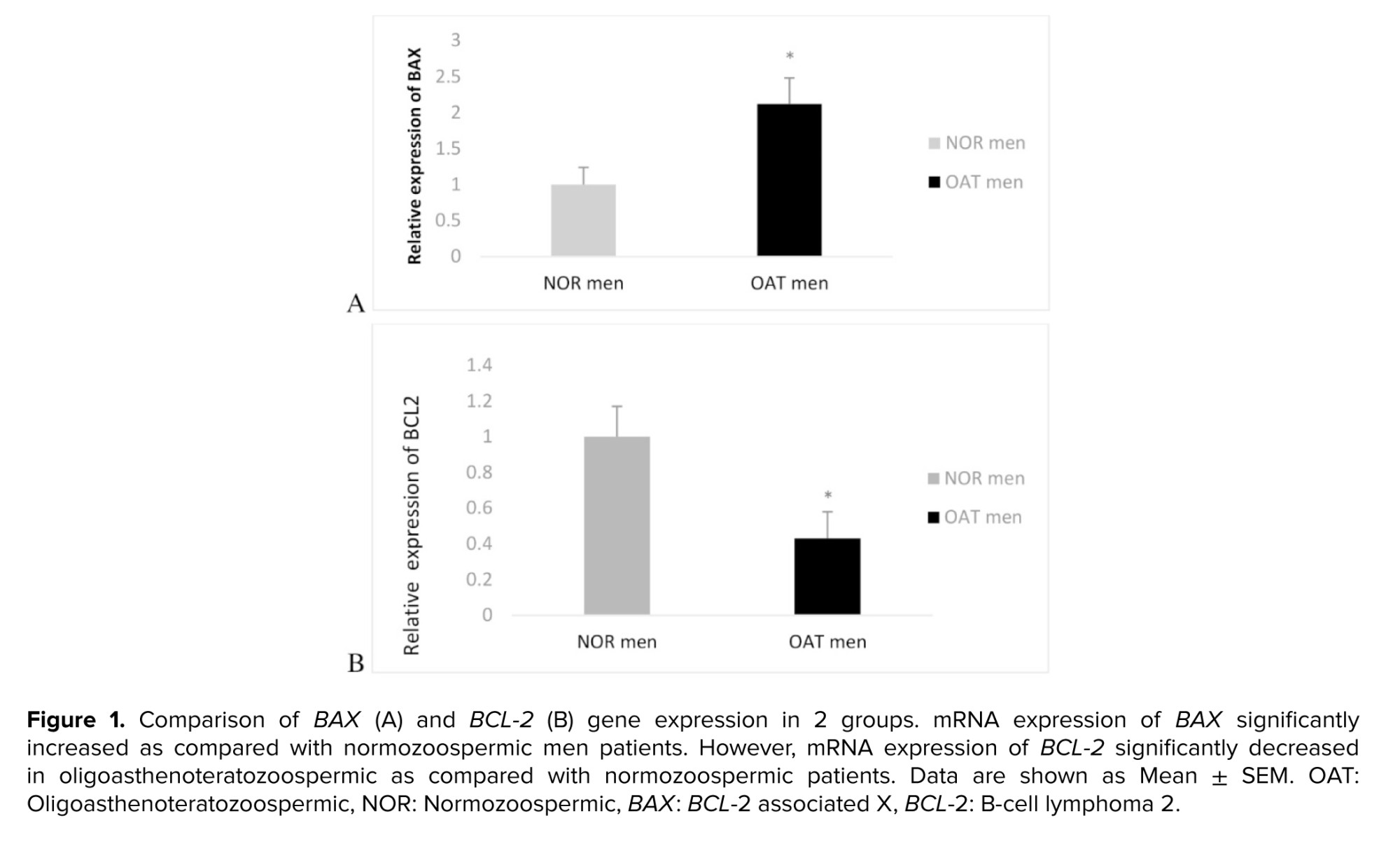
Figure 1A, B presents the evaluation of apoptosis-related genes showed that mRNA expression of BAX significantly increased, compared to fertile patients (p = 0.012). However, mRNA expression of BCL-2 significantly decreased in oligoasthenoteratozoospermic as compared with normozoospermic men (p = 0.032). As expected, the BAX/BCL-2 ratio was significantly higher in oligoasthenoteratozoospermic compared to the normozoospermic group (p = 0.033).
3.4. Correlation of BAX, BCL-2 with sperm parameters
The correlations analysis between BAX and BCL-2 gene expression with sperm parameters and DNA fragmentation (Table IV) shows that there is a significant negative correlation with sperm motility (p = 0.001), sperm count (p = 0.001), and sperm normal forms with BAX gene expression (p = 0.001), and whether, there is any significant positive correlation with sperm DNA fragmentation (p = 0.01; Table II). Seminal BCL-2 demonstrated a significant correlation with sperm count (p = 0.001), sperm motility (p = 0.001), and sperm morphology (p = 0.014) while a negative correlation was observed with sperm DNA fragmentation (p = 0.010; Table IV).
3.5. Fertilization rate and embryo quality after ICSI
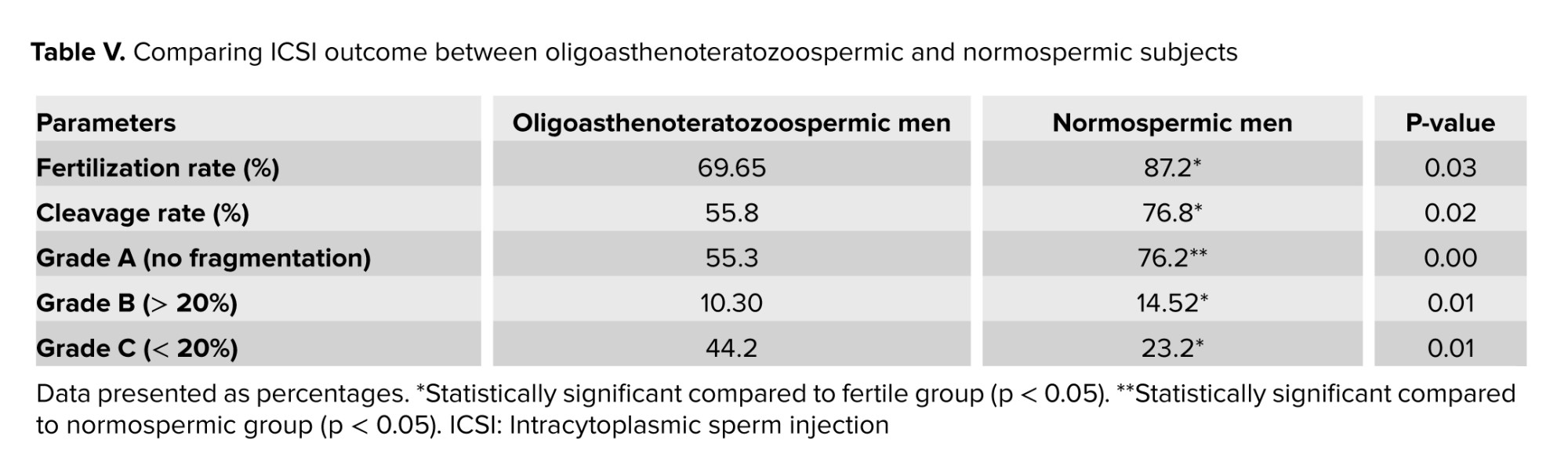
As shown in table V, oligoasthenoteratozoospermic men had a considerably lower proportion of fertilization rate and good-quality embryos at the cleavage stage than the normozoospermic subjects. A significant upper fertilization rate was observed in fertile men compared to oligoasthenoteratozoospermic (p = 0.030). Also, the cleavage rate was significantly higher in fertile men compared to oligoasthenoteratozoospermic men (p = 0.024). The mean percentages of embryo quality scores were substantially different between groups (A, B) (p = 0.001) and (p = 0.015), respectively. A significant difference in embryo grade C was observed between normozoospermic and oligoasthenoteratozoospermic men (p = 0.012).
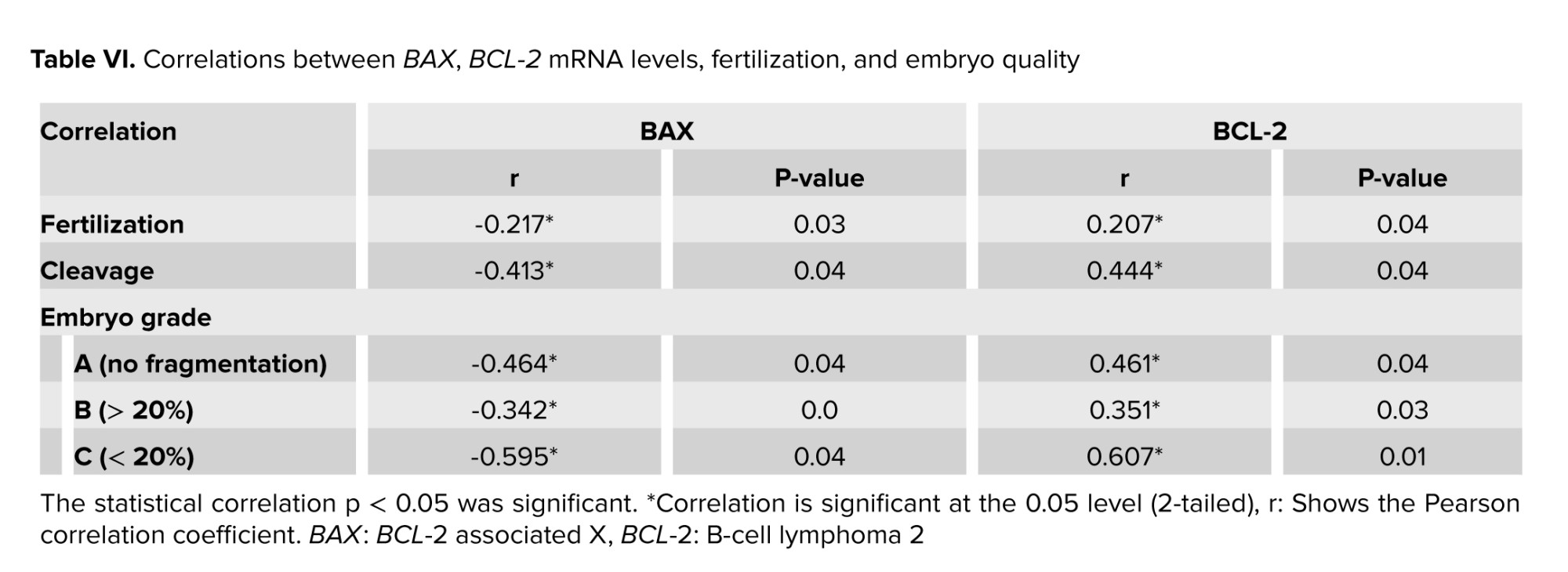
3.6. Correlation of BAX, BCL-2 with fertilization rate and embryo quality
Sperm BAX gene expression had a significantly negative correlation with fertilization rate (p = 0.036), cleavage rate (p = 0.046), embryo quality (grade A) (p = 0.044) embryo quality (grade B) (p = 0.05), and embryo quality (grade C) (p = 0.043). Whether, sperm BCL-2 gene expression demonstrated a significant positive correlation with fertilization rate (p = 0.041), cleavage rate (p = 0.048), and embryo quality (grade A) (p = 0.041), embryo quality (grade B) (p = 0.034), and embryo quality (grade C) (p = 0.011; Table VI).

4. Discussion
The main aim of this study was to investigate the increase of apoptosis in ART results based on sperm quality and DNA damage level. Our study showed that the mRNA expression of BAX were significantly increased in the oligoasthenoteratozoospermic group compared to normozoospermic individuals. Whereas, BCL-2 mRNA expression was significantly decreased in oligoasthenoteratozoospermic men compared to normozoospermic men. Also, the BAX/BCL-2 ratio of sperm in infertile men increased significantly compared to fertile men. Sperm BAX gene expression had a significant and negative correlation with sperm motility, concentration and normal shapes, while sperm BCL-2 gene expression had a positive and significant correlation with these parameters. Also, we detected a significant correlation between BAX and BCL-2 expression with DNA denaturation.
The higher expression of BAX compared to BCL-2 leads to forming pores associated with apoptosis and possibly triggers apoptotic reaction cascades (20). The cascade was initiated by the release of mitochondrial cytochrome-c into the cytosol and interacted with Apaf-1 to generate apoptosomes to recruit and activate procaspase-9 (21). Caspase effectors could destroy several substrates in the cell leading to morphological alterations in apoptosis (22). Activation of caspase-9 in ejaculated sperm occurs in the post-acrosomal stage in mature sperm (23), and the midpiece, in which residual cytoplasm and mitochondria can be found (24). Kamal et al. reported a negative association between seminal clusterin (involved in the cellular debris clearance and apoptosis) gene expression and sperm motility, concentration, and acrosin activity, linearity index and velocity, and a positive association with sperm DNA fragmentation and abnormal forms (25). Roshdy and Mostafa reported an association between survivin protein (an apoptosis inhibitor) in the seminal plasma of fertile and infertile males and sperm motility processes and spermatogenesis (26). These results are consistent with our findings regarding the interdependence between the expression of genes involved in apoptosis (BAX, BCL-2), and decreased sperm quality.
The current study describes the correlation between apoptosis markers such as BAX, and BCL-2 mRNA expression with fertilization rate, cleavage rate, and embryo quality in oligoasthenoteratozoospermic men under ICSI processing. Based on this study, changes in the expression of BAX, and BCL-2 genes indirectly through reducing the sperm quality and increasing the sperm DNA damage level might compromise the progression of embryo development, resulting in arrested embryos. Sperm DNA integrity is of great importance as the genetic material quality in a sperm population is important for the subsequent and embryonic development of children, successful fertilization, and the reproductive potential in males. Apoptosis is necessary for male gametes from the gonadal anlagen appearance in the embryo to fertilization. It can maintain the proper ratio between the Sertoli cells and germ cells during prenatal development (27).
Apoptosis removes damaged cells from the testicles in the samples of adults. Mature spermatozoa experience apoptosis and phagocytosis in the reproductive tract of females to avoid the inflammatory reaction due to the dead gamete destruction (28). The apoptosis impairment leads to developmental abnormalities in infertility, gametes of males, and oocyte fertilization by sperm with DNA damage leading to ART failure (29). Thus, apoptosis is an important cause of sperm DNA fragmentation, leading to poor embryo quality at day 3, slower rate of cleavage, poor blastocyst development, and implantation for the widespread use of assisted reproductive technologies (30). On the other hand, apoptosis shows a significant correlation with sperm morphology. Sperm head impairments are linked to the last phases of embryonic development, sperm midpiece impairments to intermediate cleaving embryos, and tail impairments to the initial embryonic development phases (31). In fact, in this study, we evaluated the sperm BCL-2 and BAX can affect the quality of the embryo through the effect on sperm morphology. Further studies are needed to confirm this hypothesis.
5. Conclusion
Moreover, according to the results, there is a relationship between apoptosis gene transcript levels sperm quality, and clinical outcomes in the oligoasthenoteratozoospermic group. However, more studies with a larger series are needed to be sure that these genes can influence reproductive outcome.
Data availability
The primary data for this study is available from the authors upon direct request.
Author contributions
R.J., F.B., and M.Kh.M., were involved in the conception, design, statistical analysis, and drafting of the manuscript. All authors have approved the final version for submission.
Acknowledgments
The authors would like to extend their gratitude Infertility Research Center, the Academic Center for Education, Culture, and Research (ACECR), Qom, Iran. Artificial intelligence has not been used in any way (translation, revision, grammar check, etc.) in this study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Apoptosis (programmed cell death) is a regulated process leading to the removal of damaged, surplus, or aged cells during normal development, and its deregulation causes many disorders (1). In the absence of specific cell surface receptors, factors that can directly penetrate the cell and modulate the apoptotic cascade may activate apoptosis (2). Such factors include: heat shock, ultraviolet radiation, reaction oxidative stress, and some drugs. Today, the presence and activity of apoptosis signals in human sperm in response to various stimuli is widely accepted (3).
There are 2 distinct mechanisms for the initiation of apoptosis: extrinsic pathway or receptor apoptosis and endogenous or mitochondrial apoptosis. Specific mechanisms include the cell DNA fragmentation through chromatin degradation during programmed cell death, is a biological mechanism critical for generating appropriate germ cells and maintaining the germ cell-to-Sertoli ratio in the testis (3). In fact, the biochemical feature of apoptosis includes the transfer of phosphatidylserine to the outer plasma membrane, caspase activation, and DNA fragmentation (4). Caspase activity is associated with sperm immaturity, low number, reduced motility, lower fertilization levels (5).
Apoptosis in spermatogenesis is a dynamic process including morphologic differentiation, mitotic divisions of spermatogonia and spermatocytes, and spermatids ending maturation in sperm formation (6). In many types of cells, there are several proteins that play a role in regulating apoptosis. It has been reported that some members of the B-cell lymphoma 2 (BCL-2) family (BCL-w, Mcl-7, BCL-xl, BCL-2) are involved in suppressing apoptosis, and some of them (Bad, Bak, BCL-xs, BAX) in promoting apoptosis (7). BCL-2 protein suppresses apoptosis, while BAX protein promotes apoptosis. BCL-2 helps maintain mitochondrial membrane integrity by binding to mitochondrial outer membrane receptors. When cells are exposed to apoptosis-inducing agents, BAX is translocated from the cytoplasm to the mitochondrial membrane, causing changes in cell membrane permeability (8). Following these changes, cytochrome C is released and induces apoptosis, which ultimately leads to sperm DNA fragmentation.
The primordial germ cells originate from the epiblast and finally migrate to the gonad. Excess cells produced at this time are killed by apoptosis, which mainly depends on coordination between BCL-xL and BAX (9). An abrogated initial wave of apoptosis in transgenic mice overexpressing BCL-2 or BCL-x leads to accumulation of spermatogonia and spermatocytes, resulting in infertile animals (10). BCL-x-knockout mice have severe defects in male germ cells during development.
The balance between proteins that are involved in the induction of apoptosis and those that suppress apoptosis in the cell affects the regulation of apoptosis in the cell. For example, a high BAX/BCL ratio indicates a pro-apoptotic tendency of the cell (11). By increasing the amount of BAX and decreasing BCL-2, a suitable pre-apoptotic environment is prepared for the cell (12). Today, the use of intracytoplasmic sperm injection (ICSI) is suggested for better selection of sperm in patients with severe male factor infertility (13). Poor seminal parameters, including increased sperm DNA fragmentation, decrease blastocyst production rates following in vitro fertilization, suggesting an effect of sperm on preimplantation embryo development in humans (14). Studies have shown that increasing the amount of apoptosis in sperm decreases the quality of sperm parameters and then increases the fragmentation rate in the embryo (15).
This study aims to explore the association of sperm BAX and BCL-2 gene expression with reproductive outcome in oligoasthenoteratozoospermic cases undergoing ICSI.
2. Materials and Methods
2.1. Study design
This study is a case-control method that was randomly sampled among 25 infertile men (oligoasthenoteratozoospermic) undergoing assisted reproductive technology (ART) cycles in the age range of 20-40 yr who were approved by a urologist and referred to the Infertility Research Center, the Academic Center for Education, Culture, and Research (ACECR), Qom, Iran from August 2022-March 2023. Men with total sperm motility < 40% and count < 15 million sperm/ml and normal morphology < 4%, participated as oligoasthenoteratozoospermic according to the analysis by the World Health Organization (WHO) (16). 25 normozoospermic men were included as healthy control in the age range of 20-40 who are healthy according to the analysis of the WHO. Those with smoking and alcohol assumption, varicoceles, systemic diseases, cryptorchidism and external genitalia abnormalities, and hormonal level abnormalities were excluded from this study. Partner’s age of patients who are oligoasthenoteratozoospermic was between 25-35 yr, and there were no female disorder factors such as ovulation disorders, tubal factor, endometrial disease, or endocrine abnormalities. Also, participants with a history of alcohol abuse, drug use, or smoking were excluded from the study.
2.2. Sperm analysis
Following 4-5 days of sexual abstinence, the ejaculates were poured into sterile containers, and more than 1 sample was prepared at an interval of 2 wk from each other. The semen examination was based on the WHO instructions (16).
Computer-assisted sperm analysis system (LABOMED, SDC313B, Germany) was used to assess sperm motility (progressive, non-progressive or quiescent). Papanicolaou staining was also used to evaluate the normal morphology of sperms. To check the number of sperm, after appropriate dilution, 100 sperm per slide were measured by Neubauer chamber through a Nikon Eclipse TE2000 microscope.
2.3. BAX and BCL-2 gene expressions
Total ribonucleic acid (RNA) was extracted from all sperm samples by total RNA extraction as well as first strand cDNA generation using QuantiTect®, and RNeasy Micro kits (Qiagen, Europe). The NanoDrop spectrophotometer was applied to determine RNA concentration, which was adjusted to 1000 ng/μl. The RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific Inc. China) was applied to synthesize cDNA on the same day as guided. The reverse transcription was done in 20 μl reactions (42oC/60 min and then 70oC/5 min) for inactivation of the reverse transcriptase and its product was directly applied in quantitative polymerase chain reaction (PCR) in an independent step to amplify the targets.
2.4. Real-time PCR
Quantitative real-time PCR was used to evaluate BCL-2 and BAX relative transcript levels using specified primers (Table I). The internal control was the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. The QuantiTect SYBR Green RT-PCR kit (Lot No.: 1201416; Applied Biosystems, UK) was used for PCR run on an ABI 7500 RT-PCR system (Applied Biosystems) considering stage 1 (95oC/5 min) and stage 2 (95oC/20 s, 58°C/30 s, 72°C/20 s) for 40 cycles, followed by a melt curve stage (95°C/15 s, 58°C/1 min, and 95°C/15 s). The specimens were run 2 times to decrease the sampling error, and the duplicates’ mean value was applied for the next calculations. Specimens minus the reverse transcriptase and no template control were run along with the original specimens. The product length, 2% agarose gel, and a melting curve analysis verified amplicon size and specificity. The ratio = (E target) ΔCt target (control sample)/(E ref) ΔCt ref (control-sample) formula was used to calculate the relative transcript levels. The target gene relative transcript levels were calculated using the RT PCR efficiency (E) as well as the threshold cycle difference related to an unfamiliar specimen compared to the control (Ct control-sample). CDNA dilution curves were constructed for each gene to determine the individual real-time PCR efficiencies (E = 10 [- 1/slope]).
2.5. Sperm DNA fragmentation
The terminal deoxynucleotidyl transferase dUTP nick end labeling assay evaluated sperm DNA fragmentation by the in-situ cell death detection kit (Roche, Germany) (17). A fluorescence microscope (BX51, Olympus, Japan) was used to assess 500 sperms for each slide at ×100 magnification. Sperm characterized by redheads had intact DNA, whereas sperm with green heads had fragmented DNA.
2.6. ICSI process, and embryo quality
A long method of ovulation induction was performed in all the cases' wives. 250 gr of recombinant human chorionic gonadotropin (OVIDREL, hCG, Merck-Serono) was injected to trigger follicular development, followed by the extraction of oocytes (18). Which were obtained from follicular fluid quickly after follicle aspiration. Oocytes with the first polar body morphology were used for ICSI. Then, 24 hr after oocyte retrieval, an injection of normal sperm was done to inseminate the oocyte. Then 16-18 hr after ICSI, 2 pronuclei observed by an Olympus inverted microscope using a Hoffmann modulation contrast system (×400) proved fertilization. Embryo quality and cleavage rate were assessed 3 days following ICSI, according to a scoring system (19): grade I, no fragmentation and symmetric blastomeres; grade II, uneven blastomeres and less than 30% fragmentation; and grade III, uneven blastomeres and more than 30% fragmentation. The resulting embryos were cryopreserved. 2 freeze-thaw embryo transfer ICSI cycles were performed in all cases.

2.7. Ethical considerations
The Ethics Committee for Human Subjects Research of Qom Azad University, Qom, Iran accepted this study (Code: IR.IAU.QOM.REC.1401.072). Patients enrolled in the study, and the proband's family gave their written approval.
2.8. Statistical analysis
We used the Statistical Package for Social Sciences (SPSS) version 16 to analyze the data. In addition, variables with a continuous normal distribution were examined using the independent sample t test, while the Mann-Whitney U test was used for data that were not normally distributed. P < 0.05 was considered statistically significant. Findings are shown as mean ± SEM.
3. Results
3.1. Clinical and demographic characteristics
No significant differences were observed in the male age (p = 0.40), female age) p = 0.49) duration of marriage (p = 0.96), or duration of infertility (p = 0.95). Also, no significant differences were observed in weight, height, or BMI between the 2 groups as presented in table II.
3.2. Sperm quality
Table III indicate that sperm concentration (p = 0.001), total motility (p = 0.001), progressive motility (p = 0.004), and normal morphology (p = 0.001), between oligoasthenoteratozoospermia, and normozoospermic fertile subjects were significantly different. Also, data were significantly different for DNA fragmentation of sperm between oligoasthenoteratozoospermic, and normozoospermic subjects (p = 0.013). No significant differences were observed in the semen volume between the 2 groups.
3.3. BAX and BCL-2 gene expression
Figure 1A, B presents the evaluation of apoptosis-related genes showed that mRNA expression of BAX significantly increased, compared to fertile patients (p = 0.012). However, mRNA expression of BCL-2 significantly decreased in oligoasthenoteratozoospermic as compared with normozoospermic men (p = 0.032). As expected, the BAX/BCL-2 ratio was significantly higher in oligoasthenoteratozoospermic compared to the normozoospermic group (p = 0.033).
3.4. Correlation of BAX, BCL-2 with sperm parameters
The correlations analysis between BAX and BCL-2 gene expression with sperm parameters and DNA fragmentation (Table IV) shows that there is a significant negative correlation with sperm motility (p = 0.001), sperm count (p = 0.001), and sperm normal forms with BAX gene expression (p = 0.001), and whether, there is any significant positive correlation with sperm DNA fragmentation (p = 0.01; Table II). Seminal BCL-2 demonstrated a significant correlation with sperm count (p = 0.001), sperm motility (p = 0.001), and sperm morphology (p = 0.014) while a negative correlation was observed with sperm DNA fragmentation (p = 0.010; Table IV).
3.5. Fertilization rate and embryo quality after ICSI
As shown in table V, oligoasthenoteratozoospermic men had a considerably lower proportion of fertilization rate and good-quality embryos at the cleavage stage than the normozoospermic subjects. A significant upper fertilization rate was observed in fertile men compared to oligoasthenoteratozoospermic (p = 0.030). Also, the cleavage rate was significantly higher in fertile men compared to oligoasthenoteratozoospermic men (p = 0.024). The mean percentages of embryo quality scores were substantially different between groups (A, B) (p = 0.001) and (p = 0.015), respectively. A significant difference in embryo grade C was observed between normozoospermic and oligoasthenoteratozoospermic men (p = 0.012).
3.6. Correlation of BAX, BCL-2 with fertilization rate and embryo quality
Sperm BAX gene expression had a significantly negative correlation with fertilization rate (p = 0.036), cleavage rate (p = 0.046), embryo quality (grade A) (p = 0.044) embryo quality (grade B) (p = 0.05), and embryo quality (grade C) (p = 0.043). Whether, sperm BCL-2 gene expression demonstrated a significant positive correlation with fertilization rate (p = 0.041), cleavage rate (p = 0.048), and embryo quality (grade A) (p = 0.041), embryo quality (grade B) (p = 0.034), and embryo quality (grade C) (p = 0.011; Table VI).






4. Discussion
The main aim of this study was to investigate the increase of apoptosis in ART results based on sperm quality and DNA damage level. Our study showed that the mRNA expression of BAX were significantly increased in the oligoasthenoteratozoospermic group compared to normozoospermic individuals. Whereas, BCL-2 mRNA expression was significantly decreased in oligoasthenoteratozoospermic men compared to normozoospermic men. Also, the BAX/BCL-2 ratio of sperm in infertile men increased significantly compared to fertile men. Sperm BAX gene expression had a significant and negative correlation with sperm motility, concentration and normal shapes, while sperm BCL-2 gene expression had a positive and significant correlation with these parameters. Also, we detected a significant correlation between BAX and BCL-2 expression with DNA denaturation.
The higher expression of BAX compared to BCL-2 leads to forming pores associated with apoptosis and possibly triggers apoptotic reaction cascades (20). The cascade was initiated by the release of mitochondrial cytochrome-c into the cytosol and interacted with Apaf-1 to generate apoptosomes to recruit and activate procaspase-9 (21). Caspase effectors could destroy several substrates in the cell leading to morphological alterations in apoptosis (22). Activation of caspase-9 in ejaculated sperm occurs in the post-acrosomal stage in mature sperm (23), and the midpiece, in which residual cytoplasm and mitochondria can be found (24). Kamal et al. reported a negative association between seminal clusterin (involved in the cellular debris clearance and apoptosis) gene expression and sperm motility, concentration, and acrosin activity, linearity index and velocity, and a positive association with sperm DNA fragmentation and abnormal forms (25). Roshdy and Mostafa reported an association between survivin protein (an apoptosis inhibitor) in the seminal plasma of fertile and infertile males and sperm motility processes and spermatogenesis (26). These results are consistent with our findings regarding the interdependence between the expression of genes involved in apoptosis (BAX, BCL-2), and decreased sperm quality.
The current study describes the correlation between apoptosis markers such as BAX, and BCL-2 mRNA expression with fertilization rate, cleavage rate, and embryo quality in oligoasthenoteratozoospermic men under ICSI processing. Based on this study, changes in the expression of BAX, and BCL-2 genes indirectly through reducing the sperm quality and increasing the sperm DNA damage level might compromise the progression of embryo development, resulting in arrested embryos. Sperm DNA integrity is of great importance as the genetic material quality in a sperm population is important for the subsequent and embryonic development of children, successful fertilization, and the reproductive potential in males. Apoptosis is necessary for male gametes from the gonadal anlagen appearance in the embryo to fertilization. It can maintain the proper ratio between the Sertoli cells and germ cells during prenatal development (27).
Apoptosis removes damaged cells from the testicles in the samples of adults. Mature spermatozoa experience apoptosis and phagocytosis in the reproductive tract of females to avoid the inflammatory reaction due to the dead gamete destruction (28). The apoptosis impairment leads to developmental abnormalities in infertility, gametes of males, and oocyte fertilization by sperm with DNA damage leading to ART failure (29). Thus, apoptosis is an important cause of sperm DNA fragmentation, leading to poor embryo quality at day 3, slower rate of cleavage, poor blastocyst development, and implantation for the widespread use of assisted reproductive technologies (30). On the other hand, apoptosis shows a significant correlation with sperm morphology. Sperm head impairments are linked to the last phases of embryonic development, sperm midpiece impairments to intermediate cleaving embryos, and tail impairments to the initial embryonic development phases (31). In fact, in this study, we evaluated the sperm BCL-2 and BAX can affect the quality of the embryo through the effect on sperm morphology. Further studies are needed to confirm this hypothesis.
5. Conclusion
Moreover, according to the results, there is a relationship between apoptosis gene transcript levels sperm quality, and clinical outcomes in the oligoasthenoteratozoospermic group. However, more studies with a larger series are needed to be sure that these genes can influence reproductive outcome.
Data availability
The primary data for this study is available from the authors upon direct request.
Author contributions
R.J., F.B., and M.Kh.M., were involved in the conception, design, statistical analysis, and drafting of the manuscript. All authors have approved the final version for submission.
Acknowledgments
The authors would like to extend their gratitude Infertility Research Center, the Academic Center for Education, Culture, and Research (ACECR), Qom, Iran. Artificial intelligence has not been used in any way (translation, revision, grammar check, etc.) in this study.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 2016; 1863: 2977-2992. [DOI:10.1016/j.bbamcr.2016.09.012]
2. Xu Zh, Hong Q, Ge K, Shi X, Wang X, Deng J, et al. Random lasing from label-free living cells for rapid cytometry of apoptosis. Nano Lett 2022; 22: 172-178. [DOI:10.1021/acs.nanolett.1c03580]
3. Ojo OA, Nwafor-Ezeh PI, Rotimi DE, Iyobhebhe M, Ogunlakin AD, Ojo AB. Apoptosis, inflammation, and oxidative stress in infertility: A mini review. Toxicol Rep 2023; 10: 448-462. [DOI:10.1016/j.toxrep.2023.04.006]
4. Gomes MT, Palasiewicz K, Gadiyar V, Lahey K, Calianese D, Birge RB, et al. Phosphatidylserine externalization by apoptotic cells is dispensable for specific recognition leading to innate apoptotic immune responses. J Biol Chem 2022; 298: 102034. [DOI:10.1016/j.jbc.2022.102034]
5. Pujianto DA, Oktarina M, Sharaswati IAS, Yulhasri. Hydrogen peroxide has adverse effects on human sperm quality parameters, induces apoptosis, and reduces survival. J Hum Reprod Sci 2021; 14: 121-128. [DOI:10.4103/jhrs.jhrs_241_20]
6. Rotgers E, Nurmio M, Pietilä E, Cisneros‐Montalvo S, Toppari J. E2F1 controls germ cell apoptosis during the first wave of spermatogenesis. Andrology 2015; 3: 1000-1014. [DOI:10.1111/andr.12090]
7. Afsari M, Talebi AR, Tafti FD, Makki M, Fesahat F. Differential apoptotic gene expression in the male partners of infertile couples with normal and abnormal sperm parameters. JBRA Assist Reprod 2022; 26: 606-611. [DOI:10.5935/1518-0557.20220004]
8. Ion B, Nicolae R, Vladimir B, Nicolae F, Alexandru D, Irina B, et al. The biological role of apoptosis in spermatogenesis: A review. Oltenia-Studii Si Comunicari Stiintele Naturii 2020; 36: 137-145.
9. Nguyen DH, Soygur B, Peng S-P, Malki S, Hu G, Laird DJ. Apoptosis in the fetal testis eliminates developmentally defective germ cell clones. Nat Cell Biol 2020; 22: 1423-1435. [DOI:10.1038/s41556-020-00603-8]
10. Li R, Wu S, Kaur M, Zong S, Abeydeera SA, Bao T, et al. Bcl2-associated X (BAX) knockout in mice resulted in persistence of neonatal testicular germ cells (Gonocytes). Sex Dev 2019; 13: 278-285. [DOI:10.1159/000508567]
11. Dome A, Dymova M, Richter V, Stepanov G. Post-transcriptional modifications of RNA as regulators of apoptosis in glioblastoma. Int J Mol Sci 2022; 23: 9272. [DOI:10.3390/ijms23169272]
12. Koohshoori YS, Marandi SM, Kargarfard M, Vaseghi G, Moshtaghian SJ. The effect of 4 weeks aerobic exercise training with detraining courses in various prevention phases on BCl-2 and BAX genes expression and proteins. Int J Prevent Med 2023; 14: 79. [DOI:10.4103/ijpvm.ijpvm_15_21]
13. Zheng D, Nguyen QN, Li R, Dang VQ. Is intracytoplasmic sperm injection the solution for all in unexplained infertility? Semin Reprod Med 2020; 38: 36-47. [DOI:10.1055/s-0040-1719085]
14. Sauerbrun-Cutler M-T, Huber WJ, Has P, Shen C, Hackett R, Alvero R, et al. Is intracytoplasmic sperm (ICSI) better than traditional in vitro fertilization (IVF): Confirmation of higher blastocyst rates per oocyte using a split insemination design. J Assist Reprod Genet 2020; 37: 1661-1667. [DOI:10.1007/s10815-020-01819-1]
15. Ammar O, Tekeya O, Hannachi I, Sallem A, Haouas Z, Mehdi M. Increased sperm DNA fragmentation in infertile men with varicocele: Relationship with apoptosis, seminal oxidative stress, and spermatic parameters. Reprod Sci 2021; 28: 909-919. [DOI:10.1007/s43032-020-00311-6]
16. World Health Organization. World health statistics 2010. Switzerland: World Health Organization Press; 2010.
17. Cankut S, Dinc T, Cincik M, Ozturk G, Selam B. Evaluation of sperm DNA fragmentation via halosperm technique and TUNEL assay before and after cryopreservation. Reprod Sci 2019; 26: 1575-1581. [DOI:10.1177/1933719119828096]
18. Stimulation EGGoO, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: Ovarian stimulation for IVF/ICSI. Hum Reprod Open 2020; 2020: hoaa009. [DOI:10.1093/hropen/hoaa009]
19. Bori L, Meseguer F, Valera MA, Galan A, Remohi J, Meseguer M. The higher the score, the better the clinical outcome: Retrospective evaluation of automatic embryo grading as a support tool for embryo selection in IVF laboratories. Hum Reprod 2022; 37: 1148-1160. [DOI:10.1093/humrep/deac066]
20. Czabotar PE, Garcia-Saez AJ. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat Rev Mol Cell Biol 2023; 24: 732-748. [DOI:10.1038/s41580-023-00629-4]
21. Wolf P, Schoeniger A, Edlich F. Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane. Biochim Biophys Acta Mol Cell Res 2022; 1869: 119317. [DOI:10.1016/j.bbamcr.2022.119317]
22. Boice A, Bouchier-Hayes L. Targeting apoptotic caspases in cancer. Biochim Biophys Acta Mol Cell Res 2020; 1867: 118688. [DOI:10.1016/j.bbamcr.2020.118688]
23. Silva JV, Freitas MJ, Correia BR, Korrodi-Gregório L, Patrício A, Pelech S, et al. Profiling signaling proteins in human spermatozoa: Biomarker identification for sperm quality evaluation. Fertil Steril 2015; 104: 845-856. [DOI:10.1016/j.fertnstert.2015.06.039]
24. Nguyen HTT, Dang HNT, Nguyen TTT, Van Nguyen T, Dang TC, Nguyen QHV, et al. Correlations between abnormalities of morphological details and DNA fragmentation in human sperm. Clin Exp Reprod Med 2022; 49: 40-48. [DOI:10.5653/cerm.2021.04777]
25. Kamal DAM, Salamt N, Yusuf ANM, Kashim M, Mokhtar MH. Potential health benefits of curcumin on female reproductive disorders: A review. Nutrients 2021; 13: 3126. [DOI:10.3390/nu13093126]
26. Mostafa T, Rashed L, Nabil N, Amin R. Seminal BAX and BCL2 gene and protein expressions in infertile men with varicocele. Urology 2014; 84: 590-595. [DOI:10.1016/j.urology.2014.05.016]
27. Nakidkina A, Kuzmina T. Apoptosis in spermatozoa and its role in deteriorating semen quality. Russian J Dev Biol 2019; 50: 165-172. [DOI:10.1134/S1062360419040064]
28. Nixon B, Schjenken JE, Burke ND, Skerrett-Byrne DA, Hart HM, De Iuliis GN, et al. New horizons in human sperm selection for assisted reproduction. Front Endocrinol 2023; 14: 1145533. [DOI:10.3389/fendo.2023.1145533]
29. Serafini S, O'Flaherty C. Redox regulation to modulate phosphorylation events in human spermatozoa. Antioxid Redox Signal 2022; 37: 437-450. [DOI:10.1089/ars.2021.0117]
30. Chi H-J, Kim S-G, Kim Y-Y, Park J-Y, Yoo C-S, Park I-H, et al. ICSI significantly improved the pregnancy rate of patients with a high sperm DNA fragmentation index. Clin Exp Reprod Med 2017; 44: 132-140. [DOI:10.5653/cerm.2017.44.3.132]
31. Nikolova S, Parvanov D, Georgieva V, Ivanova I, Ganeva R, Stamenov G. Impact of sperm characteristics on time‐lapse embryo morphokinetic parameters and clinical outcome of conventional in vitro fertilization. Andrology 2020; 8: 1107-1116. [DOI:10.1111/andr.12781]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







