Sat, Jul 12, 2025
[Archive]
Volume 22, Issue 9 (September 2024)
IJRM 2024, 22(9): 689-700 |
Back to browse issues page
Ethics code: IR.SSU.SPH.REC.1399.145
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zohrabi T, Nadjarzadeh A, Jambarsang S, Sheikhha M H, Aflatoonian A, Mozaffari-Khosravi H. Effect of dietary approaches to stop hypertension and curcumin co-administration on glycemic parameters in polycystic ovary syndrome: An RCT. IJRM 2024; 22 (9) :689-700
URL: http://ijrm.ir/article-1-3281-en.html
URL: http://ijrm.ir/article-1-3281-en.html
Tayebeh Zohrabi *1 

 , Azadeh Nadjarzadeh2
, Azadeh Nadjarzadeh2 

 , Sara Jambarsang3
, Sara Jambarsang3 

 , Mohammad Hasan Sheikhha4
, Mohammad Hasan Sheikhha4 

 , Abbas Aflatoonian5
, Abbas Aflatoonian5 

 , Hassan Mozaffari-Khosravi2
, Hassan Mozaffari-Khosravi2 




 , Azadeh Nadjarzadeh2
, Azadeh Nadjarzadeh2 

 , Sara Jambarsang3
, Sara Jambarsang3 

 , Mohammad Hasan Sheikhha4
, Mohammad Hasan Sheikhha4 

 , Abbas Aflatoonian5
, Abbas Aflatoonian5 

 , Hassan Mozaffari-Khosravi2
, Hassan Mozaffari-Khosravi2 


1- Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. , zohrabitayebeh@yahoo.com
2- Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Center for Healthcare Data Modeling, Department of Biostatistics and Epidemiology, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Center for Healthcare Data Modeling, Department of Biostatistics and Epidemiology, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Genetics, Faculty of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Abortion Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Keywords: Polycystic ovary syndrome, Dietary approaches to stop hypertension, Curcumin, Insulin resistance, Glycemic control.
Full-Text [PDF 320 kb]
(720 Downloads)
| Abstract (HTML) (626 Views)
4. Discussion
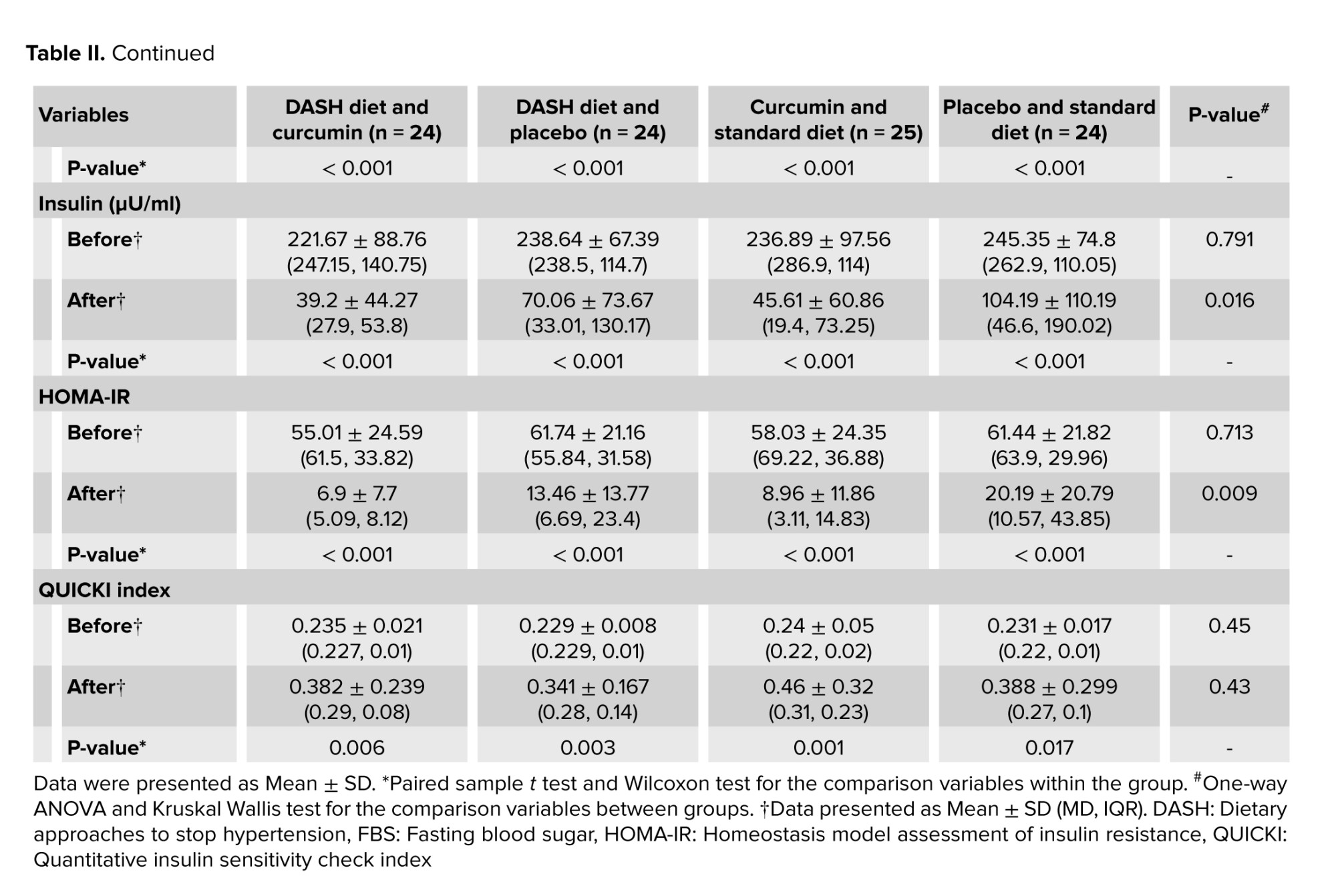
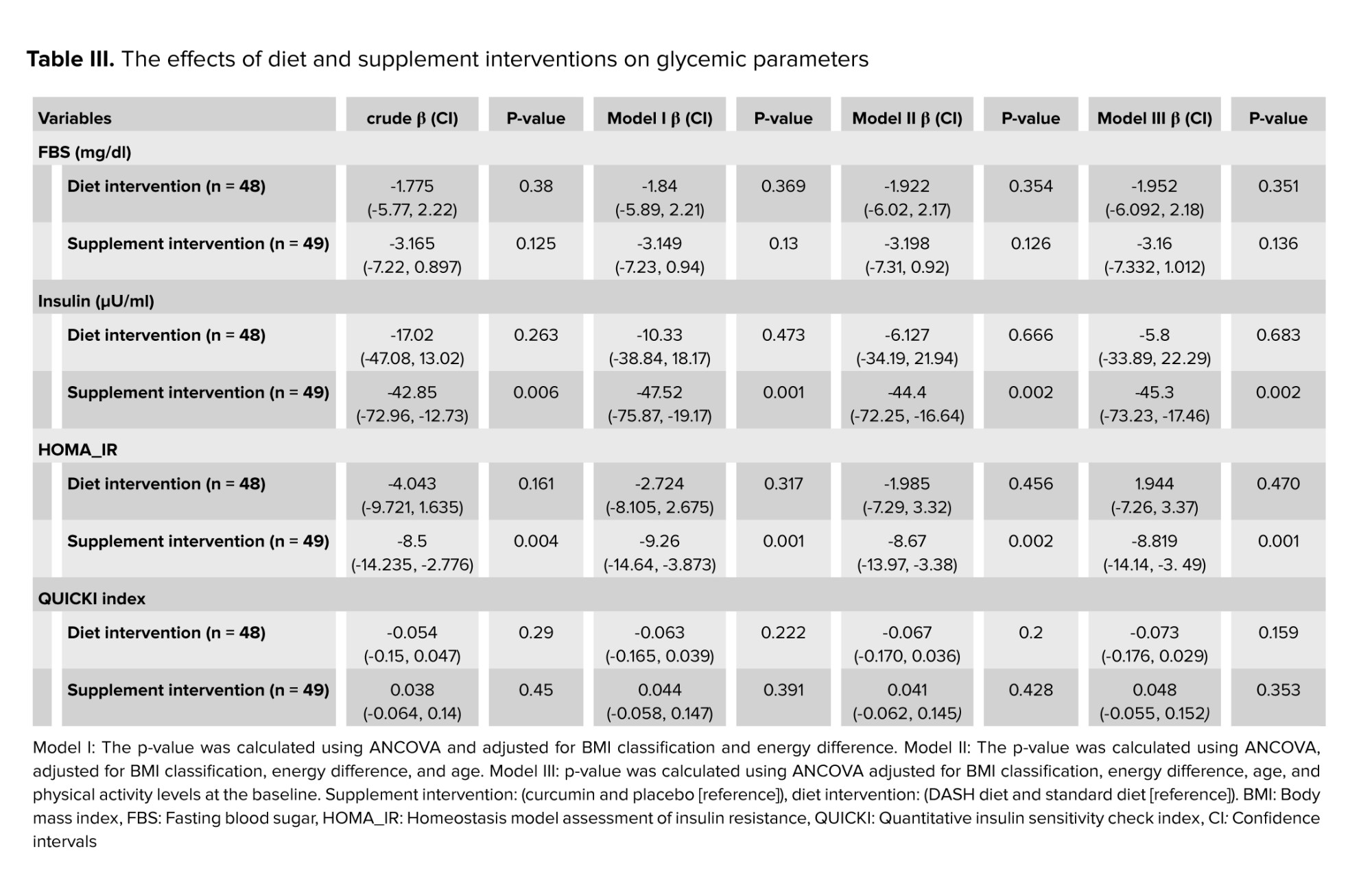
The results of this study showed that the 12 wk DASH diet has no significant effect on glycemic parameters. Still, the simultaneous use of curcumin (1000 mg/day) can significantly reduce insulin levels (ß = -45.3, 95% CI [-73.23, -17.46], p = 0.002) and IR (ß = -8.819, 95% CI [-14.14, -3.49], p = 0.001). However, curcumin administration had no effect on FBS levels and QUICKI index.
IR and compensatory hyperinsulinemia play a major role in causing reproductive and metabolic disorders (3). Therefore, treatment by inducing insulin sensitivity and reducing IR effectively manages PCOS. According to meta-analysis results, curcumin supplementation may have beneficial effects on glycemic parameters (18). Previously, no randomized clinical trial has investigated the combined effect of the DASH diet and curcumin supplementation on glycemic parameters in normal-weight and overweight/obese women with PCOS who are candidates for IVF treatment. PCOS and obesity may have a synergistic detrimental effect on IR (19). However, not all PCOS patients with hyperinsulinemia are overweight or obese. The effects of diet and curcumin independent of weight status has not been investigated so far. In this study, mean insulin levels in normal weight groups were reduced to normal levels with curcumin intake. After adding curcumin to the intervention, the mean reduction of HOMA-IR was also higher in normal BMI groups. The amount of insulin clearance is low in obese people compared to lean people (20). The mechanisms enhancing the effect of curcumin can be explained by the insulin clearance hypothesis (21). Although decreased insulin clearance occurs to compensate and adapt to IR in the body, this condition is associated with dysglycemia. It may contribute to IR and diabetes through hyperinsulinemia-induced desensitization (5). Curcumin, with its antioxidant properties and its effect on increasing circulating adiponectin as an anti-inflammatory cytokine, can play an essential role in reducing the metabolic complications of PCOS (22). In patients with high adiponectin status, hepatic clearance of insulin is increased (23). Low circulating adiponectin levels were associated with IR, BMI, adiposity, and metabolic syndrome (22). In women with PCOS, the level of adiponectin is lower, while this change occurs regardless of adiposity (24). Another possible mechanism is related to the low-grade inflammation caused by PCOS and the antioxidant protective effects of curcumin (14).
Given that obesity and unbalanced dietary composition cause more disruption in insulin metabolism and inflammation (25), we therefore adjusted for BMI and energy difference and other relevant factors such as age (26) and physical activity (27). Based on the results of umbrella reviews of meta-analyses of RCTs, there was no high-conclusive evidence of an effect of diets alone on the metabolic and endocrine outcomes of PCOS. However, curcumin supplementation showed favorable effects on HOMA-IR (28). In the present study, after controlling for BMI, energy difference, age, and physical activity levels at baseline, the effect of curcumin on insulin levels and HOMA-IR remained significant. This indicates that the effect of curcumin on serum insulin and IR is independent of the effect of these factors.
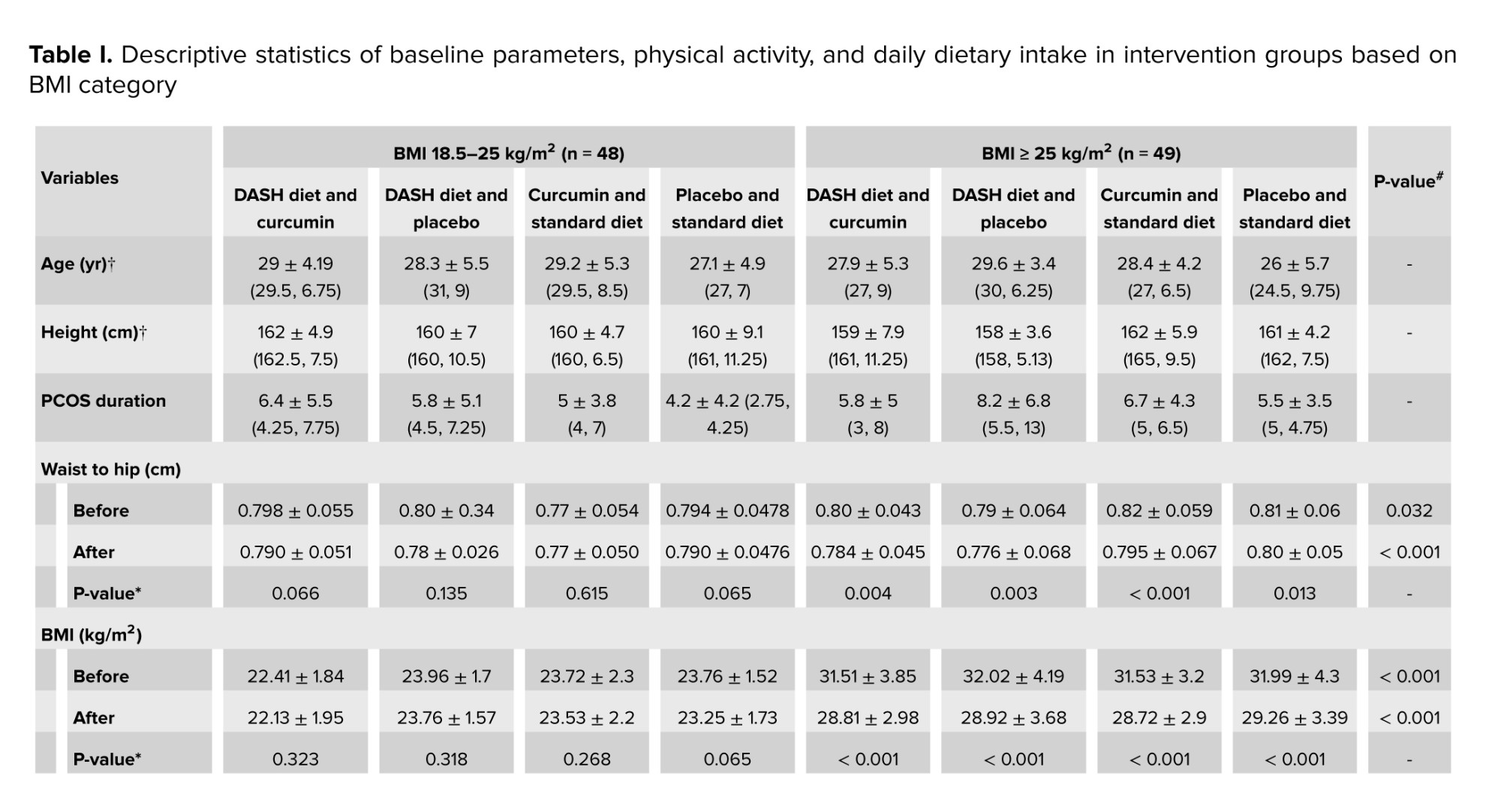
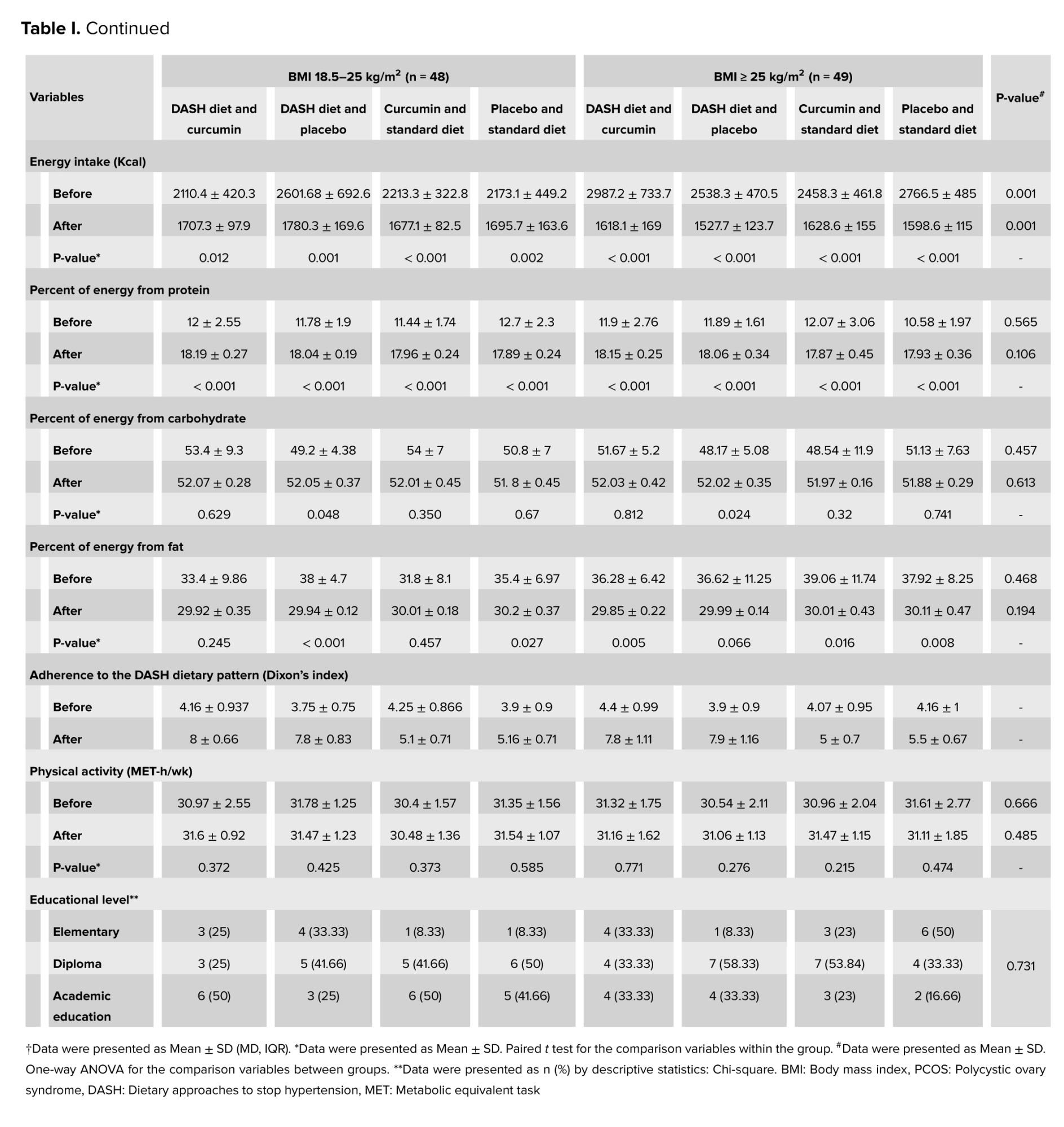
In the present study, the same macronutrient components were considered for both diets. Regarding the macronutrient composition of the participant's diet at baseline, the mean protein intake in all groups ranged from 10.58-12.7, whereas we considered 18% protein in both diets. There is no optimal strategy for macronutrient distribution or dietary interventions in nutritional recommendations for PCOS patients (29). Recent evidence suggests that high protein intake can improve IR (30).
However, protein intake should not exceed 20% of total calorie intake (29). From the perspective of dietary interventions in our study, the results of Dixon’s DASH diet index showed good adherence to the DASH diet. A meta-analysis of RCTs showed that the DASH diet can improve some glycemic parameters when implemented as a long-term intervention (> 16 wk) (31). Therefore, the addition of curcumin may enhance the anti-inflammatory effects of the DASH diet leading to faster recovery of hyperinsulinemia, and improve IR.
All participants in both diets received personalized nutrition with identical macronutrient components and sodium prescription, while being offered several carefully analyzed menu items. Fortunately, participants showed good adherence to diet and curcumin intake. On the other hand, several adjustment models intend to confound factors of glycemic parameters to prevent misleading conclusions in data analysis and interpretation. On the other hand, several adjustment models for confounding factors of glycemic parameters have been considered to avoid misleading conclusions in data analysis and interpretation.
4.1. Limitation
Dietary patterns can have long-term effects that could not be assessed due to the short duration of this study, which could lead to bias. Another source of bias could be related to seasonal food availability and preparation.
5. Conclusion
In conclusion, the present randomized clinical trials indicated that the co-administration of (1000 mg/day) curcumin and DASH diet for 12 wk, can have positive effects on reducing insulin levels, improving IR, and leading to faster recovery of hyperinsulinemia. Overall, the information presented here may highlight the potential of curcumin as a bioactive compound and future therapeutic target alongside a healthy diet for endocrine and metabolic diseases.
Data Availability
The data and materials of the current study are available from the corresponding author on reasonable request.
Author Contributions
H. Mozaffari-Khosravi, A. Aflatoonian, and T. Zohrabi designed and conducted the trial. A. Nadjaradeh and MH. Sheikhha provided scientific and technical support. H. Mozaffari-Khosravi and A. Aflatoonian supervised the study. S. Jambarsang carried out statistical analyses. T. Zohrabi interpreted the findings and drafted the manuscript. H. Mozaffari-Khosravi critically revised the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgments
The authors would like to acknowledge the “M/s Arjuna Natural Pvt Ltd., India”, for providing the curcumin supplement capsules (BCM95/Curcugreen) as a gift. The authors also thank the scientific support of “Yazd Reproductive Sciences Institute and Gynecology Clinic of Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Yazd, Iran”. This study was funded by Shahid Sadoughi University of Medical Sciences, Yazd, Iran (grant number: 8229, dated August 12, 2020). This trial has also been supported by Iran National, Science Foundation (INSF), (under Project No. 99019137 dated July 6, 2021). AI was not used in this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
a
Full-Text: (91 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disorder that can lead to major reproductive and metabolic consequences (1). This complex disorder, which can be recognized by menstrual disorder, polycystic ovarian morphology, hirsutism, acne, and infertility, significantly reduces the patient's quality of life (2).
About 50-70% of people with PCOS are at high risk for insulin resistance (IR) (3). Insulin plays an important role in regulating glucose homeostasis by stimulating the uptake of glucose into skeletal muscles and fat tissues, and inhibiting glucose production in the liver (4). IR is a condition in which the body's cells do not respond properly to insulin (5). In these conditions, the pancreas produces more insulin to overcome the resistance, resulting in hyperinsulinemia, which can be the primary mediator of metabolic syndrome, leading to type 2 diabetes and cardiovascular diseases (6).
Various methods have been proposed to control IR (2, 7). Lifestyle intervention is the first line of IR prevention in prediabetic and diabetic conditions (8). Dietary intervention can include a wide variety of caloric restriction patterns or the use of a low glycemic index diet (7). It has been suggested that dietary approaches to stop hypertension (DASH) is an effective diet with a low glycemic index and high antioxidant content that can have beneficial effects in reducing IR (9). The DASH diet is rich in whole grains, vegetables, fruits, low-fat dairy products, poultry, fish, and nuts while limiting the intake of foods high in saturated fat, cholesterol, sodium, refined grains, red and processed meat (10). Consuming the DASH dietary pattern in overweight and obese women with PCOS had favorable effects on IR, abdominal fat accumulation, and high-sensitivity C-reactive protein serum level (11).
A variety of other interventions and many potential compounds with different therapeutic activities have been investigated in recent years. In particular, curcumin's therapeutic properties and antioxidant effects as natural plant polyphenols have received more attention (12). Curcumin (diferroylmethane) is a lipophilic yellow pigment extracted from the rhizomes of turmeric (Curcuma longa L.) (13). Findings show that curcumin supplementation can affect glucose homeostasis, improve lipid metabolism and antioxidant capacity, and reduce oxygen radical species in PCOS patients (14).
Investigating the combined effects of using healthy dietary patterns and natural bioactive compounds will help researchers, nutritionists, and policymakers prescribe optimal modifications to improve quality of life and reduce PCOS-related complications. Therefore, the objective of the present clinical trial was to investigate the effect of the DASH diet along with curcumin supplementation on glycemic parameters in normal weight and overweight/obese PCOS patients undergoing in vitro fertilization (IVF) treatment. The preprint version of present article has been uploaded to the Research Square (https://www.researchsquare.com/article/rs-3610838/v1, https://doi.org/10.21203/rs.3.rs-3610838/v1).
2. Materials and Methods
This double blind, randomized clinical study was conducted on 104 infertile women with PCOS at the Gynecology Clinic of Research and Clinical Center for Infertility affiliated to Yazd Reproductive Sciences Institute of Shahid Sadoughi University of Medical Sciences, Yazd, Iran from August 2022 to April 2023.
Infertile women with PCOS, according to Rotterdam's criteria (15), based on the inclusion criteria (without a previous IVF history, between 18 and 45 yr, and with body mass index [BMI] between 18.5 and 25 kg and BMI ≥ 25 kg/m²), and exclusion criteria were included in the study.
This study was a 12-wk, randomized, placebo-controlled clinical trial with a factorial design. Participants were assigned unique codes to conceal the allocation and avoid “selection bias”. They were arranged in 4 blocks of equal size using the stratified permuted block randomization method by the “blockrand” package in R software, version 4.0.2.
Treatment conditions and BMI (18.5-25 and ≥ 25), were included in the random allocation list. Supplemental and placebo capsules were serially placed in numbered and sealed envelopes by a person unrelated to the study. These capsules were packaged in similar bottles with the same appearance (in terms of color and shape).
The bottles had different marks (A and B). Participants and researchers were not aware of the contents of the bottles. Randomization and allocation were kept secret from participants and researchers until the end of the study and data analysis. Participants were selected using the available sampling method and 2 × 2 factorial design, and randomly divided into 4 parallel treatment groups (n = 26/each group): 1) DASH diet + curcumin supplement, 2) standard diet + curcumin supplement, 3) DASH diet + placebo, and 4) standard diet + placebo.
Details of the method have been published previously. This manuscript is part of a larger clinical trial that includes part of the data. Other parameters (genetic, androgenic, nutritional, etc.) were evaluated according to the protocol. But, due to the high volume of data and to prevent the increase in the number of tables and words, they will be discussed in other articles. It should also be noted that according to the sample size formula that is fully explained in the study protocol (considering an effect size = 1, α = 0.05, power of 80%, and dropout rate of 20%) (16), the optimal sample size was 24 people in each group. However, after the start of the study, due to the distance problems of many participants and the concern of follow-up, the research team decided to add 2 people to the sample size of each group. The final volume was considered to be 104 people.
2.1. Intervention
Participants received 500 mg of placebo (roasted rice powder) or curcumin capsules (BCM95/Curcugreen) twice daily, once after breakfast and once after dinner with water. Participants received 60 capsules each month. Each 500 mg capsule contains 475 mg of curcuminoids along with natural turmeric essential oil, which improves the bioavailability and bioactivity of oral curcuminoids. All capsules were manufactured by M/s Arjuna Natural Pvt Ltd., India. Compliance was estimated by collecting bottles at the end of the month and counting the remaining capsules. In addition, all volunteers followed 2 healthy eating patterns (DASH or standard diet). These individual diets were designed in the form of diets containing 18% protein, 52% carbohydrates, and 30% total fat. Daily caloric needs were estimated by calculating physical activity levels and resting energy expenditure. Calculated energy was reduced by 300 and 500 kcal, respectively, if a person was overweight (BMI 25.0-30.0 kg/m²) or obese (> 30.0 kg/m²). A similar amount of sodium (about one teaspoon of salt) was prescribed for each diet. Macronutrients and energy intake were obtained using 3-day dietary records (including 2 nonconsecutive weekdays and one weekend) and analyzed by Nutritionist IV software. Adherence to the DASH dietary pattern was evaluated based on Dixon's DASH dietary index (17). Dixon's recommendations for cut-off values based on 1600 kcal per day for women in 9 components are as follows:
Whole grains ≥ 4 servings per day (67%* 6 servings of grains), ≥ 4 servings per day of fruits, ≥ 2 servings per day of dairy products, ≥ 3 servings per day of vegetables, ≥ 3 servings per day of seeds, nuts and legumes, < 6 ounces per day of meat and meat equivalents, average daily intake of saturated fat < 5% of total energy intake, added sugar intake ≤ 3% of total energy intake, alcohol intake < one drink per day. The minimum score is 0, and the maximum score is 9 points. The sum of 9 components was considered as the total score of people's adherence to the DASH diet.
Physical activity was assessed with the metabolic equivalents questionnaire at the beginning and end of the study, although all volunteers were advised not to change their physical activity during the study.
2.2. Anthropometric and biochemical measurements
Biochemical and anthropometric assessments were done at the beginning and after 12 wk of intervention. After a 2 wk run period and considering 12 hr overnight fasting, 5 ml of blood sample was collected from each participant in the morning. The serum was removed from centrifuged samples and immediately stored at -70°C. The serum levels of fasting insulin were assessed using an enzyme-linked immunosorbent assay kit (Monobind, California, United States). Fasting blood sugar (FBS) was measured using an autoanalyzer (AVIDA 1800 chemistry system; Siemens, United Kingdom, with Pars Azmoon Kits). Homeostasis model assessment of IR (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were also calculated.
Body composition and weight were determined using a segmental body composition analyzer (Tanita BC-418, Tokyo, Japan). Height, hip, and waist circumference measurements were done according to the standard protocol. BMI was calculated by dividing weight in kilograms by the square of height in meters (BMI = kg/m²).
2.3. Ethical Considerations
This research was approved by the Medical Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Code: IR.SSU.SPH.REC.1399.145). The protocol of this trial with the registration ID: IRCT20200915048731N1 and URL: https://www.irct.ir/trial/50970 was registered on the Iran Clinical Trials website on 09/29/2020. It has been updated on 02 May 2024. In this research, all methods and experiments were performed in accordance with the Declaration of Helsinki, and the relevant guidelines and regulations. This study was also conducted in accordance with the consolidated standards for reporting trials (CONSORT). Written informed consent was signed by all participants before the start of the study.
2.4. Statistical Analysis
Before statistical analysis, all data were checked for completeness and accuracy. The Kolmogorov-Smirnov test evaluated the normal distribution of the data. The Chi-square test was used to compare the frequency between the studied groups. The changes before and after the intervention in each group were performed using the paired t test/Wilcoxon test. Comparison between groups was evaluated through one-way analysis of variance (ANOVA)/Kruskal Wallis. Analysis of covariance (ANCOVA) was performed to compare the mean of quantitative data between groups after adjustment for potential confounders. The values of quantitative variables were expressed as mean ± standard deviation/MD, IQR and qualitative variables were expressed as numbers and percentages. A p ≤ 0.05 was defined as statistically significant. All statistical analyses were performed using SPSS (SPSS, Inc, Chicago, IL, USA), version 25.0.
3. Results
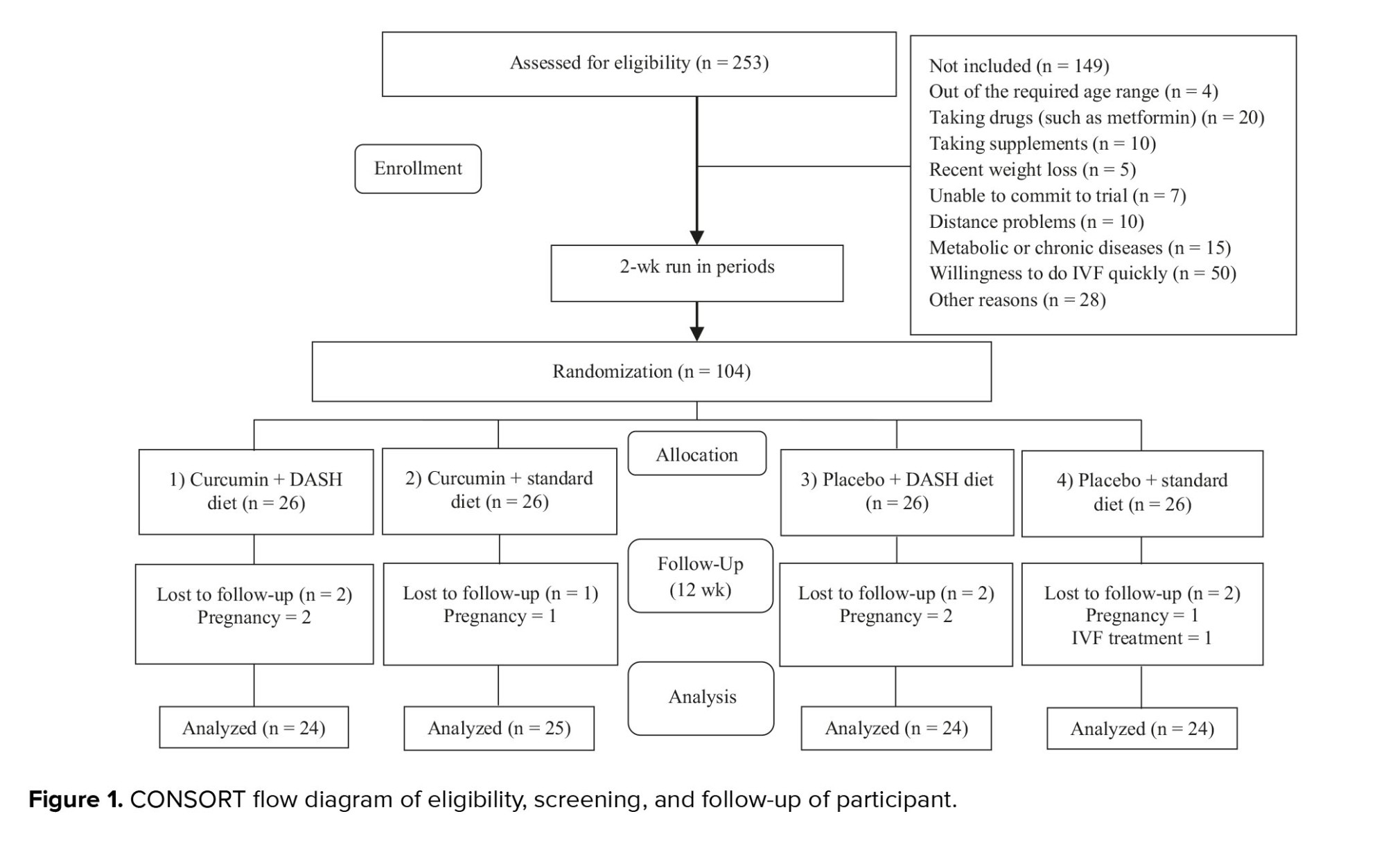
253 participants were evaluated for eligibility. Based on the inclusion and exclusion criteria, 104 volunteers were included in the study. 149 participants were not included due to reasons such as being out of the required age range (n = 4), taking some drugs (n = 20) or supplements (n = 10), having recent weight loss (n = 5), unable to commit to trial (n = 7), distance problems (n = 10), metabolic or chronic diseases (n = 15), desire to do IVF quickly (n = 50), other reasons (n = 28). 7 participants were excluded from the study due to loss of follow-up (6 were excluded due to pregnancy and 1 due to IVF). Ultimately, a total of 97 participants completed the trial (Figure 1).
Adherence to the DASH diet based on the Dixon index in the DASH diet groups was, on average, between 7.8 and 8 (86.6-88.8%). All participants consumed the capsules completely, except one who did not consume 2 capsules due to a delay in returning at the appointed time. No complications were reported due to the use of capsules. Overall, no serious adverse events were reported during the study.
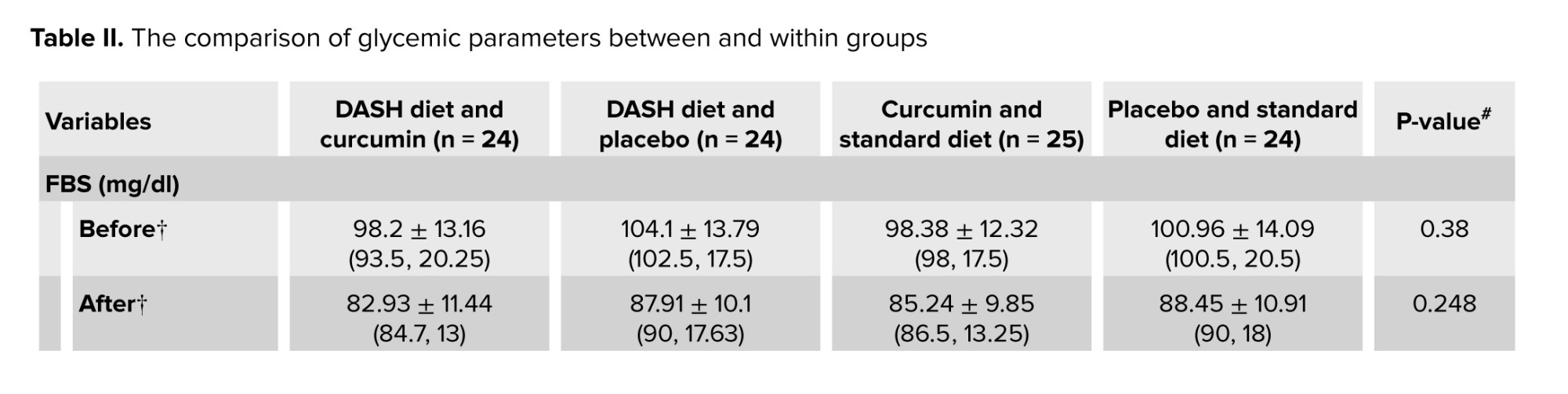
No significant difference was observed between the groups in terms of basic parameters for age examination, disease duration, education level, and height. Descriptive statistics of baseline parameters, physical activity, and daily dietary intake in intervention groups based on BMI classification are presented in table I. No significant difference was observed between the groups in terms of macronutrient composition including carbohydrates, protein, and fat with proportions of 52, 18, and 30%. Table II shows the comparison of glycemic parameters between and within groups. These parameters decreased significantly in each group compared to baseline (p < 0.05). The comparison of the average serum levels of glycemic parameters between the studied groups before the intervention did not show a statistically significant difference (p ≥ 0.05). However, a significant difference was observed regarding serum insulin and HOMA-IR after the intervention.
The effects of diet and supplement interventions on glycemic parameters are shown in (Table III). Using an unadjusted model, insulin levels were significantly reduced in the supplement intervention (p = 0.006). After controlling for “energy difference” and “BMI classification” in the first model (model I) (p = 0.001), they were significant even with further adjustments for age in model II (p = 0.002) and for baseline levels of physical activity in model III (p = 0.002). HOMA-IR decreased significantly in the supplement intervention (p = 0.004). The reduction of IR in the supplement intervention was significant even with further adjustments in model I (p = 0.001), model II (p = 0.002), and model III (p = 0.001). However, no significant difference was observed in glycemic parameters in the diet intervention in crude or adjusted models (p ≥ 0.05). Changes in FBS and QUICKI index were not significant in either the crude or adjusted models in the supplement intervention (p ≥ 0.05).
Polycystic ovary syndrome (PCOS) is an endocrine disorder that can lead to major reproductive and metabolic consequences (1). This complex disorder, which can be recognized by menstrual disorder, polycystic ovarian morphology, hirsutism, acne, and infertility, significantly reduces the patient's quality of life (2).
About 50-70% of people with PCOS are at high risk for insulin resistance (IR) (3). Insulin plays an important role in regulating glucose homeostasis by stimulating the uptake of glucose into skeletal muscles and fat tissues, and inhibiting glucose production in the liver (4). IR is a condition in which the body's cells do not respond properly to insulin (5). In these conditions, the pancreas produces more insulin to overcome the resistance, resulting in hyperinsulinemia, which can be the primary mediator of metabolic syndrome, leading to type 2 diabetes and cardiovascular diseases (6).
Various methods have been proposed to control IR (2, 7). Lifestyle intervention is the first line of IR prevention in prediabetic and diabetic conditions (8). Dietary intervention can include a wide variety of caloric restriction patterns or the use of a low glycemic index diet (7). It has been suggested that dietary approaches to stop hypertension (DASH) is an effective diet with a low glycemic index and high antioxidant content that can have beneficial effects in reducing IR (9). The DASH diet is rich in whole grains, vegetables, fruits, low-fat dairy products, poultry, fish, and nuts while limiting the intake of foods high in saturated fat, cholesterol, sodium, refined grains, red and processed meat (10). Consuming the DASH dietary pattern in overweight and obese women with PCOS had favorable effects on IR, abdominal fat accumulation, and high-sensitivity C-reactive protein serum level (11).
A variety of other interventions and many potential compounds with different therapeutic activities have been investigated in recent years. In particular, curcumin's therapeutic properties and antioxidant effects as natural plant polyphenols have received more attention (12). Curcumin (diferroylmethane) is a lipophilic yellow pigment extracted from the rhizomes of turmeric (Curcuma longa L.) (13). Findings show that curcumin supplementation can affect glucose homeostasis, improve lipid metabolism and antioxidant capacity, and reduce oxygen radical species in PCOS patients (14).
Investigating the combined effects of using healthy dietary patterns and natural bioactive compounds will help researchers, nutritionists, and policymakers prescribe optimal modifications to improve quality of life and reduce PCOS-related complications. Therefore, the objective of the present clinical trial was to investigate the effect of the DASH diet along with curcumin supplementation on glycemic parameters in normal weight and overweight/obese PCOS patients undergoing in vitro fertilization (IVF) treatment. The preprint version of present article has been uploaded to the Research Square (https://www.researchsquare.com/article/rs-3610838/v1, https://doi.org/10.21203/rs.3.rs-3610838/v1).
2. Materials and Methods
This double blind, randomized clinical study was conducted on 104 infertile women with PCOS at the Gynecology Clinic of Research and Clinical Center for Infertility affiliated to Yazd Reproductive Sciences Institute of Shahid Sadoughi University of Medical Sciences, Yazd, Iran from August 2022 to April 2023.
Infertile women with PCOS, according to Rotterdam's criteria (15), based on the inclusion criteria (without a previous IVF history, between 18 and 45 yr, and with body mass index [BMI] between 18.5 and 25 kg and BMI ≥ 25 kg/m²), and exclusion criteria were included in the study.
This study was a 12-wk, randomized, placebo-controlled clinical trial with a factorial design. Participants were assigned unique codes to conceal the allocation and avoid “selection bias”. They were arranged in 4 blocks of equal size using the stratified permuted block randomization method by the “blockrand” package in R software, version 4.0.2.
Treatment conditions and BMI (18.5-25 and ≥ 25), were included in the random allocation list. Supplemental and placebo capsules were serially placed in numbered and sealed envelopes by a person unrelated to the study. These capsules were packaged in similar bottles with the same appearance (in terms of color and shape).
The bottles had different marks (A and B). Participants and researchers were not aware of the contents of the bottles. Randomization and allocation were kept secret from participants and researchers until the end of the study and data analysis. Participants were selected using the available sampling method and 2 × 2 factorial design, and randomly divided into 4 parallel treatment groups (n = 26/each group): 1) DASH diet + curcumin supplement, 2) standard diet + curcumin supplement, 3) DASH diet + placebo, and 4) standard diet + placebo.
Details of the method have been published previously. This manuscript is part of a larger clinical trial that includes part of the data. Other parameters (genetic, androgenic, nutritional, etc.) were evaluated according to the protocol. But, due to the high volume of data and to prevent the increase in the number of tables and words, they will be discussed in other articles. It should also be noted that according to the sample size formula that is fully explained in the study protocol (considering an effect size = 1, α = 0.05, power of 80%, and dropout rate of 20%) (16), the optimal sample size was 24 people in each group. However, after the start of the study, due to the distance problems of many participants and the concern of follow-up, the research team decided to add 2 people to the sample size of each group. The final volume was considered to be 104 people.
2.1. Intervention
Participants received 500 mg of placebo (roasted rice powder) or curcumin capsules (BCM95/Curcugreen) twice daily, once after breakfast and once after dinner with water. Participants received 60 capsules each month. Each 500 mg capsule contains 475 mg of curcuminoids along with natural turmeric essential oil, which improves the bioavailability and bioactivity of oral curcuminoids. All capsules were manufactured by M/s Arjuna Natural Pvt Ltd., India. Compliance was estimated by collecting bottles at the end of the month and counting the remaining capsules. In addition, all volunteers followed 2 healthy eating patterns (DASH or standard diet). These individual diets were designed in the form of diets containing 18% protein, 52% carbohydrates, and 30% total fat. Daily caloric needs were estimated by calculating physical activity levels and resting energy expenditure. Calculated energy was reduced by 300 and 500 kcal, respectively, if a person was overweight (BMI 25.0-30.0 kg/m²) or obese (> 30.0 kg/m²). A similar amount of sodium (about one teaspoon of salt) was prescribed for each diet. Macronutrients and energy intake were obtained using 3-day dietary records (including 2 nonconsecutive weekdays and one weekend) and analyzed by Nutritionist IV software. Adherence to the DASH dietary pattern was evaluated based on Dixon's DASH dietary index (17). Dixon's recommendations for cut-off values based on 1600 kcal per day for women in 9 components are as follows:
Whole grains ≥ 4 servings per day (67%* 6 servings of grains), ≥ 4 servings per day of fruits, ≥ 2 servings per day of dairy products, ≥ 3 servings per day of vegetables, ≥ 3 servings per day of seeds, nuts and legumes, < 6 ounces per day of meat and meat equivalents, average daily intake of saturated fat < 5% of total energy intake, added sugar intake ≤ 3% of total energy intake, alcohol intake < one drink per day. The minimum score is 0, and the maximum score is 9 points. The sum of 9 components was considered as the total score of people's adherence to the DASH diet.
Physical activity was assessed with the metabolic equivalents questionnaire at the beginning and end of the study, although all volunteers were advised not to change their physical activity during the study.
2.2. Anthropometric and biochemical measurements
Biochemical and anthropometric assessments were done at the beginning and after 12 wk of intervention. After a 2 wk run period and considering 12 hr overnight fasting, 5 ml of blood sample was collected from each participant in the morning. The serum was removed from centrifuged samples and immediately stored at -70°C. The serum levels of fasting insulin were assessed using an enzyme-linked immunosorbent assay kit (Monobind, California, United States). Fasting blood sugar (FBS) was measured using an autoanalyzer (AVIDA 1800 chemistry system; Siemens, United Kingdom, with Pars Azmoon Kits). Homeostasis model assessment of IR (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were also calculated.
Body composition and weight were determined using a segmental body composition analyzer (Tanita BC-418, Tokyo, Japan). Height, hip, and waist circumference measurements were done according to the standard protocol. BMI was calculated by dividing weight in kilograms by the square of height in meters (BMI = kg/m²).
2.3. Ethical Considerations
This research was approved by the Medical Ethics Committee of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Code: IR.SSU.SPH.REC.1399.145). The protocol of this trial with the registration ID: IRCT20200915048731N1 and URL: https://www.irct.ir/trial/50970 was registered on the Iran Clinical Trials website on 09/29/2020. It has been updated on 02 May 2024. In this research, all methods and experiments were performed in accordance with the Declaration of Helsinki, and the relevant guidelines and regulations. This study was also conducted in accordance with the consolidated standards for reporting trials (CONSORT). Written informed consent was signed by all participants before the start of the study.
2.4. Statistical Analysis
Before statistical analysis, all data were checked for completeness and accuracy. The Kolmogorov-Smirnov test evaluated the normal distribution of the data. The Chi-square test was used to compare the frequency between the studied groups. The changes before and after the intervention in each group were performed using the paired t test/Wilcoxon test. Comparison between groups was evaluated through one-way analysis of variance (ANOVA)/Kruskal Wallis. Analysis of covariance (ANCOVA) was performed to compare the mean of quantitative data between groups after adjustment for potential confounders. The values of quantitative variables were expressed as mean ± standard deviation/MD, IQR and qualitative variables were expressed as numbers and percentages. A p ≤ 0.05 was defined as statistically significant. All statistical analyses were performed using SPSS (SPSS, Inc, Chicago, IL, USA), version 25.0.
3. Results
253 participants were evaluated for eligibility. Based on the inclusion and exclusion criteria, 104 volunteers were included in the study. 149 participants were not included due to reasons such as being out of the required age range (n = 4), taking some drugs (n = 20) or supplements (n = 10), having recent weight loss (n = 5), unable to commit to trial (n = 7), distance problems (n = 10), metabolic or chronic diseases (n = 15), desire to do IVF quickly (n = 50), other reasons (n = 28). 7 participants were excluded from the study due to loss of follow-up (6 were excluded due to pregnancy and 1 due to IVF). Ultimately, a total of 97 participants completed the trial (Figure 1).
Adherence to the DASH diet based on the Dixon index in the DASH diet groups was, on average, between 7.8 and 8 (86.6-88.8%). All participants consumed the capsules completely, except one who did not consume 2 capsules due to a delay in returning at the appointed time. No complications were reported due to the use of capsules. Overall, no serious adverse events were reported during the study.
No significant difference was observed between the groups in terms of basic parameters for age examination, disease duration, education level, and height. Descriptive statistics of baseline parameters, physical activity, and daily dietary intake in intervention groups based on BMI classification are presented in table I. No significant difference was observed between the groups in terms of macronutrient composition including carbohydrates, protein, and fat with proportions of 52, 18, and 30%. Table II shows the comparison of glycemic parameters between and within groups. These parameters decreased significantly in each group compared to baseline (p < 0.05). The comparison of the average serum levels of glycemic parameters between the studied groups before the intervention did not show a statistically significant difference (p ≥ 0.05). However, a significant difference was observed regarding serum insulin and HOMA-IR after the intervention.
The effects of diet and supplement interventions on glycemic parameters are shown in (Table III). Using an unadjusted model, insulin levels were significantly reduced in the supplement intervention (p = 0.006). After controlling for “energy difference” and “BMI classification” in the first model (model I) (p = 0.001), they were significant even with further adjustments for age in model II (p = 0.002) and for baseline levels of physical activity in model III (p = 0.002). HOMA-IR decreased significantly in the supplement intervention (p = 0.004). The reduction of IR in the supplement intervention was significant even with further adjustments in model I (p = 0.001), model II (p = 0.002), and model III (p = 0.001). However, no significant difference was observed in glycemic parameters in the diet intervention in crude or adjusted models (p ≥ 0.05). Changes in FBS and QUICKI index were not significant in either the crude or adjusted models in the supplement intervention (p ≥ 0.05).






4. Discussion
The results of this study showed that the 12 wk DASH diet has no significant effect on glycemic parameters. Still, the simultaneous use of curcumin (1000 mg/day) can significantly reduce insulin levels (ß = -45.3, 95% CI [-73.23, -17.46], p = 0.002) and IR (ß = -8.819, 95% CI [-14.14, -3.49], p = 0.001). However, curcumin administration had no effect on FBS levels and QUICKI index.
IR and compensatory hyperinsulinemia play a major role in causing reproductive and metabolic disorders (3). Therefore, treatment by inducing insulin sensitivity and reducing IR effectively manages PCOS. According to meta-analysis results, curcumin supplementation may have beneficial effects on glycemic parameters (18). Previously, no randomized clinical trial has investigated the combined effect of the DASH diet and curcumin supplementation on glycemic parameters in normal-weight and overweight/obese women with PCOS who are candidates for IVF treatment. PCOS and obesity may have a synergistic detrimental effect on IR (19). However, not all PCOS patients with hyperinsulinemia are overweight or obese. The effects of diet and curcumin independent of weight status has not been investigated so far. In this study, mean insulin levels in normal weight groups were reduced to normal levels with curcumin intake. After adding curcumin to the intervention, the mean reduction of HOMA-IR was also higher in normal BMI groups. The amount of insulin clearance is low in obese people compared to lean people (20). The mechanisms enhancing the effect of curcumin can be explained by the insulin clearance hypothesis (21). Although decreased insulin clearance occurs to compensate and adapt to IR in the body, this condition is associated with dysglycemia. It may contribute to IR and diabetes through hyperinsulinemia-induced desensitization (5). Curcumin, with its antioxidant properties and its effect on increasing circulating adiponectin as an anti-inflammatory cytokine, can play an essential role in reducing the metabolic complications of PCOS (22). In patients with high adiponectin status, hepatic clearance of insulin is increased (23). Low circulating adiponectin levels were associated with IR, BMI, adiposity, and metabolic syndrome (22). In women with PCOS, the level of adiponectin is lower, while this change occurs regardless of adiposity (24). Another possible mechanism is related to the low-grade inflammation caused by PCOS and the antioxidant protective effects of curcumin (14).
Given that obesity and unbalanced dietary composition cause more disruption in insulin metabolism and inflammation (25), we therefore adjusted for BMI and energy difference and other relevant factors such as age (26) and physical activity (27). Based on the results of umbrella reviews of meta-analyses of RCTs, there was no high-conclusive evidence of an effect of diets alone on the metabolic and endocrine outcomes of PCOS. However, curcumin supplementation showed favorable effects on HOMA-IR (28). In the present study, after controlling for BMI, energy difference, age, and physical activity levels at baseline, the effect of curcumin on insulin levels and HOMA-IR remained significant. This indicates that the effect of curcumin on serum insulin and IR is independent of the effect of these factors.
In the present study, the same macronutrient components were considered for both diets. Regarding the macronutrient composition of the participant's diet at baseline, the mean protein intake in all groups ranged from 10.58-12.7, whereas we considered 18% protein in both diets. There is no optimal strategy for macronutrient distribution or dietary interventions in nutritional recommendations for PCOS patients (29). Recent evidence suggests that high protein intake can improve IR (30).
However, protein intake should not exceed 20% of total calorie intake (29). From the perspective of dietary interventions in our study, the results of Dixon’s DASH diet index showed good adherence to the DASH diet. A meta-analysis of RCTs showed that the DASH diet can improve some glycemic parameters when implemented as a long-term intervention (> 16 wk) (31). Therefore, the addition of curcumin may enhance the anti-inflammatory effects of the DASH diet leading to faster recovery of hyperinsulinemia, and improve IR.
All participants in both diets received personalized nutrition with identical macronutrient components and sodium prescription, while being offered several carefully analyzed menu items. Fortunately, participants showed good adherence to diet and curcumin intake. On the other hand, several adjustment models intend to confound factors of glycemic parameters to prevent misleading conclusions in data analysis and interpretation. On the other hand, several adjustment models for confounding factors of glycemic parameters have been considered to avoid misleading conclusions in data analysis and interpretation.
4.1. Limitation
Dietary patterns can have long-term effects that could not be assessed due to the short duration of this study, which could lead to bias. Another source of bias could be related to seasonal food availability and preparation.
5. Conclusion
In conclusion, the present randomized clinical trials indicated that the co-administration of (1000 mg/day) curcumin and DASH diet for 12 wk, can have positive effects on reducing insulin levels, improving IR, and leading to faster recovery of hyperinsulinemia. Overall, the information presented here may highlight the potential of curcumin as a bioactive compound and future therapeutic target alongside a healthy diet for endocrine and metabolic diseases.
Data Availability
The data and materials of the current study are available from the corresponding author on reasonable request.
Author Contributions
H. Mozaffari-Khosravi, A. Aflatoonian, and T. Zohrabi designed and conducted the trial. A. Nadjaradeh and MH. Sheikhha provided scientific and technical support. H. Mozaffari-Khosravi and A. Aflatoonian supervised the study. S. Jambarsang carried out statistical analyses. T. Zohrabi interpreted the findings and drafted the manuscript. H. Mozaffari-Khosravi critically revised the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgments
The authors would like to acknowledge the “M/s Arjuna Natural Pvt Ltd., India”, for providing the curcumin supplement capsules (BCM95/Curcugreen) as a gift. The authors also thank the scientific support of “Yazd Reproductive Sciences Institute and Gynecology Clinic of Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Yazd, Iran”. This study was funded by Shahid Sadoughi University of Medical Sciences, Yazd, Iran (grant number: 8229, dated August 12, 2020). This trial has also been supported by Iran National, Science Foundation (INSF), (under Project No. 99019137 dated July 6, 2021). AI was not used in this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
a
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
References
1. Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab 2020; 35: 100937. [DOI:10.1016/j.molmet.2020.01.001] [PMID] [PMCID]
2. Manique MES, Ferreira AMAP. Polycystic ovary syndrome in adolescence: Challenges in diagnosis and management. Rev Bras Ginecol Obstet 2022; 44: 425-433. [DOI:10.1055/s-0042-1742292] [PMID] [PMCID]
3. Zhao H, Zhang J, Cheng X, Nie X, He B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J Ovarian Res 2023; 16: 9. [DOI:10.1186/s13048-022-01091-0] [PMID] [PMCID]
4. Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch 2020; 472: 1273-1298. [DOI:10.1007/s00424-020-02417-x] [PMID] [PMCID]
5. Wood AC, Jensen ET, Bertoni AG, Ramesh G, Rich SS, Rotter JI, et al. Defining the relative role of insulin clearance in early dysglycemia in relation to insulin sensitivity and insulin secretion: The microbiome and insulin longitudinal evaluation study (MILES). Metabolites 2021; 11: 420. [DOI:10.3390/metabo11070420] [PMID] [PMCID]
6. Thomas DD, Corkey BE, Istfan NW, Apovian CM. Hyperinsulinemia: An early indicator of metabolic dysfunction. J Endocr Soc 2019; 3: 1727-1747. [DOI:10.1210/js.2019-00065] [PMID] [PMCID]
7. Lewgood J, Oliveira B, Korzepa M, Forbes SC, Little JP, Breen L, et al. Efficacy of dietary and supplementation interventions for individuals with type 2 diabetes. Nutrients 2021; 13: 2378. [DOI:10.3390/nu13072378] [PMID] [PMCID]
8. Wolosowicz M, Prokopiuk S, Kaminski TW. Recent advances in the treatment of insulin resistance targeting molecular and metabolic pathways: Fighting a losing battle? Medicina 2022; 58: 472. [DOI:10.3390/medicina58040472] [PMID] [PMCID]
9. Esfandyari S, Bahadoran Z, Mirmiran P, Tohidi M, Azizi F. Adherence to the dietary approaches to stop hypertension trial (DASH) diet is inversely associated with incidence of insulin resistance in adults: The Tehran lipid and glucose study. J Clin Biochem Nutr 2017; 61: 123-129. [DOI:10.3164/jcbn.16-95] [PMID] [PMCID]
10. Campbell AP. DASH eating plan: An eating pattern for diabetes management. Diabetes Spectr 2017; 30: 76-81. [DOI:10.2337/ds16-0084] [PMID] [PMCID]
11. Asemi Z, Esmaillzadeh A. DASH diet, insulin resistance, and serum hs-CRP in polycystic ovary syndrome: A randomized controlled clinical trial. Horm Metab Res 2015; 47: 232-238. [DOI:10.1055/s-0034-1376990] [PMID]
12. Jakubczyk K, Drużga A, Katarzyna J, Skonieczna-Żydecka K. Antioxidant potential of curcumin-a meta-analysis of randomized clinical trials. Antioxidants (Basel) 2020; 9: 1092. [DOI:10.3390/antiox9111092] [PMID] [PMCID]
13. Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, et al. Turmeric and Its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol 2020; 11: 01021. [DOI:10.3389/fphar.2020.01021] [PMID] [PMCID]
14. Shen W, Qu Y, Jiang H, Wang H, Pan Y, Zhang Y, et al. Therapeutic effect and safety of curcumin in women with PCOS: A systematic review and meta-analysis. Front Endocrinol (Lausanne) 2022; 13: 1051111. [DOI:10.3389/fendo.2022.1051111] [PMID] [PMCID]
15. Yasmin A, Roychoudhury S, Paul Choudhury A, Ahmed ABF, Dutta S, Mottola F, et al. Polycystic ovary syndrome: an updated overview foregrounding impacts of ethnicities and geographic variations. Life (Basel) 2022; 12: 1974. [DOI:10.3390/life12121974] [PMID] [PMCID]
16. Zohrabi T, Sheikhha MH, Jambarsang S, Nadjarzadeh A, Aflatoonian A, Mozaffari-Khosravi H. Effect of dietary approaches to stop hypertension, and standard diets with and without curcumin on interleukin-1 alpha, 5-alpha reductase gene expressions, and androgenic and glycemic profile in polycystic ovary syndrome women undergoing in vitro fertilization treatment: A study protocol. Int J Reprod BioMed 2023; 21: 433-442. [DOI:10.18502/ijrm.v21i5.13496] [PMID] [PMCID]
17. Steinberg D, Kay M, Burroughs J, Svetkey LP, bennett GG. The effect of a digital behavioral weight loss intervention on adherence to the dietary approaches to stop hypertension (dash) dietary pattern in medically vulnerable primary care patients: Results from a randomized controlled trial. J Acad Nutr Diet 2019; 119: 574-584. [DOI:10.1016/j.jand.2018.12.011] [PMID] [PMCID]
18. Chien YJ, Chang CY, Wu MY, Chen CH, Horng YS, Wu HC. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: Systematic review with meta-analysis and trial sequential analysis. Nutrients 2021; 13: 684. [DOI:10.3390/nu13020684] [PMID] [PMCID]
19. Barber TM, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2021; 95: 531-541. [DOI:10.1111/cen.14421] [PMID]
20. Koh H-CE, Cao C, Mittendorfer B. Insulin clearance in obesity and type 2 diabetes. Int J Mol Sci 2022; 23: 596. [DOI:10.3390/ijms23020596] [PMID] [PMCID]
21. Kim Y, Rouse M, González-Mariscal I, Egan JM, O'Connell JF. Dietary curcumin enhances insulin clearance in diet-induced obese mice via regulation of hepatic PI3K-AKT axis and IDE, and preservation of islet integrity. Nutr Metab (Lond) 2019; 16: 48. [DOI:10.1186/s12986-019-0377-0] [PMID] [PMCID]
22. Clark CCT, Ghaedi E, Arab A, Pourmasoumi M, Hadi A. The effect of curcumin supplementation on circulating adiponectin: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr 2019; 13: 2819-2825. [DOI:10.1016/j.dsx.2019.07.045] [PMID]
23. Okura T, Fujioka Y, Nakamura R, Anno M, Ito Y, Kitao S, et al. Hepatic insulin clearance is increased in patients with high HbA1c type 2 diabetes: A preliminary report. BMJ Open Diabetes Res Care 2020; 8: e001149. [DOI:10.1136/bmjdrc-2019-001149] [PMID] [PMCID]
24. Patil S, Veerabhadra Goud GK, Shivashankar RN, Anusuya SK, Ganesh V. Association of adiponectin levels with polycystic ovary syndrome among Indian women. Bioinformation 2022; 18: 864-869. [DOI:10.6026/97320630018864] [PMID] [PMCID]
25. Li H, Meng Y, He S, Tan X, Zhang Y, Zhang X, et al. Macrophages, chronic inflammation, and insulin resistance. Cells 2022; 11: 3001. [DOI:10.3390/cells11193001] [PMID] [PMCID]
26. Ou M-Y, Zhang H, Tan P-C, Zhou S-B, Li Q-F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis 2022; 13: 300. [DOI:10.1038/s41419-022-04752-6] [PMID] [PMCID]
27. Dewi RC, Rimawati N, Purbodjati P. Body mass index, physical activity, and physical fitness of adolescence. J Public Health Res 2021; 10: 2230. [DOI:10.4081/jphr.2021.2230] [PMID] [PMCID]
28. Moslehi N, Zeraattalab-Motlagh Sh, Rahimi Sakak F, Shab-Bidar S, Ramezani Tehrani F, Mirmiran P. Effects of nutrition on metabolic and endocrine outcomes in women with polycystic ovary syndrome: An umbrella review of meta-analyses of randomized controlled trials. Nutr Rev 2023; 81: 555-577. [DOI:10.1093/nutrit/nuac075] [PMID]
29. Hmedeh C, Ghazeeri G, Tewfik I. Nutritional management in polycystic ovary syndrome: Challenges and opportunities. International Journal of Food Safety, Nutrition and Public Health 2021; 6: 120-130. [DOI:10.1504/IJFSNPH.2021.113389]
30. Akhavan NS, Pourafshar S, Johnson SA, Foley EM, George KS, Munoz J, et al. The relationship between Protein Intake and Source on Factors Associated with Glycemic Control in Individuals with Prediabetes and Type 2 Diabetes. Nutrients 2020; 12: 2031. [DOI:10.3390/nu12072031] [PMID] [PMCID]
31. Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of dietary approaches to stop hypertension (DASH) diet on some risk for developing type 2 diabetes: A systematic review and meta-analysis on controlled clinical trials. Nutrition 2013; 29: 939-947. [DOI:10.1016/j.nut.2012.12.021] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




