Sun, Jul 13, 2025
[Archive]
Volume 22, Issue 9 (September 2024)
IJRM 2024, 22(9): 727-738 |
Back to browse issues page
Ethics code: IR.SUMS.MED.REC.1396.S184
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Akbarzadeh-Jahromi M, Taheri T, Sari Aslani F, Safaei A, Pouraminaee F, Zare M. Diagnosis of hydatidiform moles using p57 immunohistochemistry and chromogenic insitu hybridization: A retrospective study. IJRM 2024; 22 (9) :727-738
URL: http://ijrm.ir/article-1-3282-en.html
URL: http://ijrm.ir/article-1-3282-en.html
Mojgan Akbarzadeh-Jahromi1 

 , Tara Taheri2
, Tara Taheri2 

 , Fatemeh Sari Aslani1
, Fatemeh Sari Aslani1 

 , Akbar Safaei2
, Akbar Safaei2 

 , Fatemeh Pouraminaee2
, Fatemeh Pouraminaee2 

 , Marjan Zare *3
, Marjan Zare *3 




 , Tara Taheri2
, Tara Taheri2 

 , Fatemeh Sari Aslani1
, Fatemeh Sari Aslani1 

 , Akbar Safaei2
, Akbar Safaei2 

 , Fatemeh Pouraminaee2
, Fatemeh Pouraminaee2 

 , Marjan Zare *3
, Marjan Zare *3 


1- Pathology Department, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran. & Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Pathology Department, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. ,marjan.zare@gmail.com
2- Pathology Department, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
3- Maternal-Fetal Medicine Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. ,
Full-Text [PDF 6962 kb]
(393 Downloads)
| Abstract (HTML) (436 Views)
2.6. Ethical Considerations
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Code: IR.SUMS.MED.REC.1396.S184). The work process was completely anonymous, and the results were reported to the patients. All the study steps agreed with the Helsinki Declaration of 1964.
2.7. Statistical Analysis
Frequency and relative frequency were used to describe quantitative variables; in addition, Chi-square and Fisher’s exact tests were used to analyze the data; Cohen's Kappa coefficient was also used to measure intra-rater reliability for qualitative items; Kappa coefficient between 0.01-0.20, 0.21-0.40, 0.41-0.60, 0.61-0.80, and 0.81-1.00 were slight, fair, moderate, substantial, and perfect agreements, respectively (https://idostatistics.com/cohen-kappa-free-calculator/#risultati), sensitivity, specificity, and accuracy with 95% CI were also estimated. IBM SPSS v.22 and MedCal v.20.015 software tools at a statistical significance level < 0.05 were used for all tests. Post hoc power analysis was done to estimate the power of a test given an observed effect size at the end of the study (https://wiki.socr.umich.edu/index.php/SMHS_PowerSensitivitySpecificity).

In agreement with the current result, it was reported that morphology by itself cannot result in an accurate diagnosis of PHM; they also showed that a genotype study is also necessary to differentiate between PHM and CHM (20).
A study done on 89 POCs containing 54 PHM and 35 HA showed that fibrillary.C was more common in HA, while T. Atypia, MTP, and cistern were more common in PHM. These findings were in line with the current results. Also, they presented an older maternal age in HA in comparison with PHM (average age of 37.2 vs. 29.5 yr); however, no difference was observed between groups regarding gestational age (10). Moreover, in our study, no significant differences were observed between groups in terms of maternal age and gestational age.
In a study performed on 45 POCs consisting of 36 CHM and 9 PHM, classified according to the morphology criteria, including T. Atypia, cistern, large-INC, small-INC, and lace-like fibrillary.C, no significant differences were observed between groups in terms of gestational age and maternal age, which is in line with our study (21).
It was revealed that flow cytometry karyotyping for detecting the presence or absence of maternal genome content was the gold standard, also CISH for DNA ploidy assessment was used. The pericentromeric area of chromosomes 11, 16, X, and Y were labeled by biotin-1. A pair of 6 primers on 4 different chromosomes were tested by genotyping 34/45 cases including 31/36 CHM and 3/9 PHM that resulted in no maternal genome content which was genetically introduced as CHM. About 11/45 cases including 5/36 CHM and 6/9 PHM, presented maternal genome content, which was genetically introduced as PHM. In the cited study, morphologic diagnosis and the genetic result showed a correlation of 88% (37/45 cases). This technique was compared with CISH, and it was concluded that all HMs with maternal genome content were presented with a triploid pattern and all HMs without maternal content were presented with a diploid pattern (21). Their findings strengthen the accuracy of the CISH technique for differentiating CHM from PHM, which is in line with the current work.
In comparison with the histology and genetic study, they concluded that morphology criteria were more reliable for diagnosis of CHM than PHM (91% or 31/34 than 55% or 6/11, p < 0.001). Our results also showed that morphology was more reliable for CHM diagnosis than PHM. In a comparison of the CISH and genetic study, a good agreement between the 2 techniques for the diagnosis of the hydatidiform mole was seen, especially in the cases with challenging morphologic pictures (21).
A study conducted on 22 POCs included 6 CHM, 10 PHM, and 6 HA, after histological evaluation according to the morphologic criteria, they used the CISH technique with a chromosome 17 probe (HER 2/CEN), dual-colored (red and blue), on all specimens. In our study, morphologic criteria and DNA ploidy techniques were the same. Their results of counting red and blue signals in the trophoblastic cells and stromal cells were as follows: 9/10 (90%) of histologically diagnosed PHM showed a HER 2 signal value of 2.92 (triploid) and 1/10 PHM had a HER 2 signal value of 2.5. Decidua tissue was selected as an internal control. A total of 11/12 remaining cases (91.7%) presented a HER 2 signal value of 2.06 and 1/12 histologically diagnosed hydropic changes presented a HER 2 signal value of 2.35. Hence, they concluded that CISH chromosome 17 to differentiate diploid from triploid had a sensitivity of 90%, a specificity of 91.6%, and an agreement of 90.9%, which was in line with our study. Also similar to our study, no significant difference was observed between the patients in terms of maternal or gestational age (9).
In a study on 51 POCs, including 18 CHMs, 24 PHMs, and 9 HAs based on morphology characters, they performed a diagnostic algorithm for the diagnosis of HM according to p57 immunohistochemistry and a DNA ploidy FISH study. All 18 (100%) cases with morphology diagnosed as CHMs demonstrated a diploid genotype. Among 24 cases with PHMs, only 9 (37.5%) cases had a triploid genotype, and the remaining (62.5%) had a diploid genotype. All cases with HA (100%) displayed a diploid genotype. In this study, after a combination of p57 immunohistochemistry, DNA ploidy FISH study, and morphology in 51 cases, the diagnostic results were as follows: 27 CHMs, 9 PHMs, and 15 HAs. The results showed that the diagnostic accuracy based on morphology alone in the diagnosis of CHM and PHM was 78.4% and 70.6%, respectively. It can lead to a misdiagnosis, so the use of other diagnostic methods such as FISH and CISH is recommended in this study, empowering the current study’s aim (22).
4.1. Strength and limitation
The utmost effort and precision have been made in defining and subtracting the variables and outcomes; all steps have been carefully monitored and checked to minimize possible bias and enhance the validity of the results. As the other strong point of the work, 3 pathologists got involved in the morphology assessment of the PHM, CHM, and HA to minimize the error. Although not all POCs were included in the study and we faced attrition of POCs, the sample size was large enough to detect the differences compared with the previous works. A limitation of the work was the retrospective design, which resulted in the unavailability of fresh specimens to conduct the gold standard methods like FISH and flow cytometry karyotyping. These supplementary tests could compare the results and improve the accuracy of the diagnosis.
5. Conclusion
Based on the current finding, CISH2 and CISH17 enjoyed perfect agreement in diagnosing HMs; in addition, their absolute power discriminating between triploid and diploid revealed that they could be used as surrogate markers for ploidy. From 44 POCs, 31, 10, and 3 cases were diagnosed as PHM, CHM, and HA based on the morphology diagnosis. It is easy to diagnose CHM by morphology unless it is in lower gestational age or early CHM which is confused with HA. Based on the current findings, it is better to evaluate and diagnose early CHM by doing p57 before sending samples suspected of PHM for CISH diagnosis; although, to increase the accuracy of the diagnosis, it is suggested to perform the test with 2 chromosomes (2 and 17). Due to the high agreement coefficient between CISH2 and CISH17, lower prevalence of trisomy of chromosome 17 in POCs, no need for fresh specimens of POCs, and CISH17 availability in laboratories, it is more cost-effective to perform only CISH17. Prospective studies on fresh specimens are suggested comparing the CISH method's accuracy with other ploidy studies, such as flow cytometry karyotyping and FISH. Some advantages and disadvantages of CISH compared with flow cytometry karyotyping and FISH are as follows:
Data Availability
Data is available at corresponding author contact (marjan.zare@gmail.com).
Author Contributions
M. Akbarzadeh-Jahromi contributed to the conception, design, conduction, supervision, and drafting the manuscript. T. Taheri, F. Sari Aslani, A. Safaei, and F. Pouraminaee contributed to the conception, design, conduction, collection of the data, and drafting the manuscript. M. Zare contributed to the design, analysis of the data, interpretation of the results, drafting the manuscript, and reviewing the work critically. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
No AI was used for writing, editing or grammar check.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (67 Views)
- Introduction
Spontaneous abortion is pregnancy loss before the 20th wk of gestational age, which occurred in 10-15% of pregnancies (1-3). In spontaneous abortions, the distinction between hydatidiform moles (HMs) including complete hydatidiform mole (CHM) and partial hydatidiform mole (PHM) and nonmolar hydropic abortion (HA) are essential (1, 4-8). HMs cannot be diagnosed by morphologic examination alone; DNA ploidy analysis is useful in the classification of PHMs as triploids and CHMs as diploids. Loss of p57 immunohistochemistry, which is a protein product of the paternally imprinted and maternally expressed Cyclin Dependent Kinase Inhibitor 1C gene, occurs in all CHMs but not in PHMs and is used to differentiate between PHM and CHM. Persistence or transformation into a malignant disease that requires chemotherapy has been estimated to occur in 0.5-5% of PHMs (9, 10); hence, an accurate diagnosis of PHM is essential (10, 11). Also, the distinction between trisomic pregnancy and PHM is difficult in early moles (9, 10, 12, 13). The PHM diagnosis is done with ploidy study; flow cytometry karyotyping is a versatile technique needing freshly frozen specimens; in addition, fluorescence in situ hybridization (FISH) is another effective DNA ploidy with accuracy in both freshly frozen and paraffin-embedded specimens (10, 14). However, FISH requires immunofluorescence staining technique which makes it costly and is not accessible in most laboratories. Since fresh tissue samples for cytogenetics are not always available and many laboratories do not perform ploidy analysis on paraffin sections (9, 15), we studied chromogenic in situ hybridization (CISH) with a probe to the chromosome 17 (HER 2) gene (ZytoDot HER-2/CEN-17) (CISH17) and chromosome 2 (CISH2) discriminating chromosomal ploidy of PHM, CHM, and non-molar HA ancillary with p57 immunohistochemistry; formalin-fixed, paraffin-embedded tissue samples were readily available in laboratory archives and have a quick turnaround time. We postulated that they could also be used as a surrogate marker for ploidy in for the evaluation of triploid and diploid in product of conception specimens (POCs).
2. Materials and Methods
2.1. Study design
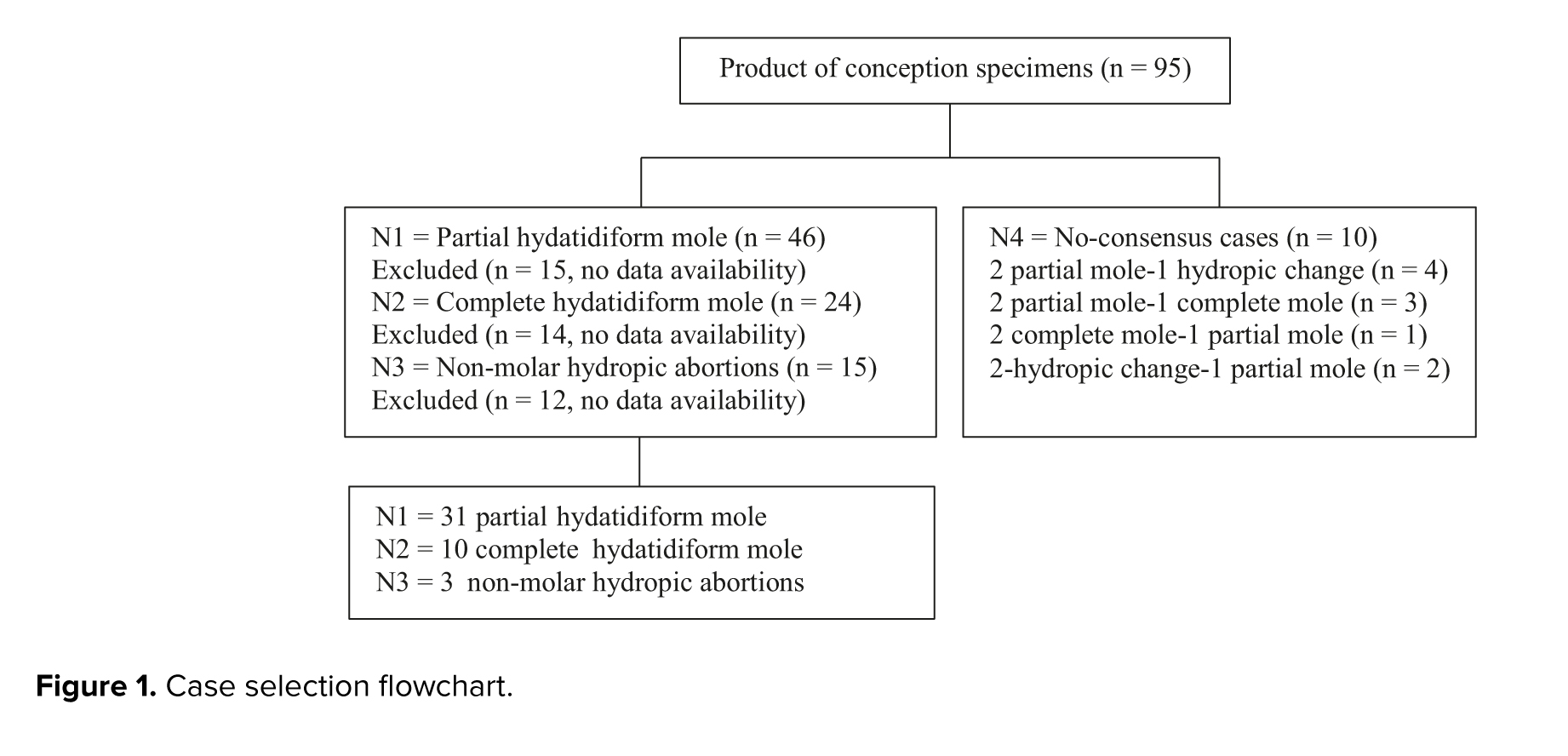
In a retrospective study, all 95 POCs having either CHM, PHM, or HA referred to Shahid Faghihi and Hazrate Zeinab hospital histopathological centers in Shiraz, Iran. Between June 2010-2015, 44 statistically significant POCs were selected based on the morphological characteristics agreed upon by 3 pathologists. Maternal and gestational ages ranged between 18 and 40 yr and 8-25 wk, respectively. The case selection flowchart is presented in figure 1.
2.2. Sample size consideration
We, retrospectively, included all statistically significant POCs diagnosed in histopathological centers over a 5 yr period.
2.3. Morphological review
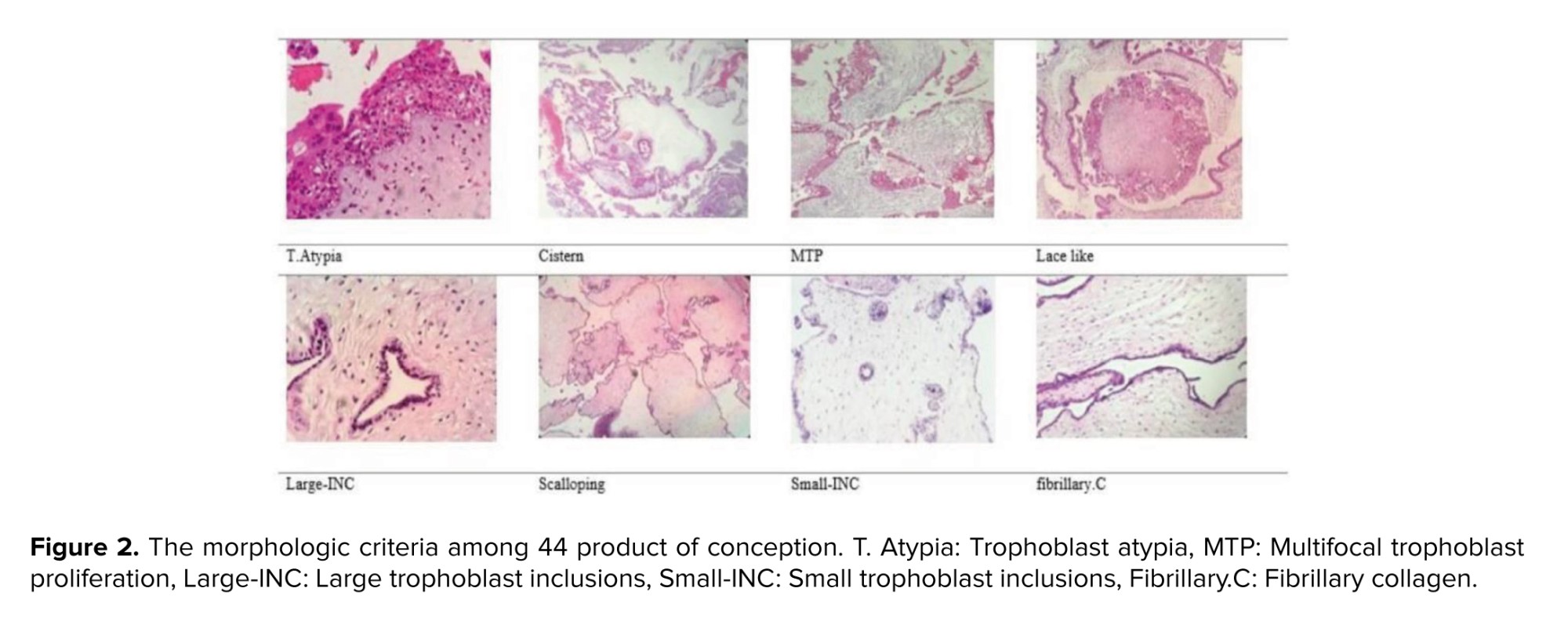
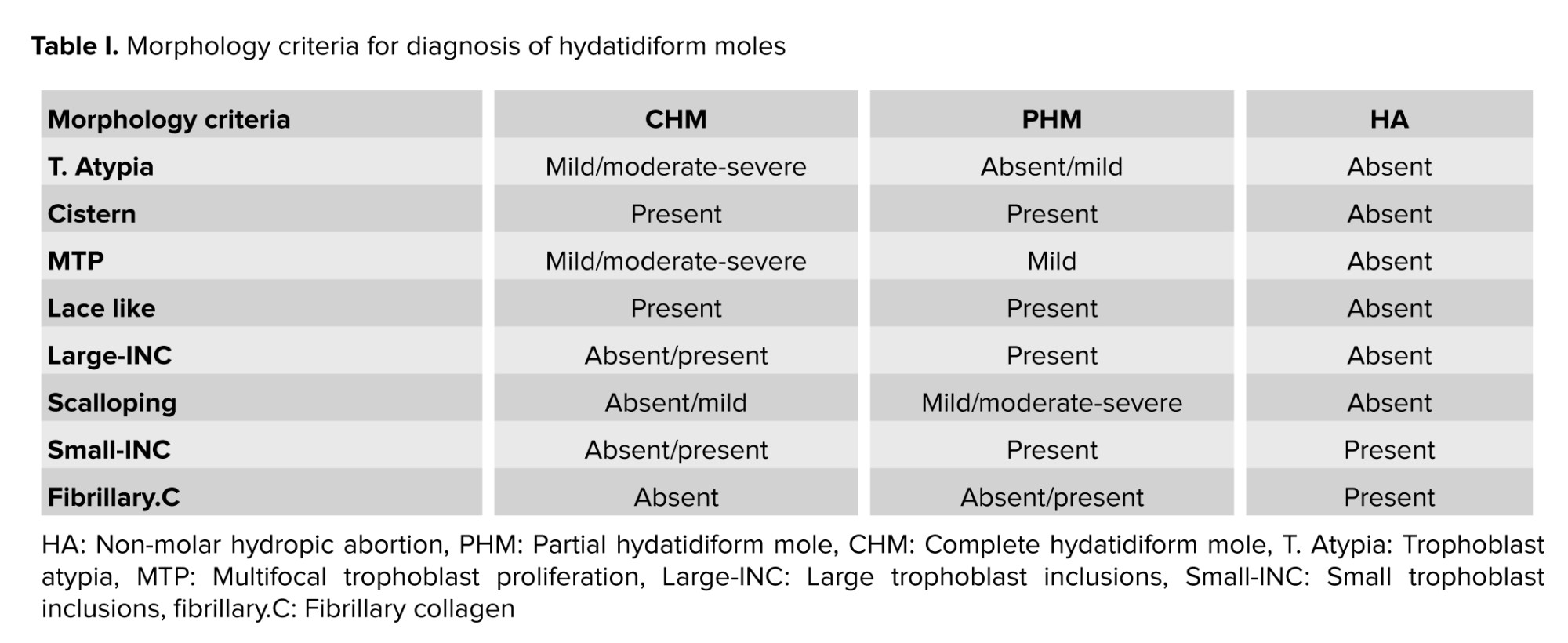
Trophoblast Atypia (T. Atypia) was described as triple variation in nuclear size, nuclear widening with pleomorphism, and/or hyperchromasia. T. Atypia was detectable at standard magnification (×100). It was measured using syncytiotrophoblast, intermediate trophoblast, cytotrophoblast, and areas. Cistern formation (Cistern) was described as completely “acellular cavities having edema fluid within the center of the villi”, which involved minimum 50% of the terminal villi. Though the villi offered deteriorating alterations, well-formed cisterns were considered. “Pseudo-cisterns subsequent bends in the stem villi or in the placental membranes” were omitted. Multifocal trophoblast proliferation (MTP) needs the manifestation of 2 or more distinct foci of trophoblast proliferation distributed along the surface of the villi. Although circumferential trophoblast was seldom identified, it was also included. Lace-like was defined as prominent intracytoplasmic lacunae developing in intermediate trophoblast and cytotrophoblast, usually in an extravillous distribution and distinguishable at ×40 to ×100 magnification. Large trophoblast inclusions (large-INC) resulted from dividing across irregular villous outlines, which resulted in irregularly shaped inclusions of various sizes but > 0.2 mm in diameter. Scalloping was described by several regular invaginations in the contours of enlarged villi. Small trophoblastic inclusions (small-INC) that did not appear in continuity with the surface trophoblast were rounded no more than 0.2 mm in diameter. Fibrillary collagen (fibrillary.C) included curvy collagen bundles leaning along the long axis of the larger villi.
All specimens were independently reviewed by 3 pathologists and classified as PHM, CHM, and HA based on morphology findings of table I (1, 16).
2.4. p57 Immunohistochemistry
Sections of 4 µm tissue were located on covered slides and immune stained using a “Leica Bond Max Autostainer (Leica Biosystems, Wetzlar, Germany), lyophilized mouse monoclonal antibody (clone 25B2 at 1:50 dilution, Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), a bond polymer refine detection/polymeric horseradish peroxidase-linker antibody conjugate system (Leica Biosystems), and against p57 protein (Kip2)”. The valuation of p57 staining was based on “the presence or absence of nuclear staining of cytotrophoblasts and villous stromal cells”. “Diffuse nuclear staining in cytotrophoblasts and villous stromal cells were considered as positive result (PHM or HA)”. “Absent or < 10% nuclear staining in the cytotrophoblasts and villous stromal cells was considered negative” (17).
2.5. CISH
Samples of 4 µm thickness tissue were prepared from paraffin-embedded blocks in which chorionic elements had been confirmed with H&E slides and sent for CISH. All specimens underwent CISH with a probe to the chromosome 17 (HER 2) gene (ZytoDot HER-2/CEN-17) and CISH2. CISH for Chr 2 and Chr 17 for PHM was defined as 3 green dots or 3 red dots in trophoblastic cells and stromal cells (triploid). CISH for Chr 2 and Chr 17 for CHM or HA change or trisomic pregnancy was defined as 2 red dots or 2 green dots in trophoblastic cells and villi stromal cells (diploid) (10, 18, 19). p57, CISH2, and CISH17 tests were used respectively as complementary tests differentiating CHM, PHM, and HA.
2. Materials and Methods
2.1. Study design
In a retrospective study, all 95 POCs having either CHM, PHM, or HA referred to Shahid Faghihi and Hazrate Zeinab hospital histopathological centers in Shiraz, Iran. Between June 2010-2015, 44 statistically significant POCs were selected based on the morphological characteristics agreed upon by 3 pathologists. Maternal and gestational ages ranged between 18 and 40 yr and 8-25 wk, respectively. The case selection flowchart is presented in figure 1.
2.2. Sample size consideration
We, retrospectively, included all statistically significant POCs diagnosed in histopathological centers over a 5 yr period.
2.3. Morphological review
Trophoblast Atypia (T. Atypia) was described as triple variation in nuclear size, nuclear widening with pleomorphism, and/or hyperchromasia. T. Atypia was detectable at standard magnification (×100). It was measured using syncytiotrophoblast, intermediate trophoblast, cytotrophoblast, and areas. Cistern formation (Cistern) was described as completely “acellular cavities having edema fluid within the center of the villi”, which involved minimum 50% of the terminal villi. Though the villi offered deteriorating alterations, well-formed cisterns were considered. “Pseudo-cisterns subsequent bends in the stem villi or in the placental membranes” were omitted. Multifocal trophoblast proliferation (MTP) needs the manifestation of 2 or more distinct foci of trophoblast proliferation distributed along the surface of the villi. Although circumferential trophoblast was seldom identified, it was also included. Lace-like was defined as prominent intracytoplasmic lacunae developing in intermediate trophoblast and cytotrophoblast, usually in an extravillous distribution and distinguishable at ×40 to ×100 magnification. Large trophoblast inclusions (large-INC) resulted from dividing across irregular villous outlines, which resulted in irregularly shaped inclusions of various sizes but > 0.2 mm in diameter. Scalloping was described by several regular invaginations in the contours of enlarged villi. Small trophoblastic inclusions (small-INC) that did not appear in continuity with the surface trophoblast were rounded no more than 0.2 mm in diameter. Fibrillary collagen (fibrillary.C) included curvy collagen bundles leaning along the long axis of the larger villi.
All specimens were independently reviewed by 3 pathologists and classified as PHM, CHM, and HA based on morphology findings of table I (1, 16).
2.4. p57 Immunohistochemistry
Sections of 4 µm tissue were located on covered slides and immune stained using a “Leica Bond Max Autostainer (Leica Biosystems, Wetzlar, Germany), lyophilized mouse monoclonal antibody (clone 25B2 at 1:50 dilution, Novocastra Laboratories Ltd., Newcastle upon Tyne, UK), a bond polymer refine detection/polymeric horseradish peroxidase-linker antibody conjugate system (Leica Biosystems), and against p57 protein (Kip2)”. The valuation of p57 staining was based on “the presence or absence of nuclear staining of cytotrophoblasts and villous stromal cells”. “Diffuse nuclear staining in cytotrophoblasts and villous stromal cells were considered as positive result (PHM or HA)”. “Absent or < 10% nuclear staining in the cytotrophoblasts and villous stromal cells was considered negative” (17).
2.5. CISH
Samples of 4 µm thickness tissue were prepared from paraffin-embedded blocks in which chorionic elements had been confirmed with H&E slides and sent for CISH. All specimens underwent CISH with a probe to the chromosome 17 (HER 2) gene (ZytoDot HER-2/CEN-17) and CISH2. CISH for Chr 2 and Chr 17 for PHM was defined as 3 green dots or 3 red dots in trophoblastic cells and stromal cells (triploid). CISH for Chr 2 and Chr 17 for CHM or HA change or trisomic pregnancy was defined as 2 red dots or 2 green dots in trophoblastic cells and villi stromal cells (diploid) (10, 18, 19). p57, CISH2, and CISH17 tests were used respectively as complementary tests differentiating CHM, PHM, and HA.


2.6. Ethical Considerations
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Code: IR.SUMS.MED.REC.1396.S184). The work process was completely anonymous, and the results were reported to the patients. All the study steps agreed with the Helsinki Declaration of 1964.
2.7. Statistical Analysis
Frequency and relative frequency were used to describe quantitative variables; in addition, Chi-square and Fisher’s exact tests were used to analyze the data; Cohen's Kappa coefficient was also used to measure intra-rater reliability for qualitative items; Kappa coefficient between 0.01-0.20, 0.21-0.40, 0.41-0.60, 0.61-0.80, and 0.81-1.00 were slight, fair, moderate, substantial, and perfect agreements, respectively (https://idostatistics.com/cohen-kappa-free-calculator/#risultati), sensitivity, specificity, and accuracy with 95% CI were also estimated. IBM SPSS v.22 and MedCal v.20.015 software tools at a statistical significance level < 0.05 were used for all tests. Post hoc power analysis was done to estimate the power of a test given an observed effect size at the end of the study (https://wiki.socr.umich.edu/index.php/SMHS_PowerSensitivitySpecificity).
- Results
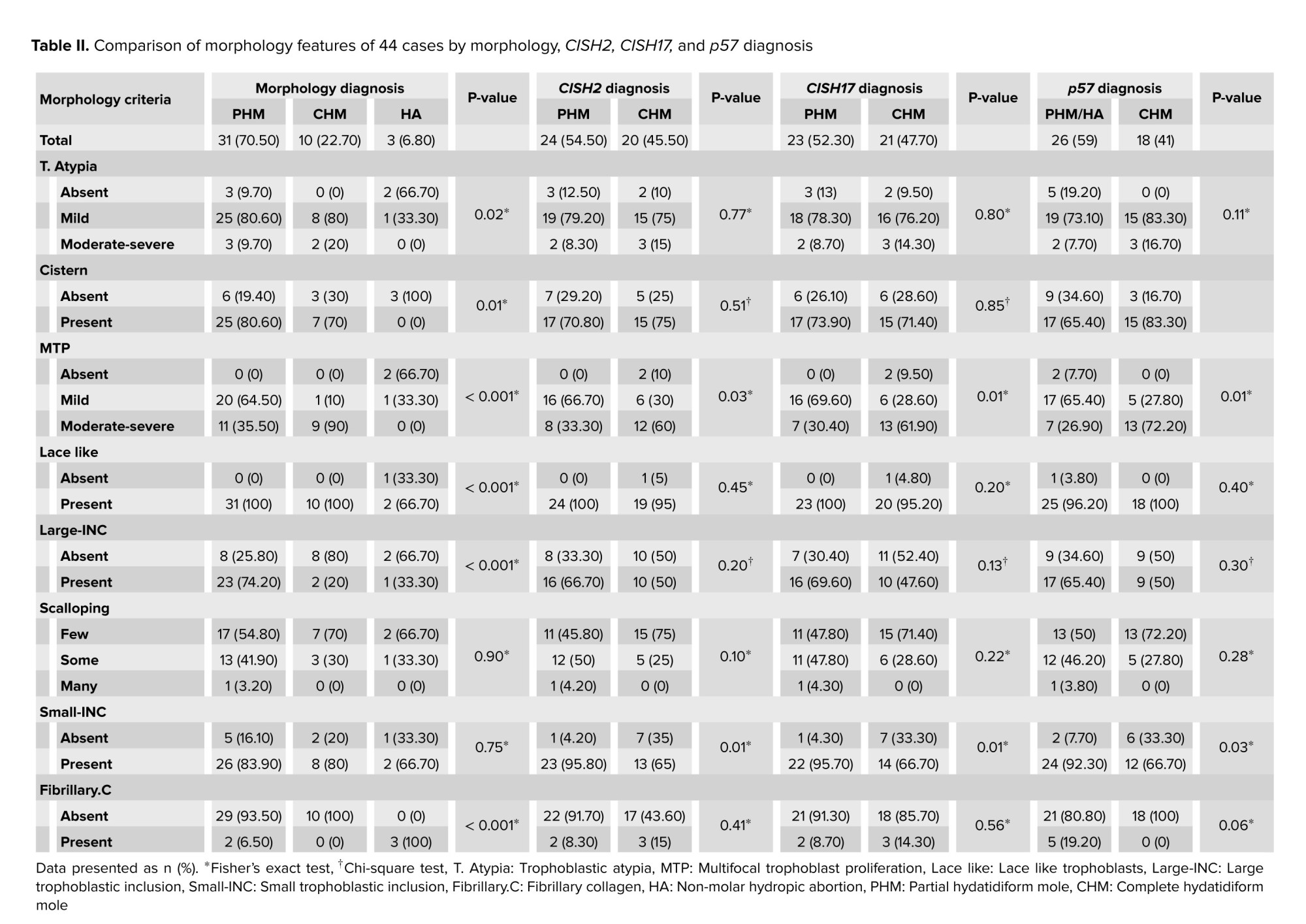
Of 44 POCs, 70.5% (31/44), 22.7% (10/44), and 6.8% (3/44) of cases were diagnosed with PHM, CHM, and HA based on morphologic diagnosis, respectively; the morphologic criteria have been presented in figure 2. In addition, no significant difference was observed between PHM, CHM, and HA in terms of maternal age (p = 0.34) and gestational age (p = 0.42). The Kappa agreement coefficient between the 3 pathologists was estimated at 95% for PHMs. The morphological features of 44 cases by morphology, CISH2, CISH17, and p57 diagnoses have been compared in table II.
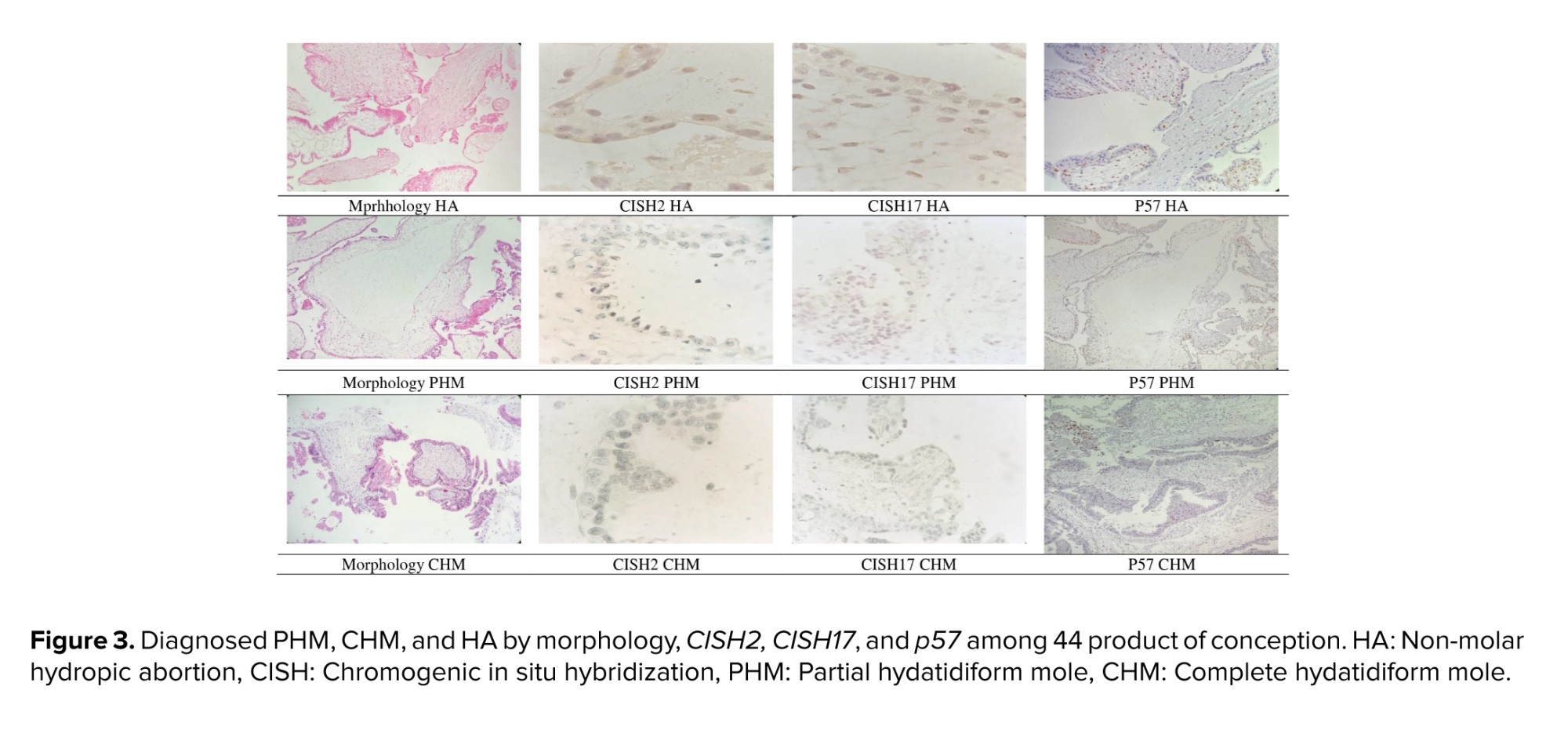
In morphologic diagnosis, T. Atypia, cistern, MTP, lace-like, large INC, and fibrillary.C were significantly different among PHM, CHM, and HA groups; however, no significant differences were observed in scalloping and small-INC. In CISH 2, CISH 17, and p57 diagnosis groups, MTP and small-INC were significantly different; however, T. Atypia, cistern, lace-like, large INC, fibrillary.C, and scalloping were not significantly different. Diagnosed PHM, CHM, and HA by morphology, CISH2, CISH17, and p57 have been presented in figure 3.
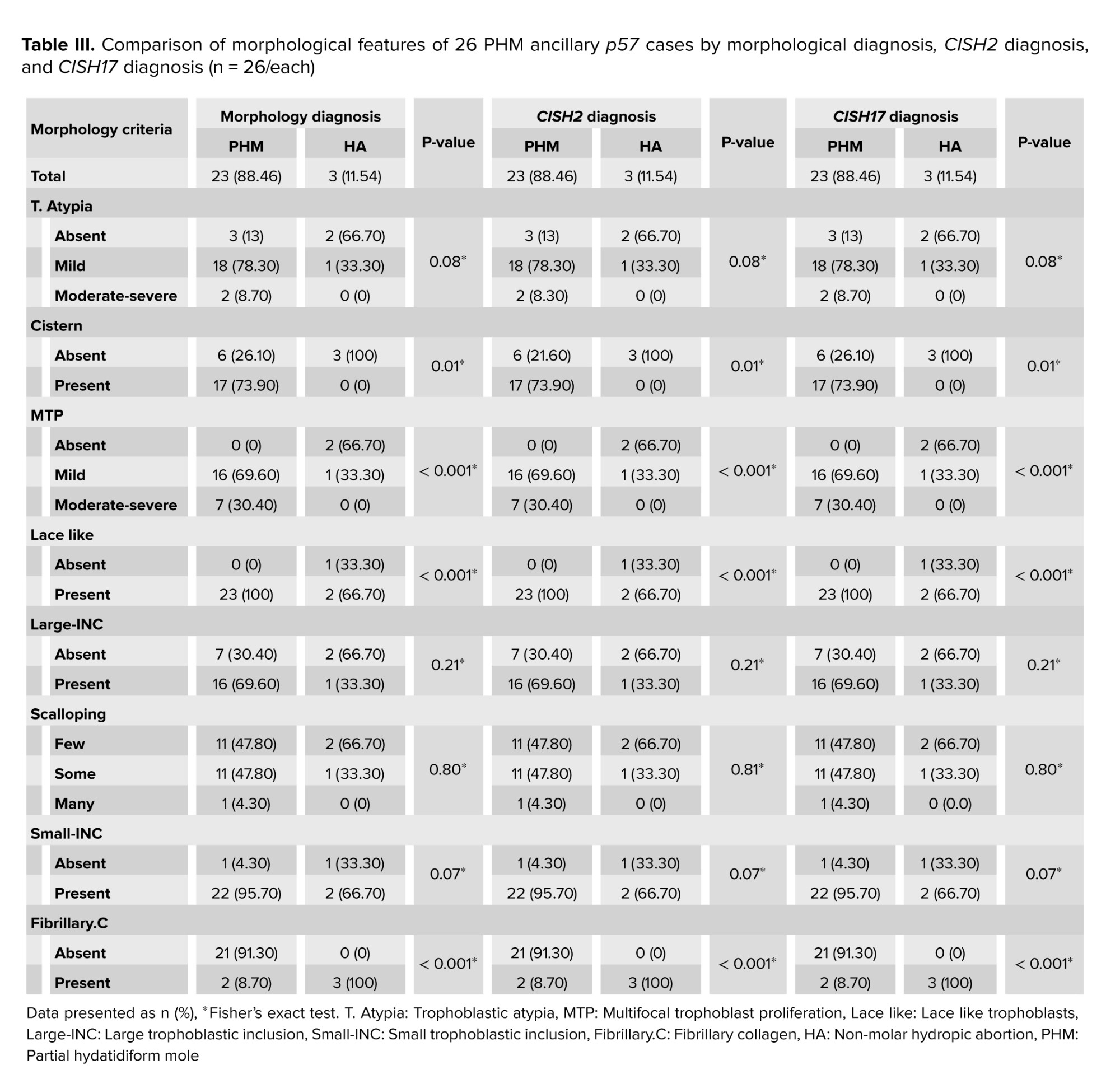
18 out of 44 (40.9%) cases were diagnosed with CHM based on p57. The morphological features of the remaining 26 PHM cases by morphology, CISH2, and CISH17 diagnosis have been compared in table III.
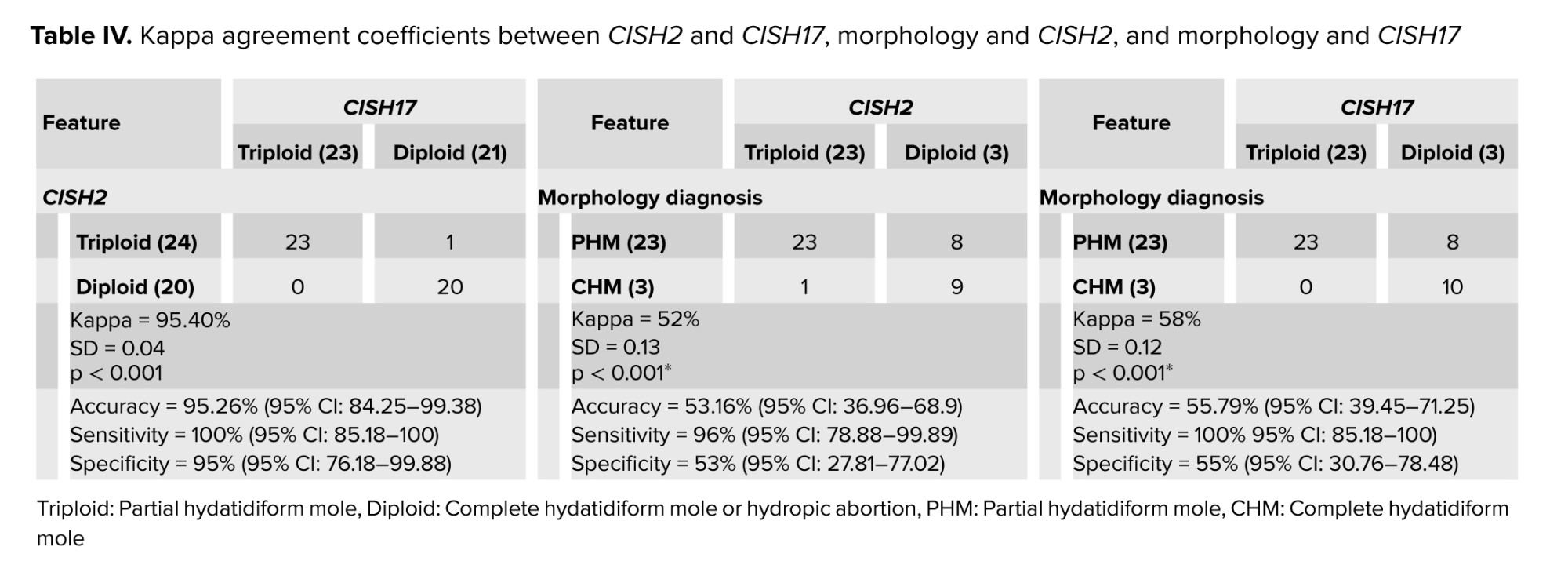
PHM and HA were 23 and 3 cases for CISH2 and CISH17. In morphology, CISH2 and CISH17 diagnostic groups, cistern, MTP, lace-like, and fibrillary.C were significantly different among PHM and HA groups; however, T. Atypia, large INC, scalloping, and small INC did not significantly differ. The Kappa agreement coefficient between CISH2 and CISH17 has been shown in table IV.
There were perfect agreements between CISH2 and CISH17 when diagnosing the chromosomal ploidy of PHM (23), CHM (20), and HA (1); however, the agreements between morphology diagnosis and CISH2 and morphology diagnosis and CISH17 were moderate.
Post hoc power analysis of CISH2 and CISH17 discriminating between triploid and diploid in POCs given the estimated sensitivity and specificity has been presented in table V.
In morphologic diagnosis, T. Atypia, cistern, MTP, lace-like, large INC, and fibrillary.C were significantly different among PHM, CHM, and HA groups; however, no significant differences were observed in scalloping and small-INC. In CISH 2, CISH 17, and p57 diagnosis groups, MTP and small-INC were significantly different; however, T. Atypia, cistern, lace-like, large INC, fibrillary.C, and scalloping were not significantly different. Diagnosed PHM, CHM, and HA by morphology, CISH2, CISH17, and p57 have been presented in figure 3.
18 out of 44 (40.9%) cases were diagnosed with CHM based on p57. The morphological features of the remaining 26 PHM cases by morphology, CISH2, and CISH17 diagnosis have been compared in table III.
PHM and HA were 23 and 3 cases for CISH2 and CISH17. In morphology, CISH2 and CISH17 diagnostic groups, cistern, MTP, lace-like, and fibrillary.C were significantly different among PHM and HA groups; however, T. Atypia, large INC, scalloping, and small INC did not significantly differ. The Kappa agreement coefficient between CISH2 and CISH17 has been shown in table IV.
There were perfect agreements between CISH2 and CISH17 when diagnosing the chromosomal ploidy of PHM (23), CHM (20), and HA (1); however, the agreements between morphology diagnosis and CISH2 and morphology diagnosis and CISH17 were moderate.
Post hoc power analysis of CISH2 and CISH17 discriminating between triploid and diploid in POCs given the estimated sensitivity and specificity has been presented in table V.






4. Discussion
From 44 POCs, 31, 10, and 3 cases were diagnosed as PHM, CHM, and HA based on the morphology diagnosis by 3 pathologists with a 95% agreement for PHM. Moreover, there was perfect agreement between CISH2 and CISH17, differentiating triploidy and diploidy. It is easy to diagnose CHM by morphology unless it is in lower gestational age or early CHM which is confused with HA. Based on the current findings, it is better to evaluate and diagnose early CHM by doing p57 before sending samples suspected of PHM for CISH diagnosis. To increase the accuracy of the diagnosis, it is suggested to perform the test with 2 chromosomes (2 and 17). Due to the high agreement coefficient between CISH2 and CISH17, lower prevalence of trisomy of chromosome 17 in POCs, and CISH17 more availability in laboratories, it is more cost-effective to do only CISH17. Further studies are suggested to compare the CISH method's accuracy with other ploidy studies, such as flow cytometry karyotyping and FISH. In agreement with the current result, it was reported that morphology by itself cannot result in an accurate diagnosis of PHM; they also showed that a genotype study is also necessary to differentiate between PHM and CHM (20).
A study done on 89 POCs containing 54 PHM and 35 HA showed that fibrillary.C was more common in HA, while T. Atypia, MTP, and cistern were more common in PHM. These findings were in line with the current results. Also, they presented an older maternal age in HA in comparison with PHM (average age of 37.2 vs. 29.5 yr); however, no difference was observed between groups regarding gestational age (10). Moreover, in our study, no significant differences were observed between groups in terms of maternal age and gestational age.
In a study performed on 45 POCs consisting of 36 CHM and 9 PHM, classified according to the morphology criteria, including T. Atypia, cistern, large-INC, small-INC, and lace-like fibrillary.C, no significant differences were observed between groups in terms of gestational age and maternal age, which is in line with our study (21).
It was revealed that flow cytometry karyotyping for detecting the presence or absence of maternal genome content was the gold standard, also CISH for DNA ploidy assessment was used. The pericentromeric area of chromosomes 11, 16, X, and Y were labeled by biotin-1. A pair of 6 primers on 4 different chromosomes were tested by genotyping 34/45 cases including 31/36 CHM and 3/9 PHM that resulted in no maternal genome content which was genetically introduced as CHM. About 11/45 cases including 5/36 CHM and 6/9 PHM, presented maternal genome content, which was genetically introduced as PHM. In the cited study, morphologic diagnosis and the genetic result showed a correlation of 88% (37/45 cases). This technique was compared with CISH, and it was concluded that all HMs with maternal genome content were presented with a triploid pattern and all HMs without maternal content were presented with a diploid pattern (21). Their findings strengthen the accuracy of the CISH technique for differentiating CHM from PHM, which is in line with the current work.
In comparison with the histology and genetic study, they concluded that morphology criteria were more reliable for diagnosis of CHM than PHM (91% or 31/34 than 55% or 6/11, p < 0.001). Our results also showed that morphology was more reliable for CHM diagnosis than PHM. In a comparison of the CISH and genetic study, a good agreement between the 2 techniques for the diagnosis of the hydatidiform mole was seen, especially in the cases with challenging morphologic pictures (21).
A study conducted on 22 POCs included 6 CHM, 10 PHM, and 6 HA, after histological evaluation according to the morphologic criteria, they used the CISH technique with a chromosome 17 probe (HER 2/CEN), dual-colored (red and blue), on all specimens. In our study, morphologic criteria and DNA ploidy techniques were the same. Their results of counting red and blue signals in the trophoblastic cells and stromal cells were as follows: 9/10 (90%) of histologically diagnosed PHM showed a HER 2 signal value of 2.92 (triploid) and 1/10 PHM had a HER 2 signal value of 2.5. Decidua tissue was selected as an internal control. A total of 11/12 remaining cases (91.7%) presented a HER 2 signal value of 2.06 and 1/12 histologically diagnosed hydropic changes presented a HER 2 signal value of 2.35. Hence, they concluded that CISH chromosome 17 to differentiate diploid from triploid had a sensitivity of 90%, a specificity of 91.6%, and an agreement of 90.9%, which was in line with our study. Also similar to our study, no significant difference was observed between the patients in terms of maternal or gestational age (9).
In a study on 51 POCs, including 18 CHMs, 24 PHMs, and 9 HAs based on morphology characters, they performed a diagnostic algorithm for the diagnosis of HM according to p57 immunohistochemistry and a DNA ploidy FISH study. All 18 (100%) cases with morphology diagnosed as CHMs demonstrated a diploid genotype. Among 24 cases with PHMs, only 9 (37.5%) cases had a triploid genotype, and the remaining (62.5%) had a diploid genotype. All cases with HA (100%) displayed a diploid genotype. In this study, after a combination of p57 immunohistochemistry, DNA ploidy FISH study, and morphology in 51 cases, the diagnostic results were as follows: 27 CHMs, 9 PHMs, and 15 HAs. The results showed that the diagnostic accuracy based on morphology alone in the diagnosis of CHM and PHM was 78.4% and 70.6%, respectively. It can lead to a misdiagnosis, so the use of other diagnostic methods such as FISH and CISH is recommended in this study, empowering the current study’s aim (22).
4.1. Strength and limitation
The utmost effort and precision have been made in defining and subtracting the variables and outcomes; all steps have been carefully monitored and checked to minimize possible bias and enhance the validity of the results. As the other strong point of the work, 3 pathologists got involved in the morphology assessment of the PHM, CHM, and HA to minimize the error. Although not all POCs were included in the study and we faced attrition of POCs, the sample size was large enough to detect the differences compared with the previous works. A limitation of the work was the retrospective design, which resulted in the unavailability of fresh specimens to conduct the gold standard methods like FISH and flow cytometry karyotyping. These supplementary tests could compare the results and improve the accuracy of the diagnosis.
5. Conclusion
Based on the current finding, CISH2 and CISH17 enjoyed perfect agreement in diagnosing HMs; in addition, their absolute power discriminating between triploid and diploid revealed that they could be used as surrogate markers for ploidy. From 44 POCs, 31, 10, and 3 cases were diagnosed as PHM, CHM, and HA based on the morphology diagnosis. It is easy to diagnose CHM by morphology unless it is in lower gestational age or early CHM which is confused with HA. Based on the current findings, it is better to evaluate and diagnose early CHM by doing p57 before sending samples suspected of PHM for CISH diagnosis; although, to increase the accuracy of the diagnosis, it is suggested to perform the test with 2 chromosomes (2 and 17). Due to the high agreement coefficient between CISH2 and CISH17, lower prevalence of trisomy of chromosome 17 in POCs, no need for fresh specimens of POCs, and CISH17 availability in laboratories, it is more cost-effective to perform only CISH17. Prospective studies on fresh specimens are suggested comparing the CISH method's accuracy with other ploidy studies, such as flow cytometry karyotyping and FISH. Some advantages and disadvantages of CISH compared with flow cytometry karyotyping and FISH are as follows:
- CISH has a quick turnaround time as FISH providing the ability to perform shorter DNA ploidy in urgent cases.
- In POCs, CISH enjoys high true positive and high true negative values in discriminating between triploid and diploid; however, “the relatively high false positive and false negative rates of FISH technique complicates its application for analyzing small population of pathological cells” (23).
- CISH correlates with HER2 status and morphological features; however, FISH is an immunofluorescence staining technique needing immunofluorescence technique, which makes it more costly and less available in pathologic laboratories.
- Flow cytometry karyotyping provides a comprehensive view of the genome, while CISH can detect cryptic or submicroscopic genetic abnormalities and identify genetic abnormalities in nondividing cells. In addition, flow cytometry karyotyping needs more time and is more costly than CISH.
Data Availability
Data is available at corresponding author contact (marjan.zare@gmail.com).
Author Contributions
M. Akbarzadeh-Jahromi contributed to the conception, design, conduction, supervision, and drafting the manuscript. T. Taheri, F. Sari Aslani, A. Safaei, and F. Pouraminaee contributed to the conception, design, conduction, collection of the data, and drafting the manuscript. M. Zare contributed to the design, analysis of the data, interpretation of the results, drafting the manuscript, and reviewing the work critically. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
No AI was used for writing, editing or grammar check.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Pattology
References
1. Bartosch C, Nadal A, Braga AC, Salerno A, Rougemont A-L, Van Rompuy A-S, et al. Practical guidelines of the EOTTD for pathological and genetic diagnosis of hydatidiform moles. Virchows Arch 2024; 484: 401-422.
https://doi.org/10.1007/s00428-023-03658-8 [DOI:10.1007/s00428-023-03715-2] [PMID]
2. Priyadarshini S, Sharma R. Disorders of sex development in office practice. Indian J Pediatr 2023; 90: 1030-1037. [DOI:10.1007/s12098-023-04640-7] [PMID]
3. Kasraeian M, Asadi N, Vafaei H, Tazang M, Faraji A, Rahimirad N, et al. The effect of 150 and 80 mg doses of aspirin on preventing preterm birth in high-risk pregnant women. J Perinat Med 2022; 50: 1264-1270. [DOI:10.1515/jpm-2021-0668] [PMID]
4. Joneborg U, Folkvaljon Y, Papadogiannakis N, Lambe M, Marions LJAO. Temporal trends in incidence and outcome of hydatidiform mole: A retrospective cohort study. Acta Oncol 2018; 57: 1094-1099. [DOI:10.1080/0284186X.2018.1438653] [PMID]
5. Kerr SE, Shahi M. Molecular testing in gynecologic cancer. In: Coleman WB, Tsongalis GJ. Diagnostic molecular pathology. 2nd Ed. Netherlands: Elsevier; 2024. [DOI:10.1016/B978-0-12-822824-1.00004-3]
6. Kavimani M, Prema RR. Pathology-II and genetics. India: Thakur Publication Pvt LTD; 2023.
7. Lund H, Nielsen S, Grove A, Vyberg M, Sunde L. p57 in hydatidiform moles: Evaluation of antibodies and expression in various cell types. Appl Immunohistochem Mol Morphol 2020; 28: 694-701. [DOI:10.1097/PAI.0000000000000807] [PMID] [PMCID]
8. Mazur M, Kurman RJ. Diagnosis of endometrial biopsies and curettings: A practical approach. 2nd Ed. Switzerland: Springer Science & Business Media; 2005. [DOI:10.1007/978-0-387-26321-2]
9. He M, Pasquariello T, Steinhoff M. Application of HER2 CISH pharmDX for DNA ploidy determination. Appl Immunohistochem Mol Morphol 2016; 24: 465-469. [DOI:10.1097/PAI.0000000000000220] [PMID]
10. Wilson Y, Bharat C, Crook ML, Kee AR, Peverall J, Ruba S, et al. Histological comparison of partial hydatidiform mole and trisomy gestation specimens. Pathology 2016; 48: 550-554. [DOI:10.1016/j.pathol.2016.06.006] [PMID]
11. Khashaba M, Arafa M, Elsalkh E, Hemida R, Kandil W. Morphological features and immunohistochemical expression of p57Kip2 in early molar pregnancies and their relations to the progression to persistent trophoblastic disease. J Pathol Transl Med 2017; 51: 381-387. [DOI:10.4132/jptm.2017.04.28] [PMID] [PMCID]
12. McMahon L, Maher GJ, Joyce C, Niemann I, Fisher R, Sunde L. When to consult a geneticist specialising in gestational trophoblastic disease. Gynecol Obstet Invest 2024; 89: 198-213. [DOI:10.1159/000531218] [PMID]
13. Ronnett BM. Hydatidiform moles: Ancillary techniques to refine diagnosis. Arch Pathol Lab Med 2018; 142: 1485-1502. [DOI:10.5858/arpa.2018-0226-RA] [PMID]
14. Lloyd RV. Pathology: Historical and contemporary aspects. Switzerland: Springer; 2023. [DOI:10.1007/978-3-031-39554-3]
15. Kerkar AN, Chinnam D, Verma A, Peters NJ, Kakkar N, Trehan A, et al. MYCN amplification, TERT rearrangements and ATRX mutations in neuroblastoma: Clinicopathological correlates-an Indian perspective. Virchows Arch 2023; 483: 477-486. [DOI:10.1007/s00428-023-03604-8] [PMID]
16. Vitale SG, Buzzaccarini G, Riemma G, Pacheco LA, Sardo ADS, Carugno J, et al. Endometrial biopsy: Indications, techniques and recommendations. An evidence-based guideline for clinical practice. J Gynecol Obstet Hum Reprod 2023; 52: 102588. [DOI:10.1016/j.jogoh.2023.102588] [PMID]
17. Usui H. Auxiliary and experimental diagnostic techniques for hydatidiform moles. J Obstet Gynaecol Res 2022; 48: 3077-3086. [DOI:10.1111/jog.15422] [PMID]
18. Kunesh JP, Kunesh JG, Jorgensen RJ, Corral CD, Blakey JD. Utilization of chromogenic in situ hybridization to assess ploidy in the diagnosis of hydatidiform mole. Am J Clin Pathol 2016; 146: 125-131. [DOI:10.1093/ajcp/aqw095] [PMID]
19. Priyadarshini S, Sharma R. Disorders of sex development in office practice. Indian J Pediatr 2023; 90: 1030-1037. [DOI:10.1007/s12098-023-04640-7] [PMID]
20. Han LM, Grenert JP, Wiita AP, Quinn M, Fujimoto VY, Rabban JT. Prevalence of partial hydatidiform mole in products of conception from gestations with fetal triploidy merits reflex genotype testing independent of the morphologic appearance of the chorionic villi. Am J Surg Pathol 2020; 44: 849-858. [DOI:10.1097/PAS.0000000000001466] [PMID]
21. Lai CY, Chan KY, Khoo U-S, Ngan HY, Xue W-C, Chiu PM, et al. Analysis of gestational trophoblastic disease by genotyping and chromosome in situ hybridization. Mod Pathol 2004; 17: 40-48. [DOI:10.1038/modpathol.3800010] [PMID]
22. Wong YP, Chia WK, Selimin A, Chia PY, Mustangin M, Shuib S, et al. Diagnostic utility of p57 immunohistochemistry and DNA ploidy analysis by fluorescence in situ hybridisation in hydatidiform moles. Malays J Pathol 2021; 43: 341-351.
23. Rosa FE, Santos RM, Rogatto SR, Domingues MAC. Chromogenic in situ hybridization compared with other approaches to evaluate HER2/neu status in breast carcinomas. Braz J Med Biol Res 2013; 46: 207-216. [DOI:10.1590/1414-431X20132483] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




