Sun, Jul 13, 2025
[Archive]
Volume 22, Issue 6 (June 2024)
IJRM 2024, 22(6): 441-450 |
Back to browse issues page
Ethics code: IR.UMSHA.REC.1400.244-Hamadan-Iran
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghorbani M, Sanoee Farimani M, Khodadadi I, Mohagheghi S, Amiri I, Tayebinia H. The regulatory roles of Smad2/3 protein and SMURF2 gene expression in granulosa cells of germinal vesicle and metaphase II oocytes in polycystic ovarian syndrome: A case-control study. IJRM 2024; 22 (6) :441-450
URL: http://ijrm.ir/article-1-3287-en.html
URL: http://ijrm.ir/article-1-3287-en.html
Marzie Ghorbani1 

 , Marzieh Sanoee Farimani2
, Marzieh Sanoee Farimani2 

 , Iraj Khodadadi3
, Iraj Khodadadi3 

 , Sina Mohagheghi3
, Sina Mohagheghi3 

 , Iraj Amiri4
, Iraj Amiri4 

 , Heidar Tayebinia *5
, Heidar Tayebinia *5 




 , Marzieh Sanoee Farimani2
, Marzieh Sanoee Farimani2 

 , Iraj Khodadadi3
, Iraj Khodadadi3 

 , Sina Mohagheghi3
, Sina Mohagheghi3 

 , Iraj Amiri4
, Iraj Amiri4 

 , Heidar Tayebinia *5
, Heidar Tayebinia *5 


1- Department of Clinical Biochemistry, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran. Fertility and Infertility Research Centre, Hamadan University of Medical Sciences, Hamadan, Iran.
2- Department of Obstetrics and Gynecology, Medicine School, Hamadan University of Medical Sciences, Hamadan, Iran. Omid Infertility Centre, Hamadan, Iran.
3- Department of Clinical Biochemistry, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
4- Fertility and Infertility Research Centre, Hamadan University of Medical Sciences, Hamadan, Iran. & Department of Anatomy and Embryology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
5- Department of Clinical Biochemistry, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran. ,tayebinia@umsha.ac.ir
2- Department of Obstetrics and Gynecology, Medicine School, Hamadan University of Medical Sciences, Hamadan, Iran. Omid Infertility Centre, Hamadan, Iran.
3- Department of Clinical Biochemistry, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
4- Fertility and Infertility Research Centre, Hamadan University of Medical Sciences, Hamadan, Iran. & Department of Anatomy and Embryology, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran.
5- Department of Clinical Biochemistry, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran. ,
Full-Text [PDF 1642 kb]
(578 Downloads)
| Abstract (HTML) (480 Views)
Full-Text: (109 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) as an endocrine disorder is seen in 8-13% of women of reproductive age. It manifests critical features, including increased androgen levels, multiple small cysts, dysregulated menstrual cycles, hirsutism, and anovulation (1, 2), together with the presence of poor-quality oocytes and infertility in 74% of these women.
The process of folliculogenesis is disturbed in PCOS, and in these women, the primary follicles stop in the early preantral stages. Women with PCOS need assisted reproductive techniques, and high-dose gonadotropins are prescribed for controlled ovarian hyperstimulation, resulting in abundant follicles with poor quality, unequal size, and maturation spectrum, immature oocytes-germinal vesicles (GV) and mature oocytes-meiosis II (3-5).
In the folliculogenesis pathway of the mammalian ovary, there is a precise biological interaction between oocytes, granulosa cells (GCs), cumulus cells, and theca cells (TCs). Investigations found that the formation of immature oocytes could be aroused from insufficient interaction between these cells. Therefore, understanding signaling pathways in ovarian development is essential for increasing the maturation rate of oocytes and managing infertility. Factors produced in the ovary such as growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15, also known as GDF9b, and anti-Müllerian hormone (AMH) as proteins of the transforming growth factor β (TGFβ) superfamily are regulators of the follicular environment and determine the fate of each follicle (6). Studies have shown that in the absence of GDF9, the TCs layer is not formed, the production of steroids decreases, and the follicles remain immature (7, 8).
One of the signaling pathways is the TGFβ/mothers against decapentaplegic homolog (Smads) pathway, which plays a role in the regulation of follicular growth, and disruption of this pathway inhibits follicular growth and ultimately results in follicular atresia (9). TGFβ phosphorylates the kinase domain of type I receptors. Phosphorylated receptors transmit the signal through the phosphorylation of proteins called Smads. Smad proteins are classified into 3 subclasses based on their intracellular functions: R-Smads (Smad1, 2, 3, 5, and 8), Co-Smads (Smad4), and I- Smads (Smad6 and 7). Phosphorylated Smad2/3 (P-Smad2/3) with the cooperation of Smad4 complexes, transmit signals to the nucleus and regulate the transcription of target genes (6). These proteins play an essential role in all stages of follicular development (10). Also, Smad anchors for receptor activation (SARA) control and facilitates the localization of Smad 2/3 to be presented to the receptors (11). Furthermore, Smad ubiquitination regulatory factor 2 (SMURF2) is one of the proteins belonging to E3 ubiquitin ligase and is known as a negative regulator in this signaling pathway (12).
Women with PCOS often have poor oocyte quality and maturation, which affects their fertility and response to assisted reproductive techniques and ovulation induction drugs. The average development and growth of the follicles depends on the complex interaction between the oocytes and the surrounding GCs, which are influenced by signaling pathways such as Smad. Relative concentrations were compared with beta-actin protein and calculated by ImageJ-1.52 software.
In this study, the levels of Smad2/3 and P-Smad2/3 proteins and the expression of SARA, Smad4, and SMURF2 genes were investigated in the GCs of GV and MII oocytes in women with PCOS. By comparing changes in the expression levels of these molecules in the GCs of GV and MII oocytes, we hope to gain more insight into the molecular mechanisms underlying the impaired oocyte maturation in women with PCOS.
2. Materials and Methods
2.1. Study population‑study design
This case-control study was conducted among 38 PCOS women aged between 25 and 38 yr who were admitted to Omid Clinic (Hamadan, Iran) as in vitro fertilization procedure candidates from August 2021-February 2022. PCOS was diagnosed based on the Rotterdam criteria (13), and all of them were under treatment with ovulation induction drugs. The exclusion criteria were the presence of hyperprolactinemia, congenital adrenal hyperplasia, and Cushing syndrome. Follicle-stimulating hormone (FSH ), luteinizing hormone, estradiol, AMH , prolactin, and testosterone were evaluated in their serum at the start of the controlled ovarian stimulation (1-3 days of menstruation).
2.2. Sample size
Considering that a similar study has not been conducted in humans in relation to the objectives of this study, a pilot study was conducted. Based on the study findings, the mean and standard deviation in the mature and immature cells of the participants were calculated considering the power of the test equal to 0.9 and the type 1 error equal to 0.05. Then using the following statistical formula, the average variances of gene expression in 2 groups of mature and immature cells, the initial sample size was estimated at 25, and 38 people were considered people in each group to increase the power of statistical tests.

2.3. Ovarian stimulation process
The ovarian stimulation process was carried out according to antagonist protocols, recombinant FSH and Cinnal-F® (CinnaGen Co., Tehran, Iran) were administered. When the dominant follicle reached a size of 14 mm, a Cetrotide antagonist (Merck, Darmstadt, Germany) was injected at a daily dose of 0.25 μg. Once at least 3 mature follicles (18 mm) were observed in the ovaries, recombinant Ovitrelle-hCG (Merck, Darmstadt, Germany) was administered. After a 36-hr interval, follicles were obtained via transvaginal ultrasound-guided puncture, and oocytes were isolated.
2.4. Isolation of GCs
Following the retrieval of oocytes, a complete denudation and maturity assessment process was carried out. The identification of MII oocytes was based on the first polar body's presence, while the GVs presence identified GV oocytes. The average MII oocyte number of women was 17.17 ± 1.34 whereas 5 ± 2 GV oocytes were retrieved from each subject. Then, GCs surrounding MII oocytes or GVs were separately collected and designated as GC-MII and GC-GV groups, respectively. The collected cells were carefully transferred into micro tubes and centrifuged before being analyzed in quantitative real-time polymerase chain reaction (RT‑qPCR) and Western blotting techniques.
2.5. Identification of GCs
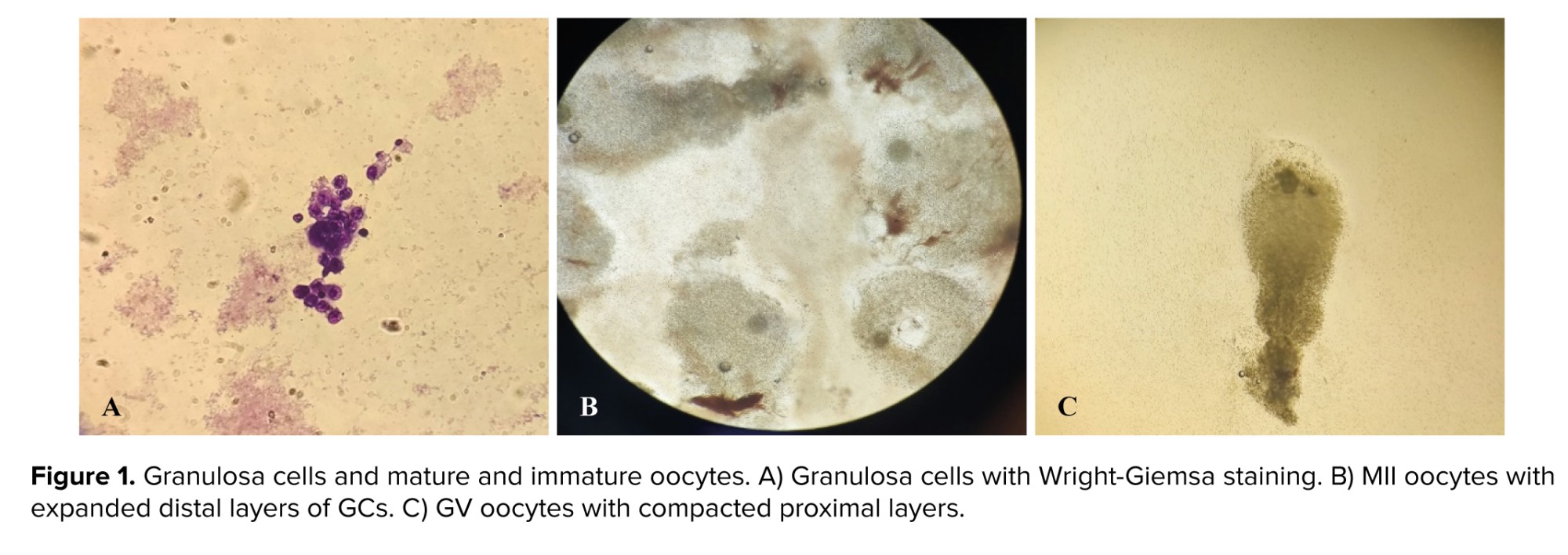
Following isolating GCs (0.1-0.6 × 106 cells per each oocyte), Wright-Giemsa staining was used (Figure 1).
2.6. RT‑qPCR for gene expression assay
After designing the primers, the sequence of each primer was checked and reconfirmed with Primer Blast software to evaluate the specificity to the corresponding gene. Total RNAs were extracted from GCs using RNX PLUS (SinaClon, Iran). The concentration of extracted RNA was evaluated using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific-USA) and its quality was evaluated by 1% agarose gel electrophoresis. The concentration of all total RNAs was adjusted to 500 ng/mL and reversely transcribed into cDNA using a cDNA synthesizing kit (Parstous Biotechnology, Iran). RT-qPCR was performed using RealQ Plus Master Mix Green (Sina SYBR Blue NO ROX HS-qPCR Kit) in a Roche LightCyclerVR96 System (Roche Life Science, Sandhofer, Germany). The relative expression of SARA, Smad4 and SMURF2 genes was calculated using the 2-ΔΔCt method. Act B (Actin Beta) was used as a reference gene. All samples were prepared in duplicate, and the mean value was used for comparative analyses (Table I).
2.7. Western blotting for the protein assay
Western blotting for detection of Smad2/3 and P-Smad2/3 was performed. A mixture of 450 μl Radioimmunoprecipitation assay buffer (Cytomatingene, Iran) and 6 μl of protease inhibitor (Cytomatingene, Iran) was used to lyse 1 × 106 GCs. The cell lysates were centrifuged at 12,000 × g for 20 min. The cell-soluble proteins were collected from the supernatants and transferred to new microtubes. Then, the total protein contents were determined using a Bicinchoninic Acid Protein Assay kit (DNAbiotech, Iran), where bovine serum albumin was used as standard. For western blotting, protein electrophoresis was performed using sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and then protein bands were transferred to polyvinylidene fluoride membrane. For blocking, 5% non-fat milk in Tris-buffered saline tween was added for 2 hr on polyvinyl chloride membrane, then primary antibodies against P-Smad2/3 (1:2,000 ab63399), Smad2/3 (1:2,000 ab63672), and β-actin (1:2,000 ab8227) were added for 12 hr. Membranes were washed 3 times with TBST buffer, then incubated with secondary antibody (Anti-rabbit IgG, HRP-Linked Antibody 7074) and stained with enhanced chemiluminescence (Cytomatingene, Iran). The protein bands were captured on film in the dark. Finally, ImageJ-1.52 software was used to quantify protein expression levels relative to β-actin as the control.

2.8. Ethical considerations
The study was approved by the Research Ethical Committee of Hamadan University of Medical Sciences, Hamadan, Iran (Code: IR.UMSHA.REC.1400.244). The main objectives of the study were explained to the women with PCOS, and written informed consents were signed. All study procedures were performed under the ethical guidelines of the Declaration of Helsinki (1967 version 2013) and with the ethical standards of the National Iranian Research Committee.
2.9. Statistical analysis
The results were shown as mean ± SEM. The normal distribution of data was evaluated using Kolmogorov-Smirnov test. Data were statistically analyzed using Student's t test for the data with normal distributions and Mann-Whitney tests were also applied for the data with abnormal distributions. Furthermore, results were analyzed using the GraphPad Prism 7.05 software (USA). Statistical significance was assumed at the p < 0.05. Statistical analysis was performed using the statistical package for social sciences logistic regression (SPSS, Inc., Chicago, IL) version 16.0. Relative concentrations were compared with beta-actin protein and calculated by ImageJ-1.52 software.
3. Results
3.1. Demographic characteristics of women with PCOS
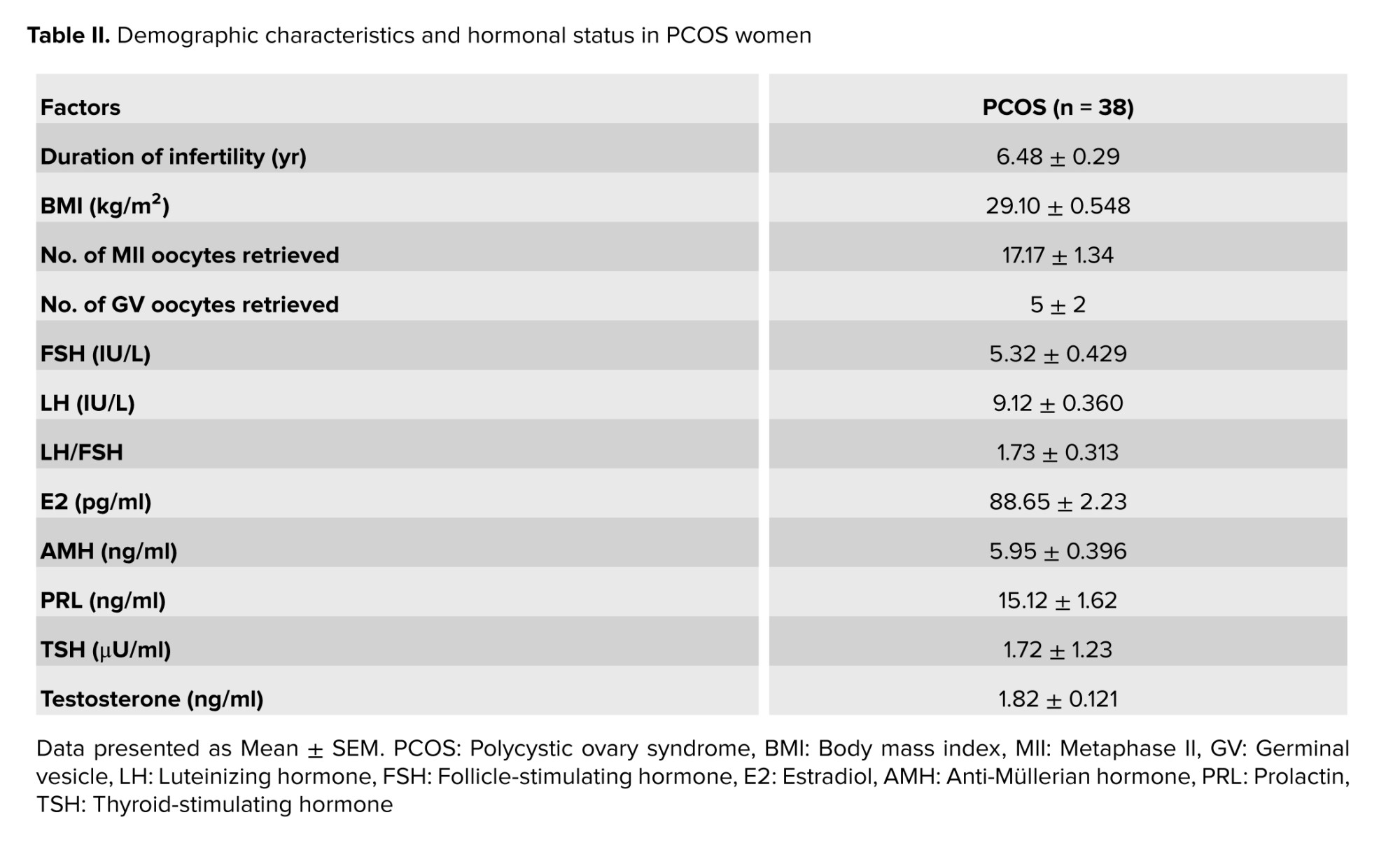
Table II shows that the studied women aged between 25 and 38 yr. It is of considerable importance to note that a comprehensive evaluation of the women’s serum hormone levels was conducted before the start of the controlled ovarian stimulation. This evaluation included FSH, luteinizing hormone, estradiol, AMH, prolactin, Thyroid-stimulating hormone, and testosterone.
3.2. The gene expression levels of SARA, Smad4, and SMURF2
RT‑qPCR was applied to determine the gene expression levels of SARA, Smad4, and SMURF2 in GCs of GV and MII oocytes. As shown in figure 2, the SARA and Smad4 expression levels did not significantly differ in GCs surrounding GV oocytes and MII oocytes (p > 0.05). However, the expression of SMURF2 was found to be considerably upregulated (p < 0.001) in GCs of GVs and rapidly increased (a few-hundred folds) compared to GCs of MII oocytes (Figure 2).
3.3. The protein expression levels of Smad2/3 and P-Smad2/3
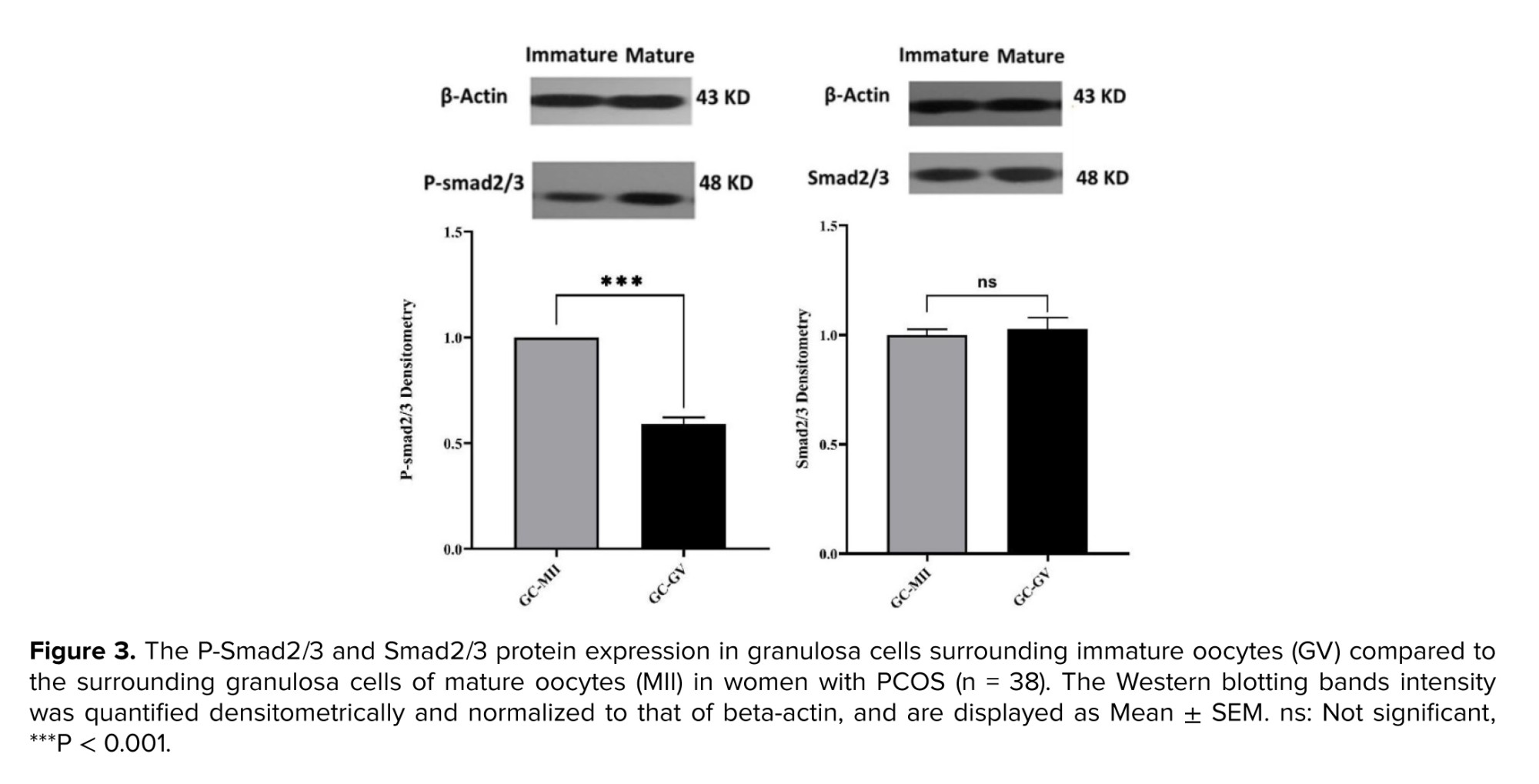
The expression of Smad2/3 and P-Smad2/3 at the protein level was evaluated by Western blotting. No significant difference was observed in Smad2/3 protein levels between GCs surrounding MII and GV oocytes. In contrast, P-Smad2/3 was significantly lower (p < 0.001) in GCs surrounding GV compared with that of GCs from MII oocytes (Figure 3).

4. Discussion
Women with PCOS often have heterogeneous follicles, a high rate of immature GV oocytes, and inappropriate fertilization rates (14). Follicle growth depends on oocytes, GCs, and TCs. Communication between GCs and oocytes is essential for follicular development, because GCs provide a variety of factors for oocytes, and the function of GCs is often directly related to the quality of the oocytes (15). GCs are considered regulators of oocyte development and can serve as biomarkers for evaluating oocyte quality, embryo competence, and pregnancy outcome (16). Our study showed that the expression level of SMURF2 was significantly higher in GCs surrounding GV oocytes compared with that of GCs encompassing MII oocytes, a lower level of P-Smad2/3 was also found in GCs of GV oocytes compared with GCs of MII oocytes. It was in line with our hypothesis that GCs of GV oocytes are different in terms of protein and gene expression.
Smads, as members of the TGFβ family, play essential roles in the reproductive system, take part in multiple aspects of ovarian functions, and have great importance during oocyte maturation. They both stimulate FSH receptors and affect the stability of follicle-stimulating hormone receptor mRNA and in the absence of Smad, ovulation, and follicle growth are impaired. Smads also affect the function of FSH. Conversely, FSH can stimulate and activate Smad2/3 and Smad4. FSH controls ovarian follicular growth and leads to maturation and selection of the dominant follicle and estradiol production (17, 18).
In this study, the protein levels of Smad2/3 did not differ between MII and GV oocyte-surrounded GCs, whereas the P-Smad2/3 was significantly lower in the GV oocyte-surrounded GCs. The binding of TGFβ to its receptor induces activation and phosphorylation of Smads 2 and 3. P-Smad2/3, with the cooperation of Smad4 complexes, transmit signals to the nucleus and regulate the transcription of target genes (19). It seems that, due to the decrease of P-Smad2/3 in GV oocyte-surrounded GCs, the effect ofFSH had decreased, and the ability of follicular growth and multiplication has reduced. Also, activation of Smad2/3 signaling machinery within the ovary mediates signals produced by the oocytes and is crucial for coordinating essential events of the ovulatory process, including granulosa expansion (20). GV oocyte-surrounded GCs did not have granulosa expansion, and their GCs remained compressed, while MII oocytes usually have a good expansion, which indicates their development (Figure 1).
It is reported that FSH reduces the apoptosis of GCs by decreasing FasL levels in vitro and protects the follicles from atresia in vivo (21, 22), and it prevents atresia of various maturing follicles, including antral follicles, pre-ovulatory follicles, and dominant follicles (23, 24). In addition, aromatase, an estradiol-producing enzyme, is stimulated by FSH in GCs, and the production of estradiol is critical for the selection of dominant follicles and follicular development (25, 26). We did not investigate the apoptosis of GCs, but it can be inferred that due to the reduction of P-Smad2/3, the function of FSH is impaired, and the apoptosis of GCs is not prevented, and these cells are stopped in the GV stages.
It is reported that the activation of Smad2/3 through phosphorylation is carried out via the TGFβ receptor type I and the adapter molecule SARA, which facilitates the presentation of Smad2/3 to the TGFβ1 receptor (19). Then Smads 2/3 phosphorylated with Smad4 translocated to the nucleus and regulated the transcription of target genes (27). During oocyte development, the Smad4 protein and mRNA expression patterns play a role in folliculogenesis (28, 29).
Our results revealed that the expression levels of SARA and Smad4 were not significantly different between the GCs surrounding MII and GV oocytes, and it can be concluded that these genes do not have a significant effect on the proliferation and development of GCs. However, the expression of SMURF2 belonging to E3 ubiquitin ligase and a negative regulator of TGFβ signaling pathways was significantly upregulated in the GCs surrounding GV oocytes, with an approximately 672-fold increase. It is revealed that SMURF2 suppresses the TGFβ signaling pathway (30). Considering that our study examined both the gene expression and protein levels, in GCs, it was found that P-Smad2/3, the functional form of this protein, contains low expression proteins in GCs surrounding GV oocytes, it does not transmit signals to the nucleus to a sufficient extent and neither does it regulate the transcription of target genes.
Therefore, the role of P-Smad2/3 protein as stimulator FSH receptors, and stabilizer FSHR mRNA is not done correctly, and follicular development, GCs expansion, and maturation of GV oocytes are disturbed, causing the oocytes to remain immature (GV). On the other hand, the SMURF2 gene is more expressed in GCs surrounding GV oocytes, which induces Smad2/3 degradation, and maybe this inhibitory factor restricts the development of the oocytes (25).
5. Conclusion
In summary, it seems that P-Smad2/3 plays an essential role in oocyte development, and the downregulation of this protein is associated with a defect in the maturation of GV oocytes. On the other hand, the upregulation of the SMURF2 gene also affects the growth process of these cells with its inhibitory effect. Additional research is necessary to reveal the activity of the Smad2/3 pathway. Due to the increase in the number of GV oocytes in women with PCOS, it is suggested that the addition of recombinant P-Smad2/3 proteins and inhibitor of SMURF2 may be effective in increasing the number of MII oocytes and fertility outcomes in these women and even treatment of human infertility using immature oocytes collected from natural cycles.
Data availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author contributions
HT, MGh, and IK designed the study and wrote the paper, MSF, IA, and MGh contributed to sample collection, MGh, SM, IK, and HT contributed to performing experiments and evaluating the results. All authors approved the final manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
The current work is a part of a research project which was financially supported by Hamadan University of Medical Sciences, Hamadan, Iran (Grant No. 14004153357). The authors declare that they did not use artificial intelligence to write this article.
Conflict of Interest
The authors declare that there is no conflict of interest.
Polycystic ovary syndrome (PCOS) as an endocrine disorder is seen in 8-13% of women of reproductive age. It manifests critical features, including increased androgen levels, multiple small cysts, dysregulated menstrual cycles, hirsutism, and anovulation (1, 2), together with the presence of poor-quality oocytes and infertility in 74% of these women.
The process of folliculogenesis is disturbed in PCOS, and in these women, the primary follicles stop in the early preantral stages. Women with PCOS need assisted reproductive techniques, and high-dose gonadotropins are prescribed for controlled ovarian hyperstimulation, resulting in abundant follicles with poor quality, unequal size, and maturation spectrum, immature oocytes-germinal vesicles (GV) and mature oocytes-meiosis II (3-5).
In the folliculogenesis pathway of the mammalian ovary, there is a precise biological interaction between oocytes, granulosa cells (GCs), cumulus cells, and theca cells (TCs). Investigations found that the formation of immature oocytes could be aroused from insufficient interaction between these cells. Therefore, understanding signaling pathways in ovarian development is essential for increasing the maturation rate of oocytes and managing infertility. Factors produced in the ovary such as growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15, also known as GDF9b, and anti-Müllerian hormone (AMH) as proteins of the transforming growth factor β (TGFβ) superfamily are regulators of the follicular environment and determine the fate of each follicle (6). Studies have shown that in the absence of GDF9, the TCs layer is not formed, the production of steroids decreases, and the follicles remain immature (7, 8).
One of the signaling pathways is the TGFβ/mothers against decapentaplegic homolog (Smads) pathway, which plays a role in the regulation of follicular growth, and disruption of this pathway inhibits follicular growth and ultimately results in follicular atresia (9). TGFβ phosphorylates the kinase domain of type I receptors. Phosphorylated receptors transmit the signal through the phosphorylation of proteins called Smads. Smad proteins are classified into 3 subclasses based on their intracellular functions: R-Smads (Smad1, 2, 3, 5, and 8), Co-Smads (Smad4), and I- Smads (Smad6 and 7). Phosphorylated Smad2/3 (P-Smad2/3) with the cooperation of Smad4 complexes, transmit signals to the nucleus and regulate the transcription of target genes (6). These proteins play an essential role in all stages of follicular development (10). Also, Smad anchors for receptor activation (SARA) control and facilitates the localization of Smad 2/3 to be presented to the receptors (11). Furthermore, Smad ubiquitination regulatory factor 2 (SMURF2) is one of the proteins belonging to E3 ubiquitin ligase and is known as a negative regulator in this signaling pathway (12).
Women with PCOS often have poor oocyte quality and maturation, which affects their fertility and response to assisted reproductive techniques and ovulation induction drugs. The average development and growth of the follicles depends on the complex interaction between the oocytes and the surrounding GCs, which are influenced by signaling pathways such as Smad. Relative concentrations were compared with beta-actin protein and calculated by ImageJ-1.52 software.
In this study, the levels of Smad2/3 and P-Smad2/3 proteins and the expression of SARA, Smad4, and SMURF2 genes were investigated in the GCs of GV and MII oocytes in women with PCOS. By comparing changes in the expression levels of these molecules in the GCs of GV and MII oocytes, we hope to gain more insight into the molecular mechanisms underlying the impaired oocyte maturation in women with PCOS.
2. Materials and Methods
2.1. Study population‑study design
This case-control study was conducted among 38 PCOS women aged between 25 and 38 yr who were admitted to Omid Clinic (Hamadan, Iran) as in vitro fertilization procedure candidates from August 2021-February 2022. PCOS was diagnosed based on the Rotterdam criteria (13), and all of them were under treatment with ovulation induction drugs. The exclusion criteria were the presence of hyperprolactinemia, congenital adrenal hyperplasia, and Cushing syndrome. Follicle-stimulating hormone (
2.2. Sample size
Considering that a similar study has not been conducted in humans in relation to the objectives of this study, a pilot study was conducted. Based on the study findings, the mean and standard deviation in the mature and immature cells of the participants were calculated considering the power of the test equal to 0.9 and the type 1 error equal to 0.05. Then using the following statistical formula, the average variances of gene expression in 2 groups of mature and immature cells, the initial sample size was estimated at 25, and 38 people were considered people in each group to increase the power of statistical tests.

2.3. Ovarian stimulation process
The ovarian stimulation process was carried out according to antagonist protocols, recombinant FSH and Cinnal-F® (CinnaGen Co., Tehran, Iran) were administered. When the dominant follicle reached a size of 14 mm, a Cetrotide antagonist (Merck, Darmstadt, Germany) was injected at a daily dose of 0.25 μg. Once at least 3 mature follicles (18 mm) were observed in the ovaries, recombinant Ovitrelle-hCG (Merck, Darmstadt, Germany) was administered. After a 36-hr interval, follicles were obtained via transvaginal ultrasound-guided puncture, and oocytes were isolated.
2.4. Isolation of GCs
Following the retrieval of oocytes, a complete denudation and maturity assessment process was carried out. The identification of MII oocytes was based on the first polar body's presence, while the GVs presence identified GV oocytes. The average MII oocyte number of women was 17.17 ± 1.34 whereas 5 ± 2 GV oocytes were retrieved from each subject. Then, GCs surrounding MII oocytes or GVs were separately collected and designated as GC-MII and GC-GV groups, respectively. The collected cells were carefully transferred into micro tubes and centrifuged before being analyzed in quantitative real-time polymerase chain reaction (RT‑qPCR) and Western blotting techniques.
2.5. Identification of GCs
Following isolating GCs (0.1-0.6 × 106 cells per each oocyte), Wright-Giemsa staining was used (Figure 1).
2.6. RT‑qPCR for gene expression assay
After designing the primers, the sequence of each primer was checked and reconfirmed with Primer Blast software to evaluate the specificity to the corresponding gene. Total RNAs were extracted from GCs using RNX PLUS (SinaClon, Iran). The concentration of extracted RNA was evaluated using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific-USA) and its quality was evaluated by 1% agarose gel electrophoresis. The concentration of all total RNAs was adjusted to 500 ng/mL and reversely transcribed into cDNA using a cDNA synthesizing kit (Parstous Biotechnology, Iran). RT-qPCR was performed using RealQ Plus Master Mix Green (Sina SYBR Blue NO ROX HS-qPCR Kit) in a Roche LightCyclerVR96 System (Roche Life Science, Sandhofer, Germany). The relative expression of SARA, Smad4 and SMURF2 genes was calculated using the 2-ΔΔCt method. Act B (Actin Beta) was used as a reference gene. All samples were prepared in duplicate, and the mean value was used for comparative analyses (Table I).
2.7. Western blotting for the protein assay
Western blotting for detection of Smad2/3 and P-Smad2/3 was performed. A mixture of 450 μl Radioimmunoprecipitation assay buffer (Cytomatingene, Iran) and 6 μl of protease inhibitor (Cytomatingene, Iran) was used to lyse 1 × 106 GCs. The cell lysates were centrifuged at 12,000 × g for 20 min. The cell-soluble proteins were collected from the supernatants and transferred to new microtubes. Then, the total protein contents were determined using a Bicinchoninic Acid Protein Assay kit (DNAbiotech, Iran), where bovine serum albumin was used as standard. For western blotting, protein electrophoresis was performed using sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and then protein bands were transferred to polyvinylidene fluoride membrane. For blocking, 5% non-fat milk in Tris-buffered saline tween was added for 2 hr on polyvinyl chloride membrane, then primary antibodies against P-Smad2/3 (1:2,000 ab63399), Smad2/3 (1:2,000 ab63672), and β-actin (1:2,000 ab8227) were added for 12 hr. Membranes were washed 3 times with TBST buffer, then incubated with secondary antibody (Anti-rabbit IgG, HRP-Linked Antibody 7074) and stained with enhanced chemiluminescence (Cytomatingene, Iran). The protein bands were captured on film in the dark. Finally, ImageJ-1.52 software was used to quantify protein expression levels relative to β-actin as the control.


2.8. Ethical considerations
The study was approved by the Research Ethical Committee of Hamadan University of Medical Sciences, Hamadan, Iran (Code: IR.UMSHA.REC.1400.244). The main objectives of the study were explained to the women with PCOS, and written informed consents were signed. All study procedures were performed under the ethical guidelines of the Declaration of Helsinki (1967 version 2013) and with the ethical standards of the National Iranian Research Committee.
2.9. Statistical analysis
The results were shown as mean ± SEM. The normal distribution of data was evaluated using Kolmogorov-Smirnov test. Data were statistically analyzed using Student's t test for the data with normal distributions and Mann-Whitney tests were also applied for the data with abnormal distributions. Furthermore, results were analyzed using the GraphPad Prism 7.05 software (USA). Statistical significance was assumed at the p < 0.05. Statistical analysis was performed using the statistical package for social sciences logistic regression (SPSS, Inc., Chicago, IL) version 16.0. Relative concentrations were compared with beta-actin protein and calculated by ImageJ-1.52 software.
3. Results
3.1. Demographic characteristics of women with PCOS
Table II shows that the studied women aged between 25 and 38 yr. It is of considerable importance to note that a comprehensive evaluation of the women’s serum hormone levels was conducted before the start of the controlled ovarian stimulation. This evaluation included FSH, luteinizing hormone, estradiol, AMH, prolactin, Thyroid-stimulating hormone, and testosterone.
3.2. The gene expression levels of SARA, Smad4, and SMURF2
RT‑qPCR was applied to determine the gene expression levels of SARA, Smad4, and SMURF2 in GCs of GV and MII oocytes. As shown in figure 2, the SARA and Smad4 expression levels did not significantly differ in GCs surrounding GV oocytes and MII oocytes (p > 0.05). However, the expression of SMURF2 was found to be considerably upregulated (p < 0.001) in GCs of GVs and rapidly increased (a few-hundred folds) compared to GCs of MII oocytes (Figure 2).
3.3. The protein expression levels of Smad2/3 and P-Smad2/3
The expression of Smad2/3 and P-Smad2/3 at the protein level was evaluated by Western blotting. No significant difference was observed in Smad2/3 protein levels between GCs surrounding MII and GV oocytes. In contrast, P-Smad2/3 was significantly lower (p < 0.001) in GCs surrounding GV compared with that of GCs from MII oocytes (Figure 3).



4. Discussion
Women with PCOS often have heterogeneous follicles, a high rate of immature GV oocytes, and inappropriate fertilization rates (14). Follicle growth depends on oocytes, GCs, and TCs. Communication between GCs and oocytes is essential for follicular development, because GCs provide a variety of factors for oocytes, and the function of GCs is often directly related to the quality of the oocytes (15). GCs are considered regulators of oocyte development and can serve as biomarkers for evaluating oocyte quality, embryo competence, and pregnancy outcome (16). Our study showed that the expression level of SMURF2 was significantly higher in GCs surrounding GV oocytes compared with that of GCs encompassing MII oocytes, a lower level of P-Smad2/3 was also found in GCs of GV oocytes compared with GCs of MII oocytes. It was in line with our hypothesis that GCs of GV oocytes are different in terms of protein and gene expression.
Smads, as members of the TGFβ family, play essential roles in the reproductive system, take part in multiple aspects of ovarian functions, and have great importance during oocyte maturation. They both stimulate FSH receptors and affect the stability of follicle-stimulating hormone receptor mRNA and in the absence of Smad, ovulation, and follicle growth are impaired. Smads also affect the function of FSH. Conversely, FSH can stimulate and activate Smad2/3 and Smad4. FSH controls ovarian follicular growth and leads to maturation and selection of the dominant follicle and estradiol production (17, 18).
In this study, the protein levels of Smad2/3 did not differ between MII and GV oocyte-surrounded GCs, whereas the P-Smad2/3 was significantly lower in the GV oocyte-surrounded GCs. The binding of TGFβ to its receptor induces activation and phosphorylation of Smads 2 and 3. P-Smad2/3, with the cooperation of Smad4 complexes, transmit signals to the nucleus and regulate the transcription of target genes (19). It seems that, due to the decrease of P-Smad2/3 in GV oocyte-surrounded GCs, the effect of
It is reported that FSH reduces the apoptosis of GCs by decreasing FasL levels in vitro and protects the follicles from atresia in vivo (21, 22), and it prevents atresia of various maturing follicles, including antral follicles, pre-ovulatory follicles, and dominant follicles (23, 24). In addition, aromatase, an estradiol-producing enzyme, is stimulated by FSH in GCs, and the production of estradiol is critical for the selection of dominant follicles and follicular development (25, 26). We did not investigate the apoptosis of GCs, but it can be inferred that due to the reduction of P-Smad2/3, the function of FSH is impaired, and the apoptosis of GCs is not prevented, and these cells are stopped in the GV stages.
It is reported that the activation of Smad2/3 through phosphorylation is carried out via the TGFβ receptor type I and the adapter molecule SARA, which facilitates the presentation of Smad2/3 to the TGFβ1 receptor (19). Then Smads 2/3 phosphorylated with Smad4 translocated to the nucleus and regulated the transcription of target genes (27). During oocyte development, the Smad4 protein and mRNA expression patterns play a role in folliculogenesis (28, 29).
Our results revealed that the expression levels of SARA and Smad4 were not significantly different between the GCs surrounding MII and GV oocytes, and it can be concluded that these genes do not have a significant effect on the proliferation and development of GCs. However, the expression of SMURF2 belonging to E3 ubiquitin ligase and a negative regulator of TGFβ signaling pathways was significantly upregulated in the GCs surrounding GV oocytes, with an approximately 672-fold increase. It is revealed that SMURF2 suppresses the TGFβ signaling pathway (30). Considering that our study examined both the gene expression and protein levels, in GCs, it was found that P-Smad2/3, the functional form of this protein, contains low expression proteins in GCs surrounding GV oocytes, it does not transmit signals to the nucleus to a sufficient extent and neither does it regulate the transcription of target genes.
Therefore, the role of P-Smad2/3 protein as stimulator FSH receptors, and stabilizer FSHR mRNA is not done correctly, and follicular development, GCs expansion, and maturation of GV oocytes are disturbed, causing the oocytes to remain immature (GV). On the other hand, the SMURF2 gene is more expressed in GCs surrounding GV oocytes, which induces Smad2/3 degradation, and maybe this inhibitory factor restricts the development of the oocytes (25).
5. Conclusion
In summary, it seems that P-Smad2/3 plays an essential role in oocyte development, and the downregulation of this protein is associated with a defect in the maturation of GV oocytes. On the other hand, the upregulation of the SMURF2 gene also affects the growth process of these cells with its inhibitory effect. Additional research is necessary to reveal the activity of the Smad2/3 pathway. Due to the increase in the number of GV oocytes in women with PCOS, it is suggested that the addition of recombinant P-Smad2/3 proteins and inhibitor of SMURF2 may be effective in increasing the number of MII oocytes and fertility outcomes in these women and even treatment of human infertility using immature oocytes collected from natural cycles.
Data availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author contributions
HT, MGh, and IK designed the study and wrote the paper, MSF, IA, and MGh contributed to sample collection, MGh, SM, IK, and HT contributed to performing experiments and evaluating the results. All authors approved the final manuscript and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
The current work is a part of a research project which was financially supported by Hamadan University of Medical Sciences, Hamadan, Iran (Grant No. 14004153357). The authors declare that they did not use artificial intelligence to write this article.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Cellular and Molecular Biology of Reproduction
References
1. Singh S, Pal N, Shubham S, Sarma DK, Verma V, Marotta F, et al. Polycystic ovary syndrome: Etiology, current management, and future therapeutics. J Clin Med 2023; 12: 1454. [DOI:10.3390/jcm12041454]
2. Zeng X, Xie Y-J, Liu Y-T, Long S-l, Mo Z-C. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta 2020; 502: 214-221. [DOI:10.1016/j.cca.2019.11.003]
3. Cunha A, Póvoa AM. Infertility management in women with polycystic ovary syndrome: A review. Porto Biomed J 2021; 6: e116. [DOI:10.1097/j.pbj.0000000000000116]
4. Fatemi H, Bilger W, Denis D, Griesinger G, La Marca A, Longobardi S, et al. Dose adjustment of follicle-stimulating hormone (FSH) during ovarian stimulation as part of medically-assisted reproduction in clinical studies: A systematic review covering 10 years (2007-2017). J Endocrinol Reprod 2021; 19: 68. [DOI:10.1186/s12958-021-00744-x]
5. Pedroso DC, Santana VP, Donaires FS, Picinato MC, Giorgenon RC, Santana BA, et al. Telomere length and telomerase activity in immature oocytes and cumulus cells of women with polycystic ovary syndrome. Reprod Sci 2020; 27: 1293-1303. [DOI:10.1007/s43032-019-00120-6]
6. de Moraes FP, Missio D, Lazzari J, Rovani MT, Ferreira R, Gonçalves PBD, et al. Local regulation of antral follicle development and ovulation in monovulatory species. Anim Reprod 2023; 19: e20220099. [DOI:10.1590/1984-3143-ar2022-0099]
7. Coxir SA, Costa GMJ, Santos CFd, Alvarenga RdLLS, Lacerda SMdSN. From in vivo to in vitro: Exploring the key molecular and cellular aspects of human female gametogenesis. Hum Cell 2023; 36: 1283-1311. [DOI:10.1007/s13577-023-00921-7]
8. Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: Role, dispute and future perspective. Autophagy 2021; 17: 2706-2733. [DOI:10.1080/15548627.2021.1938914]
9. Wang Y, Teng X, Liu J. Research progress on the effect of traditional Chinese medicine on signal pathway related to premature ovarian insufficiency. Evid Based Complement Alternat Med 2022; 2022: 7012978. [DOI:10.1155/2022/7012978]
10. Wang Z, Wei H, Wu Z, Zhang X, Sun Y, Gao L, et al. The oocyte cumulus complex regulates mouse sperm migration in the oviduct. Commun Biol 2022; 5: 1327. [DOI:10.1038/s42003-022-04287-8]
11. Rozés-Salvador V, Siri SO, Musri MM, Conde C. New player in endosomal trafficking: Differential roles of Smad anchor for receptor activation (SARA) protein. Mol Cell Biol 2018; 38: e00446. [DOI:10.1128/MCB.00446-18]
12. Tan Y, Chen Y, Du M, Peng Z, Xie P. USF2 inhibits the transcriptional activity of Smurf1 and SMURF2 to promote breast cancer tumorigenesis. Cell Signal 2019; 53: 49-58. [DOI:10.1016/j.cellsig.2018.09.013]
13. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41-47. [DOI:10.1093/humrep/deh098]
14. Gilchrist RB, Smitz J. Oocyte in vitro maturation: Physiological basis and application to clinical practice. Fertil Steril 2023; 119: 524-539. [DOI:10.1016/j.fertnstert.2023.02.010]
15. Li Y, Xiong G, Tan J, Wang S, Wu Q, Wan L, et al. Aberrant activation of the Hedgehog signaling pathway in granulosa cells from patients with polycystic ovary syndrome. Bioengineered 2021; 12: 12123-12134. [DOI:10.1080/21655979.2021.2003943]
16. Huang R-H, Zhou W-H. Granulosa cell biomarkers to predict oocyte and embryo quality in assisted reproductive technology. Reprod Dev Med 2021; 5: 30-37. [DOI:10.4103/2096-2924.313684]
17. Qin N, Fan X-C, Xu X-X, Tyasi TL, Li S-J, Zhang Y-Y, et al. Cooperative effects of FOXL2 with the members of TGF-β superfamily on FSH receptor mRNA expression and granulosa cell proliferation from hen prehierarchical follicles. PLoS One 2015; 10: e0141062. [DOI:10.1371/journal.pone.0141062]
18. Bai L, Chang HM, Zhang L, Zhu YM, Leung PC. BMP2 increases the production of BDNF through the upregulation of proBDNF and furin expression in human granulosa‐lutein cells. FASEB J 2020; 34: 16129-16143. [DOI:10.1096/fj.202000940R]
19. Tzavlaki K, Moustakas A. TGF-β Signaling. Biomolecules 2020; 10: 487. [DOI:10.3390/biom10030487]
20. Clark KL, George JW, Przygrodzka E, Plewes MR, Hua G, Wang C, et al. Hippo signaling in the ovary: Emerging roles in development, fertility, and disease. Endocr Rev 2022; 43: 1074-1096. [DOI:10.1210/endrev/bnac013]
21. Gong Z, Yang J, Bai S, Wei S. MicroRNAs regulate granulosa cells apoptosis and follicular development- A review. Asian-Australas J Anim Sci 2020; 33: 1714-1724. [DOI:10.5713/ajas.19.0707]
22. Shi XY, Guan ZQ, Yu JN, Liu HL. Follicle stimulating hormone inhibits the expression of p53 up-regulated modulator of apoptosis induced by reactive oxygen species through PI3K/AKT in mouse granulosa cells. Physiol Res 2020; 69: 687-694. [DOI:10.33549/physiolres.934421]
23. Shen M, Liu Z, Li B, Teng Y, Zhang J, Tang Y, et al. Involvement of FoxO1 in the effects of follicle-stimulating hormone on inhibition of apoptosis in mouse granulosa cells. Cell Death Dis 2014; 5: e1475. [DOI:10.1038/cddis.2014.400]
24. Bhardwaj JK, Paliwal A, Saraf P, Sachdeva SN. Role of autophagy in follicular development and maintenance of primordial follicular pool in the ovary. J Cell Physiol 2022; 237: 1157-1170. [DOI:10.1002/jcp.30613]
25. de Ceuninck van Capelle C, Spit M, Ten Dijke P. Current perspectives on inhibitory Smad7 in health and disease. Crit Rev Biochem Mol 2020; 55: 691-715. [DOI:10.1080/10409238.2020.1828260]
26. Baddela VS, Michaelis M, Sharma A, Plinski C, Viergutz T, Vanselow J. Estradiol production of granulosa cells is unaffected by the physiological mix of nonesterified fatty acids in follicular fluid. Biol Chem 2022; 298: 691-715. [DOI:10.1016/j.jbc.2022.102477]
27. Du X, Li Q, Yang L, Liu L, Cao Q, Li Q. Smad4 activates Wnt signaling pathway to inhibit granulosa cell apoptosis. Cell Death Dis 2020; 11: 373. [DOI:10.1038/s41419-020-2578-x]
28. Liu L, Li Q, Yang L, Li Q, Du X. Smad4 feedback activates the canonical TGF-β family signaling pathways. Int J Mol Sci 2021; 22: 10024. [DOI:10.3390/ijms221810024]
29. Piotrowska H, Kempisty B, Sosinska P, Ciesiolka S, Bukowska D, Antosik P, et al. The role of TGF superfamily gene expression in the regulation of folliculogenesis and oogenesis in mammals: A review. Vet Med 2013; 58: 505-515. [DOI:10.17221/7082-VETMED]
30. Chandhoke AS, Karve K, Dadakhujaev S, Netherton S, Deng L, Bonni S. The ubiquitin ligase SMURF2 suppresses TGFβ-induced epithelial- mesenchymal transition in a sumoylation-regulated manner. Cell Death Differ 2016; 23: 876-888. [DOI:10.1038/cdd.2015.152]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




