Fri, Feb 20, 2026
[Archive]
Volume 22, Issue 11 (November 2024)
IJRM 2024, 22(11): 845-856 |
Back to browse issues page
Ethics code: IR.SSU.MEDICINE.REC.1400.381
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tabatabaei R S, Mirjalili S R, Ashrafi A, Shamsi F. The relationship between maternal vitamin D levels and osteopenia development in preterm infants: A cross-sectional study. IJRM 2024; 22 (11) :845-856
URL: http://ijrm.ir/article-1-3338-en.html
URL: http://ijrm.ir/article-1-3338-en.html
1- Department of Obstetrics and Gynecology, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Mother and Newborn Health Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Mother and Newborn Health Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,Dr_sr_mirjalili@yahoo.com
3- Department of Obstetrics and Gynecology, Shahid Beheshti Hospital, Esfahan University of Medical Sciences, Ardestan, Iran.
4- Department of Biostatistics and Epidemiology, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Mother and Newborn Health Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,
3- Department of Obstetrics and Gynecology, Shahid Beheshti Hospital, Esfahan University of Medical Sciences, Ardestan, Iran.
4- Department of Biostatistics and Epidemiology, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Full-Text [PDF 245 kb]
(918 Downloads)
| Abstract (HTML) (1044 Views)
1. Introduction
Premature neonates are at risk of developing osteopenia, a condition marked by reduced mineralization of the skeletal system compared to those who reach full term in the womb (1). This typically occurs 3-12 months after birth, with a prevalence of up to 60% in extremely low birth weight neonates and 20% in very low birth weight neonates (2). Moreover, evidence has shown defined rickets in 10-20% of preterm neonates with birth weights ≤ 1000 gr (3).
Risk factors for osteopenia of prematurity (OP) include low gestational age (GA) and birth weight, extreme prematurity, maternal chorioamnionitis, prolonged total parenteral nutrition, use of corticosteroids, methylxanthines, diuretics, immobilization, and sedation (4-6). Placental calcium and phosphate transfer to the fetus significantly rise after 24 wk of gestation. Inadequate calcium and phosphorus intake post-birth or through parenteral nutrition can lead to bone deformities and fractures in neonates (7-9). Additionally, factors such as lack of phosphate intake, prolonged parenteral nutrition, high alkaline phosphatase (ALP) levels, onset of vitamin D supplementation, and premature rupture of membranes are associated with the incidence of OP (7).
ALP is a commonly used marker for OP. Studies have shown that elevated ALP levels are linked to osteopenia occurrence in neonates. Serum calcium and phosphorus levels have also been studied as markers for OP (4, 10-12). Low serum inorganic phosphate levels have been significantly associated with osteopenia in premature neonates (11). Other markers such as parathyroid hormone (PTH) and vitamin D concentrations have also been investigated (6). Maternal or neonatal vitamin D deficiency can exacerbate osteopenia in preterm and very low birth weight neonates by affecting calcium and bone metabolism (9, 13).
Thus, this study aimed to establish the occurrence of osteopenia in premature neonates and examine the influence of maternal vitamin D on their bone health.
2. Materials and Methods
2.1. Participants
In this cross-sectional study, data of 49 pregnant women with premature labor and their neonates, referred to the gynecology and neonatology wards, Shahid Sadoughi hospital, Yazd, Iran, from June 2022 to September 2023, was extracted from their medical records.
The inclusion criteria were 18 and 33 yr pregnant women without maternal chorioamnionitis who experienced preterm labor (GA ≤ 34 wk based on the last menstrual period and ultrasound) and took 1000 units of vitamin D supplements daily until delivery, who has their first delivery.
Moreover, exclusion criteria were GA > 34 wk, congenital skeletal anomalies, multiple gestations, and refusal of follow-up visits.
Data on maternal and neonatal demographics, and clinical status including: complications, GA, delivery type, neonate gender, medication use, mechanical ventilation, feeding status, and hospitalization duration were extracted from newborn medical records. Routine laboratory tests included measuring serum vitamin D levels in mothers and preterm neonates at birth and 4th wk were conducted at Shahid Sadoughi hospital, Yazd, Iran. Diagnostic biomarkers for osteopenia, including serum phosphorus, calcium, and ALP levels were also recorded. Neonates were classified into 2 groups as healthy (non-osteopenic n = 38) or at risk for osteopenia (Osteopenic n = 11) based on established biochemical marker cut-off points. Serum vitamin D and calcium levels were compared between the 2 groups to investigate potential relationships with osteopenia.
2.2. Sample size
The sample size was estimated to be a minimum of 49 participants by considering a 95% confidence level, a power of 80%, and a standard deviation of 6.8 for the vitamin D level in mothers of infants with OP. A bias of 2 units in the estimations was also considered. The calculation utilized PASS15 software (PASS 15 Power Analysis and Sample Size Software, 2017, NCSS, LLC, Kaysville, Utah, USA, ncss.com/software/pass) and followed the following formula:
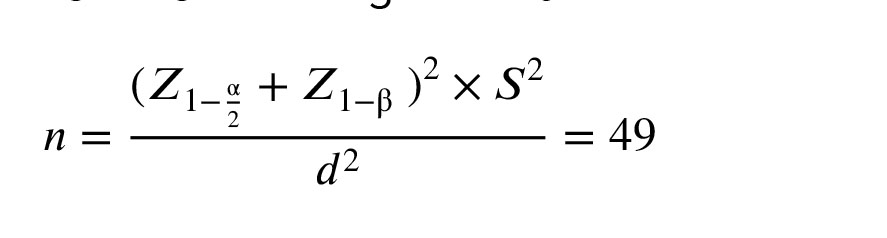
2.3. Biochemical analysis
All participating mothers received a daily 1000 unit vitamin D supplement under the supervision of a gynecologist until delivery. Following delivery, a 5 ml venous blood sample was obtained to assess maternal serum 25-(OH)-vitamin D3 (Vitamin D) levels using the enzyme-linked immunosorbent assay method. Moreover, 3 ml of umbilical cord blood was collected for neonatal serum vitamin D analysis. Subsequently, all neonates were administered total parenteral nutrition therapy in the 1st wk, which included calcium gluconate, glycophos, and 7000 units of vitamin D per week. To evaluate blood biochemical markers related to OP, serum calcium, ALP, and phosphorus levels were measured at birth and again at 4 wk of age using the spectrophotometric method. Based on the manufacturer's recommendation, serum vitamin D levels are categorized as deficiency (< 20 ng/ml), insufficiency (20-30 ng/ml), and sufficiency (> 30 ng/ml). Additionally, the cut-off points for P < 4 mmol/L or ALP > 900 IU/L were designated as predictive values for OP (14).
2.4. Ethical Considerations
The study protocol was approved by the Ethics Committee of the Institutional Review Board of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Code: IR.SSU.MEDICINE.REC.1400.381). Before enrollment, written informed consent was obtained from all participants to use their data. Participants privacy and confidentiality were maintained, and they were not subjected to any additional costs.
2.5. Statistical Analysis
The data analysis was performed using SPSS 26.0, a software developed by SPSS Inc. in Chicago, Illinois, USA. Quantitative variables were presented as mean ± SD, while qualitative variables were presented as numbers (percent). To establish relationships between biomarker values for OP, vitamin D concentration, and other variables, statistical tests such as independent Student t test, Chi-square test, and Fisher’s exact tests were used. The predetermined level of significance was p < 0.05.
3. Results
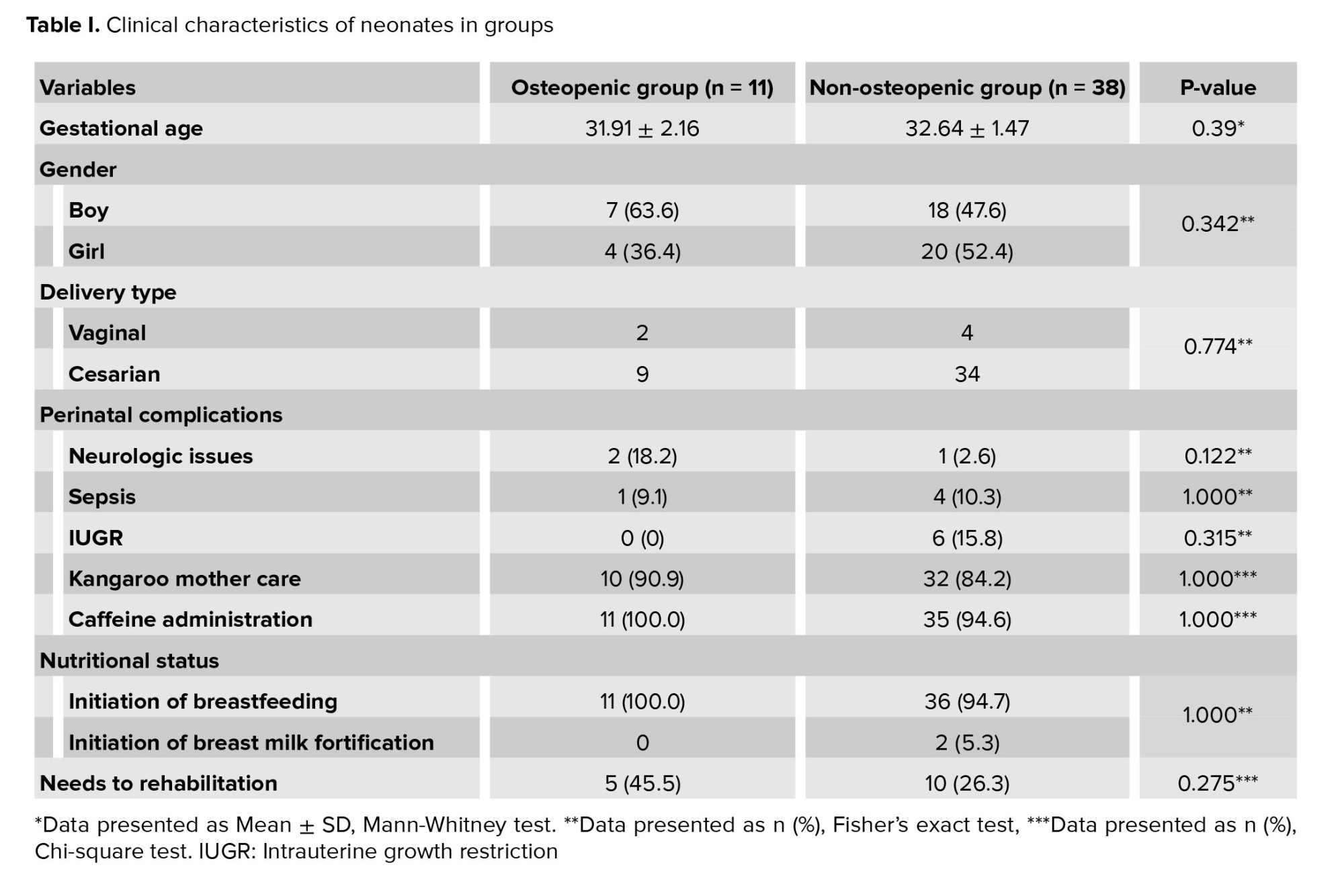
Of the 57 infants, 8 infants were excluded due to incomplete data, congenital skeletal anomalies, and twins, resulting in 49 neonates selected for the study. They had almost identical gender distribution and GA of 27-33+6 wk, with most delivered via cesarean section (8.1% vaginally). Only one mother experienced pre-eclampsia. Among the neonates, 14 (28%) faced complications, including sepsis, intrauterine growth restriction, and neurological issues. After birth, the majority received a caffeine regimen and were on "non-per oral" status, with breast milk initiated for all. Clinical characteristics are summarized in table I. Neurologic disease occurred in 2.6% of non-osteopenic and 18.2% of osteopenic infants, with no significant difference. Sepsis rates were comparable: 10.3% in non-osteopenic and 9.1% in osteopenic infants (p = 1.00). Intrauterine growth restriction was noted in 15.8% of non-osteopenic infants, with none of the osteopenic infants affected (p = 0.315). Kangaroo mother care was provided to 84.2% of non-osteopenic and 90.9% of osteopenic infants (p = 1.000), showing no association. Caffeine was given to 94.6% of non-osteopenic and all osteopenic neonates (p = 1.00). All subjects were non-per oral and mechanically intubated. High rates of breastfeeding initiation were observed in both groups (94.7% non-osteopenic vs. 100% osteopenic; p = 1.00), while breast milk fortification was rare (5.3% non-osteopenic vs. 0% osteopenic). Rehabilitation was utilized by 26.3% of non-osteopenic and 45.5% of osteopenic infants (p = 0.275), with no statistical significance.
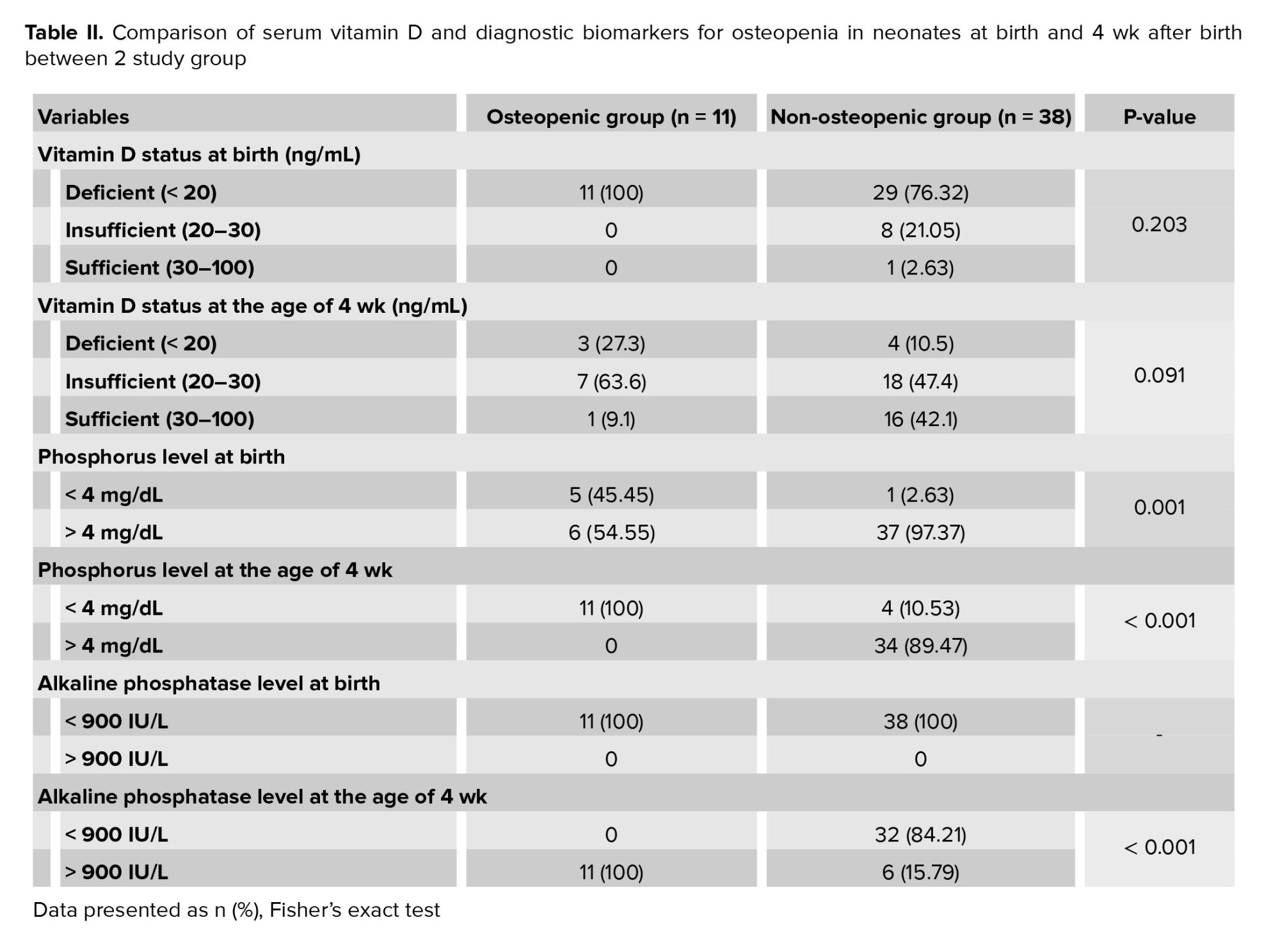
A total of 40 neonates (81.6%) were found to have vitamin D deficiency at birth, which decreased to 14.28% 4 week after birth. Among the neonates without osteopenia, 76.32% had vitamin D deficiency at birth. In contrast, 100% of those with osteopenia showed vitamin D deficiency at birth. However, this difference was not statistically significant (p = 0.203). By week 4, vitamin D deficiency was observed in 10.5% of the non-osteopenic group compared to 27.3% of the osteopenic group, but this difference was not statistically significant (p = 0.091).
At birth, only 2.63% of neonates without osteopenia had phosphorus levels below 4 mg/dL, compared to 45.45% of those with osteopenia (p = 0.001). By 4th wk, 10.53% of non-osteopenic neonates had phosphorus levels below 4 mg/dL, while all osteopenic neonates did (p < 0.001). For ALP levels, all neonates had levels below 900 IU/L at birth. However, by 4 wk, 15.79% of non-osteopenic neonates had levels above 900 IU/L, whereas all osteopenic neonates exceeded this level (p < 0.001) (Table II).
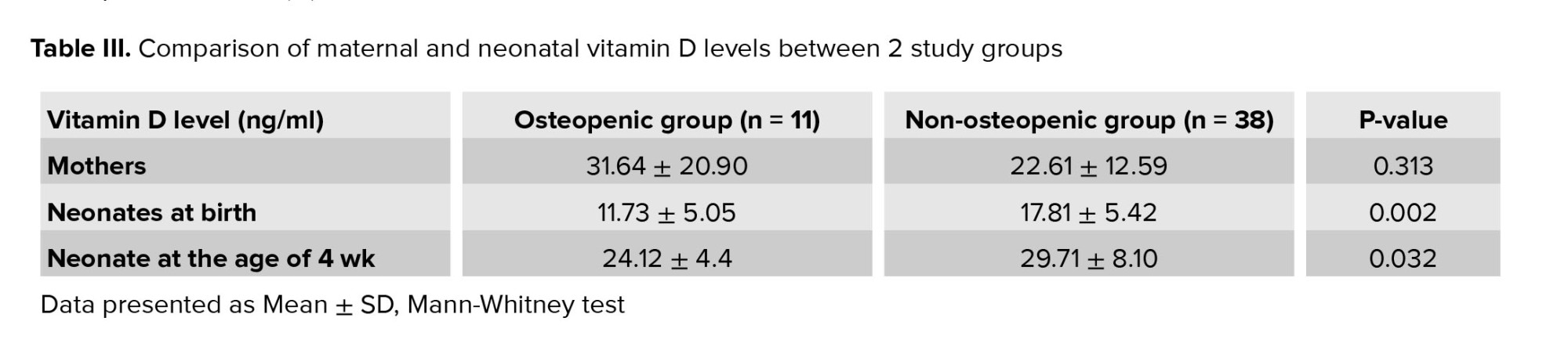
The mean level of vitamin D in mothers was 24.9 ± 15.09 ng/ml. Neonates had vitamin D levels of 16.44 ± 5.80 ng/ml at birth, which increased to 28.45 ± 7.70 ng/ml at 4 wk old. Among the mothers, 36% were vitamin D deficient, and 40% had insufficient levels. At 4th wk, neonates whose mothers had insufficient vitamin D levels faced odds of osteopenia that were 12 times higher compared to those with adequate levels (OR = 12, p = 0.053). Table III shows the vitamin D levels for both groups (determined by ALP and phosphorus cut-off points) for mothers and neonates. Interestingly, mothers of neonates with osteopenia had higher mean vitamin D levels compared to those whose neonates did not have osteopenia, though this difference was not statistically significant (p = 0.313). At birth, neonates in the osteopenia group had significantly lower vitamin D levels than those without osteopenia (p = 0.002). Furthermore, in 4th wk, neonates without osteopenia exhibited higher vitamin D levels compared to those with osteopenia (p = 0.032), suggesting a trend toward better vitamin D status in the non-osteopenic group.
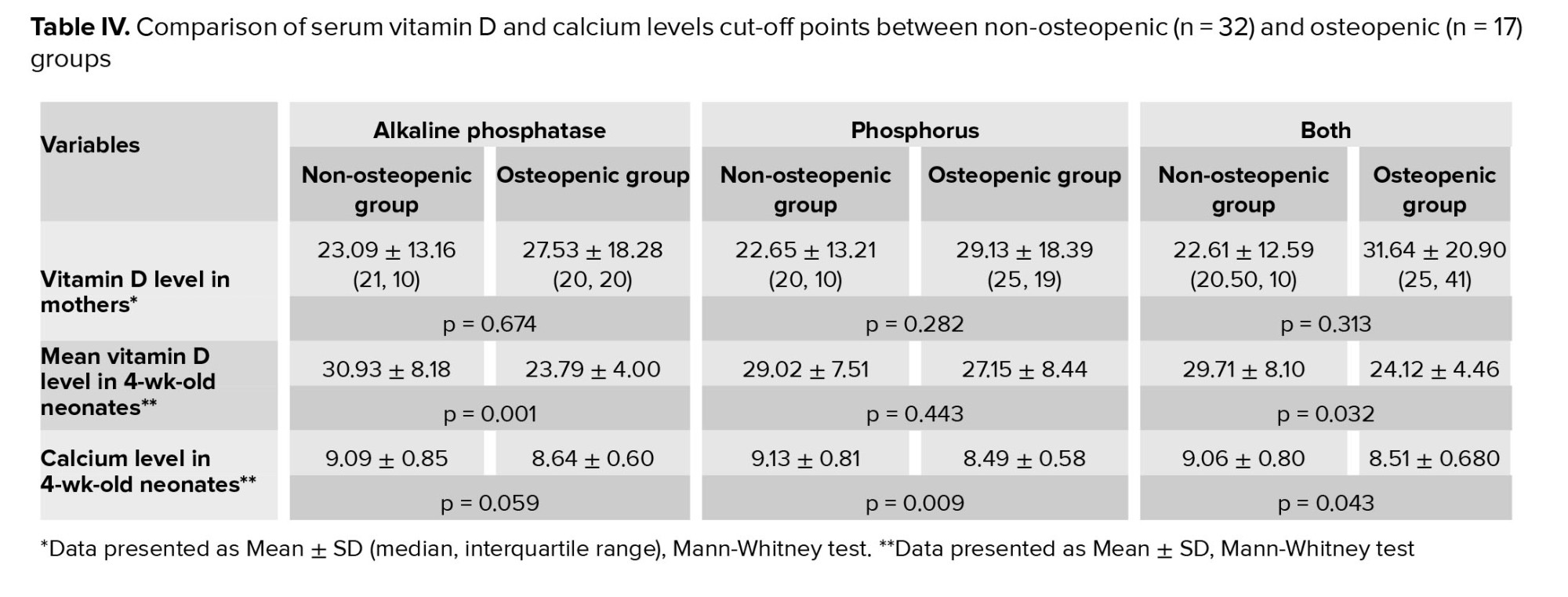
Table IV shows significant disparities in vitamin D and calcium levels between the 2 groups, highlighting their potential effects on neonatal health. Maternal vitamin D levels were similar across groups, with non-osteopenic levels at 23.09 ± 13.16 compared to 27.53 ± 18.28 in osteopenic neonates (p = 0.674 for ALP). Both phosphorus levels and overall comparisons reflected similar patterns. However, for 4-wk-old neonates, non-osteopenic infants had higher vitamin D levels (30.93 ± 8.18) than osteopenic ones (23.79 ± 4.00, p = 0.001 for ALP). Calcium levels also showed notable differences; non-osteopenic neonates had a mean level of 9.09 ± 0.85, while osteopenic neonates had lower levels at 8.64 ± 0.60 (p = 0.059 for ALP).
4. Discussion
The current study examined the effect of maternal vitamin D status on OP by measuring phosphorus and ALP levels as biomarkers. Among the 49 neonates, 11 (22%) showed signs of osteopenia at the age of 4 wk, which aligns with the reported prevalence of OP in low birth weight neonates, typically between 23% and 32% (11). Osteopenia in this study was defined by phosphorus levels below 4 mmol/L and ALP levels above 900 IU/L at 4 wk. ALP production in neonatal bone tissue increases due to limited mineral reserves, peaking during the first 3 wk of life before stabilizing at 5-6 wk (15). Contributing factors to preterm neonates' vulnerability to hypophosphatemia (phosphorus level < 4 mg/dL) and mineral bone disorders include inadequate phosphorus intake, gastrointestinal immaturity, reliance on parenteral nutrition, and the limited solubility of phosphorus (14).
Our study indicates that the incidence of hypophosphatemia increases from 12.24% at birth to 30.61% by the 4th wk of life. Notably, elevated ALP levels were not observed in neonates immediately after birth; however, by 4 wk, 34% of participants had ALP levels over 900 IU/L. These findings support a comprehensive approach that considers both serum biomarkers and assessments at 4 wk of age. This strategy may be more effective in identifying at-risk cases of osteopenia than relying on a single biochemical marker or measurement at birth. Previous research has identified a combination of high ALP levels and low phosphate concentrations as predictive factors for metabolic bone disease of prematurity (MBDP) (16, 17). Supportive evidence highlights a sensitivity of 100% and specificity > 70% when using cut-off values for phosphorus level < 4 and ALP > 900 IU/L at 3rd wk of age to diagnose MBDP (9). In another study, 61% of premature infants experienced hypophosphatemia in the early days of life, with 45% of these infants showing a subsequent decrease in phosphate levels (18). Furthermore, it is reported that 37% of preterm neonates exhibited metabolic bone disease potentially linked to hypophosphatemia by 4th wk of age. A notably high prevalence of hypophosphatemia (91%) was documented in premature neonates receiving intensive parenteral nutrition, with an average phosphorus level of 2.52 mg/dL (19, 20). Various studies have explored different diagnostic methods and cut-off values for biochemical markers. One study utilized cut-off values of ALP > 900 IU/L and phosphorus level < 5.5 mmol/L for screening MBDP in neonates aged between 3 and 5 wk (17). Another research found that combining serum ALP > 900 IU/L and phosphorus level < 1.8 mmol/L yielded a sensitivity of 100% and specificity of 70% for screening MBDP (16). Additionally, ALP levels around 800 IU/L can indicate bone metabolic disorders (15), emphasizing the importance of evaluating ALP alongside phosphorus concentrations to improve screening sensitivity. An ALP level of 344 IU/L has been identified as the most effective predictive value for MBDP among preterm cases for those aged between 3 and 30 days (21). Moreover, MBDP has been defined using criteria of ALP > 450 IU/L and phosphorus level < 4 mg/dL or through radiographic findings in neonates aged 4 wk or younger, specifically for those born at a GA of ≤ 32 wk and a birth weight of ≤ 1500 gr (11). A study involving 120 preterm and low-birth-weight neonates assessed serum ALP levels alongside wrist or knee radiographic findings to diagnose osteopenia, identifying an ALP cut-off value of 500 IU/L as indicative of the condition (22). Overall, these findings underscore the prevalence of hypophosphatemia in preterm neonates, which may persist or worsen during the early weeks of life.
In our study, using the ALP cut-off criterion, we found that serum vitamin D and calcium levels at the age of 4 wk were significantly lower in osteopenic neonates compared to their non-osteopenic counterparts. This suggests that measuring calcium and vitamin D concentrations could serve as a convenient and effective method for the rapid diagnosis of MBDP in preterm neonates. Furthermore, the early initiation of vitamin D supplementation for at-risk neonates may enhance their outcomes (23). The relationship between osteopenia and low serum calcium levels may stem from the stimulation of PTH in preterm neonates. PTH regulates blood calcium levels, potentially at the expense of bone demineralization (24). A study by Alizadeh Taheri et al. from Iran concluded that a low dose of vitamin D (200 IU/day) effectively prevents osteopenia (25). Additionally, another study found a weak positive correlation between serum 25-hydroxy vitamin D levels and serum ionized calcium and phosphorus levels, along with a negative correlation with ALP levels (26). A case-control study indicated that higher calcium intake increased the likelihood of osteopenia, while having a partner and a shorter duration of sedative use were identified as protective factors (4). Consistent with these results, Chauhan et al. found an inverse correlation between serum calcium and ALP levels in 27 preterm neonates (28-36 wk). They also observed a positive relationship between calcium and phosphorus levels within the study population (27). A prospective observational cohort study reported no significant difference in bone mineral density or content between neonates with and without osteopenia, despite both groups receiving recommended vitamin D supplementation (28). Another study indicated a correlation between MBDP and low vitamin D and calcium levels assessed at the 3rd wk of life, although it was confirmed that these factors alone could not serve as specific biochemical markers of osteopenia (29). Ercan et al. also showed lower serum vitamin D levels in neonates with craniotabes (a form of MBDP) compared to healthy counterparts (30). Conversely, an investigation found no significant difference in serum calcium levels between 2 preterm groups (27-32 wk) with and without osteopenia at the age of 3 wk (7). Overall, these studies suggest that while vitamin D and calcium levels may influence the development of osteopenia, further research is required to fully understand the connection.
Neonatal vitamin D storage at birth is dependent on maternal levels, and a deficiency can lead to bone disease in premature neonates, particularly with an incidence of up to 55% in those weighing ≤ 1000 gr. Low vitamin D status during the first trimester of pregnancy is also a risk factor for neonatal intensive care unit admission (31). However, our research findings revealed no significant correlation between maternal vitamin D levels and the incidence of osteopenia. Saleem et al. did not find notable differences in maternal vitamin D levels between groups with and without biochemical evidence of osteopenia (29.80 vs. 28.12 ng/ml; p = 0.073) (32). Similarly, Alizadeh Taheri et al. demonstrated no significant relationship between maternal vitamin D status and OP prevalence (25). However, other studies have indicated a significant association between maternal vitamin D insufficiency and MBDP during the early postnatal period (33). Discrepancies in these findings may arise from differences in the demographic and clinical characteristics of the studied populations, such as GA, criteria for defining vitamin D statuses, methods used, biochemical marker values indicating osteopenia, and sample sizes.
In our current study, no statistically significant relationships were observed between the cut-off points of biochemical markers and other neonatal variables such as: gender, non-per oral period, durations of neonatal intensive care unit admission, intubation period, or intrauterine growth restriction. To strengthen future investigations, larger sample sizes are needed. One strength of this study is the consideration of ALP alongside phosphorus and the assessment of both biochemical markers at 4 wk of age, which may help identify more cases at risk for osteopenia.
4.1. Strengths and limitations
The study boasts several strengths, including extensive data collection on clinical and biochemical parameters in neonates, highlighting the prevalence of osteopenia and vitamin D deficiency. Results are organized based on osteopenia presence, facilitating clear comparisons. It emphasizes the importance of vitamin D for bone health and the impact of maternal health on neonatal outcomes. Additionally, vitamin D levels were measured at birth and again at 4 wk, adding a temporal aspect to the analysis. However, the study has significant limitations. The small sample size of 49 neonates may limit the generalizability of the findings and reduce the power of statistical tests, hindering definitive conclusions. Incomplete data management and lack of cooperation from mothers may introduce biases affecting validity. Moreover, potential confounding factors, such as socioeconomic status and maternal diet, are not addressed, which could influence vitamin D levels and outcomes. A 4-wk follow-up may be insufficient for assessing the long-term effects of vitamin D levels and osteopenia. Finally, redundant reporting of p-value and statistical comparisons in both text and tables may obscure key findings and lead to misinterpretations. The primary focus on biochemical markers also restricts understanding of long-term clinical outcomes related to osteopenia or vitamin D deficiency in neonates.
5. Conclusion
The study highlights that maternal vitamin D levels do not have a significant correlation with the incidence of osteopenia in preterm infants. While neonates exhibit low vitamin D levels at birth, these levels improve notably by 4 wk, yet many continue to show osteopenia characteristics, as indicated by low ALP and phosphorus levels. Infants diagnosed with osteopenia presented lower serum concentrations of both vitamin D and calcium, underscoring the importance of these nutrients for bone health. Despite the significant differences in vitamin D and calcium levels which could inform the identification and management of osteopenia, this research suggests that maternal vitamin D status does not directly affect the development of this condition in preterm infants. Therefore, it is evident that additional factors beyond maternal vitamin D are influential in determining bone health outcomes. The study emphasizes the necessity of regular monitoring of vitamin D, ALP, and phosphorus levels in preterm infants to evaluate and potentially reduce the risk of osteopenia. Future research should focus on examining other maternal and neonatal factors that may impact bone health, which could lead to a deeper understanding of this issue and ultimately improve management strategies for preterm infants.
Data Availability
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
RS. Tabatabaie and SR. Mirjalili designed and conducted the research. A. Ashrafi and SR. Mirjalili monitored and evaluated the findings, while F. Shamsi analyzed the results. All authors reviewed and approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
The work was extracted from a thesis by Atefeh Ashrafi for obtaining a degree in obstetrics and gynecology. We sincerely thank all mothers who participated in this study. This study was financially supported by Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Thesis number: 12126). We confirm that we did not use AI in this context and provide information and support independently, without AI influence.
Conflict of Interest
The authors conducted this research transparently and have no financial or personal connections that could influence the work presented in this manuscript.
Full-Text: (215 Views)
1. Introduction
Premature neonates are at risk of developing osteopenia, a condition marked by reduced mineralization of the skeletal system compared to those who reach full term in the womb (1). This typically occurs 3-12 months after birth, with a prevalence of up to 60% in extremely low birth weight neonates and 20% in very low birth weight neonates (2). Moreover, evidence has shown defined rickets in 10-20% of preterm neonates with birth weights ≤ 1000 gr (3).
Risk factors for osteopenia of prematurity (OP) include low gestational age (GA) and birth weight, extreme prematurity, maternal chorioamnionitis, prolonged total parenteral nutrition, use of corticosteroids, methylxanthines, diuretics, immobilization, and sedation (4-6). Placental calcium and phosphate transfer to the fetus significantly rise after 24 wk of gestation. Inadequate calcium and phosphorus intake post-birth or through parenteral nutrition can lead to bone deformities and fractures in neonates (7-9). Additionally, factors such as lack of phosphate intake, prolonged parenteral nutrition, high alkaline phosphatase (ALP) levels, onset of vitamin D supplementation, and premature rupture of membranes are associated with the incidence of OP (7).
ALP is a commonly used marker for OP. Studies have shown that elevated ALP levels are linked to osteopenia occurrence in neonates. Serum calcium and phosphorus levels have also been studied as markers for OP (4, 10-12). Low serum inorganic phosphate levels have been significantly associated with osteopenia in premature neonates (11). Other markers such as parathyroid hormone (PTH) and vitamin D concentrations have also been investigated (6). Maternal or neonatal vitamin D deficiency can exacerbate osteopenia in preterm and very low birth weight neonates by affecting calcium and bone metabolism (9, 13).
Thus, this study aimed to establish the occurrence of osteopenia in premature neonates and examine the influence of maternal vitamin D on their bone health.
2. Materials and Methods
2.1. Participants
In this cross-sectional study, data of 49 pregnant women with premature labor and their neonates, referred to the gynecology and neonatology wards, Shahid Sadoughi hospital, Yazd, Iran, from June 2022 to September 2023, was extracted from their medical records.
The inclusion criteria were 18 and 33 yr pregnant women without maternal chorioamnionitis who experienced preterm labor (GA ≤ 34 wk based on the last menstrual period and ultrasound) and took 1000 units of vitamin D supplements daily until delivery, who has their first delivery.
Moreover, exclusion criteria were GA > 34 wk, congenital skeletal anomalies, multiple gestations, and refusal of follow-up visits.
Data on maternal and neonatal demographics, and clinical status including: complications, GA, delivery type, neonate gender, medication use, mechanical ventilation, feeding status, and hospitalization duration were extracted from newborn medical records. Routine laboratory tests included measuring serum vitamin D levels in mothers and preterm neonates at birth and 4th wk were conducted at Shahid Sadoughi hospital, Yazd, Iran. Diagnostic biomarkers for osteopenia, including serum phosphorus, calcium, and ALP levels were also recorded. Neonates were classified into 2 groups as healthy (non-osteopenic n = 38) or at risk for osteopenia (Osteopenic n = 11) based on established biochemical marker cut-off points. Serum vitamin D and calcium levels were compared between the 2 groups to investigate potential relationships with osteopenia.
2.2. Sample size
The sample size was estimated to be a minimum of 49 participants by considering a 95% confidence level, a power of 80%, and a standard deviation of 6.8 for the vitamin D level in mothers of infants with OP. A bias of 2 units in the estimations was also considered. The calculation utilized PASS15 software (PASS 15 Power Analysis and Sample Size Software, 2017, NCSS, LLC, Kaysville, Utah, USA, ncss.com/software/pass) and followed the following formula:
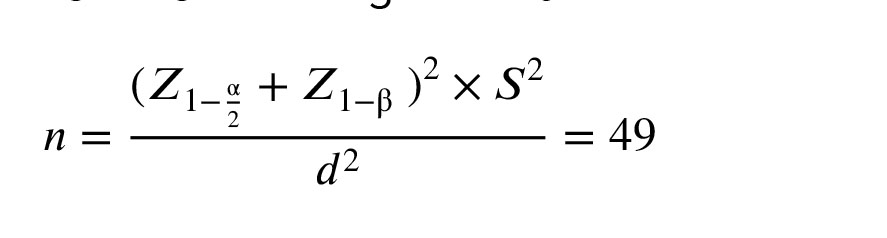
2.3. Biochemical analysis
All participating mothers received a daily 1000 unit vitamin D supplement under the supervision of a gynecologist until delivery. Following delivery, a 5 ml venous blood sample was obtained to assess maternal serum 25-(OH)-vitamin D3 (Vitamin D) levels using the enzyme-linked immunosorbent assay method. Moreover, 3 ml of umbilical cord blood was collected for neonatal serum vitamin D analysis. Subsequently, all neonates were administered total parenteral nutrition therapy in the 1st wk, which included calcium gluconate, glycophos, and 7000 units of vitamin D per week. To evaluate blood biochemical markers related to OP, serum calcium, ALP, and phosphorus levels were measured at birth and again at 4 wk of age using the spectrophotometric method. Based on the manufacturer's recommendation, serum vitamin D levels are categorized as deficiency (< 20 ng/ml), insufficiency (20-30 ng/ml), and sufficiency (> 30 ng/ml). Additionally, the cut-off points for P < 4 mmol/L or ALP > 900 IU/L were designated as predictive values for OP (14).
2.4. Ethical Considerations
The study protocol was approved by the Ethics Committee of the Institutional Review Board of Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Code: IR.SSU.MEDICINE.REC.1400.381). Before enrollment, written informed consent was obtained from all participants to use their data. Participants privacy and confidentiality were maintained, and they were not subjected to any additional costs.
2.5. Statistical Analysis
The data analysis was performed using SPSS 26.0, a software developed by SPSS Inc. in Chicago, Illinois, USA. Quantitative variables were presented as mean ± SD, while qualitative variables were presented as numbers (percent). To establish relationships between biomarker values for OP, vitamin D concentration, and other variables, statistical tests such as independent Student t test, Chi-square test, and Fisher’s exact tests were used. The predetermined level of significance was p < 0.05.
3. Results
Of the 57 infants, 8 infants were excluded due to incomplete data, congenital skeletal anomalies, and twins, resulting in 49 neonates selected for the study. They had almost identical gender distribution and GA of 27-33+6 wk, with most delivered via cesarean section (8.1% vaginally). Only one mother experienced pre-eclampsia. Among the neonates, 14 (28%) faced complications, including sepsis, intrauterine growth restriction, and neurological issues. After birth, the majority received a caffeine regimen and were on "non-per oral" status, with breast milk initiated for all. Clinical characteristics are summarized in table I. Neurologic disease occurred in 2.6% of non-osteopenic and 18.2% of osteopenic infants, with no significant difference. Sepsis rates were comparable: 10.3% in non-osteopenic and 9.1% in osteopenic infants (p = 1.00). Intrauterine growth restriction was noted in 15.8% of non-osteopenic infants, with none of the osteopenic infants affected (p = 0.315). Kangaroo mother care was provided to 84.2% of non-osteopenic and 90.9% of osteopenic infants (p = 1.000), showing no association. Caffeine was given to 94.6% of non-osteopenic and all osteopenic neonates (p = 1.00). All subjects were non-per oral and mechanically intubated. High rates of breastfeeding initiation were observed in both groups (94.7% non-osteopenic vs. 100% osteopenic; p = 1.00), while breast milk fortification was rare (5.3% non-osteopenic vs. 0% osteopenic). Rehabilitation was utilized by 26.3% of non-osteopenic and 45.5% of osteopenic infants (p = 0.275), with no statistical significance.
A total of 40 neonates (81.6%) were found to have vitamin D deficiency at birth, which decreased to 14.28% 4 week after birth. Among the neonates without osteopenia, 76.32% had vitamin D deficiency at birth. In contrast, 100% of those with osteopenia showed vitamin D deficiency at birth. However, this difference was not statistically significant (p = 0.203). By week 4, vitamin D deficiency was observed in 10.5% of the non-osteopenic group compared to 27.3% of the osteopenic group, but this difference was not statistically significant (p = 0.091).
At birth, only 2.63% of neonates without osteopenia had phosphorus levels below 4 mg/dL, compared to 45.45% of those with osteopenia (p = 0.001). By 4th wk, 10.53% of non-osteopenic neonates had phosphorus levels below 4 mg/dL, while all osteopenic neonates did (p < 0.001). For ALP levels, all neonates had levels below 900 IU/L at birth. However, by 4 wk, 15.79% of non-osteopenic neonates had levels above 900 IU/L, whereas all osteopenic neonates exceeded this level (p < 0.001) (Table II).
The mean level of vitamin D in mothers was 24.9 ± 15.09 ng/ml. Neonates had vitamin D levels of 16.44 ± 5.80 ng/ml at birth, which increased to 28.45 ± 7.70 ng/ml at 4 wk old. Among the mothers, 36% were vitamin D deficient, and 40% had insufficient levels. At 4th wk, neonates whose mothers had insufficient vitamin D levels faced odds of osteopenia that were 12 times higher compared to those with adequate levels (OR = 12, p = 0.053). Table III shows the vitamin D levels for both groups (determined by ALP and phosphorus cut-off points) for mothers and neonates. Interestingly, mothers of neonates with osteopenia had higher mean vitamin D levels compared to those whose neonates did not have osteopenia, though this difference was not statistically significant (p = 0.313). At birth, neonates in the osteopenia group had significantly lower vitamin D levels than those without osteopenia (p = 0.002). Furthermore, in 4th wk, neonates without osteopenia exhibited higher vitamin D levels compared to those with osteopenia (p = 0.032), suggesting a trend toward better vitamin D status in the non-osteopenic group.
Table IV shows significant disparities in vitamin D and calcium levels between the 2 groups, highlighting their potential effects on neonatal health. Maternal vitamin D levels were similar across groups, with non-osteopenic levels at 23.09 ± 13.16 compared to 27.53 ± 18.28 in osteopenic neonates (p = 0.674 for ALP). Both phosphorus levels and overall comparisons reflected similar patterns. However, for 4-wk-old neonates, non-osteopenic infants had higher vitamin D levels (30.93 ± 8.18) than osteopenic ones (23.79 ± 4.00, p = 0.001 for ALP). Calcium levels also showed notable differences; non-osteopenic neonates had a mean level of 9.09 ± 0.85, while osteopenic neonates had lower levels at 8.64 ± 0.60 (p = 0.059 for ALP).




4. Discussion
The current study examined the effect of maternal vitamin D status on OP by measuring phosphorus and ALP levels as biomarkers. Among the 49 neonates, 11 (22%) showed signs of osteopenia at the age of 4 wk, which aligns with the reported prevalence of OP in low birth weight neonates, typically between 23% and 32% (11). Osteopenia in this study was defined by phosphorus levels below 4 mmol/L and ALP levels above 900 IU/L at 4 wk. ALP production in neonatal bone tissue increases due to limited mineral reserves, peaking during the first 3 wk of life before stabilizing at 5-6 wk (15). Contributing factors to preterm neonates' vulnerability to hypophosphatemia (phosphorus level < 4 mg/dL) and mineral bone disorders include inadequate phosphorus intake, gastrointestinal immaturity, reliance on parenteral nutrition, and the limited solubility of phosphorus (14).
Our study indicates that the incidence of hypophosphatemia increases from 12.24% at birth to 30.61% by the 4th wk of life. Notably, elevated ALP levels were not observed in neonates immediately after birth; however, by 4 wk, 34% of participants had ALP levels over 900 IU/L. These findings support a comprehensive approach that considers both serum biomarkers and assessments at 4 wk of age. This strategy may be more effective in identifying at-risk cases of osteopenia than relying on a single biochemical marker or measurement at birth. Previous research has identified a combination of high ALP levels and low phosphate concentrations as predictive factors for metabolic bone disease of prematurity (MBDP) (16, 17). Supportive evidence highlights a sensitivity of 100% and specificity > 70% when using cut-off values for phosphorus level < 4 and ALP > 900 IU/L at 3rd wk of age to diagnose MBDP (9). In another study, 61% of premature infants experienced hypophosphatemia in the early days of life, with 45% of these infants showing a subsequent decrease in phosphate levels (18). Furthermore, it is reported that 37% of preterm neonates exhibited metabolic bone disease potentially linked to hypophosphatemia by 4th wk of age. A notably high prevalence of hypophosphatemia (91%) was documented in premature neonates receiving intensive parenteral nutrition, with an average phosphorus level of 2.52 mg/dL (19, 20). Various studies have explored different diagnostic methods and cut-off values for biochemical markers. One study utilized cut-off values of ALP > 900 IU/L and phosphorus level < 5.5 mmol/L for screening MBDP in neonates aged between 3 and 5 wk (17). Another research found that combining serum ALP > 900 IU/L and phosphorus level < 1.8 mmol/L yielded a sensitivity of 100% and specificity of 70% for screening MBDP (16). Additionally, ALP levels around 800 IU/L can indicate bone metabolic disorders (15), emphasizing the importance of evaluating ALP alongside phosphorus concentrations to improve screening sensitivity. An ALP level of 344 IU/L has been identified as the most effective predictive value for MBDP among preterm cases for those aged between 3 and 30 days (21). Moreover, MBDP has been defined using criteria of ALP > 450 IU/L and phosphorus level < 4 mg/dL or through radiographic findings in neonates aged 4 wk or younger, specifically for those born at a GA of ≤ 32 wk and a birth weight of ≤ 1500 gr (11). A study involving 120 preterm and low-birth-weight neonates assessed serum ALP levels alongside wrist or knee radiographic findings to diagnose osteopenia, identifying an ALP cut-off value of 500 IU/L as indicative of the condition (22). Overall, these findings underscore the prevalence of hypophosphatemia in preterm neonates, which may persist or worsen during the early weeks of life.
In our study, using the ALP cut-off criterion, we found that serum vitamin D and calcium levels at the age of 4 wk were significantly lower in osteopenic neonates compared to their non-osteopenic counterparts. This suggests that measuring calcium and vitamin D concentrations could serve as a convenient and effective method for the rapid diagnosis of MBDP in preterm neonates. Furthermore, the early initiation of vitamin D supplementation for at-risk neonates may enhance their outcomes (23). The relationship between osteopenia and low serum calcium levels may stem from the stimulation of PTH in preterm neonates. PTH regulates blood calcium levels, potentially at the expense of bone demineralization (24). A study by Alizadeh Taheri et al. from Iran concluded that a low dose of vitamin D (200 IU/day) effectively prevents osteopenia (25). Additionally, another study found a weak positive correlation between serum 25-hydroxy vitamin D levels and serum ionized calcium and phosphorus levels, along with a negative correlation with ALP levels (26). A case-control study indicated that higher calcium intake increased the likelihood of osteopenia, while having a partner and a shorter duration of sedative use were identified as protective factors (4). Consistent with these results, Chauhan et al. found an inverse correlation between serum calcium and ALP levels in 27 preterm neonates (28-36 wk). They also observed a positive relationship between calcium and phosphorus levels within the study population (27). A prospective observational cohort study reported no significant difference in bone mineral density or content between neonates with and without osteopenia, despite both groups receiving recommended vitamin D supplementation (28). Another study indicated a correlation between MBDP and low vitamin D and calcium levels assessed at the 3rd wk of life, although it was confirmed that these factors alone could not serve as specific biochemical markers of osteopenia (29). Ercan et al. also showed lower serum vitamin D levels in neonates with craniotabes (a form of MBDP) compared to healthy counterparts (30). Conversely, an investigation found no significant difference in serum calcium levels between 2 preterm groups (27-32 wk) with and without osteopenia at the age of 3 wk (7). Overall, these studies suggest that while vitamin D and calcium levels may influence the development of osteopenia, further research is required to fully understand the connection.
Neonatal vitamin D storage at birth is dependent on maternal levels, and a deficiency can lead to bone disease in premature neonates, particularly with an incidence of up to 55% in those weighing ≤ 1000 gr. Low vitamin D status during the first trimester of pregnancy is also a risk factor for neonatal intensive care unit admission (31). However, our research findings revealed no significant correlation between maternal vitamin D levels and the incidence of osteopenia. Saleem et al. did not find notable differences in maternal vitamin D levels between groups with and without biochemical evidence of osteopenia (29.80 vs. 28.12 ng/ml; p = 0.073) (32). Similarly, Alizadeh Taheri et al. demonstrated no significant relationship between maternal vitamin D status and OP prevalence (25). However, other studies have indicated a significant association between maternal vitamin D insufficiency and MBDP during the early postnatal period (33). Discrepancies in these findings may arise from differences in the demographic and clinical characteristics of the studied populations, such as GA, criteria for defining vitamin D statuses, methods used, biochemical marker values indicating osteopenia, and sample sizes.
In our current study, no statistically significant relationships were observed between the cut-off points of biochemical markers and other neonatal variables such as: gender, non-per oral period, durations of neonatal intensive care unit admission, intubation period, or intrauterine growth restriction. To strengthen future investigations, larger sample sizes are needed. One strength of this study is the consideration of ALP alongside phosphorus and the assessment of both biochemical markers at 4 wk of age, which may help identify more cases at risk for osteopenia.
4.1. Strengths and limitations
The study boasts several strengths, including extensive data collection on clinical and biochemical parameters in neonates, highlighting the prevalence of osteopenia and vitamin D deficiency. Results are organized based on osteopenia presence, facilitating clear comparisons. It emphasizes the importance of vitamin D for bone health and the impact of maternal health on neonatal outcomes. Additionally, vitamin D levels were measured at birth and again at 4 wk, adding a temporal aspect to the analysis. However, the study has significant limitations. The small sample size of 49 neonates may limit the generalizability of the findings and reduce the power of statistical tests, hindering definitive conclusions. Incomplete data management and lack of cooperation from mothers may introduce biases affecting validity. Moreover, potential confounding factors, such as socioeconomic status and maternal diet, are not addressed, which could influence vitamin D levels and outcomes. A 4-wk follow-up may be insufficient for assessing the long-term effects of vitamin D levels and osteopenia. Finally, redundant reporting of p-value and statistical comparisons in both text and tables may obscure key findings and lead to misinterpretations. The primary focus on biochemical markers also restricts understanding of long-term clinical outcomes related to osteopenia or vitamin D deficiency in neonates.
5. Conclusion
The study highlights that maternal vitamin D levels do not have a significant correlation with the incidence of osteopenia in preterm infants. While neonates exhibit low vitamin D levels at birth, these levels improve notably by 4 wk, yet many continue to show osteopenia characteristics, as indicated by low ALP and phosphorus levels. Infants diagnosed with osteopenia presented lower serum concentrations of both vitamin D and calcium, underscoring the importance of these nutrients for bone health. Despite the significant differences in vitamin D and calcium levels which could inform the identification and management of osteopenia, this research suggests that maternal vitamin D status does not directly affect the development of this condition in preterm infants. Therefore, it is evident that additional factors beyond maternal vitamin D are influential in determining bone health outcomes. The study emphasizes the necessity of regular monitoring of vitamin D, ALP, and phosphorus levels in preterm infants to evaluate and potentially reduce the risk of osteopenia. Future research should focus on examining other maternal and neonatal factors that may impact bone health, which could lead to a deeper understanding of this issue and ultimately improve management strategies for preterm infants.
Data Availability
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
RS. Tabatabaie and SR. Mirjalili designed and conducted the research. A. Ashrafi and SR. Mirjalili monitored and evaluated the findings, while F. Shamsi analyzed the results. All authors reviewed and approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
The work was extracted from a thesis by Atefeh Ashrafi for obtaining a degree in obstetrics and gynecology. We sincerely thank all mothers who participated in this study. This study was financially supported by Shahid Sadoughi University of Medical Sciences, Yazd, Iran (Thesis number: 12126). We confirm that we did not use AI in this context and provide information and support independently, without AI influence.
Conflict of Interest
The authors conducted this research transparently and have no financial or personal connections that could influence the work presented in this manuscript.
Type of Study: Original Article |
Subject:
Perinatology
References
1. Llorente-Pelayo S, Docio P, Lavín-Gómez BA, García-Unzueta MT, De Las Cuevas I, De La Rubia L, et al. Modified serum ALP values and timing of apparition of knee epiphyseal ossification centers in preterm infants with cholestasis and risk of concomitant metabolic bone disease of prematurity. Nutrients 2020; 12: 3854. [DOI:10.3390/nu12123854] [PMID] [PMCID]
2. Kehinde F, Marinescu A, Turchi R. Catch it before it breaks!: Managing metabolic bone disease of prematurity. Curr Opin Pediatr 2021; 33: 676-683. [DOI:10.1097/MOP.0000000000001060] [PMID]
3. Panda M, McIntosh J, Chaudhari T, Kent AL. Do maternal vitamin D levels influence vitamin D levels in preterm neonates? Int J Pediatr 2019; 2019: 8613414. [DOI:10.1155/2019/8613414] [PMID] [PMCID]
4. Pinto MRC, Machado MMT, de Azevedo DV, Correia LL, Leite ÁJM, Rocha HAL. Osteopenia of prematurity and associated nutritional factors: Case-control study. BMC Pediatr 2022; 22: 519. [DOI:10.1186/s12887-022-03581-y] [PMID] [PMCID]
5. Dan AM, Vasilescu DI. A severe case of osteopenia of prematurity. Obstet Şi Ginecol 2022; 70: 40. [DOI:10.26416/ObsGin.70.1.2022.6516]
6. Wang J, Zhao Q, Chen B, Sun J, Huang J, Meng J, et al. Risk factors for metabolic bone disease of prematurity: A meta-analysis. PLoS One 2022; 17: e0269180. [DOI:10.1371/journal.pone.0269180] [PMID] [PMCID]
7. Angelika D, Etika R, Mapindra MP, Utomo MT, Rahardjo P, Ugrasena IDG. Associated neonatal and maternal factors of osteopenia of prematurity in low resource setting: A cross-sectional study. Ann Med Surg (Lond) 2021; 64: 102235. [DOI:10.1016/j.amsu.2021.102235] [PMID] [PMCID]
8. Salehi M, Leung-Pineda V. Disorders of calcium and phosphate metabolism in infants and children. In: Wong EC, Dietsen DJ, Benneth MJ, Haymond S. Biochemical and molecular basis of pediatric disease. 5th Ed. USA: Academic Press; 2021; 379-410. [DOI:10.1016/B978-0-12-817962-8.00015-9]
9. Chacham S, Pasi R, Chegondi M, Ahmad N, Mohanty SB. Metabolic bone disease in premature neonates: An unmet challenge. J Clin Res Pediatr Endocrinol 2020; 12: 332-339. [DOI:10.4274/jcrpe.galenos.2019.2019.0091] [PMID] [PMCID]
10. Kumar M, Ali A, Khan MA, Sohail S, Saleem SM, Khan M, et al. Relationship of caffeine regimen with osteopenia of prematurity in preterm neonates: A cohort retrospective study. BMC Pediatr 2022; 22: 437. [DOI:10.1186/s12887-022-03493-x] [PMID] [PMCID]
11. Kavurt S, Demirel N, Yücel H, Unal S, Yıldız YT, Bas AY. Evaluation of radiologic evidence of metabolic bone disease in very low birth weight infants at fourth week of life. J Perinatol 2021; 41: 2668-2673. [DOI:10.1038/s41372-021-01065-y] [PMID] [PMCID]
12. Simsek GK, Buyuktiryaki M, Kanmaz Kutman HG, Canpolat FE. Alkaline phosphatase levels of preterm infants under 30 weeks of gestational age and its role in the diagnosis of osteopenia of prematurity. Ann Med Res 2019; 26: 1688-1691. [DOI:10.5455/annalsmedres.2019.07.421]
13. Kanike N, Hospattankar KG, Sharma A, Worley S, Groh-Wargo S. Prevalence of vitamin D deficiency in a large newborn cohort from Northern United States and effect of intrauterine drug exposure. Nutrients 2020; 12: 2085. [DOI:10.3390/nu12072085] [PMID] [PMCID]
14. Tan YL, Tsao PN, Chou HC, Yen TA, Chen CY. Hypophosphatemia as an early metabolic bone disease marker in extremely low-birth-weight infants after prolonged parenteral nutrition exposure. J Parenter Enter Nutr 2021; 45: 1268-1274. [DOI:10.1002/jpen.2010] [PMID]
15. Graça MI, Silva J, Guimarães H. Metabolic bone disease of prematurity- a report of five cases. J Pediatr Neonatal Individ Med 2019; 8: e080120.
16. Rayannavar A, Calabria AC. Screening for metabolic bone disease of prematurity. Semin Fetal Neonatal Med 2020; 25: 101086. [DOI:10.1016/j.siny.2020.101086] [PMID]
17. Avila-Alvarez A, Urisarri A, Fuentes-Carballal J, Mandiá N, Sucasas-Alonso A, Couce ML. Metabolic bone disease of prematurity: Risk factors and associated short-term outcomes. Nutrients 2020; 12: 3786. [DOI:10.3390/nu12123786] [PMID] [PMCID]
18. Pajak A, Królak-Olejnik B, Szafranska A. Early hypophosphatemia in very low birth weight preterm infants. Adv Clin Exp Med 2018; 27: 841-847. [DOI:10.17219/acem/70081] [PMID]
19. Brener Dik PH, Galletti MF, Fernández Jonusas SA, Alonso G, Mariani GL, Fustiñana CA. Early hypophosphatemia in preterm infants receiving aggressive parenteral nutrition. J Perinatol 2015; 35: 712-715. [DOI:10.1038/jp.2015.54] [PMID]
20. Bradford CV, Cober MP, Miller JL. Refeeding syndrome in the neonatal intensive care unit. J Pediatr Pharmacol Ther 2021; 26: 771-782. [DOI:10.5863/1551-6776-26.8.771] [PMID] [PMCID]
21. Lü KL, Xie SS, Hu Q, Yang ZY, Fan QLI, Liu E, et al. Diagnostic markers of metabolic bone disease of prematurity in preterm infants. Bone 2023; 169: 116656. [DOI:10.1016/j.bone.2022.116656] [PMID]
22. Abdallah EAA, Said RN, Mosallam DS, Moawad EMI, Kamal NM, Fathallah MGED. Serial serum alkaline phosphatase as an early biomarker for osteopenia of prematurity. Medicine (Baltimore) 2016; 95: e4837. [DOI:10.1097/MD.0000000000004837] [PMID] [PMCID]
23. Perrone S, Caporilli C, Grassi F, Ferrocino M, Biagi E, Dell'Orto V, et al. Prenatal and neonatal bone health: Updated review on early identification of newborns at high risk for osteopenia. Nutrients 2023; 15: 3515. [DOI:10.3390/nu15163515] [PMID] [PMCID]
24. O'Reilly P, Saviani M, Tou A, Tarrant A, Capra L, McCallion N. Do preterm bones still break? Incidence of rib fracture and osteopenia of prematurity in very low birth weight infants. J Paediatr Child Health 2020; 56: 959-963. [DOI:10.1111/jpc.14852] [PMID]
25. Alizadeh Taheri P, Sajjadian N, Beyrami B, Shariat M. Prophylactic effect of low dose vitamin D in osteopenia of prematurity: A clinical trial study. Acta Med Iran 2014; 52: 671-674.
26. Manish G, Uday Kumar M, Sukriti K. Prediction of rise in alkaline phosphatase at different vitamin D levels. CHRISMED J Health Res 2023; 10: 55-59. [DOI:10.4103/cjhr.cjhr_3_22]
27. Chauhan SS, Sarkar PD, Bhimte B. Prematurity and related biochemical outcomes: Study of bone mineralization and renal function parameters in preterm infants. Biochem Res Int 2011; 2011: 740370. [DOI:10.1155/2011/740370] [PMID] [PMCID]
28. Malone Jenkins S, Chan G, Weaver-Lewis K, Bardsley T, Felix J, Grinsell M. Vitamin D, bone density, and nephrocalcinosis in preterm infants: A prospective study. Pediatr Nephrol 2022; 37: 1325-1332. [DOI:10.1007/s00467-021-05300-8] [PMID]
29. Faienza MF, D'Amato E, Natale MP, Grano M, Chiarito M, Brunetti G, et al. Metabolic bone disease of prematurity: Diagnosis and management. Front Pediatr 2019; 7: 143. [DOI:10.3389/fped.2019.00143] [PMID] [PMCID]
30. Ercan M, Ozcetin M, Karaci M, Ozgurhan G, Yasar A, Guven B. Relationship between newborn craniotabes and vitamin D status. North Clin Istanbul 2016; 3: 15-21. [DOI:10.14744/nci.2016.48403] [PMID] [PMCID]
31. Kołodziejczyk A, Borszewska-Kornacka MK, Seliga-Siwecka J. Monitored supplementation of vitamin D in preterm infants (MOSVID trial): Study protocol for a randomised controlled trial. Trials 2017; 18: 424. [DOI:10.1186/s13063-017-2141-y] [PMID] [PMCID]
32. Saleem AM, Alsayed LM, Ahmed IA, Rezk NA. Biochemical markers and radiological findings in premature osteopenic neonates. Egypt J Hosp Med 2022; 87: 2038-2045. [DOI:10.21608/ejhm.2022.232262]
33. Moon RJ, Curtis EM, Woolford SJ, Ashai S, Cooper C, Harvey NC. The importance of maternal pregnancy vitamin D for offspring bone health: Learnings from the MAVIDOS trial. Ther Adv Musculoskelet Dis 2021; 13: 1759720X211006979. [DOI:10.1177/1759720X211006979] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







