Sat, Feb 21, 2026
[Archive]
Volume 22, Issue 6 (June 2024)
IJRM 2024, 22(6): 451-462 |
Back to browse issues page
Ethics code: IR.ZAUMS.REC.1401.073
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Edalatian Kharrazi A, Forghani F, Jahantigh D, Ghazaey Zidanloo S, Rezaei M, Taheri M. Positive correlation between Bax and Bcl-2 gene polymorphisms with the risk of endometriosis: A case-control study. IJRM 2024; 22 (6) :451-462
URL: http://ijrm.ir/article-1-3246-en.html
URL: http://ijrm.ir/article-1-3246-en.html
Arefe Edalatian Kharrazi1 

 , Forough Forghani *2
, Forough Forghani *2 

 , Danial Jahantigh3
, Danial Jahantigh3 

 , Saeedeh Ghazaey Zidanloo4
, Saeedeh Ghazaey Zidanloo4 

 , Mahnaz Rezaei5
, Mahnaz Rezaei5 

 , Mohsen Taheri6
, Mohsen Taheri6 




 , Forough Forghani *2
, Forough Forghani *2 

 , Danial Jahantigh3
, Danial Jahantigh3 

 , Saeedeh Ghazaey Zidanloo4
, Saeedeh Ghazaey Zidanloo4 

 , Mahnaz Rezaei5
, Mahnaz Rezaei5 

 , Mohsen Taheri6
, Mohsen Taheri6 


1- Department of Obstetrics and Gynecology, Zahedan University of Medical Sciences, Zahedan, Iran.
2- Department of Obstetrics and Gynecology, Zahedan University of Medical Sciences, Zahedan, Iran. ,forghani_f@yahoo.com
3- Department of Biology, Faculty of Sciences, University of Sistan and Baluchestan, Zahedan, Iran. & Genetics of Non-Communicable Disease Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
4- Department of Cell and Molecular Biology, Kosar University of Bojnord, Bojnord, Iran.
5- Department of Clinical Biochemistry, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
6- Genetics of Non-Communicable Disease Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
2- Department of Obstetrics and Gynecology, Zahedan University of Medical Sciences, Zahedan, Iran. ,
3- Department of Biology, Faculty of Sciences, University of Sistan and Baluchestan, Zahedan, Iran. & Genetics of Non-Communicable Disease Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
4- Department of Cell and Molecular Biology, Kosar University of Bojnord, Bojnord, Iran.
5- Department of Clinical Biochemistry, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran.
6- Genetics of Non-Communicable Disease Research Center, Zahedan University of Medical Sciences, Zahedan, Iran.
Full-Text [PDF 812 kb]
(892 Downloads)
| Abstract (HTML) (1081 Views)
Full-Text: (233 Views)
- Introduction
Endometriosis is a chronic, benign, gynecological, heritable, condition that is considered by extrauterine growth of endometrial tissue, and it has been shown by mysterious etiology (1). The disease influences 6-10% of women of reproductive age (2) and results in severe and chronic pelvic pain, dyspareunia, dysmenorrhea infertility, and dyspareunia (3). The occurrence of endometriosis is 20-40% in infertile women, showing a harmful impact on the pregnancy rate of in vitro fertilization. Twin and family studies have revealed that genetic factors are related to endometriosis pathogenesis (4). Our knowledge about the etiology and pathophysiology of endometriosis is unclear yet. Nevertheless, the genetic aspects of endometriosis might assist in around half the variation in the susceptibility to the disease.
Genes related to apoptosis have been revealed to be deregulated in endometriosis. Electron microscopic analyses have determined the existence of apoptotic bodies in endometrial tissue through the late-secretory phase with a significant reduction of endometrial cell apoptosis in endometriosis.
It has been shown that spontaneous apoptosis had decreased in the ectopic endometrium in comparison to the eutopic endometrium of endometriosis individuals. Besides, the apoptosis level were reduced in the eutopic endometrium of endometriosis women compared with control group. Therefore, the decrease response of endometrial tissue to the apoptosis pathway might be related to the development of endometriosis (5).
The possible mechanism for the reduction of apoptosis is associated with the well-known regulators of apoptosis, anti-apoptotic B-cell lymphoma-2 (Bcl-2) protein, which is bound to pro-apoptotic Bcl-2 associated X protein (Bax). Bax is a death-promoting member of the Bcl-2 family and induces apoptosis via hetero-dimerization with Bcl-2 protein. The apoptosis rate is determined by the relative level of expression of these 2 proteins, which interact physically with each other (6). The Bax gene is located on chromosome 19q13.3 with a promoter with four p53 protein binding sites and 6 exons (7). The Bcl-2 gene is another member of the Bcl-2 family, which unlike the Bax protein inhibits apoptosis. It is mapped on chromosome 18q21.3, with 2 promoters, 3 exons, and 2 introns. Bcl-2 protein is situated on the mitochondrial outer membrane and prevents Bax from changing the membrane permeability (8).
Previous studies have identified novel single-nucleotide polymorphisms (SNP) in Bax and Bcl-2 genes which are crucial factors in cell-cycle control and cell survival. A promoter SNP in the Bcl-2 gene, the -938C˃A genotype (rs2279115), is identified to be associated with high Bcl-2 gene expression and is related to some cancer developments (9-12). Another SNP mapped on the 5′ untranslated region of the Bax promoter, -248G˃A (rs4645878), has been defined to be linked to both downregulation of Bax protein (12) and different susceptibility to numerous cancers (13-17). To our knowledge, there is no report examining the association of Bax -248G˃A and Bcl-2 -938C˃A SNPs with endometriosis. This work aimed to study the polymorphic variants of these proapoptotic and antiapoptotic genes, Bax -248G˃A, rs4645878 and Bcl-2 -938C˃A, rs2279115 SNPs, with endometriosis susceptibility in a sample of the Iranian population.
Genes related to apoptosis have been revealed to be deregulated in endometriosis. Electron microscopic analyses have determined the existence of apoptotic bodies in endometrial tissue through the late-secretory phase with a significant reduction of endometrial cell apoptosis in endometriosis.
It has been shown that spontaneous apoptosis had decreased in the ectopic endometrium in comparison to the eutopic endometrium of endometriosis individuals. Besides, the apoptosis level were reduced in the eutopic endometrium of endometriosis women compared with control group. Therefore, the decrease response of endometrial tissue to the apoptosis pathway might be related to the development of endometriosis (5).
The possible mechanism for the reduction of apoptosis is associated with the well-known regulators of apoptosis, anti-apoptotic B-cell lymphoma-2 (Bcl-2) protein, which is bound to pro-apoptotic Bcl-2 associated X protein (Bax). Bax is a death-promoting member of the Bcl-2 family and induces apoptosis via hetero-dimerization with Bcl-2 protein. The apoptosis rate is determined by the relative level of expression of these 2 proteins, which interact physically with each other (6). The Bax gene is located on chromosome 19q13.3 with a promoter with four p53 protein binding sites and 6 exons (7). The Bcl-2 gene is another member of the Bcl-2 family, which unlike the Bax protein inhibits apoptosis. It is mapped on chromosome 18q21.3, with 2 promoters, 3 exons, and 2 introns. Bcl-2 protein is situated on the mitochondrial outer membrane and prevents Bax from changing the membrane permeability (8).
Previous studies have identified novel single-nucleotide polymorphisms (SNP) in Bax and Bcl-2 genes which are crucial factors in cell-cycle control and cell survival. A promoter SNP in the Bcl-2 gene, the -938C˃A genotype (rs2279115), is identified to be associated with high Bcl-2 gene expression and is related to some cancer developments (9-12). Another SNP mapped on the 5′ untranslated region of the Bax promoter, -248G˃A (rs4645878), has been defined to be linked to both downregulation of Bax protein (12) and different susceptibility to numerous cancers (13-17). To our knowledge, there is no report examining the association of Bax -248G˃A and Bcl-2 -938C˃A SNPs with endometriosis. This work aimed to study the polymorphic variants of these proapoptotic and antiapoptotic genes, Bax -248G˃A, rs4645878 and Bcl-2 -938C˃A, rs2279115 SNPs, with endometriosis susceptibility in a sample of the Iranian population.
- Materials and Methods
2.1. Study population
In this case-control association study, from May 2022 to February 2023, 252 women were enrolled (127 case and 125 controls) from the Department of Obstetrics and Gynecology of Ali-ibn-Abi Taleb Educational hospital, Zahedan, Iran. The control group was age and sex-matched healthy women of the same ethnic background with regular menstrual cycles and had not received any hormone therapy in the past 6 months. The medical records, including laparoscopy, laparotomy for diagnosis, therapy of endometriosis, and other complementary diagnostic tests were collected by the clinicians and pathologists. The diagnosis of endometriosis was histologically inveterate via the presence of an endometrial gland and/or stroma in the lesions. The characteristics of the endometriosis group and controls are shown in table I. The mean ages of the endometriosis and control groups were 30.14 ± 7.22 and 31.28 ± 6.87 yr, respectively. The participant in the healthy group had no previous medical record of endometriosis. Individuals were excluded if they withdrew consent, non-Iranian citizenship, and/or previous medical history of chronic pelvic pain, dysmenorrhea, or dyspareunia.
In this case-control association study, from May 2022 to February 2023, 252 women were enrolled (127 case and 125 controls) from the Department of Obstetrics and Gynecology of Ali-ibn-Abi Taleb Educational hospital, Zahedan, Iran. The control group was age and sex-matched healthy women of the same ethnic background with regular menstrual cycles and had not received any hormone therapy in the past 6 months. The medical records, including laparoscopy, laparotomy for diagnosis, therapy of endometriosis, and other complementary diagnostic tests were collected by the clinicians and pathologists. The diagnosis of endometriosis was histologically inveterate via the presence of an endometrial gland and/or stroma in the lesions. The characteristics of the endometriosis group and controls are shown in table I. The mean ages of the endometriosis and control groups were 30.14 ± 7.22 and 31.28 ± 6.87 yr, respectively. The participant in the healthy group had no previous medical record of endometriosis. Individuals were excluded if they withdrew consent, non-Iranian citizenship, and/or previous medical history of chronic pelvic pain, dysmenorrhea, or dyspareunia.
2.2. Extraction of DNA and genotyping analysis
The whole blood of the subjects, case and controls, were collected in ethylenediaminetetraacetic acid-containing tubes. After DNA extraction using a commercial kit (DynaBio, Takapoozist, Iran) as followed by manufacturer's instructions, the all of extracted DNA was stored preoperatively in microtubes at -20oC until analyzed. The allelic discrimination of the Bax -248G˃A and Bcl-2 -938C˃A gene SNPs, rs4645878 and rs2279115, respectively, were analyzed by the polymerase chain reaction (PCR)-restriction fragment length polymorphism. The PCR primers of 2 SNPs were synthesized The PCR program detail has described in the our previous study (18). The MspI and BccI restriction enzymes were utilized to digest the Bax and Bcl-2 PCR products, as followed by separation via 3% agarose gel electrophoresis. Each assay was run with negative controls. A random 20% of the samples were genotyped again to obtain accurate and reproducible results. The PCR products are shown in figures 1 and 2.
2.3. Sample size
The sample size was calculated by online version of sample size calculator server (https://clincalc.com/stats/SampleSize.aspx), where P1 was 3%, representing the frequency of the mutant allele in control, P2 was 14%, which signifies the frequency of the mutant allele in case, with study power set to 80% and 95% of confidence intervals with the obtained critical value of < 2.0. P1 and P2 were determined based on the previously reported percentages (19). The minimum sample size was adjusted for a total of 200 subjects.


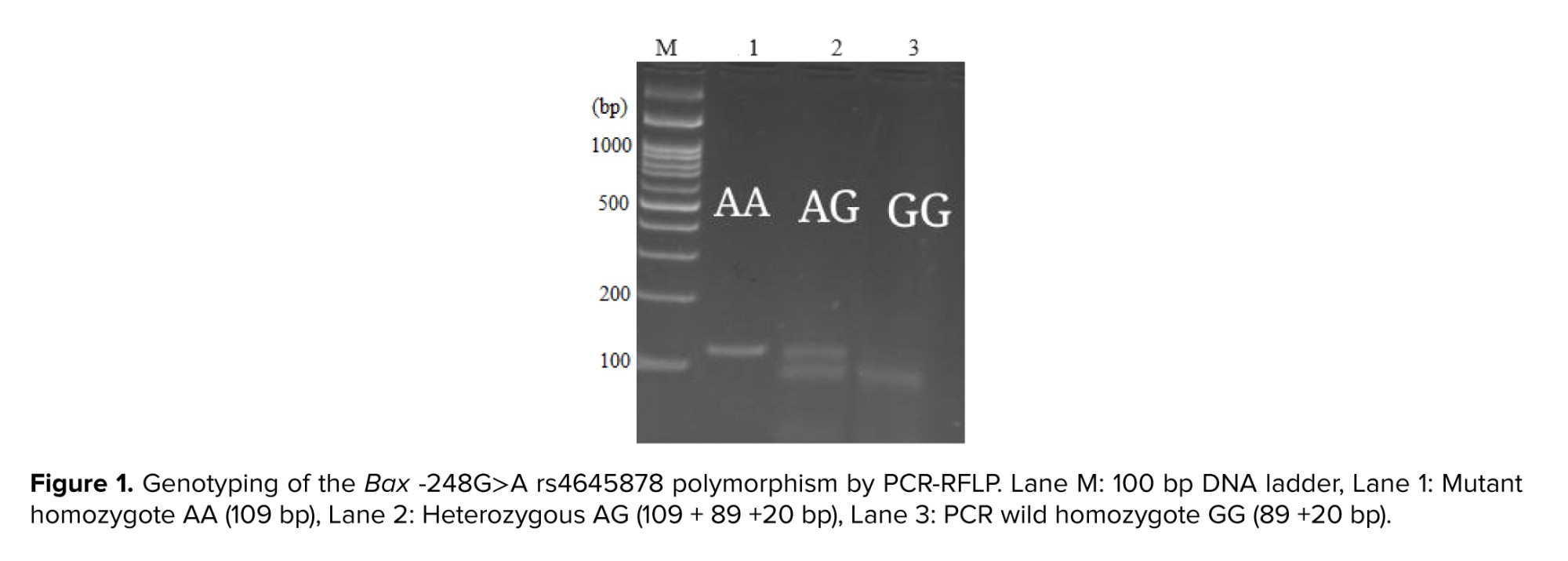
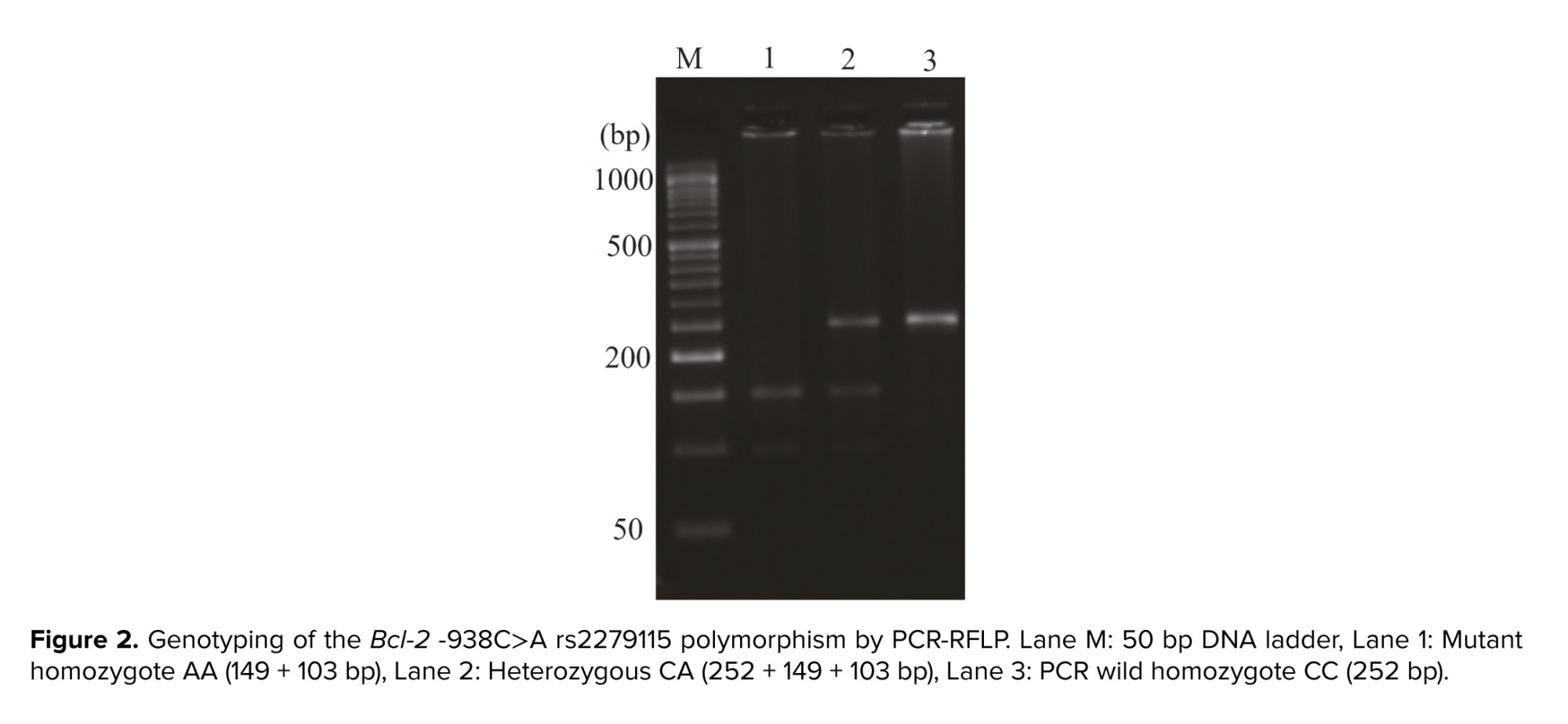
The relationship between SNPs and the development of diseases has long been accepted. Recent improvements in molecular biology techniques have resulted in attention to SNPs and their impact on the risk assessment and development of diseases (20). Female fertility is strongly affected by genetic causes, and several reports have intended to recognize important genes in diseases that influence female fertility such as recurrent pregnancy loss, pre-eclampsia, fibroids, polycystic ovary syndrome, endometriosis, option rates (21), with a strong genetic basis (22). Apoptosis as a form of programmed cell death is very important to women's reproduction according to its involvement in the turnover of endometrial cells, remodeling of the uterine spiral arteries, embryonic development, and immunologic tolerance of maternal placental (23). The rate of apoptosis is reduced in the endometrial cells of women with endometriosis (24). Apoptosis is triggered by 2 different sources, intrinsic and extrinsic pathways, which ultimately lead to irreversible cell death (25).
Bcl-2 has 2 promoters with different functions in the regulation of its expression, that is, the P2 promoter is mapped on the translation start site and performs as a negative regulator of the P1 promoter (26). The common SNP in the Bcl-2 gene was -938C>A (rs2279115) in P2 and its mutant allele displayed a significant increase in Bcl-2 promoter activity. High Bcl-2 over-expression saves cells from programmed death via enhancement of the stability of the mitochondrial outer membrane; therefore, stopping the intrinsic apoptotic pathway (27). The different Bcl-2 gene expression, in the eutopic endometrium of women with endometriosis, caused reduced apoptotic cells and subsequently resulted in abnormal endometrial cells survival in the ectopic site (28).
The expression of Bax, as a pro-apoptotic gene in the Bcl-2 family, with a pro-apoptotic role in the mitochondrial pathway, has a significant impact on the release of apoptosis-related proteins. Although some reports revealed that Bax -248G>A was associated with the Bax protein expression (7), the exact effect of the SNP on protein expression is controversial. Related to the A allele, higher Bax mRNA and protein expression were reported to be associated with G alleles (29). Besides, another study revealed it caused lower transcriptional activity for Bax (30). As well as another study proposed that there was no association between the SNP and Bax transcriptional activity (31). Recent meta-analyses proposed that the downregulation of Bax protein is not connected to the poor prognosis for some cancers. Therefore, its polymorphism might not enhance cancer susceptibility and affect the prognosis of the tumors. In addition, Bax -248G>A and Bcl-2 -938C>A SNPs have no direct association with overall cancer susceptibility nevertheless the relationship might have established ethnic specificity, and might be associated with increasing adverse prognosis of some cancers (32). Another study reported an increased susceptibility to leukemia having Bcl-2 -938C>A and Bax -248G>A SNPs that influenced their response to treatment and overall survival. The data specified that there was an association between the mutant allele of Bcl-2 -938C>AC>A and the Bax -248G>A gene and a significantly increased risk of developing leukemia, compared to the wild-type allele (17). There are few studies on the relation of these polymorphisms to fertility-associated diseases. There are only 2 reports that study the effect of Bcl-2 -938C>A C>A and Bax -248G>A on women's fertility-related disorders.
In the first report, it was assessed if these SNPs are related to unexplained recurrent pregnancy loss risk in a Brazilian population (33). They propose that the Bax -248G>A AG genotype is a protective genotype for primary recurrent pregnancy loss and that the Bcl-2 -938 CC and C are risk factors for secondary recurrent pregnancy loss. Another study investigated the probable impact of the placental Bax and Bcl-2 polymorphisms on pre-eclampsia susceptibility and found that there was no relationship between pre-eclampsia and placental Bax rs4645878 and Bcl-2 rs2279115 SNPs (18).
The main limitation of our report is the relatively small sample size of the endometriosis group, which could lead to reduction in the statistical power for identification of relations between the studied SNPs and the disease. Nevertheless, the limitation was the consequence of selection criteria because all endometriosis women who participated in this analysis were confirmed via laparoscopy and were classified based on the endometriosis stage with histologic confirmation. However, another study with larger sample sizes is needed to validate our results.
Data availability
All related data and materials are available from the corresponding authors upon request.
Author contributions
DJ, FF, and MT conceived and designed the study. AEKh and MR did the data curation. SGhZ and DJ conducted the data analysis and drafted the initial manuscript. AEKh, FF, MR, and MT helped with the interpretation of the results and gave critical comments on the manuscript. All authors contributed to the final version of the manuscript.
Acknowledgments
This work is based on the Residency's thesis (code: 10338) at Zahedan University of Medical Sciences, Zahedan, Iran. The authors would like to thank the study participants in this study. No artificial intelligence was used (translation, revision, grammar check, etc.), while preparing this paper.
Conflict of Interest
The authors declare that there is no conflict of interest.
2.3. Sample size
The sample size was calculated by online version of sample size calculator server (https://clincalc.com/stats/SampleSize.aspx), where P1 was 3%, representing the frequency of the mutant allele in control, P2 was 14%, which signifies the frequency of the mutant allele in case, with study power set to 80% and 95% of confidence intervals with the obtained critical value of < 2.0. P1 and P2 were determined based on the previously reported percentages (19). The minimum sample size was adjusted for a total of 200 subjects.




2.4. Ethical considerations
The study protocol was approved by the Ethics Committee of the Zahedan University of Medical Sciences, Zahedan, Iran (Code: IR.ZAUMS.REC.1401.073), and performed according to the guidelines of the Declaration of Helsinki. Informed consent was obtained from all participants. 2.5. Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences, version 21.0 (SPSS Inc, Chicago, IL). Student’s t test was used to detect differences in baseline clinical characteristics between cases and healthy women. Hardy-Weinberg equilibrium in both groups was examined with the Chi-square test to ensure they were representative. Differences in genetic distribution between cases and healthy women were compared using Pearson’s Chi-square test. We calculated OR and 95% CI to reveal the relative risk degree. The p-values < 0.05 were regarded as significant.- Results
3.1. Demographic and clinical characteristics
This study enrolled 125 infertile endometriosis women and 127 healthy controls without any history of endometriosis. The average ages of the case and the control group were not significantly different. A study of endometriosis-associated symptoms and the number of deliveries among the case and the control group revealed significant differences between them. Symptoms such as chronic pelvic pain, dysmenorrhea, and dyspareunia, were more frequent among endometriosis women (p < 0.001). No significant difference was found in body mass index, menstrual cycle length and period length, and spontaneous abortions among case and control groups. According to the data in the table, a significant difference was observed in the number of deliveries between the patients and the control group. Especially in the delivery of one child (p < 0.001). Based on the clinical stages, 58.26% of cases had minimal/mild (I and II) endometriosis and 41.73% of them had moderate/severe (stage III and IV) endometriosis (Table I). 3.2. Frequency distributions of Bax -248 G>A genotypes and alleles among endometriosis and control groups
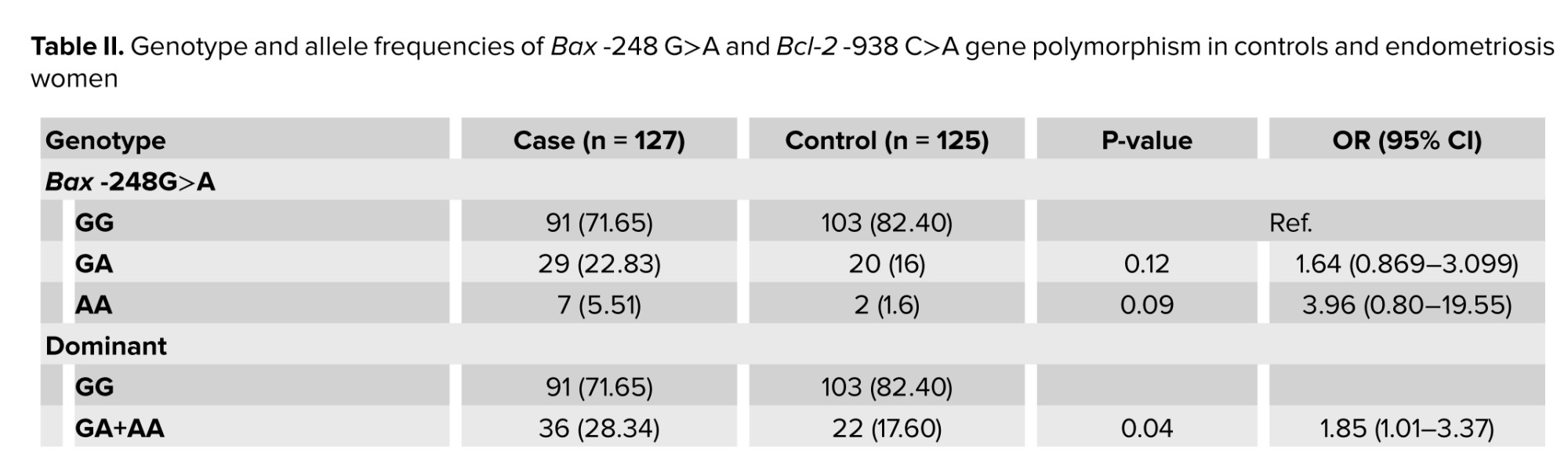
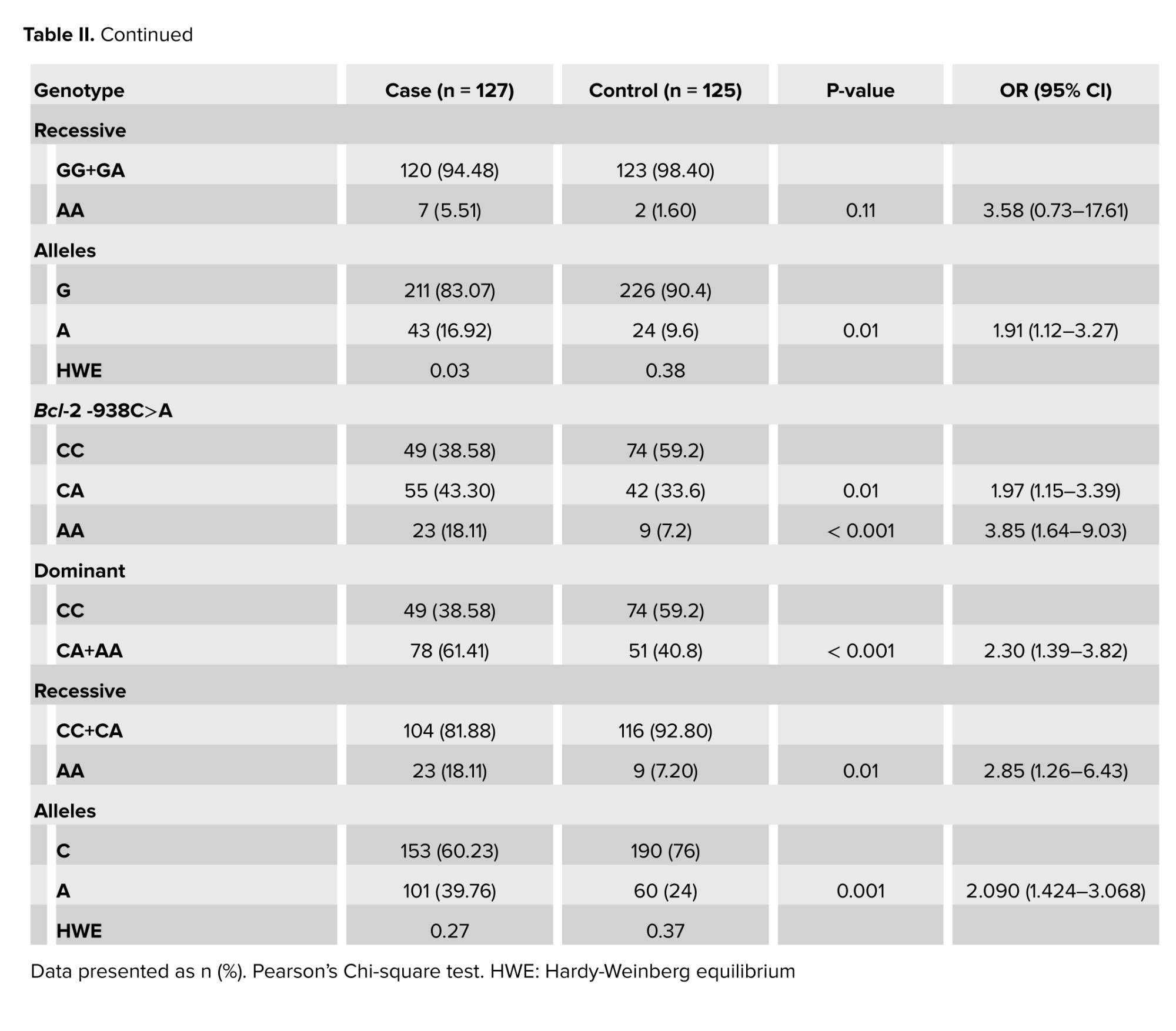
The genotypes and allele distributions of Bcl-2 and Bax SNPs in endometriosis women and controls are listed in table II. The genotype frequencies of this SNP were in agreement with Hardy-Weinberg equilibrium in the case and healthy women (p = 0.03, and 0.38, respectively). No association in genotypes GG, GA, and AA was observed between case and control groups. Under the dominant model (GG vs. GA+AA), we observed a significant association between Bax -248G>A variant and about 2-fold higher risk of endometriosis in contrasted genetic models. Interestingly, investigation of allele frequencies at Bax -248G>A, rs4645878, revealed significant increases of the mutant allele A, approximately 2-fold, in cases compared with the controls (p = 0.01) (Table II).3.3. Frequency distributions of Bcl-2 -938C>A genotypes and alleles among endometriosis and control groups
Our data showed that the frequency of the Bcl-2 -938C>A, rs2279115, homozygous mutant variant AA was significantly higher in cases compared to controls (OR: 3.859; p = 0.001). In addition, under the dominant CA and AA combined vs. CC model, a significantly higher risk of endometriosis was observed. Furthermore, under the recessive model (AA vs. CC+CA), a significant difference of Bcl-2 -938C>A gene polymorphism with approximately 3-fold increase of endometriosis risk was observed. Interestingly, investigation of allele frequencies revealed significant increases, approximately 2-fold, of the mutant allele A in endometriosis women compared with the controls (Table II).3.4. Combination effects of Bax -248G>A and Bcl-2 -938C>A gene polymorphism on endometriosis risk
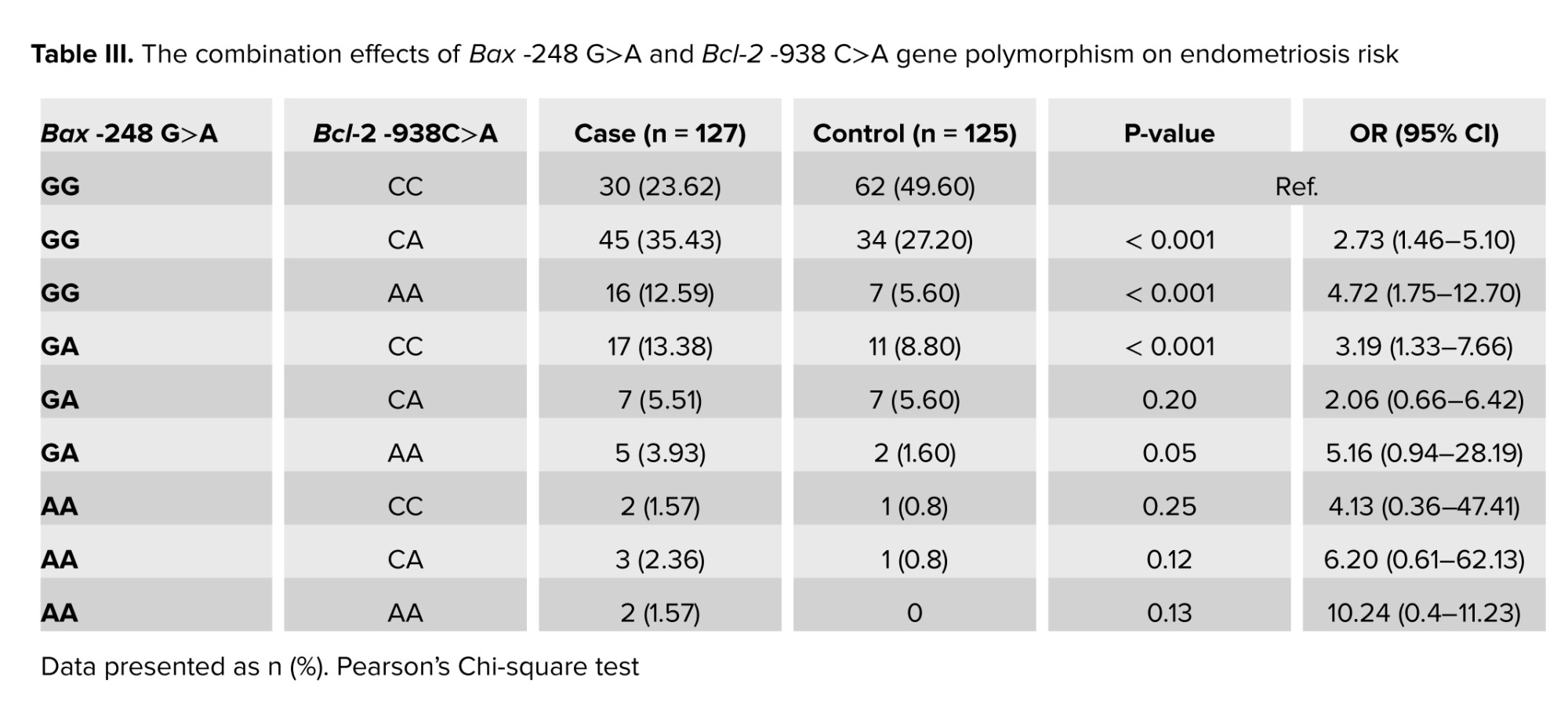
After that we combined these 2 SNPs for association with endometriosis. Our data showed that the Bax -248G>A rs4645878 GG and, the Bcl-2 -938C>A rs2279115 AA were classified as adverse genotype combinations based on association with about 5 times higher risk of endometriosis. In addition, we found that the combination of the rs4645878 GG with, the rs2279115 CC or rs2279115 CA, significantly increased the risk of endometriosis. No statistically significant association was observed between other genotypic combinations of 2 SNPs (Table III).3.5. Association between Bax -248G>A and Bcl-2 -938C>A gene polymorphisms with clinical stages in endometriosis women
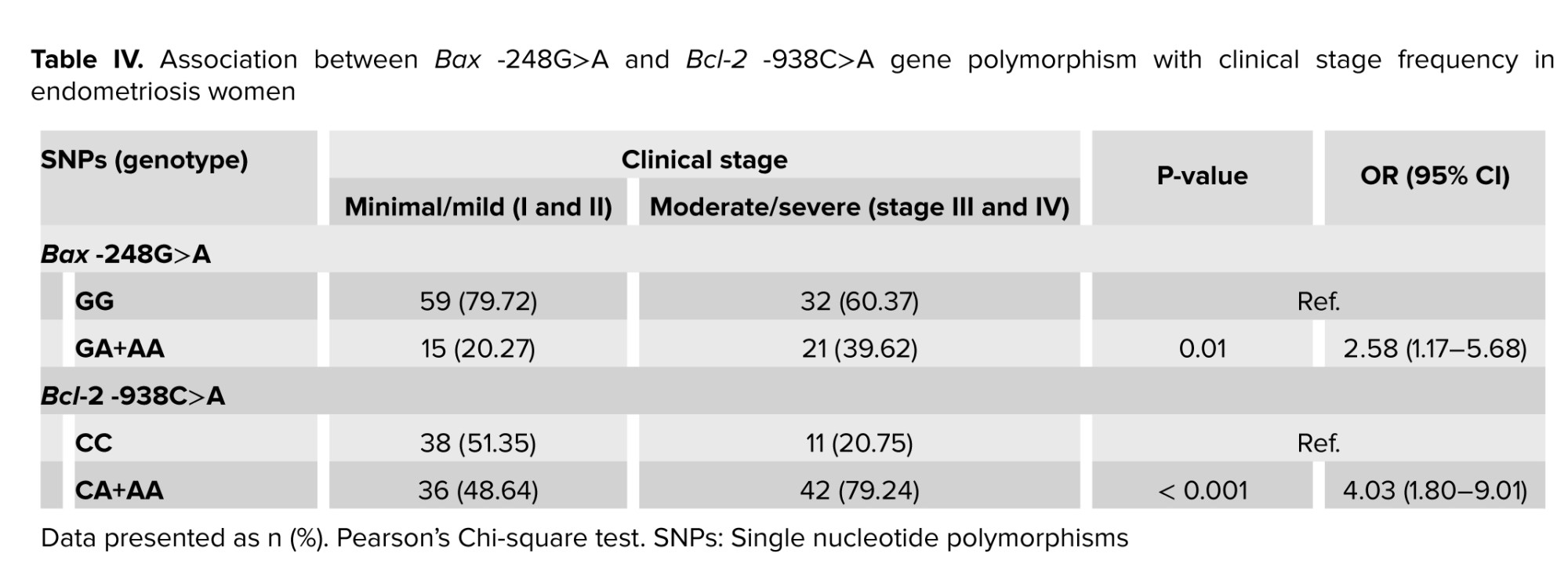
While women with minimal/mild endometriosis and moderate/severe endometriosis were considered distinctly, there were some differences for Bax -248G>A rs4645878 and, Bcl-2 -938C>A rs2279115 SNPs. Considering the Bax -248G>A SNP, there was a significant difference in A allele distribution between minimal/mild (stage I and II) and moderate/severe (stage III and IV) women which revealed that A allele was found about 2.5-fold frequent in moderate/severe endometriosis than minimal/mild one. On the other side, moderate/severe endometriosis risk was about 5 times greater in cases with Bcl-2 -938 C>A CA+AA genotype, which proposed that A allele might be a high-risk allele and could have a special effect on the severity of endometriosis disease (Table IV).



4. Discussion
In the current study, we investigated the association of the SNPs in Bax and Bcl-2 genes, involved in apoptosis, with the risk of endometriosis. The presented study is the first attempt to evaluate the role of Bax -248G/A and Bcl-2 -938C/A SNPs on the susceptibility to the disease. A statistically significant increased endometriosis rate was observed in cases with the Bax -248G>A variant (A allele) and GA+AA dominant genotype. Furthermore, cases with the Bcl-2 -938C>A mutant A allele or AA variant and CA+AA vs. CC model exhibited an elevated risk of endometriosis. We also reported about 3 times or greater risk of endometriosis when a combination of Bcl-2 -938C> A and Bax -248G> A were studied. There was a statistical association between Bcl-2 -938C/A and Bax -248G> A polymorphism and moderate/severe (stage III and IV) disease in these cases. It is worth noting that the percentage of cases studied is limited to stage III/IV endometriosis instead of stage I/II, which indicates that moderate to severe stages of the disease have a higher genetic burden compared to minimal or mild endometriosis.The relationship between SNPs and the development of diseases has long been accepted. Recent improvements in molecular biology techniques have resulted in attention to SNPs and their impact on the risk assessment and development of diseases (20). Female fertility is strongly affected by genetic causes, and several reports have intended to recognize important genes in diseases that influence female fertility such as recurrent pregnancy loss, pre-eclampsia, fibroids, polycystic ovary syndrome, endometriosis, option rates (21), with a strong genetic basis (22). Apoptosis as a form of programmed cell death is very important to women's reproduction according to its involvement in the turnover of endometrial cells, remodeling of the uterine spiral arteries, embryonic development, and immunologic tolerance of maternal placental (23). The rate of apoptosis is reduced in the endometrial cells of women with endometriosis (24). Apoptosis is triggered by 2 different sources, intrinsic and extrinsic pathways, which ultimately lead to irreversible cell death (25).
Bcl-2 has 2 promoters with different functions in the regulation of its expression, that is, the P2 promoter is mapped on the translation start site and performs as a negative regulator of the P1 promoter (26). The common SNP in the Bcl-2 gene was -938C>A (rs2279115) in P2 and its mutant allele displayed a significant increase in Bcl-2 promoter activity. High Bcl-2 over-expression saves cells from programmed death via enhancement of the stability of the mitochondrial outer membrane; therefore, stopping the intrinsic apoptotic pathway (27). The different Bcl-2 gene expression, in the eutopic endometrium of women with endometriosis, caused reduced apoptotic cells and subsequently resulted in abnormal endometrial cells survival in the ectopic site (28).
The expression of Bax, as a pro-apoptotic gene in the Bcl-2 family, with a pro-apoptotic role in the mitochondrial pathway, has a significant impact on the release of apoptosis-related proteins. Although some reports revealed that Bax -248G>A was associated with the Bax protein expression (7), the exact effect of the SNP on protein expression is controversial. Related to the A allele, higher Bax mRNA and protein expression were reported to be associated with G alleles (29). Besides, another study revealed it caused lower transcriptional activity for Bax (30). As well as another study proposed that there was no association between the SNP and Bax transcriptional activity (31). Recent meta-analyses proposed that the downregulation of Bax protein is not connected to the poor prognosis for some cancers. Therefore, its polymorphism might not enhance cancer susceptibility and affect the prognosis of the tumors. In addition, Bax -248G>A and Bcl-2 -938C>A SNPs have no direct association with overall cancer susceptibility nevertheless the relationship might have established ethnic specificity, and might be associated with increasing adverse prognosis of some cancers (32). Another study reported an increased susceptibility to leukemia having Bcl-2 -938C>A and Bax -248G>A SNPs that influenced their response to treatment and overall survival. The data specified that there was an association between the mutant allele of Bcl-2 -938C>AC>A and the Bax -248G>A gene and a significantly increased risk of developing leukemia, compared to the wild-type allele (17). There are few studies on the relation of these polymorphisms to fertility-associated diseases. There are only 2 reports that study the effect of Bcl-2 -938C>A C>A and Bax -248G>A on women's fertility-related disorders.
In the first report, it was assessed if these SNPs are related to unexplained recurrent pregnancy loss risk in a Brazilian population (33). They propose that the Bax -248G>A AG genotype is a protective genotype for primary recurrent pregnancy loss and that the Bcl-2 -938 CC and C are risk factors for secondary recurrent pregnancy loss. Another study investigated the probable impact of the placental Bax and Bcl-2 polymorphisms on pre-eclampsia susceptibility and found that there was no relationship between pre-eclampsia and placental Bax rs4645878 and Bcl-2 rs2279115 SNPs (18).
The main limitation of our report is the relatively small sample size of the endometriosis group, which could lead to reduction in the statistical power for identification of relations between the studied SNPs and the disease. Nevertheless, the limitation was the consequence of selection criteria because all endometriosis women who participated in this analysis were confirmed via laparoscopy and were classified based on the endometriosis stage with histologic confirmation. However, another study with larger sample sizes is needed to validate our results.
5. Conclusion
The results suggest that the Bax -248G˃A, rs4645878 and Bcl-2 -938C˃A, rs2279115 single nucleotide polymorphisms play a significant role in the pathogenesis of endometriosis in Iranian women. Our current study is just the first of this kind for proof-of-principle. Possibly, polymorphic variants of Bax -248G>A and Bcl-2 -938C>A promoter regions might result in the altercations in transcription factor binding to the promoter, leading to the change of gene expressions, which might be associated with endometriosis. Data availability
All related data and materials are available from the corresponding authors upon request.
Author contributions
DJ, FF, and MT conceived and designed the study. AEKh and MR did the data curation. SGhZ and DJ conducted the data analysis and drafted the initial manuscript. AEKh, FF, MR, and MT helped with the interpretation of the results and gave critical comments on the manuscript. All authors contributed to the final version of the manuscript.
Acknowledgments
This work is based on the Residency's thesis (code: 10338) at Zahedan University of Medical Sciences, Zahedan, Iran. The authors would like to thank the study participants in this study. No artificial intelligence was used (translation, revision, grammar check, etc.), while preparing this paper.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Genetics
References
1. Lamceva J, Uljanovs R, Strumfa I. The main theories on the pathogenesis of endometriosis. Int J Mol Sci 2023; 24: 4254. [DOI:10.3390/ijms24054254]
2. Gurgan T, Bektas G. Endometriosis and infertility. In: Lagana AS, Guglielmino A. Management of infertility. US: Academic Press; 2023. [DOI:10.1016/B978-0-323-89907-9.00014-4]
3. Amro B, Ramirez Aristondo ME, Alsuwaidi S, Almaamari B, Hakim Z, Tahlak M, et al. New understanding of diagnosis, treatment and prevention of endometriosis. Int J Environment Res Public Health 2022; 19: 6725. [DOI:10.3390/ijerph19116725]
4. Koninckx PhR, Ussia A, Adamyan L, Tahlak M, Keckstein J, wattiez A, et al. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract Res Clin Obstet Gynaecol 2021; 71: 14-26. [DOI:10.1016/j.bpobgyn.2020.08.005]
5. Azam INA, Abdul Wahab N, Mokhtar MH, Shafiee MN, Mokhtar NM. Roles of microRNAs in regulating apoptosis in the pathogenesis of endometriosis. Life 2022; 12: 1321. [DOI:10.3390/life12091321]
6. Laganà AS, Garzon S, Götte M, Viganò P, Franchi M, Ghezzi F, et al. The pathogenesis of endometriosis: Molecular and cell biology insights. Int J Mol Sci 2019; 20: 5615. [DOI:10.3390/ijms20225615]
7. Saxena A, Moshynska O, Sankaran K, Viswanathan S, Sheridan DP. Association of a novel single nucleotide polymorphism, G(-248)A, in the 5′-UTR of Bax gene in chronic lymphocytic leukemia with disease progression and treatment resistance. Cancer Lett 2002; 187: 199-205. [DOI:10.1016/S0304-3835(02)00378-6]
8. Czabotar PE, Garcia-Saez AJ. Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat Rev Mol Cell Biol 2023; 24: 732-748. [DOI:10.1038/s41580-023-00629-4]
9. da Silva Lawisch GK, Biolchi V, Kaufmann G, Nicolai G, Capitaneo E, Rosembach TR, et al. The role of FASL, BCL-2 and BAX polymorphisms in brazilian patients with prostate cancer and benign prostatic hyperplasia. Mol Biol Rep 2022; 49: 9445-9451. [DOI:10.1007/s11033-022-07805-3]
10. D'Aguanno S, Del Bufalo D. Inhibition of anti-apoptotic Bcl-2 proteins in preclinical and clinical studies: Current overview in cancer. Cells 2020; 9: 1287-1299. [DOI:10.3390/cells9051287]
11. Hafezi S, Rahmani M. Targeting BCL-2 in cancer: Advances, challenges, and perspectives. Cancers 2021; 13: 1292-1298. [DOI:10.3390/cancers13061292]
12. Kawiak A, Kostecka A. Regulation of Bcl-2 family proteins in estrogen receptor-positive breast cancer and their implications in endocrine therapy. Cancers 2022; 14: 279. [DOI:10.3390/cancers14020279]
13. Abdel-Maksoud MA, Ullah S, Nadeem A, Shaikh A, Zia MK, Zakri AM, et al. Unlocking the diagnostic, prognostic roles, and immune implications of BAX gene expression in pan-cancer analysis. Am J Transl Res 2024; 16: 63-69. [DOI:10.62347/TWOY1681]
14. Alam M, Kashyap T, Mishra P, Panda AK, Nagini S, Mishra R. Role and regulation of proapoptotic Bax in oral squamous cell carcinoma and drug resistance. Head Neck 2019; 41: 185-197. [DOI:10.1002/hed.25471]
15. Fernandes ATG, Rocha NP, Vendrame E, Russomano F, Grinsztejn BJ, Friedman RK, et al. Polymorphism in apoptotic BAX (-248G>A) gene but not in anti-apoptotic BCL-2 (-938C>A) gene and its protein and mRNA expression are associated with cervical intraepithelial neoplasia. Apoptosis 2015; 20: 1347-1357. [DOI:10.1007/s10495-015-1156-7]
16. Dholariya S, Mir R, Zuberi M, Yadav P, Gandhi G, Khurana N, et al. Potential impact of (rs 4645878) BAX promoter −248G>A and (rs 1042522) TP53 72Arg>pro polymorphisms on epithelial ovarian cancer patients. Clin Transl Oncol 2016; 18: 73-81. [DOI:10.1007/s12094-015-1338-3]
17. Cardoso-Duarte LC, Fratelli CF, Pereira AS, Souza JN, Freitas RD, Morais RM, et al. BAX gene (−248 G> A) polymorphism in a sample of patients diagnosed with thyroid cancer in the Federal District, Brazil. Int J Biol Marker 2021; 36: 21-26. [DOI:10.1177/17246008211057576]
18. Mohammadpour-Gharehbagh A, Jahantigh D, Eskandari M, Sadegh MH, Nematollahi MH, Rezaei M, et al. Genetic and epigenetic analysis of the BAX and BCL-2 in the placenta of pregnant women complicated by preeclampsia. Apoptosis 2019; 24: 301-311. [DOI:10.1007/s10495-018-1501-8]
19. Chatterjee K, De S, Deb Roy S, Sahu SK, Chakraborty A, Ghatak S, et al. BAX -248 G>A and BCL-2 -938 C>A variant lowers the survival in patients with nasopharyngeal carcinoma and could be associated with tissue-specific malignancies: A multi-method approach. Asian Pac J Cancer Prev 2021; 22: 1171-1181. [DOI:10.31557/APJCP.2021.22.4.1171]
20. Degtyareva AO, Antontseva EV, Merkulova TI. Regulatory SNPs: Altered transcription factor binding sites implicated in complex traits and diseases. Int J Mol Sci 2021; 22: 6454. [DOI:10.3390/ijms22126454]
21. Gadducci A, Zannoni GF. Endometriosis-associated extraovarian malignancies: A challenging question for the clinician and the pathologist. Anticancer Res 2020; 40: 2429-2438. [DOI:10.21873/anticanres.14212]
22. Chiorean DM, Mitranovici MI, Toru HS, Cotoi TC, Tomuț AN, Turdean SG, et al. New insights into genetics of endometriosis: A comprehensive literature review. Diagnostics 2023; 13: 2265. [DOI:10.3390/diagnostics13132265]
23. Turesheva A, Aimagambetova G, Ukybassova T, Marat A, Kanabekova P, Kaldygulova L, et al. Recurrent pregnancy loss etiology, risk factors, diagnosis, and management: Fresh look into a full box. J Clin Med 2023; 12: 4074. [DOI:10.3390/jcm12124074]
24. Samimi M, Pourhanifeh MH, Mehdizadehkashi A, Eftekhar T, Asemi Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J Cell Physiol 2019; 234: 19384-19392. [DOI:10.1002/jcp.28666]
25. Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol 2020; 21: 678-695. [DOI:10.1038/s41580-020-0270-8]
26. Rahman N, Khan H, Zia A, Khan A, Fakhri S, Aschner M, et al. Bcl-2 modulation in p53 signaling pathway by flavonoids: A potential strategy towards the treatment of cancer. Int J Mol Sci 2021; 22: 11315-11343. [DOI:10.3390/ijms222111315]
27. Moawadh MS, Mir R, Tayeb FJ, Asim O, Ullah MF. Molecular evaluation of the impact of polymorphic variants in apoptotic (Bcl-2/Bax) and proinflammatory cytokine (TNF-α/IL-8) genes on the susceptibility and progression of myeloproliferative neoplasms: A case-control biomarker study. Curr Issues Mol Biol 2023; 45: 3933-3952. [DOI:10.3390/cimb45050251]
28. Kolahdouz-Mohammadi R, Delbandi AA, Khodaverdi S, Arefi S, Arablou T, Shidfar F. The effects of resveratrol treatment on Bcl-2 and bax gene expression in endometriotic compared with non-endometriotic stromal cells. Iran J Public Health 2020; 49: 1546-1554. [DOI:10.18502/ijph.v49i8.3900]
29. Nikmohammadi N, Sarbakhsh P, Goudarzi MM, Talebi M, Farshdousti-Hagh M, Malakouti J, et al. The effect of B-cell lymphoma 2 and BCL2-associated X polymorphisms on the survival of acute lymphoblastic leukemia patients: Application of frailty survival models. Iran J Public Health 2022; 51: 895-899. [DOI:10.18502/ijph.v51i4.9251]
30. Jiang P, Tang J, Shao Q, Cai Y. Interactive effect of BCL2 and BAX polymorphisms with cigarette smoking on the risk of non-small cell lung cancer: A case-control study in China. Acta Biochimica et Biophysica Sinica 2019; 51: 1178-1181. [DOI:10.1093/abbs/gmz103]
31. Moazami-Goudarzi M, Farshdousti-Hagh M, Hoseinpour-Feizi A, Talebi M, Movassaghpour-Akbari AA, Shams-Asanjan K, et al. The acute lymphoblastic leukemia prognostic scoring whether it is possible by BCL-2, BAX gene promoter genotyping. Caspian J Intern Med 2016; 7: 105-112.
32. Feng Y, Chen X, Zheng Y, Liu Q, Chen H, Cai Y, et al. Prognostic value and susceptibility of BAX rs4645878 polymorphism in cancer: A systematic review and meta-analysis. Medicine 2018; 97: 1151-1159. [DOI:10.1097/MD.0000000000011591]
33. Michita RT, Zambra FMB, Fraga LR, Sanseverino MT, Schuler-Faccini L, Chies JAB, et al. The role of FAS, FAS-L, BAX, and BCL-2 gene polymorphisms in determining susceptibility to unexplained recurrent pregnancy loss. J Assist Reprod Genet 2019; 36: 995-1002. [DOI:10.1007/s10815-019-01441-w]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




