Fri, Feb 13, 2026
[Archive]
Volume 22, Issue 8 (August 2024)
IJRM 2024, 22(8): 661-672 |
Back to browse issues page
Ethics code: IR.KAUMS.MEDNT.REC.1396.45
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Almasi M, Shafiei G, Nikzad H, Karimian M, Moshkdanian G. The effect of L-carnitine in reactive oxygen species reduction and apoptotic gene expression in mice after cyclophosphamide: An experimental study. IJRM 2024; 22 (8) :661-672
URL: http://ijrm.ir/article-1-3335-en.html
URL: http://ijrm.ir/article-1-3335-en.html
1- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran.
2- Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran.
3- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran. Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran. & Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran.
4- Department of Molecular and Cell Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran.
5- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran. & Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran. ,G_moshkdanian@yahoo.com
2- Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran.
3- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran. Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran. & Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran.
4- Department of Molecular and Cell Biology, Faculty of Basic Sciences, University of Mazandaran, Babolsar, Iran.
5- Gametogenesis Research Center, Kashan University of Medical Sciences, Kashan, Iran. & Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran. ,
Full-Text [PDF 830 kb]
(889 Downloads)
| Abstract (HTML) (1236 Views)
Full-Text: (183 Views)
- Introduction
The surprising competence of cancer chemotherapy has recently resulted in significantly improved survival and life expectancy in cancer patients. Thereby, young cancer patients have been considerably interested in preserving their fertility after cancer treatment. On the other hand, all the chemotherapy medicines, no doubt, cause harmful effects on reproductive performance. Among different drug types, alkylating agents are generally coupled with reproductive issues (1). Cyclophosphamide (CP), an “alkylating agent”, has been extensively used in anticancer drugs (2). Even though cancer patients who received the CP treatment experienced oocyte damage, primordial follicle destruction, and meiotic spindle abnormalities. It also adversely affected their offspring outcomes and fertility (3, 4).
Thus, evaluating and preserving the quality of fertility after CP exposure is crucial (5). Accumulating studies declared that reactive oxygen species (ROS) generated by CP is considered the most pivotal event that leads to infertility; however, the mechanisms contributing to CP causing oocyte damage remain unclear. A summary evaluation of the published studies indicates that excessive ROS generation plays an important role in activating the apoptotic pathways via various undesirable effects such as membrane and DNA damage, lipid peroxidation, spindle morphology, and microtubule arrangement (6).
Scientists demonstrated that antioxidant supplementation is associated with overcoming harmful effects caused by ROS and alleviating apoptosis in oocytes (7, 8). L-carnitine (LC) is a small, highly polar, water-soluble quaternary amine essential for fat metabolism. The active form of carnitine, LC (3-hydroxy-4-N-trimethyl amino butyrate), is primarily synthesized from the amino acids lysine and methionine in the liver (9). As an antioxidant, LC neutralizes free radicals, particularly superoxide anions, safeguarding cells from oxidative damage-induced apoptosis and enhancing the quality of oocytes and embryos (10). Previous research has shown that LC protects female reproductive health by mitigating oxidative stress in oocytes, thereby improving their quality (11, 12). In sheep models, supplementing with LC during in vitro maturation mitigated embryo toxicity caused by oxidative stress. This was achieved by reducing intracellular ROS levels and boosting intracellular glutathione levels, which consequently enhanced the developmental potential of oocytes and embryos and altered the gene expression levels for antioxidant enzymes (9).
As to scientific evidence, the correlation between CP and the apoptotic pathway in oocytes is not fully understood, and the restriction of generated ROS from chemotherapy drugs is the most important counteraction. Also, there is not enough data available that indicates the effects of LC after exposure of oocytes to CP. Therefore, this study has been initiated to examine the possible protective effects of LC against oocyte injury, and to investigate the apoptosis pathway in oocytes after exposure to CP.
Thus, evaluating and preserving the quality of fertility after CP exposure is crucial (5). Accumulating studies declared that reactive oxygen species (ROS) generated by CP is considered the most pivotal event that leads to infertility; however, the mechanisms contributing to CP causing oocyte damage remain unclear. A summary evaluation of the published studies indicates that excessive ROS generation plays an important role in activating the apoptotic pathways via various undesirable effects such as membrane and DNA damage, lipid peroxidation, spindle morphology, and microtubule arrangement (6).
Scientists demonstrated that antioxidant supplementation is associated with overcoming harmful effects caused by ROS and alleviating apoptosis in oocytes (7, 8). L-carnitine (LC) is a small, highly polar, water-soluble quaternary amine essential for fat metabolism. The active form of carnitine, LC (3-hydroxy-4-N-trimethyl amino butyrate), is primarily synthesized from the amino acids lysine and methionine in the liver (9). As an antioxidant, LC neutralizes free radicals, particularly superoxide anions, safeguarding cells from oxidative damage-induced apoptosis and enhancing the quality of oocytes and embryos (10). Previous research has shown that LC protects female reproductive health by mitigating oxidative stress in oocytes, thereby improving their quality (11, 12). In sheep models, supplementing with LC during in vitro maturation mitigated embryo toxicity caused by oxidative stress. This was achieved by reducing intracellular ROS levels and boosting intracellular glutathione levels, which consequently enhanced the developmental potential of oocytes and embryos and altered the gene expression levels for antioxidant enzymes (9).
As to scientific evidence, the correlation between CP and the apoptotic pathway in oocytes is not fully understood, and the restriction of generated ROS from chemotherapy drugs is the most important counteraction. Also, there is not enough data available that indicates the effects of LC after exposure of oocytes to CP. Therefore, this study has been initiated to examine the possible protective effects of LC against oocyte injury, and to investigate the apoptosis pathway in oocytes after exposure to CP.
- Materials and Methods
2.1. Animal preparation
In this experimental study, 24 NMRI female mice (6-8 wk, 30 ± 5 gr) were purchased from the Research Center of Kashan University of Medical Sciences (Kashan, Isfahan, Iran). After 7 days of adapting, animals were maintained to access the controlled conditions of temperature (25 ± 2oC), good ventilation, and lighting (12 hr light/dark cycle).
In this experimental study, 24 NMRI female mice (6-8 wk, 30 ± 5 gr) were purchased from the Research Center of Kashan University of Medical Sciences (Kashan, Isfahan, Iran). After 7 days of adapting, animals were maintained to access the controlled conditions of temperature (25 ± 2oC), good ventilation, and lighting (12 hr light/dark cycle).
2.2.Experimental design
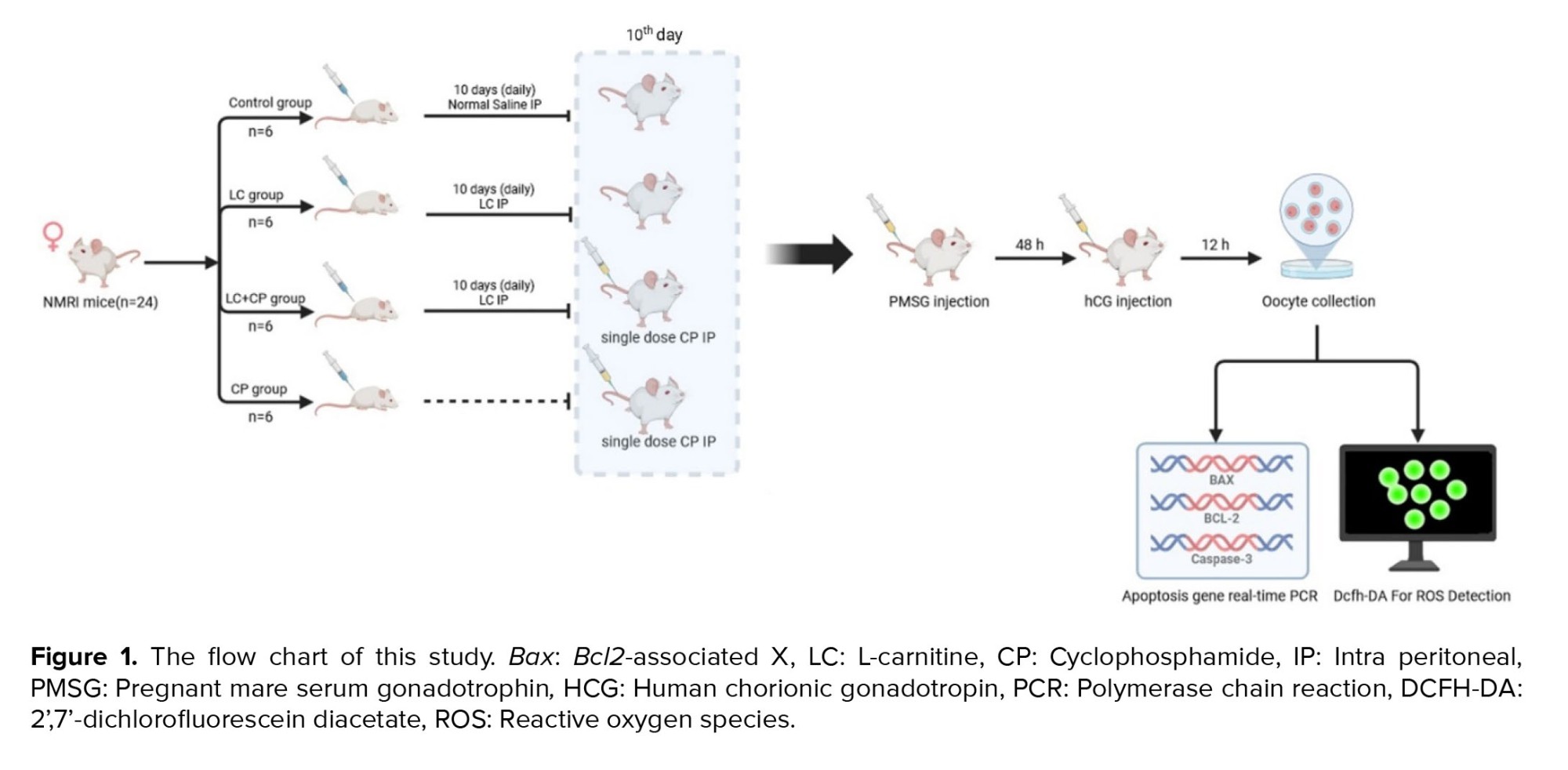
The sample size was selected based on the previous study (Figure 1) (13). The female NMRI mice were divided into 4 groups randomly (n = 6/group) and housed in polycarbonate cages (n = 3/cage). Design groups are in the following:
Control group: Received 2.5 ml/kg normal saline intraperitoneal (IP) injection for 10 days.
LC group: Received 200 mg/kg of LC (Sigma-Aldrich Co., St. Louis, MO, USA) IP injection for 10 days (10, 14).
LC+CP group: Received 200 mg/kg IP injection for 10 days and CP (Endoxan, Baxter Oncology GmbH, Halle, Germany) 75 mg/kg single IP injection on the 10th day of the experiment.
CP group: Received 75 mg/kg of CP as a single IP injection on 10th day of the experiment (10).
2.3. Oocyte collection
Female mice were superovulated by IP injection of 8 IU pregnant mare serum gonadotropin (Sigma-Aldrich, China), after 10 days injection of LC, CP, or saline. Then, 48 hr later, a similar dose of human chorionic gonadotropin (Pregnyl; Organon, Germany) was also given to the females.
Mice were sacrificed by cervical dislocation 13.5 hr later and oviducts were flushed in Ham’s F10 media (Thermo Fisher Scientific, USA) under a stereomicroscope (Olympus SZX9, Japan), the cumulus cells were removed enzymatically using 0.3 mg/ml hyaluronidase (Sigma-Aldrich, USA) and only mature oocytes with homogeneous cytoplasm, normal perivitelline space, and zona pellucida were considered morphologically normal. Selected oocytes were incubated in media drops under liquid paraffin oil at 37°C, 5% CO2 incubator to be used in experiments (10, 14).
2.4. RNA isolation and real-time polymerase chain reaction (RT-PCR)
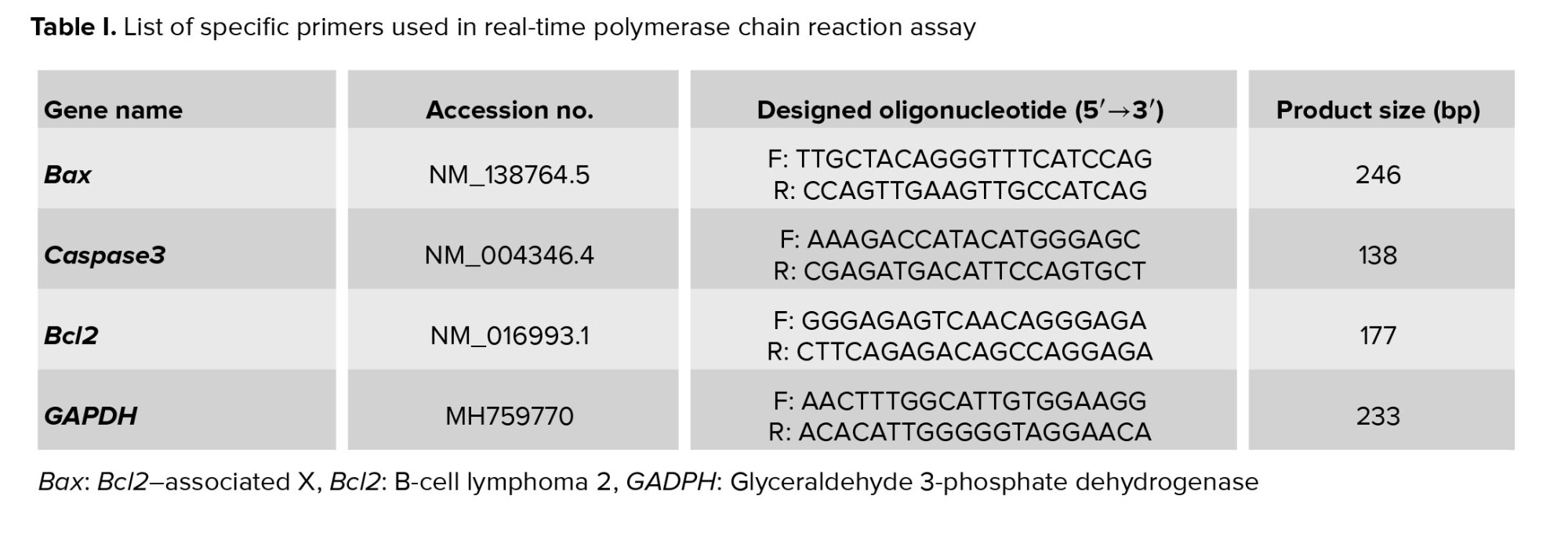
The expression levels of Bcl2-associated X (Bax), Caspase3, and B-cell lymphoma 2 (Bcl2) genes in oocytes (n = 60/each) were quantified using RT-PCR. RNA was extracted from each group utilizing the RNeasy Micro kit (74004, Qiagen, USA), adhering to the manufacturer’s guidelines (10). Subsequently, the isolated RNA served as a template for cDNA synthesis, performed with the Prime Script™ RT reagent kit (Takara Bio Inc., Shiga, Japan), using 500 ng of total RNA under the provided protocol. The RT-PCR analysis involved 2 µl of the cDNA, combined with the SYBR green master mix (BioFACT, Korea), in a final volume of 10 µl. This reaction was executed on the IQ5 RT-PCR system (Bio-Rad, Germany). Primer sequences employed for each gene are detailed in table I. To normalize the expression of long non-coding RNAs, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels were used as a reference, and the relative changes were calculated using the 2-ΔΔc method.
2.5. Measurement of ROS in oocytes
To quantify the relative levels of ROS in oocytes, we employed the fluorescent dye 2’,7’-dichlorofluorescein diacetate (DCFH-DA, 2 µM, Sigma, D6883, USA). A group of 5-10 oocytes were incubated for 20 min in Ham’s F10 medium enriched with DCFH-DA (2 µM) at 37°C in darkness. Postincubation, the oocytes were rinsed with Ham’s F10 medium supplemented with 10% human serum albumin (N6908, Sigma, USA) and then placed on glass slides. Fluorescence measurements were conducted using a Nikon Eclipse Ti fluorescence microscope (533450, Japan), utilizing excitation and emission filters with wavelengths of 450-490 nm and 520 nm, respectively. The fluorescence intensity, indicative of ROS levels, was quantified for each oocyte using Image Quant software (TotalLab Quant, UK) (15).
2.6. Ethical considerations
The procedures were approved by the Kashan University of Medical Sciences, Kashan, Iran (Code: IR.KAUMS.MEDNT.REC.1396.45). The animal experimental protocols were supported by the Iranian Council’s guidelines for the Use and Care of Animals.
2.7. Statistical analysis
Each experiment was performed in triplicate, and statistical analysis was conducted using GraphPad Prism 8 (San Diego, CA, USA) and SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). A one-way ANOVA test (comparison between 3 or more experimental groups) was performed followed by post hoc Tukey’s test. The results were expressed as the mean ± standard deviation. The mean differences between groups for ROS and gene expression levels were compared using analysis of variance (ANOVA), followed by post hoc Tukey’s test. P < 0.05 were considered statistically significant.
3. Results
3.1. The effect of LC and CP on changes in the expression of apoptosis genes (Bax, Caspase3, Bcl2)
To investigate whether apoptosis genes are up- or downregulated among studied groups (control, LC, LC+CP, CP), we monitored the relative change in mRNA expression of proapoptotic genes (Bax and Caspase3) and antiapoptotic gene (Bcl2) with RT-PCR.
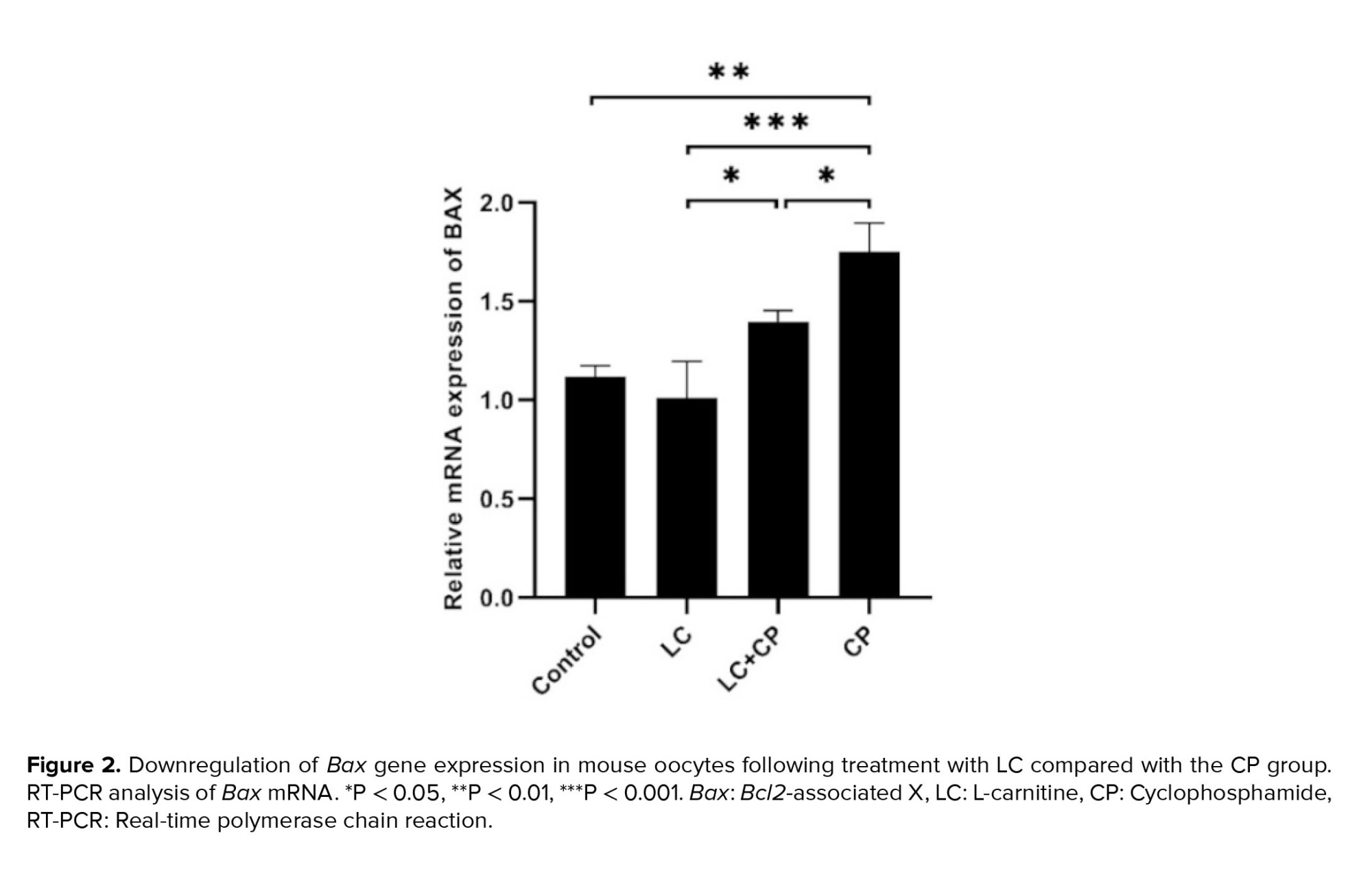
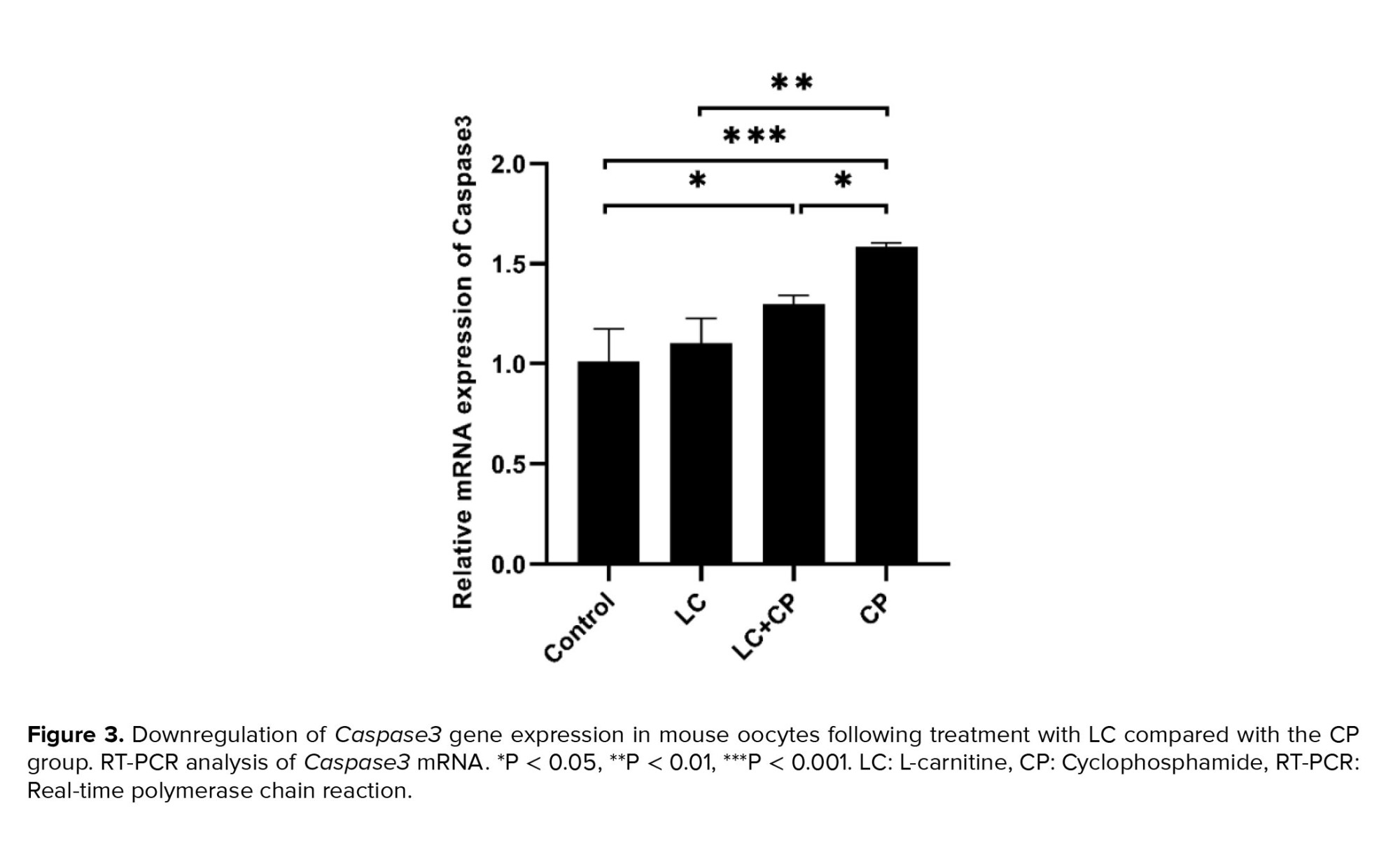
As shown in figures 2 and 3, the expression of the Bax and Caspase3 genes significantly upregulated in the CP group in comparison to the LC+CP (p = 0.03, p = 0.04), LC (p ˂ 0.001) and control (p ˂ 0.001) groups, respectively. The lowest significant expression of the Bax gene was found in the LC group in comparison to the LC+CP (p = 0.02) group. Interestingly, the expression level of the Bax gene in the LC+CP group was nonsignificant in comparison to the control group (Figure 2). The LC+CP group had a significantly (p = 0.04) higher expression of Caspase3 genes than control group (Figure 3).
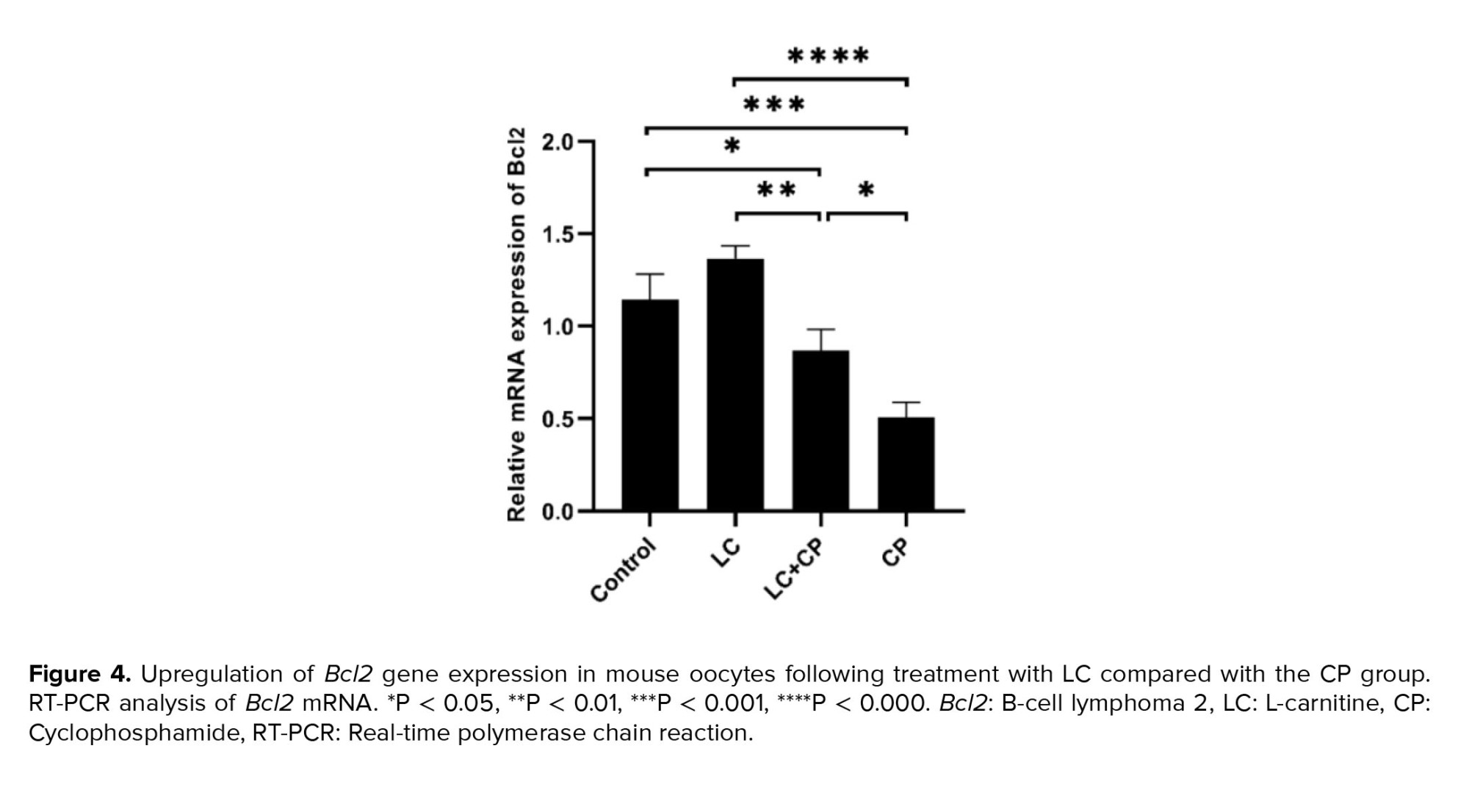
As depicted in figure 4 a significant upregulation in Bcl2 expression was observed in the LC group compared to LC+CP (p ˂ 0.001) and CP (p ˂ 0.001) groups. In addition, a significant decrease was observed in the expression level of Bcl2 in the CP group in comparison to the LC+CP (p = 0.01), and control (p ˂ 0.001) groups. Moreover, the expression of the Bcl2 gene in the LC+CP group significantly decreased compared to control group (p = 0.04) (Figure 4).
3.2. The effect of LC and CP on the intracellular ROS level
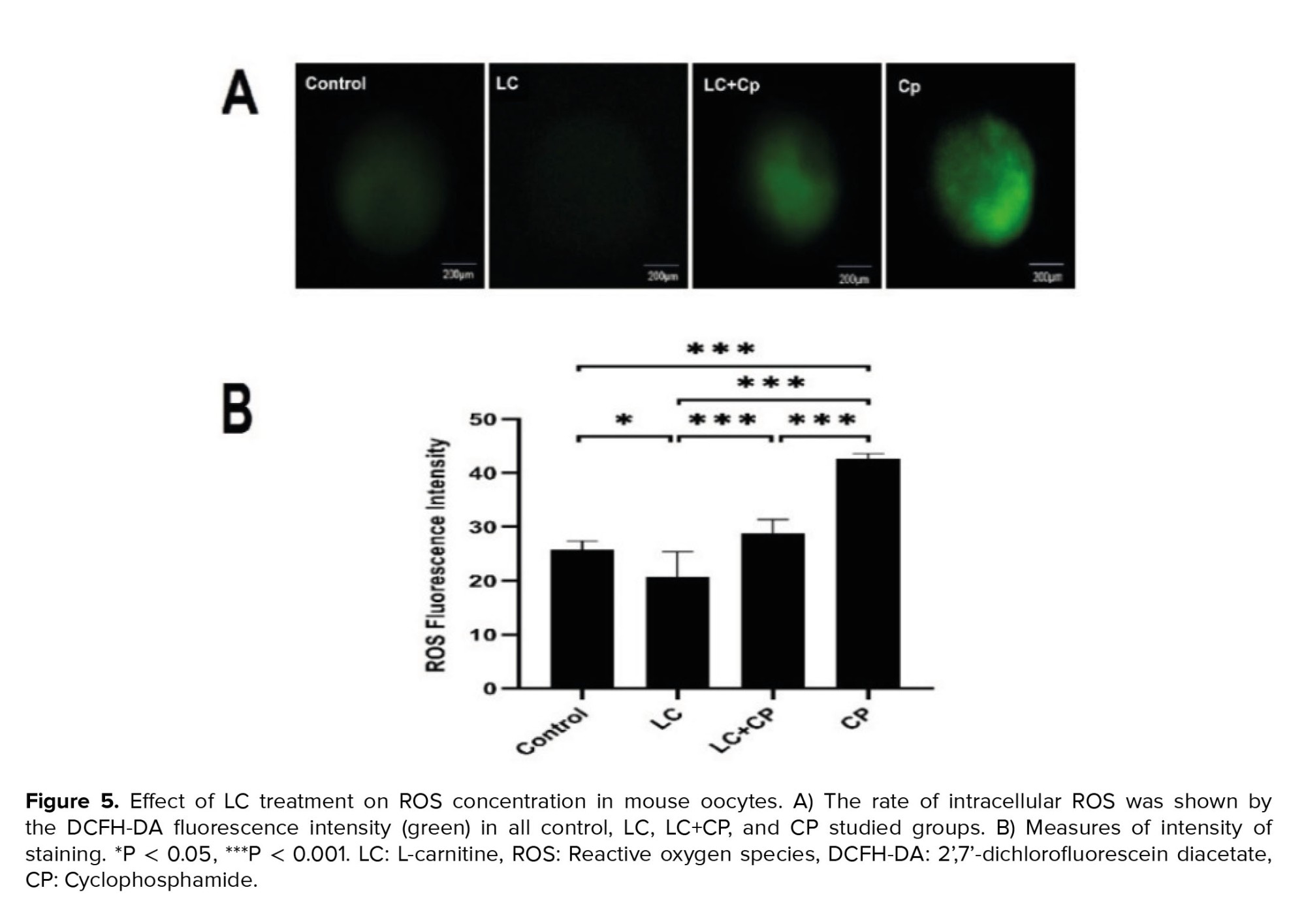
Oocyte intracellular ROS levels were assessed using a DCFH-DA fluorescence assay based on the protocol previously described (15). As described in figure 5, LC could markedly diminish the generation of ROS in the LC group in comparison to the CP (p < 0.001), LC+CP (p ˂ 0.001), and control (p = 0.01) groups. Also, administration of LC significantly decreased the ROS level in the LC+CP group compared to that of the CP group (p < 0.001). Furthermore, a significant (p < 0.001) increase in ROS level was detected in the CP group compared to control group (Figure 5), no statistical difference was observed between control and LC+CP groups.
4. Discussion
Our findings indicated a direct correlation between increasing the production of ROS and activating apoptotic pathways following the treatment with CP. We have shown notable alterations in ROS level and the expression of pro-apoptotic genes (Bax and Caspase3) and an anti-apoptotic gene (Bcl2) in the oocytes of mice exposed to CP group when compared to that of the other groups which can improve by LC. Many reports imply the adverse effect of CP on oocytes. However, the mechanisms presenting how the CP makes oocyte incompetency are still unknown (6).
In this study, we found the upregulation of gene expression of Bax and Caspase3 and downregulation of gene expression of the Bcl2 in the CP group in comparison to the other groups. This modification can trigger the apoptotic pathway associated with oxidative stress and the induction of ROS and reactive nitrogen species. This, in turn, can cause DNA damage, ultimately leading to the mitochondrial apoptotic pathway by reducing the levels of anti-apoptotic proteins (16). One study showed that the expression levels of Bax were elevated in the CP group. This elevation exerts a toxic effect by causing abnormal DNA base pairings, which leads to malformed and dysfunctional cells, resulting in irreversible damage to the ovary (17). So, CP as the most successful and frequently used anticancer drug can cause side effects on the ovaries and oocytes and eventually result in low fertility rates (3, 18). Also, CP can increase the apoptosis of granulosa cells, leading to the depletion of the ovarian reserve of primordial follicles and, consequently, ovarian function failure (19). Active metabolites of CP can prevent DNA synthesis and disrupt the antioxidant defense system of the ovary and ultimately, lead to excessive production of ROS (20).
Furthermore, the generated ROS within the mitochondria can induce lipid peroxidation in the mitochondrial membrane during CP metabolism. This can affect the mitochondrial membrane potential and lead to the release of cytochrome c, triggering apoptosis through the activation of Caspase3 (21). The release of mitochondrial cytochrome c can be further enhanced by p53-induced Bax activation in a caspase-dependent manner (16).
Extracted data from this study showed that the injection of 75 ml/kg CP could significantly increase the level of ROS. In agreement with our results, previous studies have stated that injection of 120 mg/kg of CP yields a significant increase in oocyte intracellular ROS in mice. Yet, most studies on oxidative injury to oocytes focus on some major cellular functions, such as impaired mitochondrial function, the formation of meiotic spindle, configuration, and DNA damage (6, 22). Consequently, the detoxification of ROS might be impressive for improving the oocyte competence to preserve fertility in women treated with chemotherapy (7, 23). Since antioxidants scavenge free radicals and reduce ROS levels in the body (24), these may also be useful in minimizing the harmful effects of free radicals on the apoptotic signaling pathway. There are several reports declaring that both enzymatic and nonenzymatic antioxidants are used to reduce the level of ROS generation (23-25). They have revealed that LC has some properties similar to nonenzymatic antioxidants, although it lacks the activity to scavenge free radicals directly (26).
This compound is widely distributed in nature, especially in red meats and dairy, and can protect loads of cells from oxidative injury and apoptosis in primary neuronal cells as well as some non-neuronal cell lines (27, 28). Many articles have also indicated the protective effects of LC against the various chemotherapy drugs and radiotherapy in different organs and tissue (29-31), but based on our broad search not enough data were available that confirms the protective effect of LC against CP-induced damage and apoptosis in the oocyte.
Additionally, LC’s role as an antioxidant is crucial in preventing the depletion of ATP reserves caused by the accumulation of ROS in follicles, which otherwise leads to diminished follicle quality (32, 33). Our result showed that treatment with 200 mg/kg LC in mice could significantly reduce the intracellular ROS accumulation in the LC group rather than that in the other groups. In confirmation of our results, studies suggested that LC can reduce the oxidative stress of cells by increasing the expression of antioxidant proteins such as superoxide dismutase 2 and improving the ability of the oocyte to reduce the probable damage (34, 35).
Likewise, treating oocytes with LC in culture can enhance their quality, potentially due to a decrease in granulosa cell apoptosis and an improvement in mitochondrial function. Additionally, it has been documented that administering LC can help mitigate delayed embryonic development, DNA fragmentation, and abnormal blastocyst formation resulting due to prolonged culture under high ROS conditions (11). Therefore, LC treatment might enhance follicle quality and improve pregnancy outcomes after in vitro fertilization and embryo transfer. Recent studies have highlighted the protective effects of LC on various organs like rat testicular toxicity (36), reducing oxidative stress in the rat brain (37) and cardiac injury in rats (38). LC shields miotic oocytes from damage induced by follicular fluid in women with mild endometriosis (39). It may inhibit apoptosis by enhancing the β-oxidation of fatty acids, thereby reducing their toxicity.
Moreover, data from our study described that LC could reduce the expression of pro-apoptotic genes (Bax and Caspase3 genes) and significantly enhance the expression of the antiapoptotic gene (Bcl2 gene) in the LC group. Interestingly, we found that using LC in the LC+CP group can significantly upregulate the expression of the antiapoptotic gene and down-regulate pro-apoptotic genes when compared with the CP group. Remarkably, the expression of the Bax gene in the LC+CP group was the same as that presented in the control group. LC has been shown to mitigate the effects of formalin and enhance Bcl2 expression while decreasing Bax expression, suggesting its potential role in inhibiting apoptosis (40).
One study demonstrated that supplementing porcine cumulus oocyte complexes with LC, at a concentration of 0.5 mg/mL during in vitro maturation enhanced nuclear maturation. After parthenogenic activation, this supplementation led to an increased number of blastocysts with a reduced number of apoptotic cells, suggesting that the positive effects of LC might be linked to its antiapoptotic properties (34). This finding was confirmed by studies conducted on a mouse model. When mouse embryos were exposed to hydrogen peroxide or tumor necrosis factor (TNF), there was a decrease in blastocyst development rates accompanied by increased apoptosis. However, the introduction of LC at concentrations of 0.3 or 0.6 mg/ml significantly mitigated the detrimental effects of both hydrogen peroxide and TNF (34). The antiapoptotic and proliferative effects of LC during embryo development were supported by changes in the expression of apoptotic genes, such as Bax, Bcl2, and Caspase3. This was further confirmed by observations that LC could counteract the proapoptotic and antiproliferative effects of actinomycin D and TNF-alpha on cleavage-stage embryos (41). Furthermore, LC reduces apoptosis induced by mitochondria in both in vivo and in vitro conditions (42). A similar study showed that LC not only stabilizes mitochondrial membranes but also enhances the energy supply to the organelle and protects cells from apoptosis (43). Data from this study confirmed that LC can potentially decrease the pro-apoptotic gene expression in mice exposed to CP.
4.1. Limitations
More research is necessary to clarify the molecular mechanisms underlying the function of LC to repair damaged oocytes and ovaries after chemotherapy by CP.
5. Conclusion
LC can alleviate the adverse effects of CP chemotherapy, including intracellular ROS accumulation, and influence the apoptosis pathway in CP-exposed mice oocytes by decreasing the ROS level and the expression of Bax and Caspase3 genes, while increasing the Bcl2 gene expression.
Data availability
The data and materials supporting this study’s findings are available from the corresponding author upon reasonable request.
Author contributions
Gh. Moshkdanian, H. Nikzad, M. Almasi, and G. Shafiei had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design Gh. Moshkdanian, H. Nikzad, and M. Almasi. Acquisition, analysis, or interpretation of data Gh. Moshkdanian, H. Nikzad, M. Karimian, M. Almasi, and G. Shafiei. Drafting of the manuscript: M. Almasi, G. Shafiei, and M. Karimian. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: M. Almasi, G. Shafiei, and Gh. Moshkdanian. Supervision: Gh. Moshkdanian and H. Nikzad.
Acknowledgments
This study was financially supported by Kashan University of Medical Sciences, Kashan, Iran (grant number: 96114). AI (GPT-4) was used to improve the language and readability of the paper.
Conflict of Interest
No conflict of interest.
Control group: Received 2.5 ml/kg normal saline intraperitoneal (IP) injection for 10 days.
LC group: Received 200 mg/kg of LC (Sigma-Aldrich Co., St. Louis, MO, USA) IP injection for 10 days (10, 14).
LC+CP group: Received 200 mg/kg IP injection for 10 days and CP (Endoxan, Baxter Oncology GmbH, Halle, Germany) 75 mg/kg single IP injection on the 10th day of the experiment.
CP group: Received 75 mg/kg of CP as a single IP injection on 10th day of the experiment (10).
2.3. Oocyte collection
Female mice were superovulated by IP injection of 8 IU pregnant mare serum gonadotropin (Sigma-Aldrich, China), after 10 days injection of LC, CP, or saline. Then, 48 hr later, a similar dose of human chorionic gonadotropin (Pregnyl; Organon, Germany) was also given to the females.
Mice were sacrificed by cervical dislocation 13.5 hr later and oviducts were flushed in Ham’s F10 media (Thermo Fisher Scientific, USA) under a stereomicroscope (Olympus SZX9, Japan), the cumulus cells were removed enzymatically using 0.3 mg/ml hyaluronidase (Sigma-Aldrich, USA) and only mature oocytes with homogeneous cytoplasm, normal perivitelline space, and zona pellucida were considered morphologically normal. Selected oocytes were incubated in media drops under liquid paraffin oil at 37°C, 5% CO2 incubator to be used in experiments (10, 14).
2.4. RNA isolation and real-time polymerase chain reaction (RT-PCR)
The expression levels of Bcl2-associated X (Bax), Caspase3, and B-cell lymphoma 2 (Bcl2) genes in oocytes (n = 60/each) were quantified using RT-PCR. RNA was extracted from each group utilizing the RNeasy Micro kit (74004, Qiagen, USA), adhering to the manufacturer’s guidelines (10). Subsequently, the isolated RNA served as a template for cDNA synthesis, performed with the Prime Script™ RT reagent kit (Takara Bio Inc., Shiga, Japan), using 500 ng of total RNA under the provided protocol. The RT-PCR analysis involved 2 µl of the cDNA, combined with the SYBR green master mix (BioFACT, Korea), in a final volume of 10 µl. This reaction was executed on the IQ5 RT-PCR system (Bio-Rad, Germany). Primer sequences employed for each gene are detailed in table I. To normalize the expression of long non-coding RNAs, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels were used as a reference, and the relative changes were calculated using the 2-ΔΔc method.
2.5. Measurement of ROS in oocytes
To quantify the relative levels of ROS in oocytes, we employed the fluorescent dye 2’,7’-dichlorofluorescein diacetate (DCFH-DA, 2 µM, Sigma, D6883, USA). A group of 5-10 oocytes were incubated for 20 min in Ham’s F10 medium enriched with DCFH-DA (2 µM) at 37°C in darkness. Postincubation, the oocytes were rinsed with Ham’s F10 medium supplemented with 10% human serum albumin (N6908, Sigma, USA) and then placed on glass slides. Fluorescence measurements were conducted using a Nikon Eclipse Ti fluorescence microscope (533450, Japan), utilizing excitation and emission filters with wavelengths of 450-490 nm and 520 nm, respectively. The fluorescence intensity, indicative of ROS levels, was quantified for each oocyte using Image Quant software (TotalLab Quant, UK) (15).


2.6. Ethical considerations
The procedures were approved by the Kashan University of Medical Sciences, Kashan, Iran (Code: IR.KAUMS.MEDNT.REC.1396.45). The animal experimental protocols were supported by the Iranian Council’s guidelines for the Use and Care of Animals.
2.7. Statistical analysis
Each experiment was performed in triplicate, and statistical analysis was conducted using GraphPad Prism 8 (San Diego, CA, USA) and SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). A one-way ANOVA test (comparison between 3 or more experimental groups) was performed followed by post hoc Tukey’s test. The results were expressed as the mean ± standard deviation. The mean differences between groups for ROS and gene expression levels were compared using analysis of variance (ANOVA), followed by post hoc Tukey’s test. P < 0.05 were considered statistically significant.
3. Results
3.1. The effect of LC and CP on changes in the expression of apoptosis genes (Bax, Caspase3, Bcl2)
To investigate whether apoptosis genes are up- or downregulated among studied groups (control, LC, LC+CP, CP), we monitored the relative change in mRNA expression of proapoptotic genes (Bax and Caspase3) and antiapoptotic gene (Bcl2) with RT-PCR.
As shown in figures 2 and 3, the expression of the Bax and Caspase3 genes significantly upregulated in the CP group in comparison to the LC+CP (p = 0.03, p = 0.04), LC (p ˂ 0.001) and control (p ˂ 0.001) groups, respectively. The lowest significant expression of the Bax gene was found in the LC group in comparison to the LC+CP (p = 0.02) group. Interestingly, the expression level of the Bax gene in the LC+CP group was nonsignificant in comparison to the control group (Figure 2). The LC+CP group had a significantly (p = 0.04) higher expression of Caspase3 genes than control group (Figure 3).
As depicted in figure 4 a significant upregulation in Bcl2 expression was observed in the LC group compared to LC+CP (p ˂ 0.001) and CP (p ˂ 0.001) groups. In addition, a significant decrease was observed in the expression level of Bcl2 in the CP group in comparison to the LC+CP (p = 0.01), and control (p ˂ 0.001) groups. Moreover, the expression of the Bcl2 gene in the LC+CP group significantly decreased compared to control group (p = 0.04) (Figure 4).
3.2. The effect of LC and CP on the intracellular ROS level
Oocyte intracellular ROS levels were assessed using a DCFH-DA fluorescence assay based on the protocol previously described (15). As described in figure 5, LC could markedly diminish the generation of ROS in the LC group in comparison to the CP (p < 0.001), LC+CP (p ˂ 0.001), and control (p = 0.01) groups. Also, administration of LC significantly decreased the ROS level in the LC+CP group compared to that of the CP group (p < 0.001). Furthermore, a significant (p < 0.001) increase in ROS level was detected in the CP group compared to control group (Figure 5), no statistical difference was observed between control and LC+CP groups.




4. Discussion
Our findings indicated a direct correlation between increasing the production of ROS and activating apoptotic pathways following the treatment with CP. We have shown notable alterations in ROS level and the expression of pro-apoptotic genes (Bax and Caspase3) and an anti-apoptotic gene (Bcl2) in the oocytes of mice exposed to CP group when compared to that of the other groups which can improve by LC. Many reports imply the adverse effect of CP on oocytes. However, the mechanisms presenting how the CP makes oocyte incompetency are still unknown (6).
In this study, we found the upregulation of gene expression of Bax and Caspase3 and downregulation of gene expression of the Bcl2 in the CP group in comparison to the other groups. This modification can trigger the apoptotic pathway associated with oxidative stress and the induction of ROS and reactive nitrogen species. This, in turn, can cause DNA damage, ultimately leading to the mitochondrial apoptotic pathway by reducing the levels of anti-apoptotic proteins (16). One study showed that the expression levels of Bax were elevated in the CP group. This elevation exerts a toxic effect by causing abnormal DNA base pairings, which leads to malformed and dysfunctional cells, resulting in irreversible damage to the ovary (17). So, CP as the most successful and frequently used anticancer drug can cause side effects on the ovaries and oocytes and eventually result in low fertility rates (3, 18). Also, CP can increase the apoptosis of granulosa cells, leading to the depletion of the ovarian reserve of primordial follicles and, consequently, ovarian function failure (19). Active metabolites of CP can prevent DNA synthesis and disrupt the antioxidant defense system of the ovary and ultimately, lead to excessive production of ROS (20).
Furthermore, the generated ROS within the mitochondria can induce lipid peroxidation in the mitochondrial membrane during CP metabolism. This can affect the mitochondrial membrane potential and lead to the release of cytochrome c, triggering apoptosis through the activation of Caspase3 (21). The release of mitochondrial cytochrome c can be further enhanced by p53-induced Bax activation in a caspase-dependent manner (16).
Extracted data from this study showed that the injection of 75 ml/kg CP could significantly increase the level of ROS. In agreement with our results, previous studies have stated that injection of 120 mg/kg of CP yields a significant increase in oocyte intracellular ROS in mice. Yet, most studies on oxidative injury to oocytes focus on some major cellular functions, such as impaired mitochondrial function, the formation of meiotic spindle, configuration, and DNA damage (6, 22). Consequently, the detoxification of ROS might be impressive for improving the oocyte competence to preserve fertility in women treated with chemotherapy (7, 23). Since antioxidants scavenge free radicals and reduce ROS levels in the body (24), these may also be useful in minimizing the harmful effects of free radicals on the apoptotic signaling pathway. There are several reports declaring that both enzymatic and nonenzymatic antioxidants are used to reduce the level of ROS generation (23-25). They have revealed that LC has some properties similar to nonenzymatic antioxidants, although it lacks the activity to scavenge free radicals directly (26).
This compound is widely distributed in nature, especially in red meats and dairy, and can protect loads of cells from oxidative injury and apoptosis in primary neuronal cells as well as some non-neuronal cell lines (27, 28). Many articles have also indicated the protective effects of LC against the various chemotherapy drugs and radiotherapy in different organs and tissue (29-31), but based on our broad search not enough data were available that confirms the protective effect of LC against CP-induced damage and apoptosis in the oocyte.
Additionally, LC’s role as an antioxidant is crucial in preventing the depletion of ATP reserves caused by the accumulation of ROS in follicles, which otherwise leads to diminished follicle quality (32, 33). Our result showed that treatment with 200 mg/kg LC in mice could significantly reduce the intracellular ROS accumulation in the LC group rather than that in the other groups. In confirmation of our results, studies suggested that LC can reduce the oxidative stress of cells by increasing the expression of antioxidant proteins such as superoxide dismutase 2 and improving the ability of the oocyte to reduce the probable damage (34, 35).
Likewise, treating oocytes with LC in culture can enhance their quality, potentially due to a decrease in granulosa cell apoptosis and an improvement in mitochondrial function. Additionally, it has been documented that administering LC can help mitigate delayed embryonic development, DNA fragmentation, and abnormal blastocyst formation resulting due to prolonged culture under high ROS conditions (11). Therefore, LC treatment might enhance follicle quality and improve pregnancy outcomes after in vitro fertilization and embryo transfer. Recent studies have highlighted the protective effects of LC on various organs like rat testicular toxicity (36), reducing oxidative stress in the rat brain (37) and cardiac injury in rats (38). LC shields miotic oocytes from damage induced by follicular fluid in women with mild endometriosis (39). It may inhibit apoptosis by enhancing the β-oxidation of fatty acids, thereby reducing their toxicity.
Moreover, data from our study described that LC could reduce the expression of pro-apoptotic genes (Bax and Caspase3 genes) and significantly enhance the expression of the antiapoptotic gene (Bcl2 gene) in the LC group. Interestingly, we found that using LC in the LC+CP group can significantly upregulate the expression of the antiapoptotic gene and down-regulate pro-apoptotic genes when compared with the CP group. Remarkably, the expression of the Bax gene in the LC+CP group was the same as that presented in the control group. LC has been shown to mitigate the effects of formalin and enhance Bcl2 expression while decreasing Bax expression, suggesting its potential role in inhibiting apoptosis (40).
One study demonstrated that supplementing porcine cumulus oocyte complexes with LC, at a concentration of 0.5 mg/mL during in vitro maturation enhanced nuclear maturation. After parthenogenic activation, this supplementation led to an increased number of blastocysts with a reduced number of apoptotic cells, suggesting that the positive effects of LC might be linked to its antiapoptotic properties (34). This finding was confirmed by studies conducted on a mouse model. When mouse embryos were exposed to hydrogen peroxide or tumor necrosis factor (TNF), there was a decrease in blastocyst development rates accompanied by increased apoptosis. However, the introduction of LC at concentrations of 0.3 or 0.6 mg/ml significantly mitigated the detrimental effects of both hydrogen peroxide and TNF (34). The antiapoptotic and proliferative effects of LC during embryo development were supported by changes in the expression of apoptotic genes, such as Bax, Bcl2, and Caspase3. This was further confirmed by observations that LC could counteract the proapoptotic and antiproliferative effects of actinomycin D and TNF-alpha on cleavage-stage embryos (41). Furthermore, LC reduces apoptosis induced by mitochondria in both in vivo and in vitro conditions (42). A similar study showed that LC not only stabilizes mitochondrial membranes but also enhances the energy supply to the organelle and protects cells from apoptosis (43). Data from this study confirmed that LC can potentially decrease the pro-apoptotic gene expression in mice exposed to CP.
4.1. Limitations
More research is necessary to clarify the molecular mechanisms underlying the function of LC to repair damaged oocytes and ovaries after chemotherapy by CP.
5. Conclusion
LC can alleviate the adverse effects of CP chemotherapy, including intracellular ROS accumulation, and influence the apoptosis pathway in CP-exposed mice oocytes by decreasing the ROS level and the expression of Bax and Caspase3 genes, while increasing the Bcl2 gene expression.
Data availability
The data and materials supporting this study’s findings are available from the corresponding author upon reasonable request.
Author contributions
Gh. Moshkdanian, H. Nikzad, M. Almasi, and G. Shafiei had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design Gh. Moshkdanian, H. Nikzad, and M. Almasi. Acquisition, analysis, or interpretation of data Gh. Moshkdanian, H. Nikzad, M. Karimian, M. Almasi, and G. Shafiei. Drafting of the manuscript: M. Almasi, G. Shafiei, and M. Karimian. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: M. Almasi, G. Shafiei, and Gh. Moshkdanian. Supervision: Gh. Moshkdanian and H. Nikzad.
Acknowledgments
This study was financially supported by Kashan University of Medical Sciences, Kashan, Iran (grant number: 96114). AI (GPT-4) was used to improve the language and readability of the paper.
Conflict of Interest
No conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Rodriguez-Wallberg KA, Jiang Y, Lekberg T, Nilsson HP. The late effects of cancer treatment on female fertility and the current status of fertility preservation-a narrative review. Life 2023; 13: 1195. [DOI:10.3390/life13051195] [PMID] [PMCID]
2. Alesi LR, Nguyen Q-N, Stringer JM, Winship AL, Hutt KJ. The future of fertility preservation for women treated with chemotherapy. Reprod Fertil 2023; 4: e220123. [DOI:10.1530/RAF-22-0123] [PMID] [PMCID]
3. Feng J, Ma W-W, Li H-X, Pei X-Y, Deng S-L, Jia H, et al. Melatonin prevents cyclophosphamide-induced primordial follicle loss by inhibiting ovarian granulosa cell apoptosis and maintaining AMH expression. Front Endocrinol (Lausanne) 2022; 13: 895095. [DOI:10.3389/fendo.2022.895095] [PMID] [PMCID]
4. Raeeszadeh M, Hosseini SMS, Amiri AA. Impact of co‐administration of N‐acetylcysteine and vitamin E on cyclophosphamide‐induced ovarian toxicity in female rats. J Toxicol 2022; 2022: 9073405. [DOI:10.1155/2022/9073405] [PMID] [PMCID]
5. Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 2019; 25: 673-693. [DOI:10.1093/humupd/dmz027] [PMID] [PMCID]
6. Yang W, Ma Y, Jin J, Ren P, Zhou H, Xu S, et al. Cyclophosphamide exposure causes long-term detrimental effect of oocytes developmental competence through affecting the epigenetic modification and maternal factors' transcription during oocyte growth. Front Cell Dev Biol 2021; 9: 682060. [DOI:10.3389/fcell.2021.682060] [PMID] [PMCID]
7. Rakha SI, Elmetwally MA, El-Sheikh Ali H, Balboula A, Mahmoud AM, Zaabel SM. Importance of antioxidant supplementation during in vitro maturation of mammalian oocytes. Vet Sci 2022; 9: 439. [DOI:10.3390/vetsci9080439] [PMID] [PMCID]
8. Yang L, Chen Y, Liu Y, Xing Y, Miao C, Zhao Y, et al. The role of oxidative stress and natural antioxidants in ovarian aging. Front Pharmacol 2021; 11: 617843. [DOI:10.3389/fphar.2020.617843] [PMID] [PMCID]
9. Jiang W, Li Y, Zhao Y, Gao Q, Jin Q, Yan C, et al. L-carnitine supplementation during in vitro culture regulates oxidative stress in embryos from bovine aged oocytes. Theriogenology 2020; 143: 64-73. [DOI:10.1016/j.theriogenology.2019.11.036] [PMID]
10. Behi Shahreza P, Ahmadpour S, Almasi M, Seyyed Hosseini E, Akhavan Taheri M, Moshkdanian Gh. The effect of L-carnitine on oocyte mitochondrial health and biomarkers on cyclophosphamide chemotherapy drug in mice. Reprod Toxicol 2023; 122: 108490. [DOI:10.1016/j.reprotox.2023.108490] [PMID]
11. Li X, Wu X, Ma T, Zhang Y, Sun P, Qi D, et al. Protective effect of L-carnitine against oxidative stress injury in human ovarian granulosa cells. Exp Ther Med 2023; 25: 161. [DOI:10.3892/etm.2023.11860] [PMID] [PMCID]
12. Li J, Liu L, Weng J, Yin T-L, Yang J, Feng HL. Biological roles of L‐carnitine in oocyte and early embryo development. Mol Reprod Dev 2021; 88: 673-685. [DOI:10.1002/mrd.23542] [PMID]
13. Sanamiri Kh, Soleimani Mehranjani M, Shahhoseini M, Shariatzadeh MA. L-carnitine improves follicular survival and function in ovarian grafts in the mouse. Reprod Fertil Dev 2022; 34: 713-721. [DOI:10.1071/RD21287] [PMID]
14. Rajab AA, Hafidh A, Al-Shmgani HS. The impact of platelet-rich plasma and L-carnitine on cyclophosphamide-induced hematotoxicity in male albino rats. Biochem Cell Arch 2022; 22: 85.
15. Shafiei G, Moghani-Ghoroghi F, Miyan J, Almasi M, Regerdi Kashani I, Nikzad H, et al. Melatonin protects against visible light-induced oxidative stress and promotes the implantation potential of mouse blastocyst in vitro. Res Vet Sci 2023; 155: 29-35. [DOI:10.1016/j.rvsc.2022.12.003] [PMID]
16. El Kiki SM, Omran MM, Mansour HH, Hasan HF. Metformin and/or low dose radiation reduces cardiotoxicity and apoptosis induced by cyclophosphamide through SIRT-1/SOD and BAX/Bcl-2 pathways in rats. Mol Biol Rep 2020; 47: 5115-5126. [DOI:10.1007/s11033-020-05582-5] [PMID]
17. Zhang B-F, Hu Y, Liu X, Cheng Z, Lei Y, Liu Y, et al. The role of AKT and FOXO3 in preventing ovarian toxicity induced by cyclophosphamide. PloS One 2018; 13: e0201136. [DOI:10.1371/journal.pone.0201136] [PMID] [PMCID]
18. Barberino RS, Silva RLS, Palheta Junior RC, Smitz JEJ, Matos MHT. Protective effects of antioxidants on cyclophosphamide-induced ovarian toxicity. Biopreserv Biobank 2023; 21: 121-141. [DOI:10.1089/bio.2021.0159] [PMID]
19. Liu W, Chen Q, Liu Z, Weng Z, Nguyen TN, Feng J, et al. Zihuai recipe alleviates cyclophosphamide-induced diminished ovarian reserve via suppressing PI3K/AKT-mediated apoptosis. J Ethnopharmacol 2021; 277: 113789. [DOI:10.1016/j.jep.2021.113789] [PMID]
20. Chen Y, Zhao Y, Miao C, Yang L, Wang R, Chen B, et al. Quercetin alleviates cyclophosphamide-induced premature ovarian insufficiency in mice by reducing mitochondrial oxidative stress and pyroptosis in granulosa cells. J Ovarian Res 2022; 15: 138. [DOI:10.1186/s13048-022-01080-3] [PMID] [PMCID]
21. Aladaileh SH, Abukhalil MH, Saghir SAM, Hanieh H, Alfwuaires MA, Almaiman AA, et al. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules 2019; 9: 346. [DOI:10.3390/biom9080346] [PMID] [PMCID]
22. Wang W, Zhang D, Sun L, Zhang Z, Zhang Y, Zhang Y, et al. Alpha-lipoic acid supplementation reverses the declining quality of oocytes exposed to cyclophosphamide. Food Chem Toxicol 2023; 181: 114090. [DOI:10.1016/j.fct.2023.114090] [PMID]
23. Zhang S, Liu Q, Chang M, Pan Y, Yahaya BH, Liu Y, et al. Chemotherapy impairs ovarian function through excessive ROS-induced ferroptosis. Cell Death Dis 2023; 14: 340. [DOI:10.1038/s41419-023-05859-0] [PMID] [PMCID]
24. Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, et al. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol 2021; 236: 7966-7983. [DOI:10.1002/jcp.30468] [PMID]
25. Hussain T. Antioxidant strategies to improve female reproduction. In: Otero Fuertes P, Fraga Corralga Corral M. Recent developments in antioxidants from natural sources. IntechOpen; 2023. Available at: www.intechopen.com/chapters/86532.
26. Shafiei G, Almasi M, Nikzad H, Miyan J, Amini Mahabadi J, Moshkdanian Gh. L-carnitine reduces the adverse effects of ROS and up-regulates the expression of implantation related genes in in vitro developed mouse embryos. Theriogenology 2020; 145: 59-66. [DOI:10.1016/j.theriogenology.2020.01.008] [PMID]
27. Lee Y-CG, Chou H-C, Chen Y-T, Tung S-Y, Ko T-L, Buyandelger B, et al. L-carnitine reduces reactive oxygen species/endoplasmic reticulum stress and maintains mitochondrial function during autophagy-mediated cell apoptosis in perfluorooctanesulfonate-treated renal tubular cells. Sci Rep 2022; 12: 4673. [DOI:10.1038/s41598-022-08771-3] [PMID] [PMCID]
28. Morid OF, Menze ET, Tadros MG, George MY. L-Carnitine modulates cognitive impairment induced by doxorubicin and cyclophosphamide in rats; insights to oxidative stress, inflammation, synaptic plasticity, liver/brain, and kidney/brain axes. J Neuroimmune Pharmacol 2023; 18: 310-326. [DOI:10.1007/s11481-023-10062-1] [PMID] [PMCID]
29. Bushra RR, Bastwrous AE. Possible protective role of L-carnitine against cisplatin-induced testicular changes in adult male albino rats: A histological and morphometric study. Egypt Acad J Biol Sci, D Histol Histochem 2022; 14: 81-94. [DOI:10.21608/eajbsd.2022.220437]
30. Alkasaby Zalat Z, Abdel-Latif MM, Alotaibi BS, Alm El-Din MA, Kohaf NA, Elewa HA. Efficacy of L-carnitine and silymarin administration on the health-related quality of life in 120 patients with cancer undergoing anthracycline-based chemotherapy. J Med Life Sci 2020; 2: 20-38. [DOI:10.21608/jmals.2020.111233]
31. Momenzadeh M, Aria A, Ghadimi K, Moghaddas A. Acetyl-L-carnitine for the prevention of taxane-induced neuropathy in patients with breast cancer: A systematic review and meta-analysis. Res Pharm Sci 2023; 18: 112-120. [DOI:10.4103/1735-5362.367791] [PMID] [PMCID]
32. Zhang Q, Wang S-M, Yao P-B, Zhang L, Zhang Y-J, Chen R-X, et al. Effects of L-carnitine on follicular survival and graft function following autotransplantation of cryopreserved-thawed ovarian tissues. Cryobiology 2015; 71: 135-140. [DOI:10.1016/j.cryobiol.2015.04.008] [PMID]
33. El-Sokary MMM, El-Naby Al-HH, Abd El Hameed AR, Mahmoud KGM, Scholkamy TH. Impact of L-carnitine supplementation on the in vitro developmental competence and cryotolerance of buffalo embryos. Vet World 2021; 14: 3164-3169. [DOI:10.14202/vetworld.2021.3164-3169] [PMID] [PMCID]
34. Placidi M, Di Emidio G, Virmani A, D'Alfonso A, Artini PG, D'Alessandro AM, et al. Carnitines as mitochondrial modulators of oocyte and embryo bioenergetics. Antioxidants 2022; 11: 745. [DOI:10.3390/antiox11040745] [PMID] [PMCID]
35. Silva BR, Silva JRV. Mechanisms of action of non-enzymatic antioxidants to control oxidative stress during in vitro follicle growth, oocyte maturation, and embryo development. Anim Reprod Sci 2023; 249: 107186. [DOI:10.1016/j.anireprosci.2022.107186] [PMID]
36. Khedr NF, Werida RH. L-carnitine modulates autophagy, oxidative stress and inflammation in trazodone induced testicular toxicity. Life Sci 2022; 290: 120025. [DOI:10.1016/j.lfs.2021.120025] [PMID]
37. Salama A, Elgohary R. L-carnitine and Co Q10 ameliorate potassium dichromate-induced acute brain injury in rats targeting AMPK/AKT/NF-κβ. Int Immunopharmacol 2021; 101: 107867. [DOI:10.1016/j.intimp.2021.107867] [PMID]
38. Farag A, Elfadadny A, Mandour AS, Ngeun SK, Aboubakr M, Kaneda M, et al. Potential protective effects of L-carnitine against myocardial ischemia/reperfusion injury in a rat model. Environ Sci Pollut Res Int 2024; 31: 18813-18825. [DOI:10.1007/s11356-024-32212-5] [PMID]
39. Giorgi VSI, Da Broi MG, Paz CCP, Ferriani RA, Navarro PA. N-acetyl-cysteine and L-carnitine prevent meiotic oocyte damage induced by follicular fluid from infertile women with mild endometriosis. Reprod Sci 2016; 23: 342-351. [DOI:10.1177/1933719115602772] [PMID]
40. Vardiyan R, Ezati D, Anvari M, Ghasemi N, Talebi A. Effect of L-carnitine on the expression of the apoptotic genes Bcl-2 and Bax. Clin Exp Reprod Med 2020; 47: 155-160. [DOI:10.5653/cerm.2019.03440] [PMID] [PMCID]
41. Mishra A, Reddy IJ, Gupta P, Mondal S. Developmental regulation and modulation of apoptotic genes expression in sheep oocytes and embryos cultured in vitro with L‐carnitine. Reprod Domest Anim 2016; 51: 1020-1029. [DOI:10.1111/rda.12789] [PMID]
42. Hassan N, Rashad M, Elleithy E, Sabry Z, Ali G, Elmosalamy S. L-carnitine alleviates hepatic and renal mitochondrial-dependent apoptotic progression induced by letrozole in female rats through modulation of Nrf-2, Cyt c and CASP-3 signaling. Drug Chem Toxicol 2023; 46: 357-368. [DOI:10.1080/01480545.2022.2039180] [PMID]
43. Li P, Xia Z, Kong W, Wang Q, Zhao Z, Arnold A, et al. Exogenous L-carnitine ameliorates burn-induced cellular and mitochondrial injury of hepatocytes by restoring CPT1 activity. Nutr Metab 2021; 18: 65. [DOI:10.1186/s12986-021-00592-x] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








