Sat, Jan 31, 2026
[Archive]
Volume 22, Issue 12 (December 2024)
IJRM 2024, 22(12): 943-962 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bahreiny S S, Ahangarpour A, Harooni E, Amraei M, Aghaei M, Mohammadpour Fard R. Closer look at circulating nitric oxide levels and their association with polycystic ovary syndrome: A meta-analytical exploration. IJRM 2024; 22 (12) :943-962
URL: http://ijrm.ir/article-1-3372-en.html
URL: http://ijrm.ir/article-1-3372-en.html
Seyed Sobhan Bahreiny1 

 , Akram Ahangarpour2
, Akram Ahangarpour2 

 , Elnaz Harooni1
, Elnaz Harooni1 

 , Mahdi Amraei3
, Mahdi Amraei3 

 , Mojtaba Aghaei3
, Mojtaba Aghaei3 

 , Reza Mohammadpour Fard *4
, Reza Mohammadpour Fard *4 




 , Akram Ahangarpour2
, Akram Ahangarpour2 

 , Elnaz Harooni1
, Elnaz Harooni1 

 , Mahdi Amraei3
, Mahdi Amraei3 

 , Mojtaba Aghaei3
, Mojtaba Aghaei3 

 , Reza Mohammadpour Fard *4
, Reza Mohammadpour Fard *4 


1- Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. & Medical Basic Sciences Research Institute, Physiology Research Center, Department of Physiology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2- Medical Basic Sciences Research Institute, Physiology Research Center, Department of Physiology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3- Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
4- Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran & Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. ,rezamohammadpour1377@gmail.com
2- Medical Basic Sciences Research Institute, Physiology Research Center, Department of Physiology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
3- Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
4- Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran & Department of Immunology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. ,
Full-Text [PDF 1476 kb]
(808 Downloads)
| Abstract (HTML) (1088 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) encompasses a spectrum of metabolic and endocrine derangements that impose significant health concerns on women of childbearing age, often serving as a precursor to a variety of life-impacting co-morbidities (1). Situated at the intersection of reproductive biology and metabolic homeostasis, PCOS challenges medical understanding with its multifactorial etiology, diverse phenotypic expressions, and complex pathophysiological pathways underpinned predominantly by hyperandrogenism and ovarian dysfunction (2).
Recent advancements in the understanding of hyperandrogenism have highlighted its intricate etiology, which may involve contributions from fetal development, maternal factors, and placental influences (3, 4).
This nuanced view of its genesis opens gateways to personalized therapeutic approaches that address the patient's unique disease matrix. The ominous constellation of obesity, insulin resistance, hyperglycemia, and associated cardiovascular risk factors further emphasizes the systemic impact of PCOS and its position as more than a reproductive disorder (5, 6).
Central to our investigation is the role of oxidative stress (OS) a pervasive player in the pathogenesis of PCOS. As the body grapples with the discord between the generation of reactive oxygen species (ROS) and its inherent antioxidant defenses, the resultant OS becomes a critical focus for therapeutic intervention (7, 8).
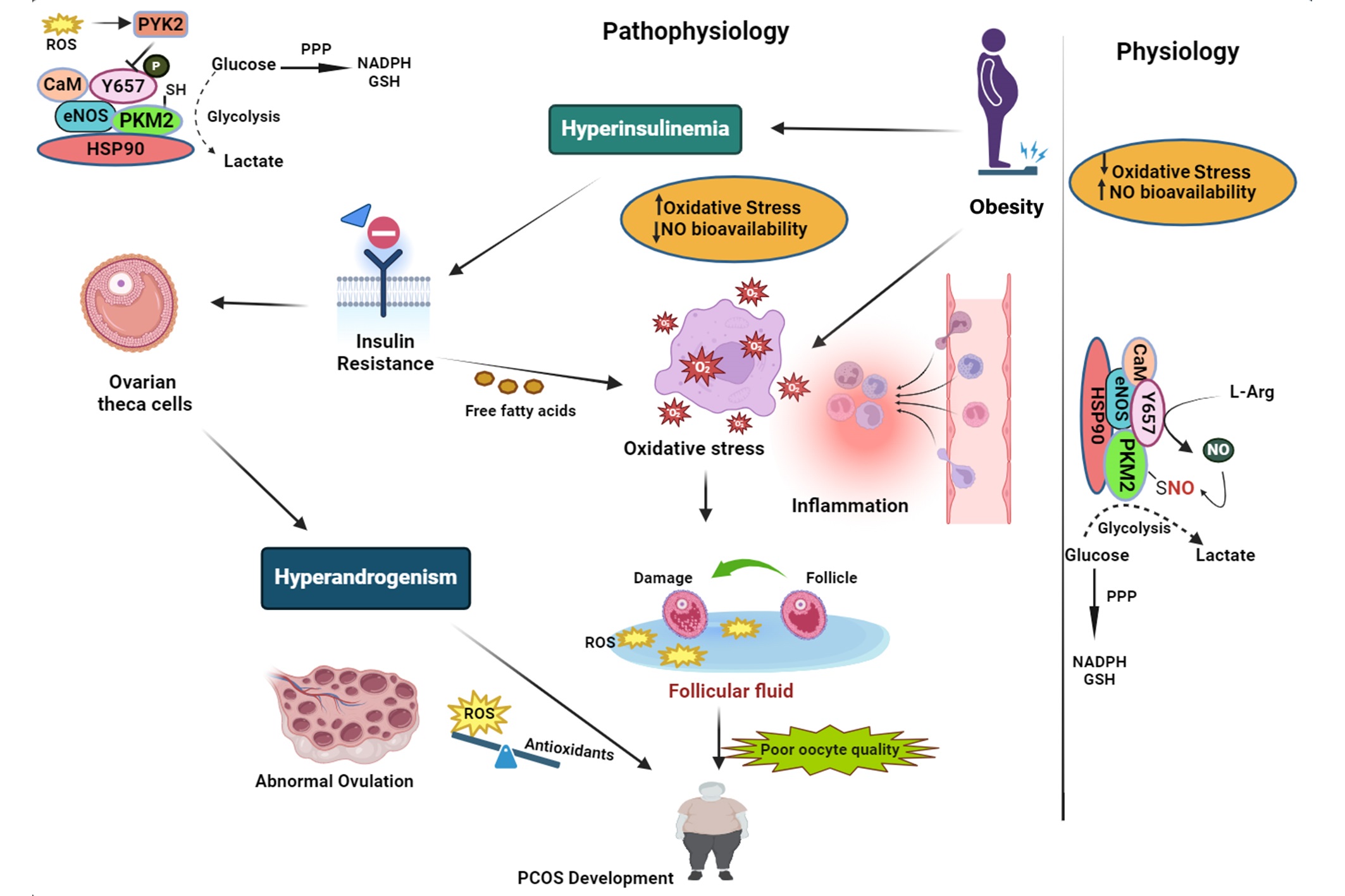
Nitric oxide (NO) emerges as a pivotal mediator within this redox landscape, straddling roles in vascular regulation and signal transduction, thus implicating it in both the symptomatic presentations and long-term sequelae of PCOS (9). Mounting evidence suggests that the dysregulation of NO synthesis pathways, encompassing the 3 isoforms of NO synthase ([NOS] endothelial, inducible, and neuronal), may have paradoxical implications for PCOS pathology (10).
This dual-faceted role of NO is highlighted by its vascular protective functions courtesy of endothelial NOS (eNOS)-derived NO, juxtaposed against the noxious sequel of inducible NOS (iNOS) over-expression under chronic inflammatory stimuli, commonly witnessed in PCOS phenotypes characterized by insulin resistance (11) (Figure 1). Thus, investigation into serum NO levels in PCOS patients has disclosed a conflicting landscape, with some studies indicating reduced levels implicating vascular dysfunction while others suggest augmented concentrations reflective of chronic inflammation (12, 13). The intricate dance between NO and ROS and their collective influence on the body's redox state presents a potential avenue for diagnostic biomarker development and therapeutic targeting. Given the pivotal role of NO in both the preservation of vascular homeostasis and the exacerbation of oxidative pathology, an opportunity arises to probe deeper into its relationship with PCOS (14-16). To elucidate the enigmatic nature of NO's dual role in PCOS pathophysiology and demystify its prognostic potential as a biomarker, we have embarked on a systematic review and meta-analysis, critically examining observational studies that hinge on this molecule's dynamic within the disorder.
This comprehensive analysis aims to sift through the contradictory evidence and provide a quantitative synthesis of NO levels in women with PCOS. This offers insights into its diagnostic robustness and prognostic value in this prevalent condition. Previous meta-analyses in this area were often based on older studies, with limited subgroup analyses, and lacked rigorous bias assessment and meta-regression techniques. In contrast, our study incorporates a larger, updated dataset. It applies advanced analytical methods including detailed subgroup analyses (age, body mass index [BMI], sample type, and ethnicity), meta-regression, and thorough bias evaluation to deepen our understanding of NO’s role in PCOS. By exploring these dimensions, this meta-analysis aims to definitively characterize the interaction between NO and PCOS, paving the way for future personalized treatment strategies that address the underlying oxidative and endothelial disruptions in this complex syndrome.
2. Materials and Methods
2.1. Protocol and registration
The systematic review and meta-analysis protocol were registered with "Prospective International Registration of Systematic Reviews" (PROSPERO) CRD42024507048. The systematic reviews were reported according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (17).
2.2. Eligibility criteria
This review only included studies that focused on humans and used observational study designs such as cross-sectional, cohort, or case-control based on population, intervention, comparator, and outcome (PICO) criteria (Table I). The scope of the review was limited to observational studies that examined women aged 18 yr or older who had been diagnosed with PCOS. The diagnostic criteria applied in identifying PCOS patients were the Rotterdam Criteria or National Institutes of Health Criteria. Studies having insufficient data, as well as those that were reviewed, letters to the editor, reports, or were not accessible, were excluded from this review.
2.3. Literature search
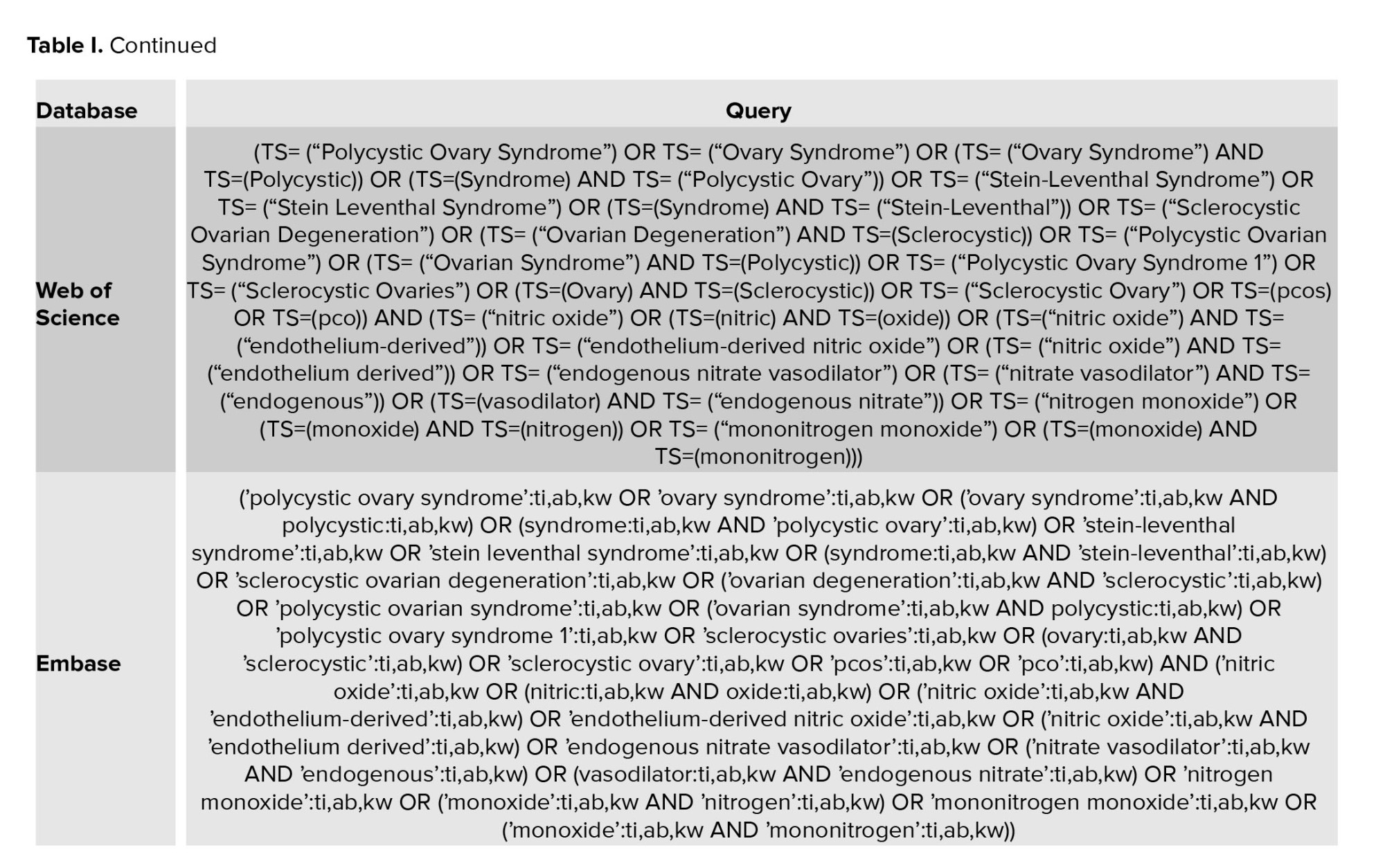
Valid medical databases such as PubMed, Web of Science, EMBASE, Scopus, and Cochrane Library (CENTRAL) were searched for the literature review in January 2024. The search terms were defined and searched by a medical librarian and medical professionals, without any time and place restrictions. Search terms were supplemented by searching for available gray studies such as governmental and academic studies. Also, the reference list of all articles related to the snowball method was searched for more comprehensiveness. The keywords "nitric oxide", "polycystic ovary syndrome", and "PCOS" were selected as the primary keywords based on mesh in PubMed. Full details of the search strategy are available in table I. All records reported in the literature search were uploaded to Comprehensive Meta-analysis v3.7z software.
2.4. Study selection
After deleting the duplicate articles, 2 independent reviewers (S. B. and M. A.) assessed the eligibility based on inclusion/exclusion criteria and reviewed the titles/abstracts of the retrieved studies. Subsequently, 2 reviewers assessed the full texts, and disagreements were resolved with arbitration by the third reviewer.
2.5. Data extraction
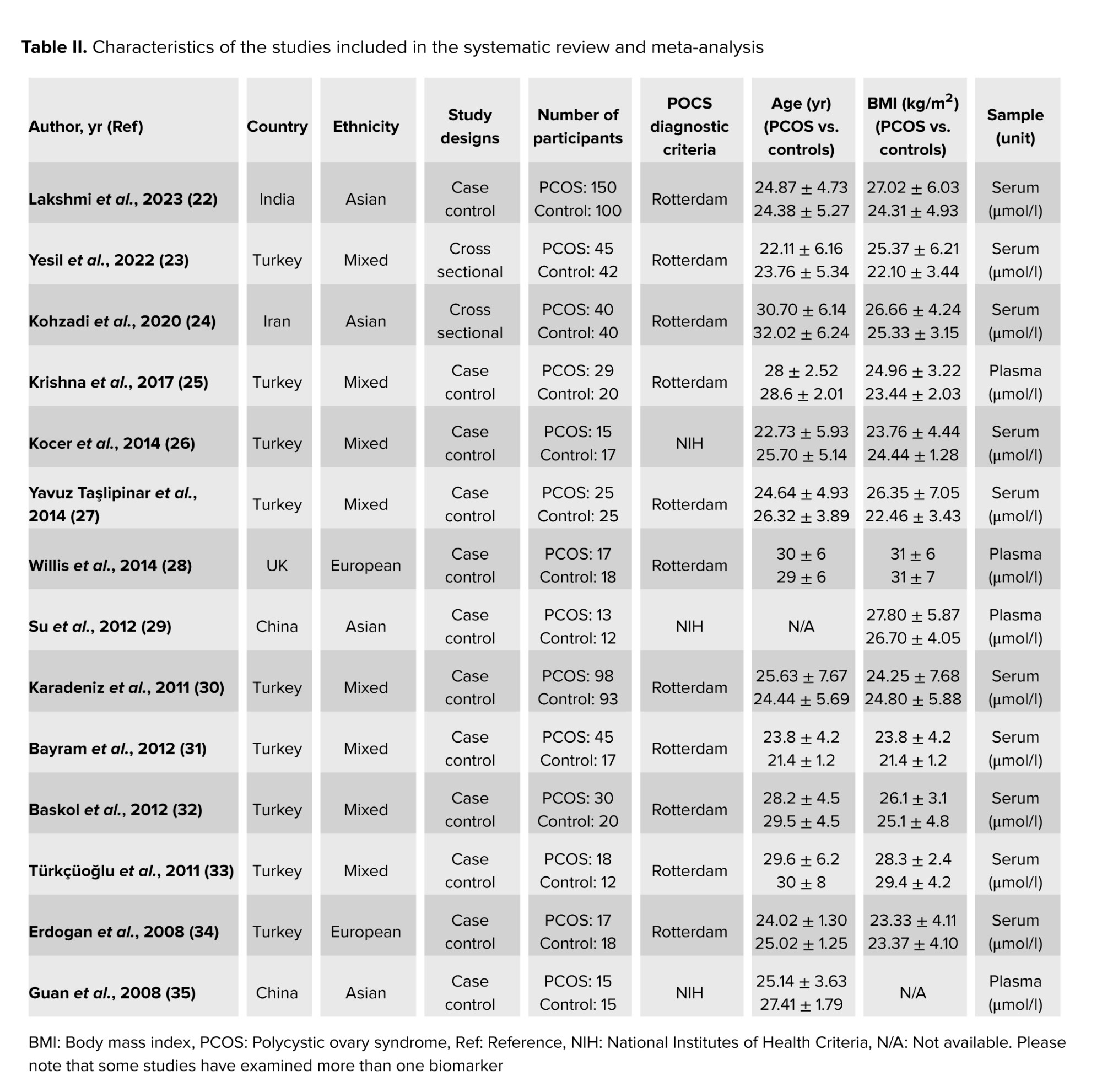
Data extraction was systematically carried out using a predefined checklist designed to capture essential elements relevant to the research question adhering to the study extraction design outlined in our previous research (18-21). The checklist included information on the study's origin, such as the first author's name, publication year, and geographic region of the study. Additionally, details on study design (cross-sectional, cohort, or case-control), participant characteristics (age, sample size, and body mass index), and measurements performed, diagnostic criteria, and reported outcomes were systematically extracted.
2.6 . Risk of bias assessment in the included studies
The Newcastle-Ottawa scale is a valuable tool for evaluating the quality of nonrandomized studies in a meta-analysis. Utilizing this scale ensures that only the most reliable studies are considered, leading to a more precise and trustworthy research analysis. The NOS is meticulously designed with 3 critical domains: the comparability of study groups, the selection of these groups, and the ascertainment of the outcome of interest. Each domain is equipped with specific criteria that guide the evaluation process. The scale ranges from 0-9, with studies scoring ≤ 4 classified as low quality and those scoring ≥ 5 as high quality. 2 independent reviewers reviewed each study and assessed it against the 3 domains. Any discrepancies were resolved with the assistance of a third reviewer.
2.7. Statistical Analysis
The meta-analysis was conducted using Comprehensive Meta-Analysis v3.7z software. Circulating NO levels in women with PCOS were compared to those in control groups using the standardized mean difference (SMD) and a 95% confidence interval (CI). The utilization of SMD proved to be pivotal as it facilitated consistent comparison of effect sizes across studies employing diverse measurement scales for NO levels. Heterogeneity among studies was assessed using Cochran's Q test and the I2 statistic, with statistical significance set at p < 0.05 or I2 > 50%. A random-effects model was employed for data synthesis, considering potential variability among studies. Publication bias was assessed using Egger's tests, with significance set at a p-value of 0.05. Subgroup analyses and meta-regression analyses were conducted to explore potential sources of heterogeneity, such as age, geographical region, and study design. Furthermore, a sensitivity analysis was performed to evaluate the impact of individual studies by systematically excluding each study from the analysis. The overall statistical significance level was set at p < 0.05 for all the analyses as well as their prediction intervals were reported.
3. Results
3.1. Characteristics of the included studies
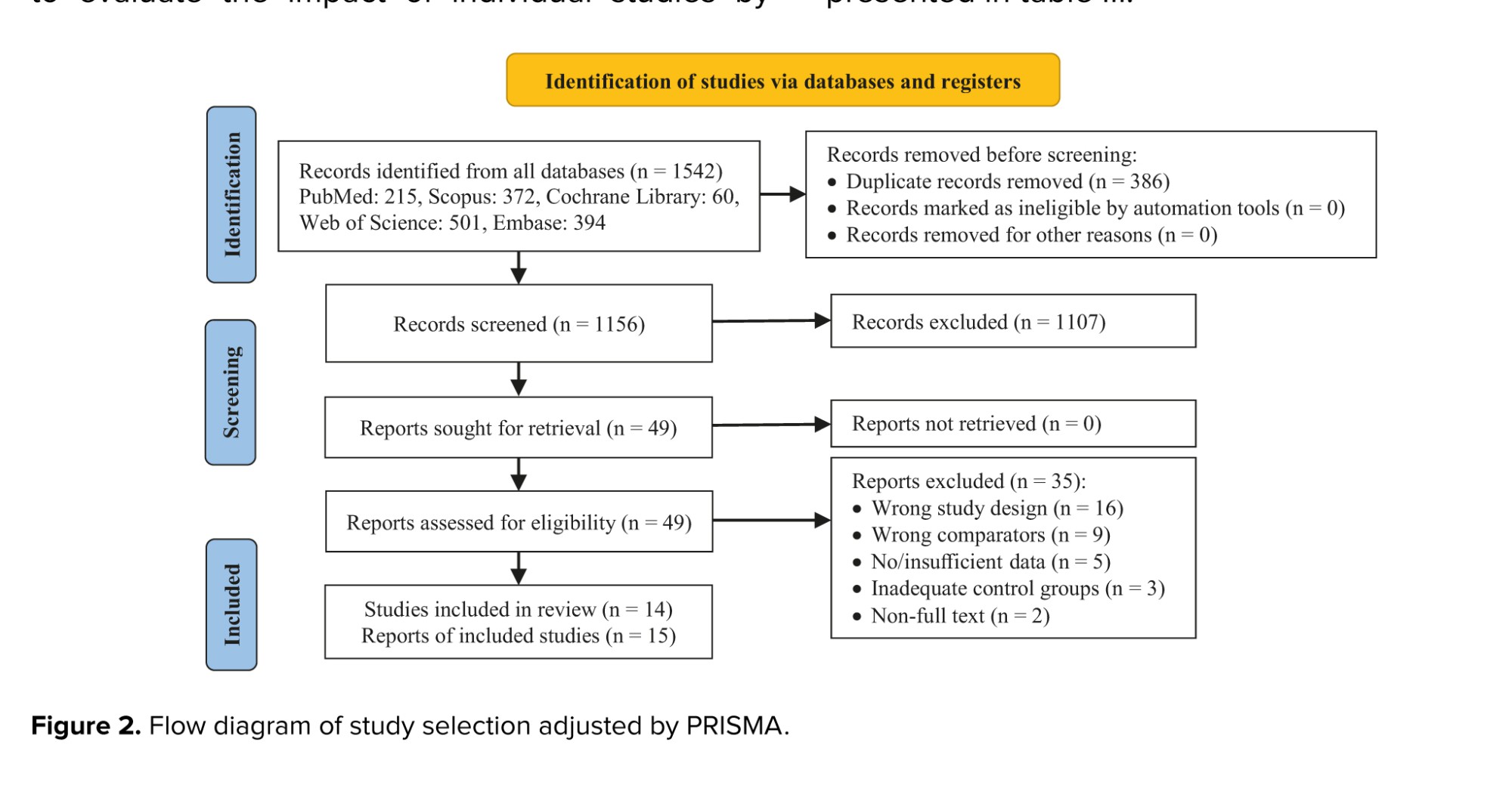
The systematic search strategy identified 1542 articles in the database. After removing 386 duplicate records and excluding 1107 articles based on title and abstract evaluation, 49 articles underwent a comprehensive full-text assessment. During this process, 35 studies were excluded for various reasons, including lack of relevant data on NO levels, insufficient sample size, failure to meet the diagnostic criteria for PCOS, and methodological flaws such as poor study design or inadequate control groups. Ultimately, 14 studies met the pre-established selection criteria involving a combined total of 1171 participants (Figure 2). The characteristics of these 14 studies, including participant demographics, sample type, and study design, are summarized in table II. Furthermore, the quality assessment of the included studies is presented in table III.

3.2 . Association and comparison details
A comprehensive investigation, encompassing 14 studies (15 reports) with a total of 1171 participants, was conducted to examine the correlation between NO levels and PCOS. Subgroup and meta-regression analyses were performed to explore potential sources of heterogeneity, including BMI, age, type of sample, diagnostic criteria for PCOS, and study year. This thorough analysis significantly enhanced our understanding of the association between NO levels and PCOS, illuminating the factors contributing to the observed heterogeneity.
3.3. Relationship between serum NO levels and PCOS
3.3.1. Meta‐analysis
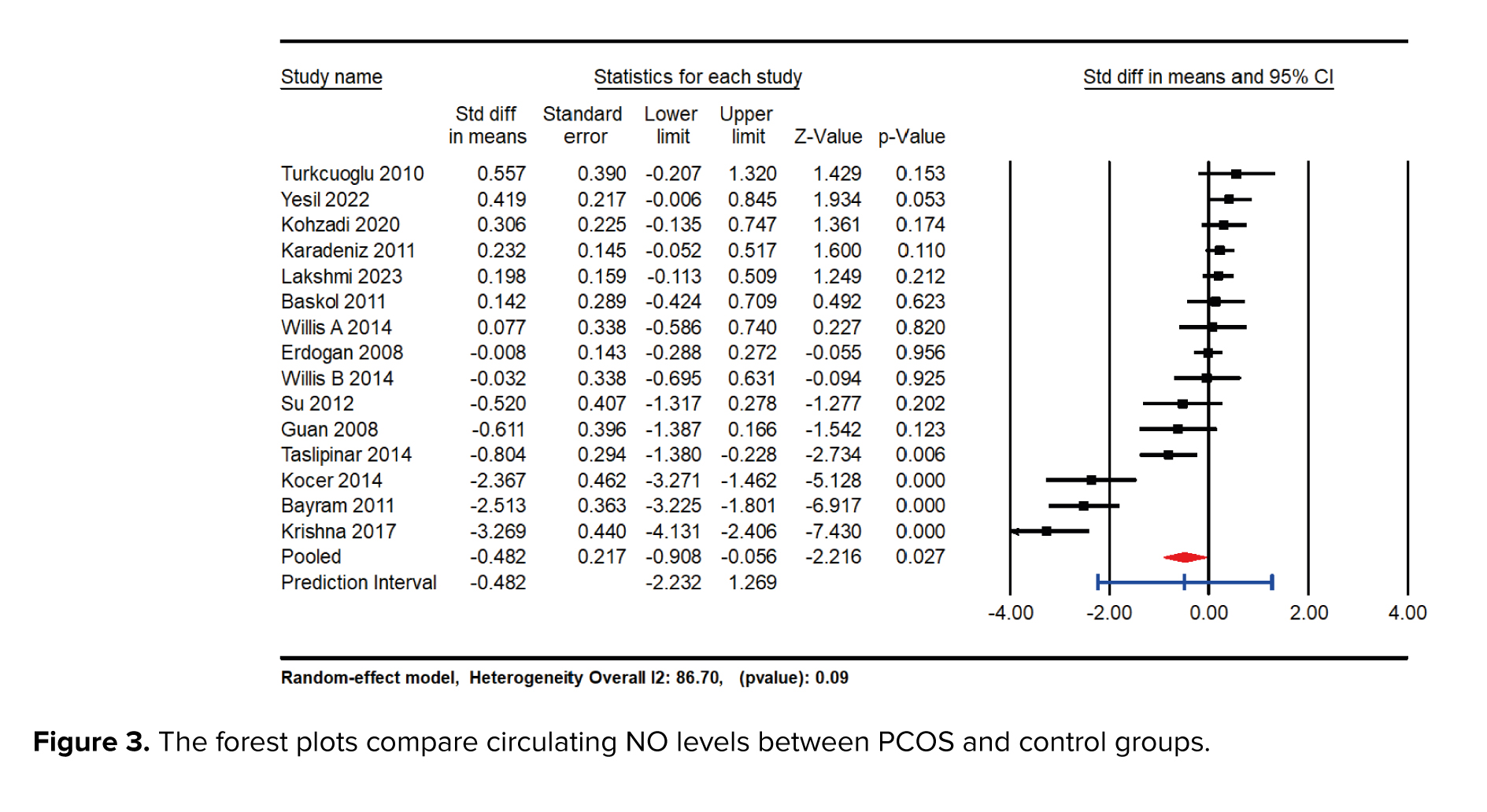
The meta-analysis results indicated a significant reduction in NO levels in the group with PCOS compared to the control group, with an SMD of -0.482 (95% CI: -0.908 to -0.056; p = 0.027). This finding suggests a substantial decrease in NO levels associated with PCOS. Furthermore, a high degree of heterogeneity was detected among the included studies, as evidenced by an I² value of 91.34% and a p-value of 0.01, indicating considerable variability in the study results (Figure 3).
3.3.2. Prediction interval
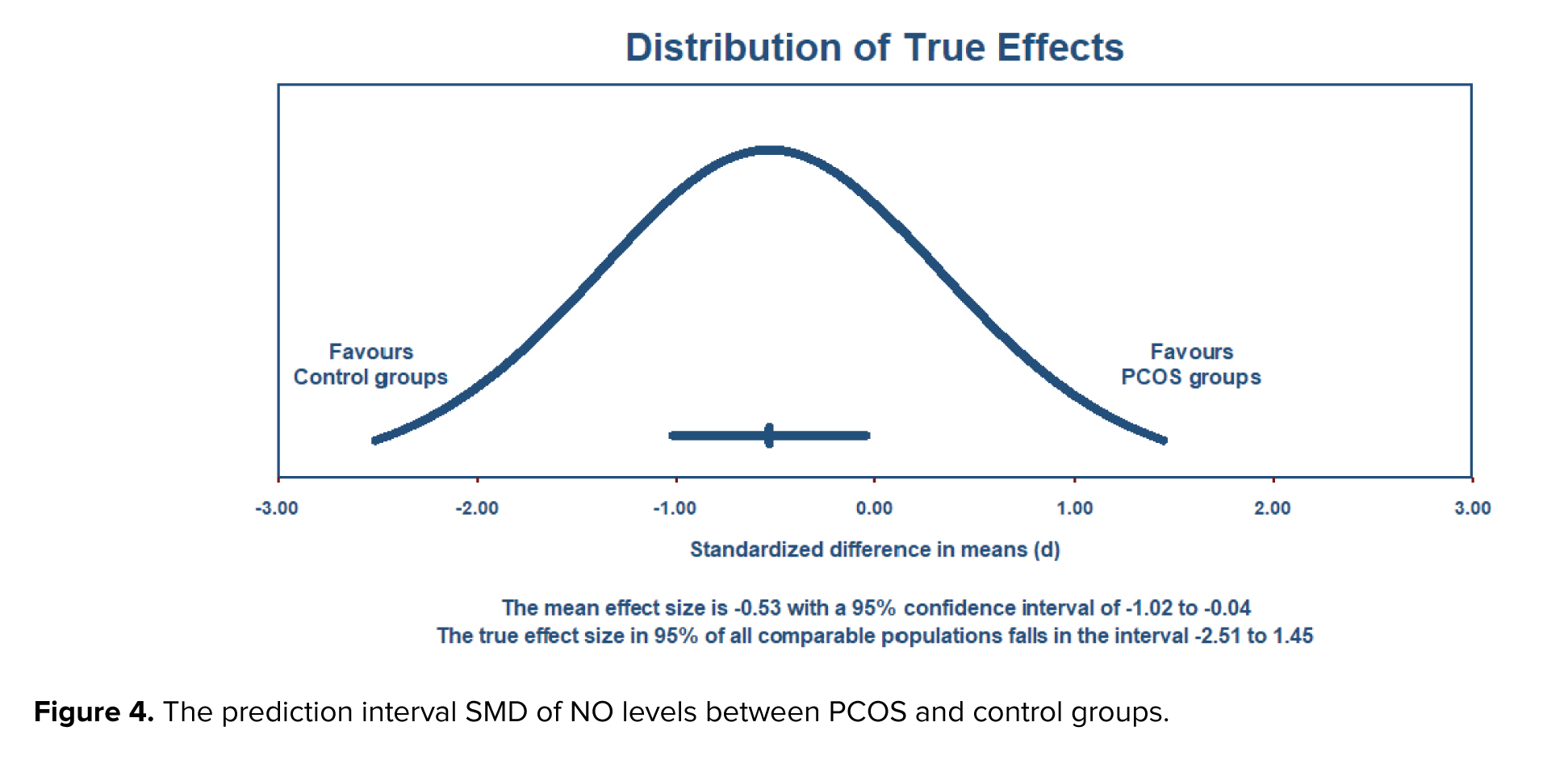
The estimation of a prediction interval revealed a range of -2.51 to 1.45 for the true effect size, assuming a normal distribution. This interval is expected to encompass the effect size in 95% of comparable populations. However, it is important to note that variations in the actual effect size may occur within this interval due to factors such as sample size and study design. Therefore, careful consideration is warranted when interpreting results within this prediction interval (Figure 4).
3.3.3. Subgroup analysis
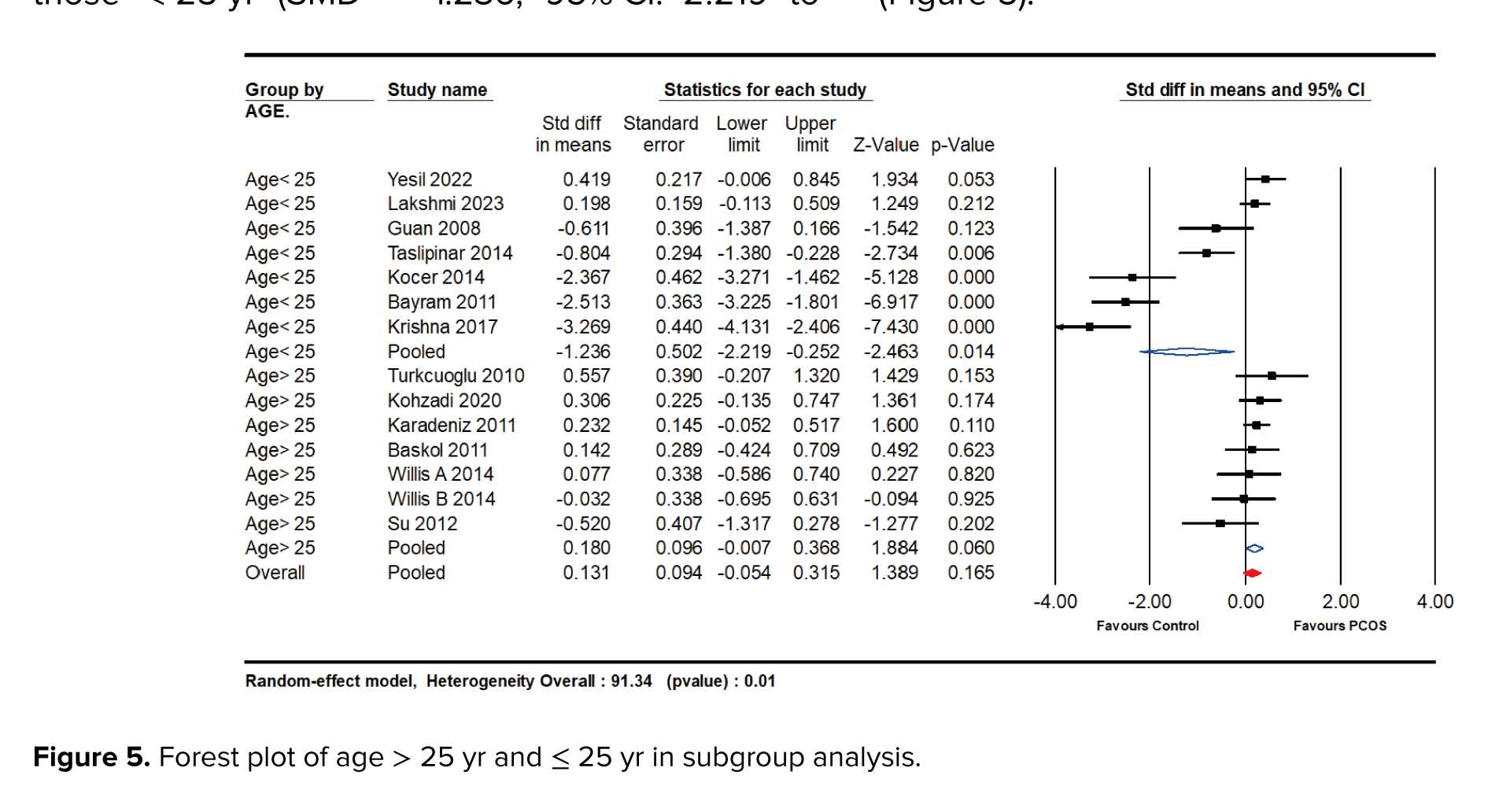
Our systematic review and meta-analysis revealed a significant association between NO levels and PCOS, with nuanced variations observed across different subgroups. The subgroup analyses focused on age, BMI, sample type, and race, providing valuable insights into the diverse manifestations of NO levels in PCOS patients. A noteworthy difference was observed between women aged 25 yr and above and those < 25 yr. The studies were analyzed in 2 subgroups, one including participants with an average age of ≥ 25 yr (SMD = 0.180; 95% CI: -0.007 to 0.368; p = 0.060), and the other including those < 25 yr (SMD = -1.236; 95% CI: -2.219 to -0.252; p = 0.014). According to the analysis, individuals aged ≥ 25 had higher levels of NO (Figure 5).
Moreover, subgroup analyses based on BMI and the sample type were conducted. The analysis of studies with a mean BMI of ≥ 25 kg/m² or < 25 kg/m² revealed that women with BMI > 25 kg/m² had higher NO levels (SMD = 0.163; 95% CI: -0.031 to 0.357; p = 0.10) compared to those with BMI < 25 kg/m² (SMD = -1.230; 95% CI: -2.213 to -0.247; p = 0.014) (Figure 6).

In addition, the study also looked at subgroups based on the type of sample collected (serum or plasma). The results showed that women with plasma samples had lower levels of NO (SMD = -0.851; 95% CI: -1.950 to 0.248; p = 0.129) compared to those with serum samples (SMD = -0.363; 95% CI: -0.906 to 0.181; p = 0.19) (Table IV, Figure 7).


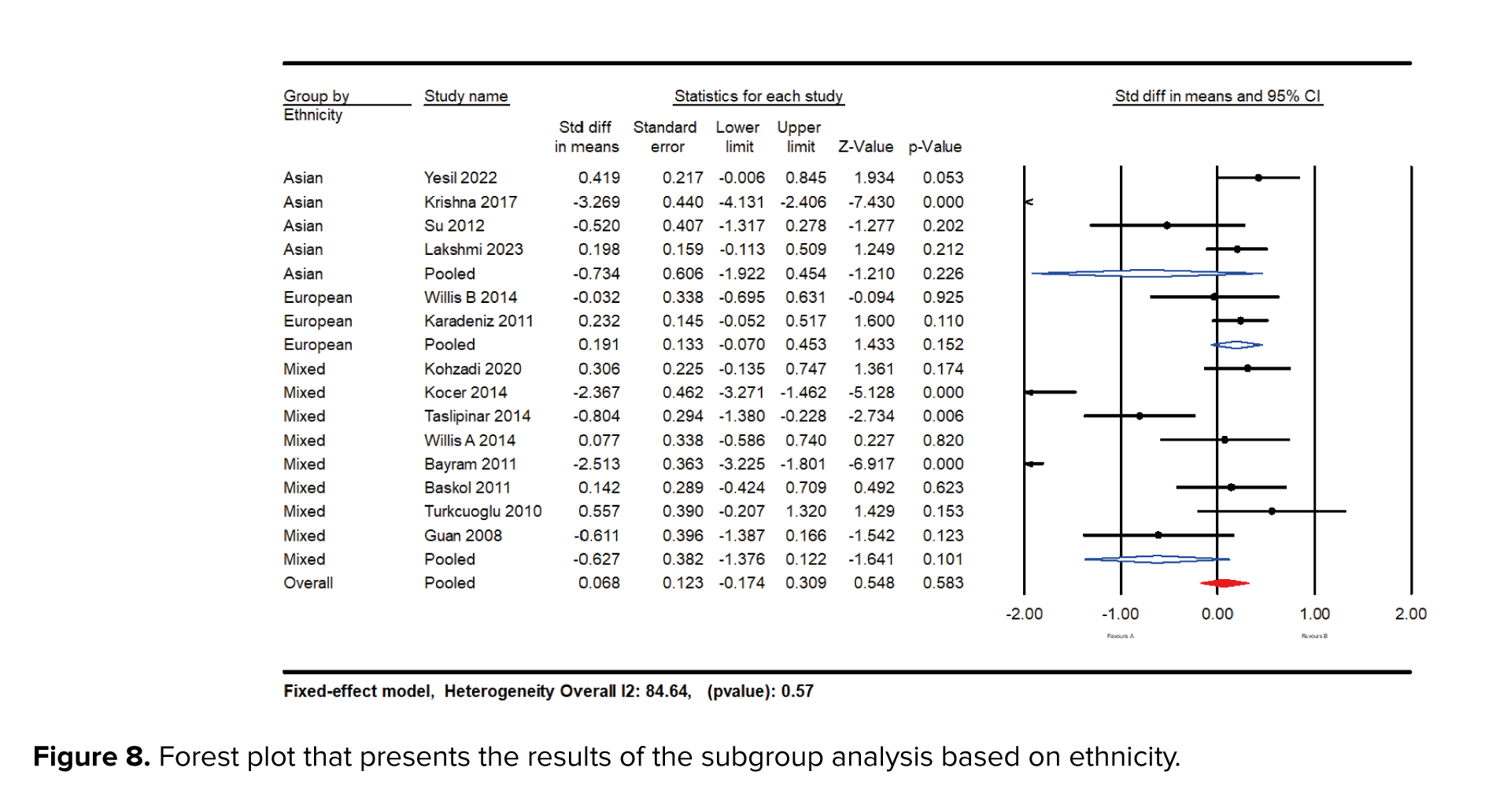
Subgroup analyses were conducted based on ethnicity, including Asian, European, and mixed races. When focusing on racial diversity, studies revealed that women of European ethnicity had higher NO levels (SMD = 0.191; 95% CI: -0.07 to 0.45; p = 0.015) compared to those of Asian (SMD = -0.734; 95% CI: -1.92 to -045; p = 0.22) and mixed race (SMD = -0.62; 95% CI: -1.641 to 0.101; p = 0.10) (Figure 8).
3.3.4. Meta-regression analysis
A meta-regression analysis was conducted to investigate the relationship between sample size, PCOS diagnostic criteria, study year, BMI, and overall effect size. The analysis results indicated no association between PCOS diagnostic criteria (meta-regression coefficient: 0.698; 95% CI: -0.619 to 2.016; p = 0.29), sample size (meta-regression coefficient: 0.064; 95% CI: -0.006 to 0.198; p = 0.11), and year of studies (meta-regression coefficient: 0.015; 95% CI: -0.138 to 0.168; p = 0.84) with effect size. However, the analysis revealed a significant positive relationship between BMI and effect sizes (meta-regression coefficient: 0.327; 95% CI: 0.051 to 0.604; p = 0.02).
These findings suggest that BMI can significantly impact on the effect size. In contrast, factors such as PCOS diagnostic criteria, sample size, and year of study did not significantly affect the results of the meta-analysis studies.
3.4. Sensitivity analysis and publication bias
Sensitivity analysis, excluding studies one by one, demonstrated a stable SMD range from -0.696 to -0.383. The 95% CI ranged from -1.326 to 0.095. The values of I² maintained clarity, indicating stable results throughout, with no significant changes observed. The funnel plot method, utilized for detecting publication bias, and Egger’s test, used for quantifying publication bias, did not provide evidence of publication bias regarding the included studies (coefficient: -6.061; ٍُ: 1.963; 95% CI: -10.901 to 1.022, p = 0.112). These results further support the credibility and robustness of the pooled findings.
4. Discussion
4.1. Meta-analysis overview
This comprehensive meta-analysis examines the association between circulating NO levels and PCOS, synthesizing data from a diverse array of studies. The analysis encompasses a significant sample size, integrating findings from 1171 participants across 14 studies. The results consistently reveal a notable decrease in NO levels among individuals with PCOS compared to healthy controls. This finding is pivotal, suggesting that NO may serve as a potential biomarker for diagnosing and managing PCOS. This discussion delves into the biological mechanisms underlying these observations, interpreting the main findings, and exploring their implications for clinical practice and future research.
4.2. Interpretation of main findings
Our meta-analysis highlights a significant reduction in circulating NO levels in women with PCOS, with an SMD of -0.482 (95% CI: -0.908 to -0.056; p = 0.027). This suggests a strong association between low NO levels and the presence of PCOS. The substantial heterogeneity observed (I² = 91.34%) indicates variability in NO levels based on demographic and clinical factors, warranting further investigation. Moreover, the prediction interval (-2.51 to 1.45) underscores the variability in effect size, suggesting that while reduced NO levels are generally associated with PCOS, the specific utility of NO as a biomarker may vary across different populations and clinical settings.
4.3. Subgroup analysis reflection: Interpreting variability and trends
4.3.1. Age-based analysis
The differential impact of NO levels across various age groups elucidates critical aspects of the pathogenesis of PCOS. Among women over 30 yr old, NO levels were significantly elevated in those with PCOS compared to the control group (SMD = 0.180 vs. -1.236). This increase may reflect age-related changes in vascular function or the prolonged exposure to metabolic and endocrine disturbances typical of PCOS. In contrast, younger women (aged 16-25) exhibited a less pronounced reduction in NO levels. This difference may indicate an alternative pathogenic mechanism or an adaptive response specific to younger individuals.
4.3.2. BMI-based analysis
BMI significantly moderated the relationship between NO levels and PCOS. Women with a BMI greater than 30 kg/m² exhibited a more substantial decrease in NO levels compared to those with a BMI below 25 kg/m² (SMD = 0.163 vs. -1.230). This suggests that obesity, a common feature of PCOS, may exacerbate endothelial dysfunction and reduce NO bioavailability. The association between obesity and NO levels underscores the importance of considering BMI in the clinical assessment and management of PCOS.
4.3.3. Sample type and ethnicity-based analysis
The analysis did not find significant differences in NO levels when comparing serum and plasma samples (SMD = -0.851 vs. -0.906). This suggests that the bioavailability of NO and its potential as a biomarker is consistent across different sample types, adding robustness to the findings and allowing flexibility in clinical assessments. Subgroup analyses based on ethnicity revealed that women of European ethnicity had higher NO levels (SMD = 0.191; p = 0.473) compared to those of Asian (SMD = -0.045; p ≤ 001) and mixed race (SMD = -0.404 p ≤ 001). These findings suggest that genetic and environmental factors related to ethnicity may influence NO levels and their association with PCOS.
4.4. Deciphering study outcome patterns: Meta-regression insights
The meta-regression analysis provided vital insights into the factors influencing circulating NO levels in patients with PCOS. The analysis examined variables such as PCOS diagnostic criteria, sample size, study year, and BMI, with significant findings, particularly regarding BMI.
The meta-regression revealed no statistically significant association between the effect size and PCOS diagnostic criteria, sample size, and study year. These results suggest that these factors do not significantly impact the heterogeneity of NO levels reported across studies, indicating a degree of consistency in findings despite variations in study designs, populations, and periods. However, a significant positive relationship was observed between BMI and effect size (meta-regression coefficient: 0.327; 95% CI: 0.051 to 0.604; p = 0.02). This finding implies that BMI is a crucial determinant of circulating NO levels in PCOS patients. Higher BMI appears to be associated with increased NO levels, which could reflect the interplay between obesity, metabolic dysfunction, and OS commonly seen in PCOS.
The role of BMI in modulating NO levels can be understood through several mechanisms. Obesity is often associated with insulin resistance, a hallmark of PCOS, which is linked to endothelial dysfunction and altered NO production. Adipose tissue in obese individuals secretes pro-inflammatory cytokines and free fatty acids, which can further impair endothelial function and exacerbate OS. This cascade of metabolic disturbances can lead to increased NO production as a compensatory mechanism or as a marker of ongoing OS.
4.5. Prior research and contextualization
PCOS is recognized as a multifactorial endocrine disorder, which represents a primary cause of infertility issues among women of reproductive age (36, 37). Considering several factors influencing NO levels, this review provides a critical analysis of the role of NO as a potential biomarker in the pathology of PCOS. The intricate association between NO levels and PCOS, as suggested by the results of this meta-analysis, underscores the complexity of the disorder. NO is a well-known endothelium-derived relaxing factor that plays a crucial role in vascular homeostasis (27).
NO is a well-established endothelium-derived relaxing factor essential for maintaining vascular homeostasis. It mediates vasodilation by stimulating the soluble guanylate cyclase in vascular smooth muscle cells, leading to cyclic guanosine monophosphate (cGMP) production and subsequent relaxation of smooth muscle fibers. This vasodilatory effect is crucial for regulating blood flow and blood pressure (38). Reduced NO bioavailability is associated with endothelial dysfunction, a precursor to atherosclerosis and cardiovascular disease (CVD) (39).
In the context of PCOS, several studies have identified a link between endothelial dysfunction and the syndrome's pathophysiology. For example, a study by Paradisi et al. found that women with PCOS exhibited impaired endothelial function, as evidenced by decreased flow-mediated dilation of the brachial artery (40). This dysfunction is thought to stem from reduced NO availability, contributing to the increased cardiovascular risk observed in PCOS patients.
Insulin resistance is a core feature of PCOS, affecting approximately 50-70% of women with the condition (41, 42). NO plays a significant role in insulin signaling and glucose metabolism. Insulin stimulates NO production via the PI3K/Akt pathway, which promotes glucose uptake in peripheral tissues (43). However, hyperinsulinemia, commonly seen in PCOS, can impair eNOS activity, reducing NO synthesis and exacerbating insulin resistance (44).
Clinical studies have demonstrated a correlation between reduced NO levels and insulin resistance in PCOS. For instance, a study by Yildirim reported significantly lower NO levels in insulin-resistant women with PCOS compared to healthy controls (45). This reduction was associated with elevated fasting insulin and glucose levels, underscoring the role of NO in metabolic regulation and its potential as a biomarker for insulin resistance in PCOS.
Chronic low-grade inflammation is a hallmark of PCOS, characterized by elevated levels of inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6 (46). These cytokines can inhibit eNOS expression and activity, leading to reduced NO production. Additionally, OS, indicated by increased ROS levels, further diminishes NO bioavailability by converting NO to peroxynitrite, a reactive nitrogen species (47). Previous research has provided mixed results regarding NO levels in PCOS, with some studies reporting decreased levels while others found no significant differences. For example, a study by Macut et al. reported significantly lower NO levels in women with PCOS, which was associated with increased insulin resistance and cardiovascular risk (48).
4.6. Elucidating the biological mechanisms of NO in the pathophysiology of PCOS
The underlying mechanisms connecting NO levels to PCOS involve both the factors that lead to reduced NO levels and the subsequent impacts on the disease’s progression. In PCOS, reduced NO bioavailability is a marker of endothelial dysfunction, which is a precursor to atherosclerosis and other CVDs (39). Several inter-related mechanisms drive this endothelial dysfunction in PCOS. Hyperinsulinemia, a common feature in PCOS, leads to reduced NO production by inhibiting eNOS activity. Elevated insulin levels in insulin-resistant PCOS patients negatively affect eNOS via the phosphoinositide 3-kinase (PI3K) pathway, thus decreasing NO synthesis (43). Insulin resistance, prevalent in PCOS, impairs the PI3K/Akt pathway, which is critical for eNOS activation and NO production. This impairment leads to decreased NO availability and endothelial dysfunction (49). Hyperandrogenism, another hallmark of PCOS, further contributes to endothelial dysfunction by downregulating eNOS expression and impairing NO-mediated vasodilation (50). Elevated androgen levels in PCOS increase the production of asymmetric dimethylarginine, an endogenous inhibitor of eNOS, thus reducing NO synthesis (51).
The relationship between NO and insulin resistance is complex and bidirectional. NO facilitates insulin-mediated glucose uptake in skeletal muscle by enhancing blood flow and glucose delivery to peripheral tissues (52). NO enhances the translocation of glucose transporter type 4 to the cell membrane in response to insulin, promoting glucose uptake (53). In PCOS, decreased NO availability due to endothelial dysfunction and OS impairs this process, exacerbating insulin resistance. This insulin resistance perpetuates hyperinsulinemia, creating a vicious cycle of metabolic dysregulation (54). Furthermore, insulin resistance in PCOS is linked to adipose tissue dysfunction.
Adipocytes in PCOS patients exhibit impaired NO synthesis, leading to reduced adiponectin secretion and increased secretion of pro-inflammatory cytokines (55). This adipose tissue inflammation reduces insulin sensitivity and increases systemic OS, subsequently reducing NO levels and exacerbating metabolic outcomes in PCOS. Elevated free fatty acids in obesity, often seen in PCOS, also play a role. Free fatty acids induce mitochondrial dysfunction and increase ROS production in skeletal muscle, which inhibits the insulin signaling pathway and reduces NO production, further contributing to insulin resistance (56, 57).
NO plays a pivotal role in ovarian physiology, particularly in follicular development and ovulation. NO regulates ovarian blood flow, follicle maturation, and oocyte quality (58). In PCOS, reduced NO levels can impair these processes, leading to anovulation and the formation of ovarian cysts, hallmark features of the syndrome. Furthermore, the presence of hyperinsulinemia and hyperandrogenism in individuals with PCOS disturbs the microenvironment of the ovaries, leading to a decrease in the synthesis of NO and hindering the process of follicular angiogenesis.
This leads to poor follicular development and increased follicular atresia (58). Insulin resistance also reduces the bioavailability of NO in the ovaries, further impairing folliculogenesis (59). The ovarian dysfunction in PCOS is also linked to the dysregulation of steroidogenesis. NO modulates the production of steroid hormones by influencing the expression of key enzymes involved in steroid biosynthesis. Reduced NO levels lead to an imbalance in androgen and estrogen production, contributing to the hyperandrogenic state seen in PCOS (60).
Additionally, the dysregulation of the NO-cGMP pathway in the ovaries of PCOS patients contributes to impaired ovulation. NO activates guanylate cyclase, increasing cGMP levels crucial for follicular expansion and oocyte maturation. Reduced NO levels disrupt this signaling pathway, leading to ovulatory dysfunction (61).
4.7. Clinical implications and future directions
The significant association between reduced NO levels and PCOS suggests that NO could serve as a valuable biomarker for the early diagnosis and management of PCOS. Monitoring NO levels could help identify individuals at risk of developing PCOS-related complications, such as CVD and insulin resistance.
Understanding the mechanistic role of NO in PCOS could open new avenues for therapeutic strategies aimed at modulating NO levels to mitigate disease severity. For instance, interventions that enhance NO production, such as lifestyle modifications (e.g., exercise and diet) or pharmacological agents (e.g., L-arginine supplementation), could improve endothelial function and insulin sensitivity in PCOS patients.
Future research should focus on longitudinal studies to track NO levels over time in women with PCOS, from initial diagnosis through treatment and disease progression. Such studies could clarify the temporal dynamics of NO expression and its prognostic utility. Additionally, interventional studies examining the effects of therapies targeting NO pathways could provide insights into their potential benefits in managing PCOS.
4.8. Strengths and limitations
This study boasts several strengths, including a rigorous search methodology, a substantial sample size, and consistent findings across multiple studies. The use of subgroup analyses and meta-regression techniques has enhanced the interpretation of results, adding depth to our understanding of the relationship between NO levels and PCOS. However, several limitations warrant acknowledgment. The variability in detection methods across included studies may introduce discrepancies in reported NO levels. Despite efforts to standardize our analysis, differences in assay sensitivity and specificity could influence the consistency of results. Additionally, the predominance of English-language articles in the search could mask relevant data in non-English publications, potentially introducing language bias.
The heterogeneity in study designs and data collection methods across studies adds another layer of complexity to data interpretation. Variations in participant lifestyles, such as diet, physical activity, and smoking status, could influence NO levels and were not uniformly accounted for in all studies. Furthermore, the evolving landscape of PCOS diagnostic criteria and treatment modalities poses additional challenges in standardizing findings.
5. Conclusion
This meta-analysis reviewed data from 14 studies and 1171 participants, highlighting the potential of NO levels as a biomarker for PCOS and suggesting an association between NO dysregulation and PCOS pathophysiology. Our findings underscore the pertinence of NO levels as a potential biomarker for PCOS, suggesting an interplay between NO dysregulation and the pathophysiology of PCOS. Significantly, our research adds a critical dimension to the clinical understanding of PCOS, aligning with the hypothesis that NO could serve not only as a marker of disease presence but also as an indicator of its severity. This relationship holds promising outcomes for developing more precise diagnostic criteria and improving the therapeutic strategies tailored to individual NO profiles. It also proposes a new vista for clinicians to prognosticate the progression and mitigate the risks associated with PCOS by monitoring NO levels. Future research should aim at longitudinal studies with larger cohorts and standardized methodologies for assessing NO levels, to advance beyond correlation toward a clearer understanding of causation.
Data Availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author Contributions
SS. Bahreiny and A. Ahangarpour contributed to data analysis, manuscript preparation, and supervision, while R. Mohammadpour Fard, M. Aghaei, and A. Ahangarpour contributed to manuscript searching, preparation, and data analysis. E. Harooni and M. Amraei contributed to the search strategy, article searching, and manuscript preparation. The manuscript has been reviewed and approved by all authors.
Acknowledgments
The authors of this research would like to express their sincere gratitude to the esteemed researchers whose scholarly articles were utilized during this study. Your invaluable contributions have been instrumental in shaping the outcome of this research, and we are deeply appreciative of your efforts. Additionally, we acknowledge the use of artificial intelligence tools for revision (OpenAI, ChatGPT-4, version 4.0), and grammar checks, which assisted in refining the manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (137 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) encompasses a spectrum of metabolic and endocrine derangements that impose significant health concerns on women of childbearing age, often serving as a precursor to a variety of life-impacting co-morbidities (1). Situated at the intersection of reproductive biology and metabolic homeostasis, PCOS challenges medical understanding with its multifactorial etiology, diverse phenotypic expressions, and complex pathophysiological pathways underpinned predominantly by hyperandrogenism and ovarian dysfunction (2).
Recent advancements in the understanding of hyperandrogenism have highlighted its intricate etiology, which may involve contributions from fetal development, maternal factors, and placental influences (3, 4).
This nuanced view of its genesis opens gateways to personalized therapeutic approaches that address the patient's unique disease matrix. The ominous constellation of obesity, insulin resistance, hyperglycemia, and associated cardiovascular risk factors further emphasizes the systemic impact of PCOS and its position as more than a reproductive disorder (5, 6).
Central to our investigation is the role of oxidative stress (OS) a pervasive player in the pathogenesis of PCOS. As the body grapples with the discord between the generation of reactive oxygen species (ROS) and its inherent antioxidant defenses, the resultant OS becomes a critical focus for therapeutic intervention (7, 8).
Nitric oxide (NO) emerges as a pivotal mediator within this redox landscape, straddling roles in vascular regulation and signal transduction, thus implicating it in both the symptomatic presentations and long-term sequelae of PCOS (9). Mounting evidence suggests that the dysregulation of NO synthesis pathways, encompassing the 3 isoforms of NO synthase ([NOS] endothelial, inducible, and neuronal), may have paradoxical implications for PCOS pathology (10).
This dual-faceted role of NO is highlighted by its vascular protective functions courtesy of endothelial NOS (eNOS)-derived NO, juxtaposed against the noxious sequel of inducible NOS (iNOS) over-expression under chronic inflammatory stimuli, commonly witnessed in PCOS phenotypes characterized by insulin resistance (11) (Figure 1). Thus, investigation into serum NO levels in PCOS patients has disclosed a conflicting landscape, with some studies indicating reduced levels implicating vascular dysfunction while others suggest augmented concentrations reflective of chronic inflammation (12, 13). The intricate dance between NO and ROS and their collective influence on the body's redox state presents a potential avenue for diagnostic biomarker development and therapeutic targeting. Given the pivotal role of NO in both the preservation of vascular homeostasis and the exacerbation of oxidative pathology, an opportunity arises to probe deeper into its relationship with PCOS (14-16). To elucidate the enigmatic nature of NO's dual role in PCOS pathophysiology and demystify its prognostic potential as a biomarker, we have embarked on a systematic review and meta-analysis, critically examining observational studies that hinge on this molecule's dynamic within the disorder.
This comprehensive analysis aims to sift through the contradictory evidence and provide a quantitative synthesis of NO levels in women with PCOS. This offers insights into its diagnostic robustness and prognostic value in this prevalent condition. Previous meta-analyses in this area were often based on older studies, with limited subgroup analyses, and lacked rigorous bias assessment and meta-regression techniques. In contrast, our study incorporates a larger, updated dataset. It applies advanced analytical methods including detailed subgroup analyses (age, body mass index [BMI], sample type, and ethnicity), meta-regression, and thorough bias evaluation to deepen our understanding of NO’s role in PCOS. By exploring these dimensions, this meta-analysis aims to definitively characterize the interaction between NO and PCOS, paving the way for future personalized treatment strategies that address the underlying oxidative and endothelial disruptions in this complex syndrome.

2. Materials and Methods
2.1. Protocol and registration
The systematic review and meta-analysis protocol were registered with "Prospective International Registration of Systematic Reviews" (PROSPERO) CRD42024507048. The systematic reviews were reported according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (17).
2.2. Eligibility criteria
This review only included studies that focused on humans and used observational study designs such as cross-sectional, cohort, or case-control based on population, intervention, comparator, and outcome (PICO) criteria (Table I). The scope of the review was limited to observational studies that examined women aged 18 yr or older who had been diagnosed with PCOS. The diagnostic criteria applied in identifying PCOS patients were the Rotterdam Criteria or National Institutes of Health Criteria. Studies having insufficient data, as well as those that were reviewed, letters to the editor, reports, or were not accessible, were excluded from this review.
2.3. Literature search
Valid medical databases such as PubMed, Web of Science, EMBASE, Scopus, and Cochrane Library (CENTRAL) were searched for the literature review in January 2024. The search terms were defined and searched by a medical librarian and medical professionals, without any time and place restrictions. Search terms were supplemented by searching for available gray studies such as governmental and academic studies. Also, the reference list of all articles related to the snowball method was searched for more comprehensiveness. The keywords "nitric oxide", "polycystic ovary syndrome", and "PCOS" were selected as the primary keywords based on mesh in PubMed. Full details of the search strategy are available in table I. All records reported in the literature search were uploaded to Comprehensive Meta-analysis v3.7z software.


2.4. Study selection
After deleting the duplicate articles, 2 independent reviewers (S. B. and M. A.) assessed the eligibility based on inclusion/exclusion criteria and reviewed the titles/abstracts of the retrieved studies. Subsequently, 2 reviewers assessed the full texts, and disagreements were resolved with arbitration by the third reviewer.
2.5. Data extraction
Data extraction was systematically carried out using a predefined checklist designed to capture essential elements relevant to the research question adhering to the study extraction design outlined in our previous research (18-21). The checklist included information on the study's origin, such as the first author's name, publication year, and geographic region of the study. Additionally, details on study design (cross-sectional, cohort, or case-control), participant characteristics (age, sample size, and body mass index), and measurements performed, diagnostic criteria, and reported outcomes were systematically extracted.
2.6 . Risk of bias assessment in the included studies
The Newcastle-Ottawa scale is a valuable tool for evaluating the quality of nonrandomized studies in a meta-analysis. Utilizing this scale ensures that only the most reliable studies are considered, leading to a more precise and trustworthy research analysis. The NOS is meticulously designed with 3 critical domains: the comparability of study groups, the selection of these groups, and the ascertainment of the outcome of interest. Each domain is equipped with specific criteria that guide the evaluation process. The scale ranges from 0-9, with studies scoring ≤ 4 classified as low quality and those scoring ≥ 5 as high quality. 2 independent reviewers reviewed each study and assessed it against the 3 domains. Any discrepancies were resolved with the assistance of a third reviewer.
2.7. Statistical Analysis
The meta-analysis was conducted using Comprehensive Meta-Analysis v3.7z software. Circulating NO levels in women with PCOS were compared to those in control groups using the standardized mean difference (SMD) and a 95% confidence interval (CI). The utilization of SMD proved to be pivotal as it facilitated consistent comparison of effect sizes across studies employing diverse measurement scales for NO levels. Heterogeneity among studies was assessed using Cochran's Q test and the I2 statistic, with statistical significance set at p < 0.05 or I2 > 50%. A random-effects model was employed for data synthesis, considering potential variability among studies. Publication bias was assessed using Egger's tests, with significance set at a p-value of 0.05. Subgroup analyses and meta-regression analyses were conducted to explore potential sources of heterogeneity, such as age, geographical region, and study design. Furthermore, a sensitivity analysis was performed to evaluate the impact of individual studies by systematically excluding each study from the analysis. The overall statistical significance level was set at p < 0.05 for all the analyses as well as their prediction intervals were reported.
3. Results
3.1. Characteristics of the included studies
The systematic search strategy identified 1542 articles in the database. After removing 386 duplicate records and excluding 1107 articles based on title and abstract evaluation, 49 articles underwent a comprehensive full-text assessment. During this process, 35 studies were excluded for various reasons, including lack of relevant data on NO levels, insufficient sample size, failure to meet the diagnostic criteria for PCOS, and methodological flaws such as poor study design or inadequate control groups. Ultimately, 14 studies met the pre-established selection criteria involving a combined total of 1171 participants (Figure 2). The characteristics of these 14 studies, including participant demographics, sample type, and study design, are summarized in table II. Furthermore, the quality assessment of the included studies is presented in table III.




3.2 . Association and comparison details
A comprehensive investigation, encompassing 14 studies (15 reports) with a total of 1171 participants, was conducted to examine the correlation between NO levels and PCOS. Subgroup and meta-regression analyses were performed to explore potential sources of heterogeneity, including BMI, age, type of sample, diagnostic criteria for PCOS, and study year. This thorough analysis significantly enhanced our understanding of the association between NO levels and PCOS, illuminating the factors contributing to the observed heterogeneity.
3.3. Relationship between serum NO levels and PCOS
3.3.1. Meta‐analysis
The meta-analysis results indicated a significant reduction in NO levels in the group with PCOS compared to the control group, with an SMD of -0.482 (95% CI: -0.908 to -0.056; p = 0.027). This finding suggests a substantial decrease in NO levels associated with PCOS. Furthermore, a high degree of heterogeneity was detected among the included studies, as evidenced by an I² value of 91.34% and a p-value of 0.01, indicating considerable variability in the study results (Figure 3).

3.3.2. Prediction interval
The estimation of a prediction interval revealed a range of -2.51 to 1.45 for the true effect size, assuming a normal distribution. This interval is expected to encompass the effect size in 95% of comparable populations. However, it is important to note that variations in the actual effect size may occur within this interval due to factors such as sample size and study design. Therefore, careful consideration is warranted when interpreting results within this prediction interval (Figure 4).

3.3.3. Subgroup analysis
Our systematic review and meta-analysis revealed a significant association between NO levels and PCOS, with nuanced variations observed across different subgroups. The subgroup analyses focused on age, BMI, sample type, and race, providing valuable insights into the diverse manifestations of NO levels in PCOS patients. A noteworthy difference was observed between women aged 25 yr and above and those < 25 yr. The studies were analyzed in 2 subgroups, one including participants with an average age of ≥ 25 yr (SMD = 0.180; 95% CI: -0.007 to 0.368; p = 0.060), and the other including those < 25 yr (SMD = -1.236; 95% CI: -2.219 to -0.252; p = 0.014). According to the analysis, individuals aged ≥ 25 had higher levels of NO (Figure 5).

Moreover, subgroup analyses based on BMI and the sample type were conducted. The analysis of studies with a mean BMI of ≥ 25 kg/m² or < 25 kg/m² revealed that women with BMI > 25 kg/m² had higher NO levels (SMD = 0.163; 95% CI: -0.031 to 0.357; p = 0.10) compared to those with BMI < 25 kg/m² (SMD = -1.230; 95% CI: -2.213 to -0.247; p = 0.014) (Figure 6).

In addition, the study also looked at subgroups based on the type of sample collected (serum or plasma). The results showed that women with plasma samples had lower levels of NO (SMD = -0.851; 95% CI: -1.950 to 0.248; p = 0.129) compared to those with serum samples (SMD = -0.363; 95% CI: -0.906 to 0.181; p = 0.19) (Table IV, Figure 7).


Subgroup analyses were conducted based on ethnicity, including Asian, European, and mixed races. When focusing on racial diversity, studies revealed that women of European ethnicity had higher NO levels (SMD = 0.191; 95% CI: -0.07 to 0.45; p = 0.015) compared to those of Asian (SMD = -0.734; 95% CI: -1.92 to -045; p = 0.22) and mixed race (SMD = -0.62; 95% CI: -1.641 to 0.101; p = 0.10) (Figure 8).

3.3.4. Meta-regression analysis
A meta-regression analysis was conducted to investigate the relationship between sample size, PCOS diagnostic criteria, study year, BMI, and overall effect size. The analysis results indicated no association between PCOS diagnostic criteria (meta-regression coefficient: 0.698; 95% CI: -0.619 to 2.016; p = 0.29), sample size (meta-regression coefficient: 0.064; 95% CI: -0.006 to 0.198; p = 0.11), and year of studies (meta-regression coefficient: 0.015; 95% CI: -0.138 to 0.168; p = 0.84) with effect size. However, the analysis revealed a significant positive relationship between BMI and effect sizes (meta-regression coefficient: 0.327; 95% CI: 0.051 to 0.604; p = 0.02).
These findings suggest that BMI can significantly impact on the effect size. In contrast, factors such as PCOS diagnostic criteria, sample size, and year of study did not significantly affect the results of the meta-analysis studies.
3.4. Sensitivity analysis and publication bias
Sensitivity analysis, excluding studies one by one, demonstrated a stable SMD range from -0.696 to -0.383. The 95% CI ranged from -1.326 to 0.095. The values of I² maintained clarity, indicating stable results throughout, with no significant changes observed. The funnel plot method, utilized for detecting publication bias, and Egger’s test, used for quantifying publication bias, did not provide evidence of publication bias regarding the included studies (coefficient: -6.061; ٍُ: 1.963; 95% CI: -10.901 to 1.022, p = 0.112). These results further support the credibility and robustness of the pooled findings.
4. Discussion
4.1. Meta-analysis overview
This comprehensive meta-analysis examines the association between circulating NO levels and PCOS, synthesizing data from a diverse array of studies. The analysis encompasses a significant sample size, integrating findings from 1171 participants across 14 studies. The results consistently reveal a notable decrease in NO levels among individuals with PCOS compared to healthy controls. This finding is pivotal, suggesting that NO may serve as a potential biomarker for diagnosing and managing PCOS. This discussion delves into the biological mechanisms underlying these observations, interpreting the main findings, and exploring their implications for clinical practice and future research.
4.2. Interpretation of main findings
Our meta-analysis highlights a significant reduction in circulating NO levels in women with PCOS, with an SMD of -0.482 (95% CI: -0.908 to -0.056; p = 0.027). This suggests a strong association between low NO levels and the presence of PCOS. The substantial heterogeneity observed (I² = 91.34%) indicates variability in NO levels based on demographic and clinical factors, warranting further investigation. Moreover, the prediction interval (-2.51 to 1.45) underscores the variability in effect size, suggesting that while reduced NO levels are generally associated with PCOS, the specific utility of NO as a biomarker may vary across different populations and clinical settings.
4.3. Subgroup analysis reflection: Interpreting variability and trends
4.3.1. Age-based analysis
The differential impact of NO levels across various age groups elucidates critical aspects of the pathogenesis of PCOS. Among women over 30 yr old, NO levels were significantly elevated in those with PCOS compared to the control group (SMD = 0.180 vs. -1.236). This increase may reflect age-related changes in vascular function or the prolonged exposure to metabolic and endocrine disturbances typical of PCOS. In contrast, younger women (aged 16-25) exhibited a less pronounced reduction in NO levels. This difference may indicate an alternative pathogenic mechanism or an adaptive response specific to younger individuals.
4.3.2. BMI-based analysis
BMI significantly moderated the relationship between NO levels and PCOS. Women with a BMI greater than 30 kg/m² exhibited a more substantial decrease in NO levels compared to those with a BMI below 25 kg/m² (SMD = 0.163 vs. -1.230). This suggests that obesity, a common feature of PCOS, may exacerbate endothelial dysfunction and reduce NO bioavailability. The association between obesity and NO levels underscores the importance of considering BMI in the clinical assessment and management of PCOS.
4.3.3. Sample type and ethnicity-based analysis
The analysis did not find significant differences in NO levels when comparing serum and plasma samples (SMD = -0.851 vs. -0.906). This suggests that the bioavailability of NO and its potential as a biomarker is consistent across different sample types, adding robustness to the findings and allowing flexibility in clinical assessments. Subgroup analyses based on ethnicity revealed that women of European ethnicity had higher NO levels (SMD = 0.191; p = 0.473) compared to those of Asian (SMD = -0.045; p ≤ 001) and mixed race (SMD = -0.404 p ≤ 001). These findings suggest that genetic and environmental factors related to ethnicity may influence NO levels and their association with PCOS.
4.4. Deciphering study outcome patterns: Meta-regression insights
The meta-regression analysis provided vital insights into the factors influencing circulating NO levels in patients with PCOS. The analysis examined variables such as PCOS diagnostic criteria, sample size, study year, and BMI, with significant findings, particularly regarding BMI.
The meta-regression revealed no statistically significant association between the effect size and PCOS diagnostic criteria, sample size, and study year. These results suggest that these factors do not significantly impact the heterogeneity of NO levels reported across studies, indicating a degree of consistency in findings despite variations in study designs, populations, and periods. However, a significant positive relationship was observed between BMI and effect size (meta-regression coefficient: 0.327; 95% CI: 0.051 to 0.604; p = 0.02). This finding implies that BMI is a crucial determinant of circulating NO levels in PCOS patients. Higher BMI appears to be associated with increased NO levels, which could reflect the interplay between obesity, metabolic dysfunction, and OS commonly seen in PCOS.
The role of BMI in modulating NO levels can be understood through several mechanisms. Obesity is often associated with insulin resistance, a hallmark of PCOS, which is linked to endothelial dysfunction and altered NO production. Adipose tissue in obese individuals secretes pro-inflammatory cytokines and free fatty acids, which can further impair endothelial function and exacerbate OS. This cascade of metabolic disturbances can lead to increased NO production as a compensatory mechanism or as a marker of ongoing OS.
4.5. Prior research and contextualization
PCOS is recognized as a multifactorial endocrine disorder, which represents a primary cause of infertility issues among women of reproductive age (36, 37). Considering several factors influencing NO levels, this review provides a critical analysis of the role of NO as a potential biomarker in the pathology of PCOS. The intricate association between NO levels and PCOS, as suggested by the results of this meta-analysis, underscores the complexity of the disorder. NO is a well-known endothelium-derived relaxing factor that plays a crucial role in vascular homeostasis (27).
NO is a well-established endothelium-derived relaxing factor essential for maintaining vascular homeostasis. It mediates vasodilation by stimulating the soluble guanylate cyclase in vascular smooth muscle cells, leading to cyclic guanosine monophosphate (cGMP) production and subsequent relaxation of smooth muscle fibers. This vasodilatory effect is crucial for regulating blood flow and blood pressure (38). Reduced NO bioavailability is associated with endothelial dysfunction, a precursor to atherosclerosis and cardiovascular disease (CVD) (39).
In the context of PCOS, several studies have identified a link between endothelial dysfunction and the syndrome's pathophysiology. For example, a study by Paradisi et al. found that women with PCOS exhibited impaired endothelial function, as evidenced by decreased flow-mediated dilation of the brachial artery (40). This dysfunction is thought to stem from reduced NO availability, contributing to the increased cardiovascular risk observed in PCOS patients.
Insulin resistance is a core feature of PCOS, affecting approximately 50-70% of women with the condition (41, 42). NO plays a significant role in insulin signaling and glucose metabolism. Insulin stimulates NO production via the PI3K/Akt pathway, which promotes glucose uptake in peripheral tissues (43). However, hyperinsulinemia, commonly seen in PCOS, can impair eNOS activity, reducing NO synthesis and exacerbating insulin resistance (44).
Clinical studies have demonstrated a correlation between reduced NO levels and insulin resistance in PCOS. For instance, a study by Yildirim reported significantly lower NO levels in insulin-resistant women with PCOS compared to healthy controls (45). This reduction was associated with elevated fasting insulin and glucose levels, underscoring the role of NO in metabolic regulation and its potential as a biomarker for insulin resistance in PCOS.
Chronic low-grade inflammation is a hallmark of PCOS, characterized by elevated levels of inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6 (46). These cytokines can inhibit eNOS expression and activity, leading to reduced NO production. Additionally, OS, indicated by increased ROS levels, further diminishes NO bioavailability by converting NO to peroxynitrite, a reactive nitrogen species (47). Previous research has provided mixed results regarding NO levels in PCOS, with some studies reporting decreased levels while others found no significant differences. For example, a study by Macut et al. reported significantly lower NO levels in women with PCOS, which was associated with increased insulin resistance and cardiovascular risk (48).
4.6. Elucidating the biological mechanisms of NO in the pathophysiology of PCOS
The underlying mechanisms connecting NO levels to PCOS involve both the factors that lead to reduced NO levels and the subsequent impacts on the disease’s progression. In PCOS, reduced NO bioavailability is a marker of endothelial dysfunction, which is a precursor to atherosclerosis and other CVDs (39). Several inter-related mechanisms drive this endothelial dysfunction in PCOS. Hyperinsulinemia, a common feature in PCOS, leads to reduced NO production by inhibiting eNOS activity. Elevated insulin levels in insulin-resistant PCOS patients negatively affect eNOS via the phosphoinositide 3-kinase (PI3K) pathway, thus decreasing NO synthesis (43). Insulin resistance, prevalent in PCOS, impairs the PI3K/Akt pathway, which is critical for eNOS activation and NO production. This impairment leads to decreased NO availability and endothelial dysfunction (49). Hyperandrogenism, another hallmark of PCOS, further contributes to endothelial dysfunction by downregulating eNOS expression and impairing NO-mediated vasodilation (50). Elevated androgen levels in PCOS increase the production of asymmetric dimethylarginine, an endogenous inhibitor of eNOS, thus reducing NO synthesis (51).
The relationship between NO and insulin resistance is complex and bidirectional. NO facilitates insulin-mediated glucose uptake in skeletal muscle by enhancing blood flow and glucose delivery to peripheral tissues (52). NO enhances the translocation of glucose transporter type 4 to the cell membrane in response to insulin, promoting glucose uptake (53). In PCOS, decreased NO availability due to endothelial dysfunction and OS impairs this process, exacerbating insulin resistance. This insulin resistance perpetuates hyperinsulinemia, creating a vicious cycle of metabolic dysregulation (54). Furthermore, insulin resistance in PCOS is linked to adipose tissue dysfunction.
Adipocytes in PCOS patients exhibit impaired NO synthesis, leading to reduced adiponectin secretion and increased secretion of pro-inflammatory cytokines (55). This adipose tissue inflammation reduces insulin sensitivity and increases systemic OS, subsequently reducing NO levels and exacerbating metabolic outcomes in PCOS. Elevated free fatty acids in obesity, often seen in PCOS, also play a role. Free fatty acids induce mitochondrial dysfunction and increase ROS production in skeletal muscle, which inhibits the insulin signaling pathway and reduces NO production, further contributing to insulin resistance (56, 57).
NO plays a pivotal role in ovarian physiology, particularly in follicular development and ovulation. NO regulates ovarian blood flow, follicle maturation, and oocyte quality (58). In PCOS, reduced NO levels can impair these processes, leading to anovulation and the formation of ovarian cysts, hallmark features of the syndrome. Furthermore, the presence of hyperinsulinemia and hyperandrogenism in individuals with PCOS disturbs the microenvironment of the ovaries, leading to a decrease in the synthesis of NO and hindering the process of follicular angiogenesis.
This leads to poor follicular development and increased follicular atresia (58). Insulin resistance also reduces the bioavailability of NO in the ovaries, further impairing folliculogenesis (59). The ovarian dysfunction in PCOS is also linked to the dysregulation of steroidogenesis. NO modulates the production of steroid hormones by influencing the expression of key enzymes involved in steroid biosynthesis. Reduced NO levels lead to an imbalance in androgen and estrogen production, contributing to the hyperandrogenic state seen in PCOS (60).
Additionally, the dysregulation of the NO-cGMP pathway in the ovaries of PCOS patients contributes to impaired ovulation. NO activates guanylate cyclase, increasing cGMP levels crucial for follicular expansion and oocyte maturation. Reduced NO levels disrupt this signaling pathway, leading to ovulatory dysfunction (61).
4.7. Clinical implications and future directions
The significant association between reduced NO levels and PCOS suggests that NO could serve as a valuable biomarker for the early diagnosis and management of PCOS. Monitoring NO levels could help identify individuals at risk of developing PCOS-related complications, such as CVD and insulin resistance.
Understanding the mechanistic role of NO in PCOS could open new avenues for therapeutic strategies aimed at modulating NO levels to mitigate disease severity. For instance, interventions that enhance NO production, such as lifestyle modifications (e.g., exercise and diet) or pharmacological agents (e.g., L-arginine supplementation), could improve endothelial function and insulin sensitivity in PCOS patients.
Future research should focus on longitudinal studies to track NO levels over time in women with PCOS, from initial diagnosis through treatment and disease progression. Such studies could clarify the temporal dynamics of NO expression and its prognostic utility. Additionally, interventional studies examining the effects of therapies targeting NO pathways could provide insights into their potential benefits in managing PCOS.
4.8. Strengths and limitations
This study boasts several strengths, including a rigorous search methodology, a substantial sample size, and consistent findings across multiple studies. The use of subgroup analyses and meta-regression techniques has enhanced the interpretation of results, adding depth to our understanding of the relationship between NO levels and PCOS. However, several limitations warrant acknowledgment. The variability in detection methods across included studies may introduce discrepancies in reported NO levels. Despite efforts to standardize our analysis, differences in assay sensitivity and specificity could influence the consistency of results. Additionally, the predominance of English-language articles in the search could mask relevant data in non-English publications, potentially introducing language bias.
The heterogeneity in study designs and data collection methods across studies adds another layer of complexity to data interpretation. Variations in participant lifestyles, such as diet, physical activity, and smoking status, could influence NO levels and were not uniformly accounted for in all studies. Furthermore, the evolving landscape of PCOS diagnostic criteria and treatment modalities poses additional challenges in standardizing findings.
5. Conclusion
This meta-analysis reviewed data from 14 studies and 1171 participants, highlighting the potential of NO levels as a biomarker for PCOS and suggesting an association between NO dysregulation and PCOS pathophysiology. Our findings underscore the pertinence of NO levels as a potential biomarker for PCOS, suggesting an interplay between NO dysregulation and the pathophysiology of PCOS. Significantly, our research adds a critical dimension to the clinical understanding of PCOS, aligning with the hypothesis that NO could serve not only as a marker of disease presence but also as an indicator of its severity. This relationship holds promising outcomes for developing more precise diagnostic criteria and improving the therapeutic strategies tailored to individual NO profiles. It also proposes a new vista for clinicians to prognosticate the progression and mitigate the risks associated with PCOS by monitoring NO levels. Future research should aim at longitudinal studies with larger cohorts and standardized methodologies for assessing NO levels, to advance beyond correlation toward a clearer understanding of causation.
Data Availability
Data supporting the findings of this study are available upon reasonable request from the corresponding author.
Author Contributions
SS. Bahreiny and A. Ahangarpour contributed to data analysis, manuscript preparation, and supervision, while R. Mohammadpour Fard, M. Aghaei, and A. Ahangarpour contributed to manuscript searching, preparation, and data analysis. E. Harooni and M. Amraei contributed to the search strategy, article searching, and manuscript preparation. The manuscript has been reviewed and approved by all authors.
Acknowledgments
The authors of this research would like to express their sincere gratitude to the esteemed researchers whose scholarly articles were utilized during this study. Your invaluable contributions have been instrumental in shaping the outcome of this research, and we are deeply appreciative of your efforts. Additionally, we acknowledge the use of artificial intelligence tools for revision (OpenAI, ChatGPT-4, version 4.0), and grammar checks, which assisted in refining the manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Endocrinology
References
1. Dennett CC, Simon J. The role of polycystic ovary syndrome in reproductive and metabolic health: Overview and approaches for treatment. Diabetes Spectr 2015; 28: 116-120. [DOI:10.2337/diaspect.28.2.116] [PMID] [PMCID]
2. Escobar-Morreale HF. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018; 14: 270-284. [DOI:10.1038/nrendo.2018.24] [PMID]
3. Abbott DH, Kraynak M, Dumesic DA, Levine JE. In utero androgen excess: A developmental commonality preceding polycystic ovary syndrome? Front Horm Res 2019; 53: 1-17. [DOI:10.1159/000494899] [PMID] [PMCID]
4. Bahreiny SS, Ahangarpour A, Amraei M, Mansouri Z, Pirsadeghi A, Kazemzadeh R, et al. Autoimmune thyroid disorders and polycystic ovary syndrome: Tracing links through systematic review and meta-analysis. J Reprod Immunol 2024; 163: 104215. [DOI:10.1016/j.jri.2024.104215] [PMID]
5. Hudecova M, Holte J, Olovsson M, Larsson A, Berne C, Sundstrom-Poromaa I. Prevalence of the metabolic syndrome in women with a previous diagnosis of polycystic ovary syndrome: Long-term follow-up. Fertil Steril 2011; 96: 1271-1274. [DOI:10.1016/j.fertnstert.2011.08.006] [PMID]
6. De Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum Reprod Update 2011; 17: 495-500. [DOI:10.1093/humupd/dmr001] [PMID]
7. Mancini A, Bruno C, Vergani E, d'Abate C, Giacchi E, Silvestrini A. Oxidative stress and low-grade inflammation in polycystic ovary syndrome: Controversies and new insights. Int J Mol Sci 2021; 22: 1667. [DOI:10.3390/ijms22041667] [PMID] [PMCID]
8. Mizgier M, Jarząbek-Bielecka G, Wendland N, Jodłowska-Siewert E, Nowicki M, Brożek A, et al. Relation between inflammation, oxidative stress, and macronutrient intakes in normal and excessive body weight adolescent girls with clinical features of polycystic ovary syndrome. Nutrients 2021; 13: 896. [DOI:10.3390/nu13030896] [PMID] [PMCID]
9. Stichtenoth DO, Frölich JC. Nitric oxide and inflammatory joint diseases. Br J Rheumatol 1998; 37: 246-257. [DOI:10.1093/rheumatology/37.3.246] [PMID]
10. Somasundaram V, Basudhar D, Bharadwaj G, No JH, Ridnour LA, Cheng RY, et al. Molecular mechanisms of nitric oxide in cancer progression, signal transduction, and metabolism. Antioxid Redox Signal 2019; 30: 1124-1143. [DOI:10.1089/ars.2018.7527] [PMID] [PMCID]
11. Stadler K. Peroxynitrite-driven mechanisms in diabetes and insulin resistance-the latest advances. Curr Med Chem 2011; 18: 280-290. [DOI:10.2174/092986711794088317] [PMID] [PMCID]
12. Rai N, Dey S. Protective response of sestrin under stressful conditions in aging. Ageing Res Rev 2020; 64: 101186. [DOI:10.1016/j.arr.2020.101186] [PMID]
13. Sun W, Wang Y, Zheng Y, Quan N. The emerging role of sestrin2 in cell metabolism, and cardiovascular and age-related diseases. Aging Dis 2020; 11: 154-163. [DOI:10.14336/AD.2019.0320] [PMID] [PMCID]
14. Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep 2017; 19: 42. [DOI:10.1007/s11883-017-0678-6] [PMID]
15. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid Med Cell Longev 2019; 2019: 7092151. [DOI:10.1155/2019/7092151] [PMID] [PMCID]
16. Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol 2019; 34: 975-991. [DOI:10.1007/s00467-018-4005-4] [PMID]
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI:10.1136/bmj.n71] [PMID] [PMCID]
18. Bahreiny SS, Ahangarpour A, Saki N, Dabbagh MR, Ebrahimi R, Mahdizade AH, et al. Association of free radical product and polycystic ovary syndrome: A systematic review and meta-analysis. Reprod Sci 2024; 31: 1486-1495. [DOI:10.1007/s43032-023-01447-x] [PMID]
19. Bahreiny SS, Aghaei M, Dabbagh MR, Ghorbani H, Javidan M, Fard RM. Exploring the relationship between ambient sulfur dioxide and semen quality parameters: A systematic review and meta-analysis. Asian Pacific J Reprod 2024; 13: 12-21. [DOI:10.4103/apjr.apjr_95_23]
20. Bahreiny SS, Bastani M-N, Aghaei M, Dabbagh MR, Mahdizade AH. Circulating galectin-3 levels in women with polycystic ovary syndrome: A meta-analysis. Taiwan J Obstet Gynecol 2024; 63: 37-45. [DOI:10.1016/j.tjog.2023.10.003] [PMID]
21. Bahreiny SS, Bastani M-N, Dabbagh MR, Ghorbani H, Aghaei M, Zahedian M, et al. Association between ambient particulate matter and semen quality parameters: A systematic review and meta-analysis. Middle East Fertil Soc J 2024; 29: 2. [DOI:10.1186/s43043-023-00162-6]
22. Lakshmi K, Malini S. Relationship between serum levels of oxidative stress markers and metabolic syndrome components in PCOS women. J Endocrinol Reprod 2023; 27: 29-39. [DOI:10.18311/jer/2023/33508]
23. Yeşil F, Deveci K, Karli P. Evaluation of the clinical significance of serum pentraxin-3 levels in patients with polycystic ovary syndrome. Clin Exp Obstet Gynecol 2022; 49: 38. [DOI:10.31083/j.ceog4902038]
24. Kohzadi M, Khazaei MR, Choobsaz F, Khazaei M. Relationship between serum levels of anti-mullerian hormone, adiponectin and oxidative stress markers in patients with polycystic ovary syndrome. Int J Fertil Steril 2020; 14: 27-33.
25. Krishna MB, Joseph A, Thomas PL, Dsilva B, Pillai SM, Laloraya M. Impaired arginine metabolism coupled to a defective redox conduit contributes to low plasma nitric oxide in polycystic ovary syndrome. Cell Physiol Biochem 2017; 43: 1880-1892. [DOI:10.1159/000484107] [PMID]
26. Kocer D, Bayram F, Diri H. The effects of metformin on endothelial dysfunction, lipid metabolism and oxidative stress in women with polycystic ovary syndrome. Gynecol Endocrinol 2014; 30: 367-371. [DOI:10.3109/09513590.2014.887063] [PMID]
27. Yavuz Taşlipinar M, Kiliç N, Bayraktar N, Güler İ, Gülcan Kurt Y, Göktaş T, et al. Endothelial dysfunction and insulin resistance in young women with polycystic ovarian syndrome. Turk J Med Sci 2014; 44: 787-791. [DOI:10.3906/sag-1401-4] [PMID]
28. Willis GR, Udiawar M, Evans WD, Blundell HL, James PE, Rees DA. Detailed characterisation of circulatory nitric oxide and free radical indices-is there evidence for abnormal cardiovascular homeostasis in young women with polycystic ovary syndrome? BJOG 2014; 121: 1596-1603. [DOI:10.1111/1471-0528.12834] [PMID]
29. Su Y. Determination and clinical application of serum NO, SOD, and MDA in patients with polycystic ovary syndrome [M.Sc. thesis]. China: Dali University; 2012.
30. Karadeniz M, Erdoğan M, Ayhan Z, Yalcin M, Olukman M, Cetinkalp S, et al. Effect of G2706A and G1051A polymorphisms of the ABCA1 gene on the lipid, oxidative stress and homocystein levels in Turkish patients with polycystıc ovary syndrome. Lipids Health Dis 2011; 10: 193. [DOI:10.1186/1476-511X-10-193] [PMID] [PMCID]
31. Bayram F, Kocer D, Ozsan M, Muhtaroglu S. Evaluation of endothelial dysfunction, lipid metabolism in women with polycystic ovary syndrome: Relationship of paraoxonase 1 activity, malondialdehyde levels, low-density lipoprotein subfractions, and endothelial dysfunction. Gynecol Endocrinol 2012; 28: 497-501. [DOI:10.3109/09513590.2011.569607] [PMID]
32. Baskol G, Aygen E, Erdem F, Caniklioğlu A, Narin F, Sahin Y, et al. Assessment of paraoxonase 1, xanthine oxidase and glutathione peroxidase activities, nitric oxide and thiol levels in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 2012; 91: 326-330. [DOI:10.1111/j.1600-0412.2011.01337.x] [PMID]
33. Türkçüoğlu I, Engin-Üstün Y, Turan F, Kali Z, Karabulut AB, Meydanli M, et al. Evaluation of asymmetric dimethylarginine, nitric oxide levels and associated independent variables in obese and lean patients with polycystic ovarian syndrome. Gynecol Endocrinol 2011; 27: 609-614. [DOI:10.3109/09513590.2010.507291] [PMID]
34. Erdogan M, Karadeniz M, Berdeli A, Alper G, Caglayan O, Yilmaz C. The relationship of the interleukin-6-174 G> C gene polymorphism with oxidative stress markers in Turkish polycystic ovary syndrome patients. J Endocrinol Invest 2008; 31: 624-629. [DOI:10.1007/BF03345614] [PMID]
35. Guan Y, Yang X, Ren F. Clinical study on the serum nitric oxide and related factors in women with PCOS. Chinese J Clin Obstet Gynecol 2008; 9: 217-219.
36. Bahreiny SS, Dabbagh MR, Ebrahimi R, Harooni E. [Prevalence of autoimmune thyroiditis in women with polycystic ovary syndrome: A systematic review and meta-analysis]. Iran J Obstet Gynecol Infertil 2023; 26: 94-106. (in Persian)
37. Bahreiny SS, Ahangarpour A, Hemmati AA, Kazemzadeh R, Bastani M-N, Dabbagh MR, et al. Circulating nesfatin-1 levels in women with polycystic ovary syndrome: A systematic review and meta-analysis. Int J Reprod BioMed 2023; 21: 777-788. [DOI:10.18502/ijrm.v21i10.14533] [PMID] [PMCID]
38. Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li PL, et al. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci 2018; 19: 2605. [DOI:10.3390/ijms19092605] [PMID] [PMCID]
39. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007; 115: 1285-1295. [DOI:10.1161/CIRCULATIONAHA.106.652859] [PMID]
40. Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 2001; 103: 1410-1415. [DOI:10.1161/01.CIR.103.10.1410] [PMID]
41. Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev 1997; 18: 774-800.
https://doi.org/10.1210/edrv.18.6.0318 [DOI:10.1210/er.18.6.774] [PMID]
42. Mahdizade AH, Bahreiny SS, Bastani M-N, Dabbagh MR, Aghaei M, Ali Malayeri F, et al. The influence of CDKAL1 (rs7754840) gene polymorphism on susceptibility to gestational diabetes mellitus in pregnant women: A systematic review and meta-analysis. Int J Diabetes Develop Countries 2024; 44: 3-12. [DOI:10.1007/s13410-023-01272-7]
43. Mather KJ, Steinberg HO, Baron AD. Insulin resistance in the vasculature. J Clin Invest 2013; 123: 1003-1004. [DOI:10.1172/JCI67166] [PMID] [PMCID]
44. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr Rev 2012; 33: 981-1030. [DOI:10.1210/er.2011-1034] [PMID] [PMCID]
45. Yildirim B, Sabir N, Kaleli B. Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril 2003; 79: 1358-1364. [DOI:10.1016/S0015-0282(03)00265-6] [PMID]
46. Mohammadpour Fard R, Rashno M, Bahreiny SS. Effects of melatonin supplementation on markers of inflammation and oxidative stress in patients with diabetes: A systematic review and meta-analysis of randomized controlled trials. Clinl Nutr ESPEN 2024; 63: 530-539. [DOI:10.1016/j.clnesp.2024.07.015] [PMID]
47. González F. Inflammation in polycystic ovary syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids 2012; 77: 300-305. [DOI:10.1016/j.steroids.2011.12.003] [PMID] [PMCID]
48. Macut D, Bjekić-Macut J, Savić-Radojević A. Dyslipidemia and oxidative stress in PCOS. Front Horm Res 2013; 40: 51-63. [DOI:10.1159/000341683] [PMID]
49. Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocrine Rev 2007; 28: 463-491. [DOI:10.1210/er.2007-0006] [PMID]
50. Orio Jr F, Palomba S, Cascella T, Milan G, Mioni R, Pagano C, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2003; 88: 2619-2623. [DOI:10.1210/jc.2002-022033] [PMID]
51. Sydow K, Münzel T. ADMA and oxidative stress. Atheroscler Suppl 2003; 4: 41-51. [DOI:10.1016/S1567-5688(03)00033-3] [PMID]
52. Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, et al. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 2002; 277: 1794-1799. [DOI:10.1074/jbc.M103728200] [PMID]
53. Etgen Jr GJ, Fryburg DA, Gibbs EM. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/ contraction- and phosphatidylinositol-3-kinase- independent pathway. Diabetes 1997; 46: 1915-1919.
https://doi.org/10.2337/diab.46.11.1915 [DOI:10.2337/diabetes.46.11.1915] [PMID]
54. Bahreiny SS, Ahangarpour A, Aghaei M. Circulating levels of advanced glycation end products in females with polycystic ovary syndrome: A meta-analysis. Reprod Dev Med 2024; 8: 93-100. [DOI:10.1097/RD9.0000000000000089]
55. Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab 2003; 88: 1055-1058. [DOI:10.1210/jc.2002-021437] [PMID]
56. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007; 87: 507-520. [DOI:10.1152/physrev.00024.2006] [PMID] [PMCID]
57. Aghaei M, Khademi R, Far MAJ, Bahreiny SS, Mahdizade AH, Amirrajab N. Genetic variants of dectin-1 and their antifungal immunity impact in hematologic malignancies: A comprehensive systematic review. Curr Res Transl Med 2024; 72: 103460. [DOI:10.1016/j.retram.2024.103460] [PMID]
58. Luo Y, Zhu Y, Basang W, Wang X, Li C, Zhou X. Roles of nitric oxide in the regulation of reproduction: A review. Front Endocrinol 2021; 12: 752410. [DOI:10.3389/fendo.2021.752410] [PMID] [PMCID]
59. Zhao H, Zhang J, Cheng X, Nie X, He B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J Ovarian Res 2023; 16: 9. [DOI:10.1186/s13048-022-01091-0] [PMID] [PMCID]
60. Meinhardt UJ, Ho KK. Modulation of growth hormone action by sex steroids. Clin Endocrinol 2006; 65: 413-422. [DOI:10.1111/j.1365-2265.2006.02676.x] [PMID]
61. Tewari D, Sah AN, Bawari S, Nabavi SF, Dehpour AR, Shirooie S, et al. Role of nitric oxide in neurodegeneration: Function, regulation, and inhibition. Curr Neuropharmacol 2021; 19: 114-126.
https://doi.org/10.2174/1570159XMTA2qMjAky
https://doi.org/10.2174/1570159X18666200429001549 [DOI:10.2174/18756190MTA2pMjApy] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |