Wed, Jan 28, 2026
[Archive]
Volume 23, Issue 8 (August 2025)
IJRM 2025, 23(8): 595-612 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Etezadi A, Aleyasin A, Heidar Z, Shirkosh P, Hazari V. The evaluation role of T helper 17 cells in pregnancy and abortion: A molecular and future perspective: Narrative review. IJRM 2025; 23 (8) :595-612
URL: http://ijrm.ir/article-1-3383-en.html
URL: http://ijrm.ir/article-1-3383-en.html
1- Reproductive Health Research Center, Department of Obstetrics and Gynecology, Alzahra Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Obstetrics and Gynecology, School of Medicine, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Obstetrics and Gynecology, Mahdieh Hospital, Medical Faculty, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Ahvaz Jundishapur University of Medical Science, Ahvaz, Iran.
5- Department of Obstetrics and Gynecology, School of Medicine, Rooyesh Infertility Center, Birjand University of Medical Sciences, Birjand, Iran. & Clinical Research Development Unit (CRDU), Valiasr Hospital, Birjand University of Medical Sciences, Birjand, Iran. ,dr.vhazari@gmail.com; vajihehazari@bums.ac.ir
2- Department of Obstetrics and Gynecology, School of Medicine, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Obstetrics and Gynecology, Mahdieh Hospital, Medical Faculty, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Ahvaz Jundishapur University of Medical Science, Ahvaz, Iran.
5- Department of Obstetrics and Gynecology, School of Medicine, Rooyesh Infertility Center, Birjand University of Medical Sciences, Birjand, Iran. & Clinical Research Development Unit (CRDU), Valiasr Hospital, Birjand University of Medical Sciences, Birjand, Iran. ,
Full-Text [PDF 628 kb]
(1121 Downloads)
| Abstract (HTML) (634 Views)
Full-Text: (107 Views)
1. Introduction
Abortion is a condition characterized by the loss of the fetus before the 20th wk of pregnancy (1, 2). Research has shown that unwanted pregnancy can be one of the main causes of abortion in patients. Therefore, its increase can lead to the spread of abortion among pregnant women (3). The proportion of miscarriages varies and depends on several aspects, such as age, health status, and lifestyle. According to the Centers for Disease Control and Prevention, approximately 31-50% of pregnancies lead to a miscarriage by 13 wk (4).
It has been shown that successful gestation needs to be balanced between anti- and pro-inflammatory immune cells (5). Hence, an essential cause of poor pregnancy outcomes may be an aberration in the immune system (6-8). During early pregnancy, the mother and fetus engage in bilateral communication that involves a complex series of immunologic events. These events are orchestrated through diverse cells, including decidual macrophages, decidual dendritic cells (DCs), T cells, and innate lymphocytes (ILC). These cells are essential for establishing a balance between the immune tolerance of the fetus from a different genetic origin and the preservation of the host’s protection against potential infections (5, 9).
T helper (Th)17 cells are known to generate defensive immunity against extracellular microorganisms during gestation (7). However, existing evidence indicates that Th17 cell response imbalance can lead to some problems, such as recurrent spontaneous abortion (RSA) (10, 11). Abnormal inflammation, trophoblast invasion into the uterine spiral arteries, and insufficient tolerance are associated with increased peripheral blood Th17 cells (12).
Th17 cells are characterized through interleukin (IL)17 and IL-22 production as signature cytokines. These cells also produce tumour necrosis factor (TNF), IL-21, granulocyte macrophage-colony stimulating factor, and interferon (IFN)γ and can express Chemokine receptors (CCR) 6. Accordingly, they guide Th17 cells to the epithelial barrier locations by binding to chemokine ligand (CCL)20 (13, 14).
The IL-17 family consists of 6 ligands, IL-17A to IL-17F, with similar structures in homo or heterodimer forms, such as IL17A/F. IL-17A and IL-17F, among others, are produced by Th17 cells. Their function depends on the presence of their heterodimer receptor-containing subunits IL-17RA and IL-17RC (15).
IL-17-producing cells can gather at mucosal surfaces to provide an immune response against extracellular pathogens by keeping barrier integrity, boosting antimicrobial factors, and driving granulopoiesis (16, 17). For example, IL-17 interaction with IL-17RA receptors on the keratinocyte cells promotes epidermal cells to increase expression of antimicrobial peptides, IL-8 (CXCL8), IL-6, granulocyte colony-stimulating factor, and CCL20 (18-21).
Research on this subset will advance our understanding of Th17 functions in maternal-fetal junction and its association with abortion. This article offers a wide range of overviews of the existing condition of knowledge on the Th17 cells’ functions in pregnancy, with a particular focus on their interactions with other immune cells and their impact on abortion.
2. The immune system and pregnancy
2.1. Maternal and fetus immunity
The immune system is vital in pregnancy control and fetal development in pregnant women (22). Despite the direct contact between fetal and maternal cells, maternal immune cells are not stimulated against the surface antigens of fetal cells (23). Changes in the expression of some genes, including immune cells, lead to the control of immune mediators by them. Consequently, gathering T cells is limited within the decidua and does not attack a developing fetus and placenta (24). A recent study on human decidual stromal cells has shown that these cells inhibit the expression of chemokines CXCL9, CXCL10, and CXCL11 binding to expressed CXCR3 on CD4+ Th1 and CD8+ T cells to prevent the arrival of T cells in the decidua (25, 26).
Improper management of this procedure may trigger inflammation and the requirement of immune cells at the maternal-fetal interface. In addition, pregnancy can raise the risk of having certain infections and can also make normally mild infections more severe (22). In this respect, trophoblast cells are specialized fetal cells and originate from a blastocyst that regulates the immune system around the implantation place to provide an immune response against outside stimulations (27). These cells boost the employment of peripheral natural killer cells (NKs), neutrophils, and monocytes to the implantation site through cytokine production of transforming growth factor (TGF) and chemokine ligands CCL2, CXCL12, and CXCL8 (28). Moreover, trophoblast cells have an active role in the recruitment and differentiation of T regulatory cells (Tregs) (CD4+ Foxp3+ cells) from naive CD4+ cells through secreting high levels of TGFβ (29-31). Also, trophoblast cells express innate immune sensors, including NOD-like receptors and Toll-like receptors, permitting them to respond against tissue injury or microbial invasion. The decidual NKs (dNK), which produce IL-15 and TGFβ12, are responsible for the decidual vascular remodeling and placental function (27). CD14-positive monocytes around the maternal-fetal interface have an M2-like macrophage phenotype (28, 32) that ca n cause tissue remodeling and degradation of apoptotic cells (33) by secreting TGFβ and type-I IFN (34).
The fetus is susceptible to infections such as cytomegalovirus, rubella, human immunodeficiency virus, chickenpox, and hepatitis, particularly during the first and second trimesters of pregnancy (35). While the adaptive immune system is not yet fully matured at that stage, several mechanisms activating innate cells are the first line of protection against infections (36). For example, intrinsically expressed IFN-stimulated genes, including IFN-induced transmembrane protein (IFITM)-1 and IFITM3 on the embryonic stem cells, protect against viral infection by inhibiting the entry and replication of viruses (37, 38). IFITM3 is a restriction factor that can obstruct the RNA-virus-triggered IFN-I production by facilitating the autophagic degradation of IFN regulatory factor 3 (39). Previous studies have reported toll-like receptor expression on neonatal granulocytes, monocytes, and phagocytosis by neonatal macrophages, DCs, and NK cells, even though they provide lower immune responses than adults (40). Fetuses are more enriched with ILCs than NK cells compared with infants (41). Fetus-derived IFN-I can activate signal transducer and activator of transcription 1 (STAT1) and STAT2 along with JAK1 and TYK2 signaling, activating numerous IFN-stimulated-related genes and promoting the response to Zika virus infection (42).
3. Th17 cells
Following exposure to a microbial pathogen or foreign antigen, the innate immune system stimulates the CD4+ T cells to become Th cells that are basic managers of adaptive (acquired) immunity. Based on certain transcription factors and cytokine expression, naive CD4 T cells distinguish one of the Th subsets and induce Treg, as defined by the cytokines they secrete and/or their specific surface markers (43). The significant cytokine moderators of Th17 are TGFβ along with IL-1β, IL-6, IL-21, and/or IL-23 (44). TGFβ, in combination with IL-1β and IL-6, activates retinoid orphan receptor gamma t (RORγt) transcription factor via STAT3 signaling (45, 46). In a recent study, Fang et al. revealed that TGFβ/SMAD and IL-6/STAT3 signaling induce RORγt expression through Rorc-CNS-1.5 and Rorc-CNS+6.5 elements, during Th17 cell differentiation (47). IL-1β and TNF-α may also synergistically elevate IL-6 expression and, consequently, RORγt expression (48). TNF-α promotes the expansion of T cell subsets, producing IL-17 through both receptors TNFR1 or TNFR2 (49). Following Th17 cell differentiation, RORγt stimulates IL-17 and IL-23 receptor expressions (50). STAT3 can also upregulate the expression of IL-17 genes via its promoter binding, although this upregulation requires RORγt existence (51).
IL-17, in turn, motivates the expression of TNF-α, IL-6, and IL-1β by human macrophages and monocytes. Expressed IL-23 on the antigen-presenting cells binds to IL-23R, which maintains and expan7ds Th17 cells. Meanwhile, a positive feedback loop upregulates IL-17 and RORγt expression. Robust phosphorylation and dimerization of STAT3 following JAK-STAT axis activation by IL-6 and IL-23 enhances functions of RORγt (52). Since IL-23R is only expressed on differentiated Th17 cells, IL-23 alone cannot induce their development (43). Therefore, the role of IL-23 in the survival and maintenance of Th17 cells is more than initiating their differentiation (53). In another positive feedback loop, IL-6-induced STAT3 joins the promoter of IL-21, and then IL-21 induces RORγt and IL-17 expression synergistically with IL-6 and TGFβ (16, 54).
4. The role of Th17 cells in pregnancy
Pregnancy is the process of growth and development of the fetus in the womb. As a requirement for this process, the fetus should be resistant to maternal rejection and external environmental challenges. Since the uterine cavity is not fully sterile, Th17 cells expand within pregnancy to protect the maternal-fetal interface from microbial infections. In support of this idea, altered numbers of Th17 cells or Th17/Treg cells ratio in the maternal circulation or uterus can be a reason for pregnancy complications such as preterm birth, spontaneous abortion, and preeclampsia (55). Research has shown the correlation of Th17-cell activities, recruitment of neutrophils and monocytes with the maternal-fetal interface for clearance of pathogens, producing cytokines that promote antimicrobial peptide production, and B-cell differentiation into plasma cells, which produce antibodies against extracellular pathogens (56, 57). Th17 cells support implantation and provoke a defensive immune response against bacteria passing through inflammation induction (55). In addition, IL-17 is a regulatory cytokine that is vital for the preservation of prolonged periods of gestation (58). Conversely, to the high levels of peripheral IL-17 at late stages of healthy pregnancy, low levels were revealed immediately after natural abortion (59). In a study of 13 healthy pregnant women, IL-17 meaningfully increased from the 1st-3rd trimester, possibly as a member of the cytokines network due to implantation, embryo development, and the birth process itself (60).
On the one hand, the IL-17-granulocyte colony-stimulating factor axis promotes neutrophil development, while the IL-17-CXCR2 axis facilitates the recruitment of neutrophils. In contrast, IL-17 increases progesterone secretion, which is essential for maintaining neutrophils during pregnancy (61). Neutrophils drive maternal tolerance in progesterone hormone exposure by inducing a distinct sub-population of CD4+ T cells, generating IL-10, IL-17, and vascular endothelial growth factor (VEGF), promoting vessel development (62). Neutrophil reduction during mouse pregnancy leads to abnormal development of the fetal-maternal unit and reduced fetal growth (46, 62, 63). Neutrophil-induced induction of T cells and their regulatory function occurs through the transfer of forkhead box protein 1 (FOXO1) to T cells. The lack of FOXO1 transfer from neutrophils to T cells prevents the activation of induced T cells, as shown by the blood and placental neutrophils of women with preeclampsia. Furthermore, reducing FOXO1 expression only in neutrophils causes poor placentation and impaired fetal growth, mimicking the effects of neutrophil depletion (62). It has been reported that neutrophil depletion led to an impairment of the junctional zone development and reduction in the number of placenta cells expressing VEGF, intercellular cell adhesion molecule, and platelet-endothelial cell adhesion molecule, which are essential for adhesiveness and angiogenesis (64).
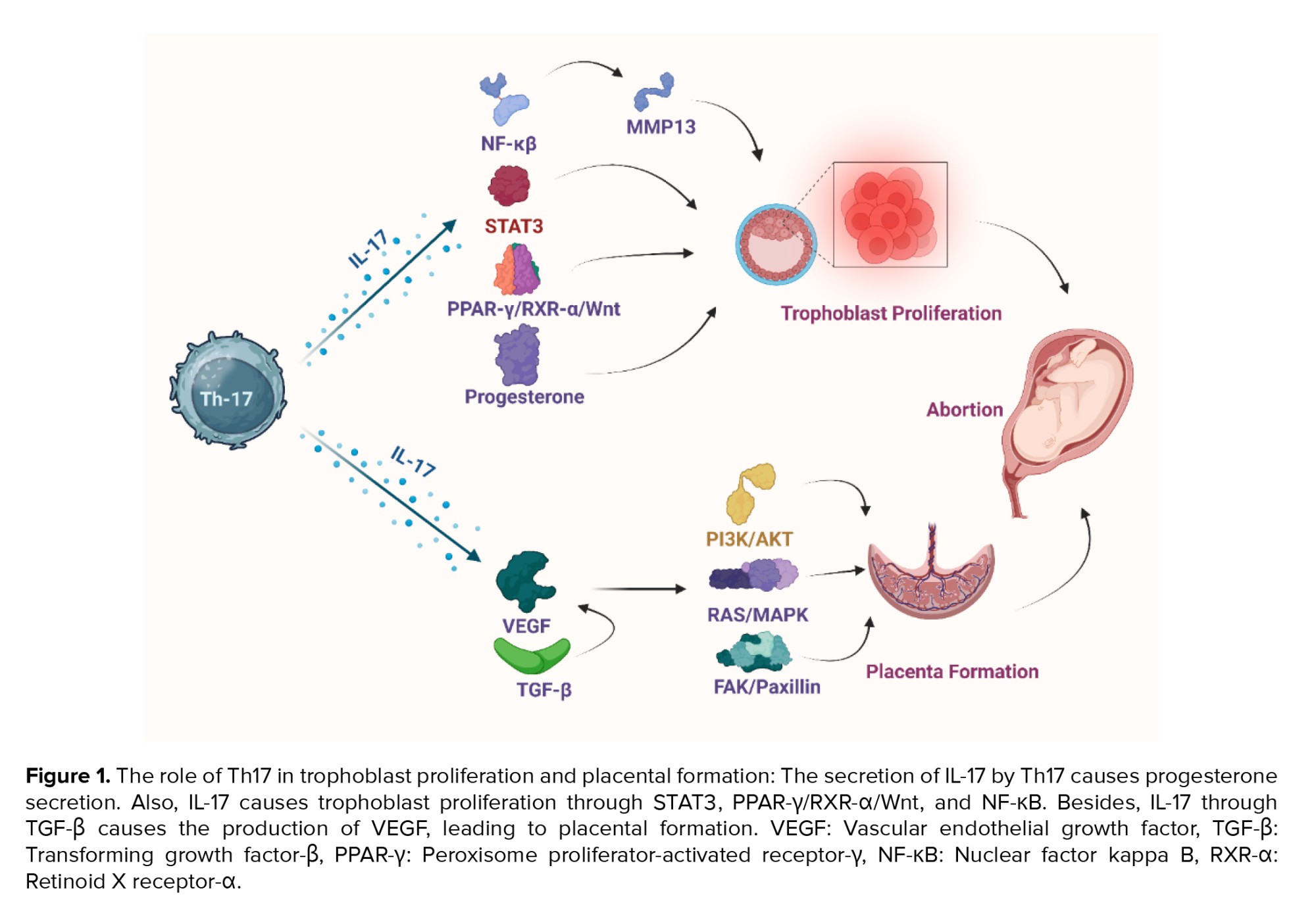
Nevertheless, Th17 cells may play a dual role in pregnancy: they can be protective or harmful depending on the context. Several studies link Th17 cells to allograft rejection and state that they contribute to adverse pregnancy outcomes, such as RSA and preeclampsia (10, 65, 66). In animal models, exogenous IL-17 can cause increased rates of abortion (67). In human studies, women with RSA have higher levels of Th17 cells and their related cytokines (e.g., IL-17 and IL-23) in both their blood and decidual tissue (68). Similarly, in patients with preeclampsia, Th17 cells and IL-6 levels are elevated, disrupting the balance between decidual Th17 and Tregs, and creating a pro-inflammatory environment (10, 65, 66, 69). The underlying mechanisms of Th17 activation in pregnancy may involve defective IL-27 signaling. IL-27 expression is reduced in the decidua of women with unexplained recurrent miscarriage compared to women with spontaneous miscarriage or normal pregnancy (70). Another possible factor is the high mobility group protein B1, a cytokine that promotes inflammation and is released by innate immune cells. High mobility group protein B1 may signal excessive Th17 responses, leading to excessive neutrophil infiltration at the maternal-fetal interface and potentially harmful pregnancy outcomes (55, 71). Additionally, the number of Th17 cells in the chorioamniotic membrane of cases with preterm labor was elevated. Amniotic fluid IL-17 upregulates TNF-α-induced IL-8 secretion from amniotic mesenchymal cells through IKK and MAPK signaling pathways (72, 73). The results of a study on mice demonstrated that Th17 and IL-17a are associated with an abnormal cortical phenotype in the fetal brain (74). Conversely, Th17, through the production of cytokines, causes the differentiation of macrophages, dendritic cells, monocytes, and other immune cells. The differentiation of these cells during inflammation can lead to spontaneous miscarriage, whereas cytokines can cause the secretion of progesterone. Progesterone stimulates Th17 and increase the differentiation of cells. Th17 also controls immune system modulator cells, including Treg. The balance between Treg cells and other T cells can play an important role in spontaneous miscarriage. This balance is maintained by cytokines produced by Th17 (75). These results indicate that Th17 cells can have a photogenic role at the feto-maternal interface in preterm delivery (Figure 1).
5. The role of Th17 cells in decidua
Decidua is the modified mucosal lining of the uterus that forms the maternal part of the placenta under the influence of progesterone and remains for the duration of the pregnancy. First, the ovary’s corpus luteum and, later, the placenta produce progesterone-inducing vascular alterations and cellular modifications within endometrial stromal cells, called the decidual reaction (76). The cellular composition of the decidua includes fibroblast-transformed stromal cells, glands, and immune cells, mostly ILCs, NKs, macrophages, Tregs, and DCs (77-79). Besides, it has already been revealed that decidual stromal cells drive Th17 cells from peripheral blood into the decidua while secreting high levels of CCL2 (80), known as monocytic chemotactic protein 1 (MCP-1), which binds to CCR2 expressed on Th17 cells (81, 82). Th17 cells in decidua express αβTCR, CD45RO, CCR7, and CD161 with effector memory phenotype (80). Th17 cells are attracted via the C-type lectin domain family (83), and CD161 is a C-type lectin-like receptor (84). Therefore, this molecule also has a role in the maternal/fetal interface by facilitating the movement of Th17 cells across the endothelium and influencing their selection in the decidua. These recruited Th17 cells can prevent human trophoblast cell apoptosis by producing IL-17 in the primary trimester of pregnancy (80). IL-17 activates the Wnt pathway through the peroxisome proliferator-activated receptor (PPAR)-γ/retinoid X receptor (RXR)-α heterodimer, thereby inhibiting the apoptosis of trophoblast cells (85).
As described earlier, IL-17 enhances hormone progesterone secretion (61), which functions fundamentally in decidua development and maintenance (86). It helps prepare the uterus for implanting the fertilized egg. It also promotes the growth of blood vessels in the endometrium, which helps supply nutrients to the developing embryo (87). Progesterone also inhibits the contraction of the uterus, contributing to the prevention of premature labor (88). It also plays a role in thickening the cervical mucus and creating a barrier to prevent bacteria from entering the uterus (89).
Another population of decidual T cells are named Th17/Th2/Th22 cells, producing IL-4, IL-22, and IL-17 during successful pregnancies. The embryo implantation site is the exclusive location of these cells and Th17/Th0/IL-22+ cells, where the mRNA expression levels of IL-4, GATA-3, IL-17A, ROR-C, IL-22, and AHR are amplified (90, 91). In patients with recurrent abortion, IFN-γ producing CD4+ T cells are predominant. These cells do not secrete IL-4 and are recognized as Th17/Th1/Th22+ cells (91). However, some studies reported an elevated frequency of Th17 cells in the decidua of pregnant women with spontaneous abortion or preeclampsia relative to their non-pregnant or healthy pregnant counterparts. This information suggests that excessive Th17 cells in decidua might adversely affect gestation (11, 92, 93). Chronic infusion of Th17 can lead to embryo resorption (94) because of the impaired vascular reactivity of uterine arteries following the increased number and activation of dNK cells (95). Th17-expressed macrophage inflammatory protein-3 alpha (MIP‐3α), a chemoattractant for NKs, could describe the expanded attendance of NK cells in the placentas (96). The main secretors of cytokines that attract angiogenesis and trophoblasts (e.g., IL-8, VEGF, and IL-10) are dNK cells with slight cytolytic activity (97). They are important in increasing tolerance to cytotrophoblasts and maternal vascular regeneration in myometrium and endometrium (98-100). The remodeling of maternal vessels by cytotrophoblasts is essential for establishing a functional uteroplacental circulation, which is necessary to deliver nutrients and oxygen to the developing fetus (101). As NKs express the IL‐17 RC receptor, IL‐17 can stimulate both their expansion and secretion of cytolytic enzymes, such as granzymes A, B, and K. These enzymes are responsible for the increased placental reactive oxygen species (ROS) in women with pre-eclampsia (95, 102). As previously mentioned, IL-17 directly recruits neutrophils and increases their activity, boosting oxidative stress (103, 104). In addition, it increases cytolytic granzymes, resulting in significant ROS production when it degrades mitochondrial proteins (105-108). Notably, dNK cells produce less VEGF following increased IL-17 levels in the placenta, which may lead to endothelial-dependent impaired uterine vasorelaxation (95).
Regarding the mentioned points, a dynamic balance of Th17 cells is required during pregnancy, and Treg cells play a central part in maintaining this balance (109). Compared to controls, women with unexplained recurrent pregnancy loss exhibited elevated levels of Th17 cells and IL-17A in peripheral blood throughout the ovulatory cycle, especially during the proliferative and secretory phases. Conversely, Treg cells and their related cytokines (i.e., IL-10 and TGFβ1) were reduced in the proliferative phase in women with unexplained recurrent pregnancy loss (11). Although the Th17 population is normal in women with preeclampsia, the Treg population is decreased, leading to an altered Th17/Treg ratio (110).
It has been reported that the population of CD4 + CD25 + and CD4 + CD25bright cells augments during pregnancy (111, 112). Also, the sustained, not transient, expression of Foxp3 in Treg cells enables them to suppress the immune response (113). Treg can participate in the regulation of Th17 cells via suppressing T cell differentiation, activation, and moderating molecular secretion patterns through the programmed death 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) axis (114). PD-1 and PD-L1 are the 2 leading immune checkpoint pathways that modulate immune responses by inhibiting PI3K/AKT and Ras-MEK-ERK signaling pathways (115, 116). In mice, blocking the PD-1/PD-L1 axis decreased Treg, leading to pregnancy loss by inducing Th17 and Th1 cell differentiation (117-119). PD-L1 expressions on Th17 cells were significantly downregulated in women with recurrent pregnancy loss (RPL), although they had similar proportions of Tregs to those of controls (120). Based on these findings, a possible cause of RPL is the reduced expression of PD-1, which can lead to an imbalance between Th17 and Treg. These cells are involved in Th17 immunity, which may affect pregnancy outcomes depending on how well they are balanced.
Moreover, progesterone administration via the vaginal route is one of the accepted strategies to lower the risk of preterm birth (121). According to Areia et al. the membrane progesterone receptor α is expressed by Treg in healthy pregnant women (122). This data suggests that progesterone’s protective effect on preterm birth conditions may be due to the activation of Treg cell proliferation; besides, progesterone considerably augments the PD-1 expression in Treg (123). Therefore, it seems that Th17-produced IL-17 creates a progesterone-rich environment that prevents them from over-activating in a negative feedback mechanism while activating Treg cell proliferation (Figure 1).
6. The role of Th17 cells in trophoblast cell invasion
Trophoblast cells are specialized cells that form the outer layer of the blastocyst, which later develops into the placenta (124). During pregnancy, trophoblast cells migrate and invade the decidua (i.e., the thickened endometrium of the uterus) to establish a connection between the fetal and maternal circulatory systems (125). The placenta needs this process to connect to the mother physically and change the uterine blood vessels to supply enough blood to the developing fetus during pregnancy (126). The exact mechanisms of trophoblast migration and invasion are not fully understood. However, studies suggest that this mechanism involves interacting with various cellular and molecular players, including decorin, a small leucine-rich proteoglycan produced by stromal cells (127).
During normal placenta development, cytokines and adhesion molecules are some factors that regulate trophoblasts’ growth and spread. By producing IL-17, Th17 cells contribute to trophoblasts’ survival, invasion, and expansion with IL-17 receptors in the first trimester of human pregnancy (80). Moreover, IL-17 increased the production of MMP-13 via the NF-kB pathway and led to the degradation of the extracellular matrix. Placental trophoblast also secretes matrix metalloproteinases (MMPs), which may play a role in trophoblast invasion mediated by IL-17. However, abnormal MMP expression can cause pregnancy complications, such as fetal intrauterine growth restriction and pre-eclampsia (128, 129).
Furthermore, crosstalk between STAT3 and the progesterone receptor in the uterus has boosted trophoblast cell proliferation, invasion, and/or differentiation (130). Since progesterone hormone secretion can increase following IL-17 expression, it is also involved in all processes mentioned for trophoblast cells. Pongcharoen and Supalap demonstrated that IL-17-increased progesterone induces invasive capacity in cells (61), which is associated with increased human leukocyte antigen G (HLA-G) expression in human trophoblast cells (131). Researchers have extensively investigated how HLA-G affects the invasion of trophoblast cells in both normal and abnormal human pregnancy and in trophoblast cell lines (132-134). This molecule increases the production of MMPs and urokinase, which are known to remodel the endometrial extracellular matrix (135, 136). The interaction between HLA-G on trophoblasts and dNKs also leads to CXCL8 and CXCL10 secretion. These cytokines elevate trophoblasts invasiveness by motivation of CXCR1 and CXCR3 expression (97).
According to a study, producing IL-17 is the main way Th17 cells prevent trophoblast cell death (80). Nevertheless, the exact processes involved are unclear. In this respect, overexpression of IL-17 can lead to excessive trophoblast cell proliferation, migration, and invasion (85), resulting in complications during pregnancy (137). Over-activation of PPAR-γ/RXR-α/Wnt signaling promotes trophoblast cell invasion due to the overexpression of IL-17 in women with preeclampsia (85). Moreover, retinoic acids (i.e., active products of vitamin A) act as a main part of cell differentiation, migration, and invasion when binding to RXR-α (i.e., a retinoic acid receptor) and improve trophoblast cell differentiation. Thus, high levels of RXR-α are related to preeclampsia (138). As IL-17 increases the expression of RXR-α, its overexpression can also participate in the pathogenesis of preeclampsia. Besides, specific DNA elements can be bound by hetero- or homodimers formed by RXRs. Research has also shown the role of PPAR-γ /RXR-α heterodimer signaling in enhancing trophoblast cell invasion capacity by IL-17 activation (85). In addition, the imbalance of Th17, Treg, and the associated cytokines induces premature ovarian failure (139). Thus, a balanced Th17/Treg cell immunity is also necessary for a successful pregnancy, as these findings suggest.
7. The role of Th17 cells in placenta formation
The placenta is a special organ that transfers substances between the mother and the fetus. It originates from tissues outside the embryo and constantly adapts its shape and activity (140). The placenta not only meets the fetus’s needs for immune tolerance, nutrition, and embryonic support but also significantly alters the mother’s metabolism by releasing various hormones into her bloodstream (141). Therefore, pregnancy is a complicated process that demands strict coordination between the fetus and the mother. As an indicator of healthy pregnancy, the placental formation involves the conversion of the uterine spiral arteries into vessels with high flow and low resistance, which enhances the blood delivery to the fetoplacental unit. The structural changes during the spiral artery remodeling resemble those in the initial phases of angiogenesis (142). It is believed that Th17 cells play a role in regulating spiral artery modification by stimulating the formation of new blood vessels and the secretion of proangiogenic factors (143, 144). IL-17 stimulates angiogenesis while regulating the production of VEGF (143, 145). The formation of new blood vessels, either by expanding existing vessels (angiogenesis) or creating new ones (vasculogenesis), depends on VEGF, a crucial factor for embryonic development and vessel repair (146). When VEGF binds to its receptors, it activates them and triggers several signaling pathways downstream. These pathways include Ras/MAPK, focal adhesion kinase/paxillin, PI3K/AKT, RhoA/ROCK, and the PLCγ/REF. These pathways regulate cell proliferation and gene expression, cytoskeleton rearrangement, cell survival, new capillary formation, and vascular permeability, respectively (147, 148).
Studies show that pregnancies with problems in the placenta, have fewer Tregs. Consequently, Th17 cells become more active and cause inflammation where the mother and the fetus connect (149, 150). Meanwhile, the levels of IL-17 positively correlated with systolic blood pressure in patients with placental insufficiency. Hypertension during pregnancy can be fatal, as it can result in fetal growth restriction, early placental detachment, and low oxygen levels, which often lead to death (151). A remarkable finding is that oxidative stress and autoantibodies that target angiotensin II receptor type 1 cause blood pressure to rise. Meanwhile, the blood pressure is reduced by either giving superoxide dismutase or blocking angiotensin II receptor type 1 (152). Therefore, it is no wonder that IL‐17 increases the placental ROS in women with preeclampsia (95, 102).
Although some studies reported elevated levels of TGFβ1 in pregnant women with hypertension or miscarriages and premature labor, this result contradicts to the results of Santner-Nanan et al. and Darmochwal-Kolarz et al. (93, 153). However, Treg activation and Th17 reduction are necessary for ensuring pregnancy success (60, 154). Through proangiogenic effects, TGFβ also helps to transform maternal T cells into T CD4+FoxP3+ Tregs. Therefore, it can restore the balance between Th17 and Treg and promote the proper development of placental blood vessels (29, 155).
8. The treatment of abortion by Th17
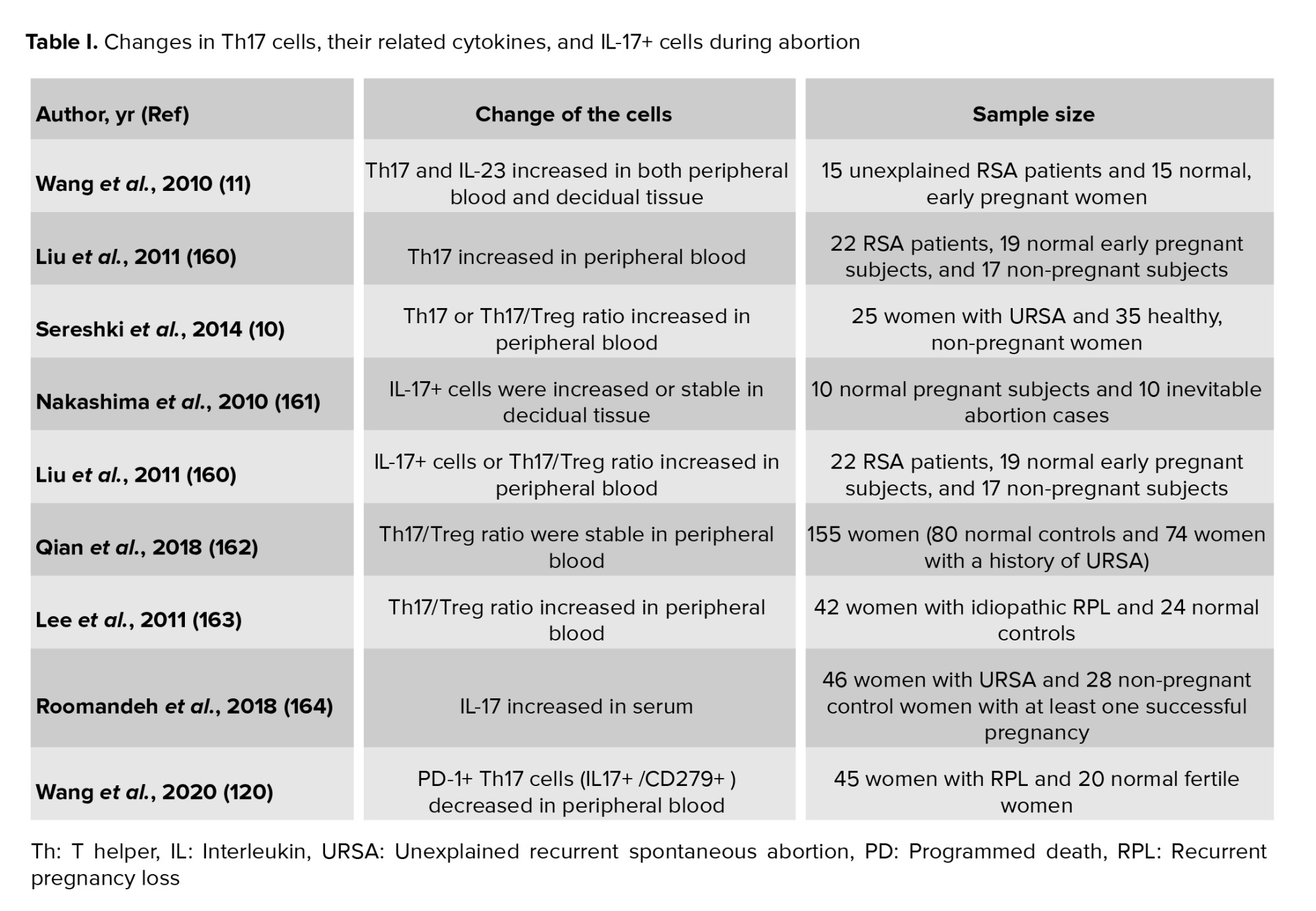
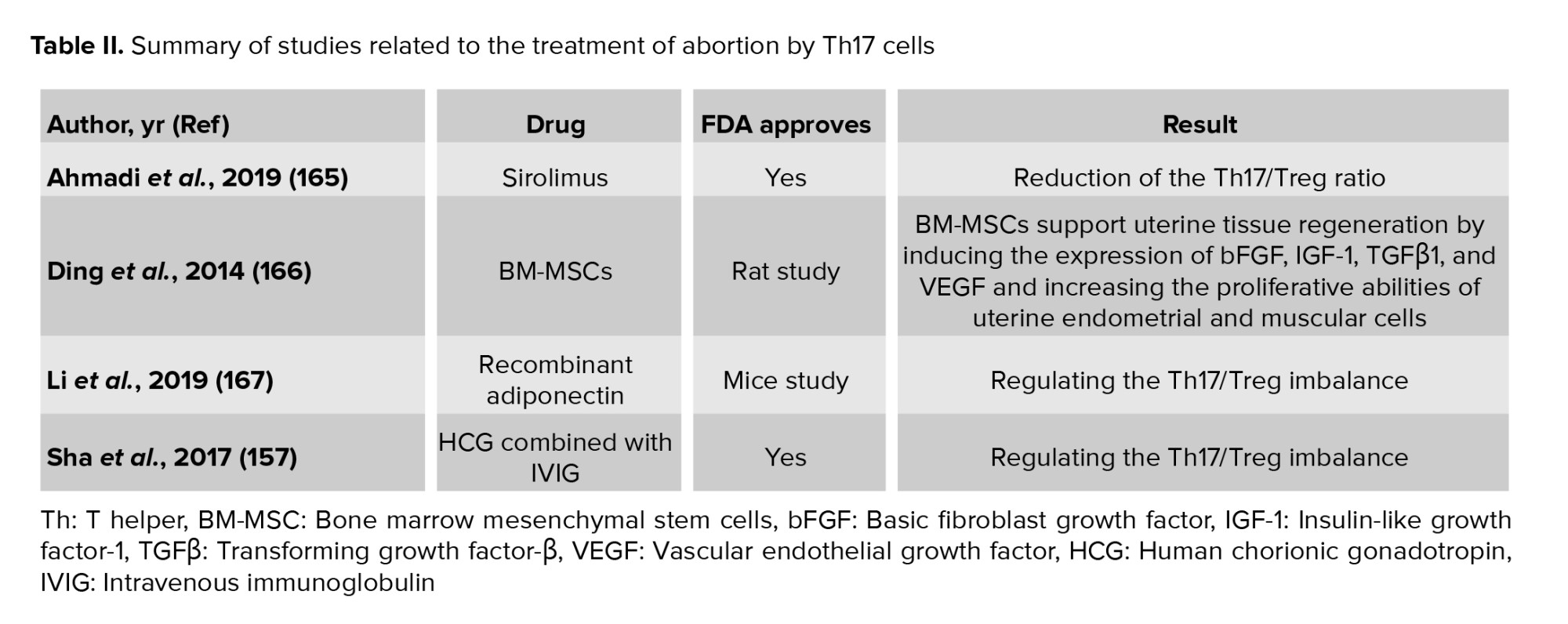
The treatment of patients at risk of abortion is determined based on their clinical conditions. The imbalance of Th17 and Treg cells that regulate immune responses can produce cytokines and inflammation, and eventually miscarriage. Accordingly, the balance of Th17/Treg and preventing the increase in Th17 levels in the peripheral blood is among the therapeutic strategies to prevent abortion (Tables I and II). Based on this data, intravenous immunoglobulin (IVIG) is injected into patients who are at risk of abortion to balance Th17/Treg (156). It has also been found that using IVIG along with human chorionic gonadotropin (hCG) can effectively balance Th17/Treg (157). Another strategy is immunotherapy to balance Th17/Treg. In other words, mononuclear cells are received from the spouse or a third person. This method can decrease the Th17 level and increase the Treg level in patients (158). Also, using hydroxychloroquine and adiponectin can be effective in balancing Th17/Treg. Adiponectin balances Th17/Treg through the P38/MAPK/STAT5 pathway (159).
Overall, it can be stated that the goal of therapeutic strategies is to balance Th17/Treg. This balance regulates immune responses and prevents miscarriage in patients.
9. Conclusion
In general, it can be said that Th17 plays an important role in modulating the immune system. Th17 regulates the inflammatory response and immune cell function through the secretion of cytokines. These functions interfere with the physiological processes of the placenta and can affect RPL in patients. In future studies, it is better to evaluate the main mechanisms of Th17 and the upstream and downstream factors that are affected so that an appropriate therapeutic strategy can be designed based on them.
Author Contributions
V. Hazari and A. Etezadi conducted the research and wrote the manuscript. P. Shirkosh designed the figure. Further, A. Aleyasin and Z. Heidar reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
The authors appreciate and thank the efforts of the Center for the Development of Clinical Researches of the Educational and Therapeutic Research Complex of Birjand University of Medical Science, Birjand, Iran. No artificial intelligence was used for search, grammar checks, or other applications.
Conflict of Interest
The authors declare that there is no conflict of interest.
Abortion is a condition characterized by the loss of the fetus before the 20th wk of pregnancy (1, 2). Research has shown that unwanted pregnancy can be one of the main causes of abortion in patients. Therefore, its increase can lead to the spread of abortion among pregnant women (3). The proportion of miscarriages varies and depends on several aspects, such as age, health status, and lifestyle. According to the Centers for Disease Control and Prevention, approximately 31-50% of pregnancies lead to a miscarriage by 13 wk (4).
It has been shown that successful gestation needs to be balanced between anti- and pro-inflammatory immune cells (5). Hence, an essential cause of poor pregnancy outcomes may be an aberration in the immune system (6-8). During early pregnancy, the mother and fetus engage in bilateral communication that involves a complex series of immunologic events. These events are orchestrated through diverse cells, including decidual macrophages, decidual dendritic cells (DCs), T cells, and innate lymphocytes (ILC). These cells are essential for establishing a balance between the immune tolerance of the fetus from a different genetic origin and the preservation of the host’s protection against potential infections (5, 9).
T helper (Th)17 cells are known to generate defensive immunity against extracellular microorganisms during gestation (7). However, existing evidence indicates that Th17 cell response imbalance can lead to some problems, such as recurrent spontaneous abortion (RSA) (10, 11). Abnormal inflammation, trophoblast invasion into the uterine spiral arteries, and insufficient tolerance are associated with increased peripheral blood Th17 cells (12).
Th17 cells are characterized through interleukin (IL)17 and IL-22 production as signature cytokines. These cells also produce tumour necrosis factor (TNF), IL-21, granulocyte macrophage-colony stimulating factor, and interferon (IFN)γ and can express Chemokine receptors (CCR) 6. Accordingly, they guide Th17 cells to the epithelial barrier locations by binding to chemokine ligand (CCL)20 (13, 14).
The IL-17 family consists of 6 ligands, IL-17A to IL-17F, with similar structures in homo or heterodimer forms, such as IL17A/F. IL-17A and IL-17F, among others, are produced by Th17 cells. Their function depends on the presence of their heterodimer receptor-containing subunits IL-17RA and IL-17RC (15).
IL-17-producing cells can gather at mucosal surfaces to provide an immune response against extracellular pathogens by keeping barrier integrity, boosting antimicrobial factors, and driving granulopoiesis (16, 17). For example, IL-17 interaction with IL-17RA receptors on the keratinocyte cells promotes epidermal cells to increase expression of antimicrobial peptides, IL-8 (CXCL8), IL-6, granulocyte colony-stimulating factor, and CCL20 (18-21).
Research on this subset will advance our understanding of Th17 functions in maternal-fetal junction and its association with abortion. This article offers a wide range of overviews of the existing condition of knowledge on the Th17 cells’ functions in pregnancy, with a particular focus on their interactions with other immune cells and their impact on abortion.
2. The immune system and pregnancy
2.1. Maternal and fetus immunity
The immune system is vital in pregnancy control and fetal development in pregnant women (22). Despite the direct contact between fetal and maternal cells, maternal immune cells are not stimulated against the surface antigens of fetal cells (23). Changes in the expression of some genes, including immune cells, lead to the control of immune mediators by them. Consequently, gathering T cells is limited within the decidua and does not attack a developing fetus and placenta (24). A recent study on human decidual stromal cells has shown that these cells inhibit the expression of chemokines CXCL9, CXCL10, and CXCL11 binding to expressed CXCR3 on CD4+ Th1 and CD8+ T cells to prevent the arrival of T cells in the decidua (25, 26).
Improper management of this procedure may trigger inflammation and the requirement of immune cells at the maternal-fetal interface. In addition, pregnancy can raise the risk of having certain infections and can also make normally mild infections more severe (22). In this respect, trophoblast cells are specialized fetal cells and originate from a blastocyst that regulates the immune system around the implantation place to provide an immune response against outside stimulations (27). These cells boost the employment of peripheral natural killer cells (NKs), neutrophils, and monocytes to the implantation site through cytokine production of transforming growth factor (TGF) and chemokine ligands CCL2, CXCL12, and CXCL8 (28). Moreover, trophoblast cells have an active role in the recruitment and differentiation of T regulatory cells (Tregs) (CD4+ Foxp3+ cells) from naive CD4+ cells through secreting high levels of TGFβ (29-31). Also, trophoblast cells express innate immune sensors, including NOD-like receptors and Toll-like receptors, permitting them to respond against tissue injury or microbial invasion. The decidual NKs (dNK), which produce IL-15 and TGFβ12, are responsible for the decidual vascular remodeling and placental function (27). CD14-positive monocytes around the maternal-fetal interface have an M2-like macrophage phenotype (28, 32) that ca n cause tissue remodeling and degradation of apoptotic cells (33) by secreting TGFβ and type-I IFN (34).
The fetus is susceptible to infections such as cytomegalovirus, rubella, human immunodeficiency virus, chickenpox, and hepatitis, particularly during the first and second trimesters of pregnancy (35). While the adaptive immune system is not yet fully matured at that stage, several mechanisms activating innate cells are the first line of protection against infections (36). For example, intrinsically expressed IFN-stimulated genes, including IFN-induced transmembrane protein (IFITM)-1 and IFITM3 on the embryonic stem cells, protect against viral infection by inhibiting the entry and replication of viruses (37, 38). IFITM3 is a restriction factor that can obstruct the RNA-virus-triggered IFN-I production by facilitating the autophagic degradation of IFN regulatory factor 3 (39). Previous studies have reported toll-like receptor expression on neonatal granulocytes, monocytes, and phagocytosis by neonatal macrophages, DCs, and NK cells, even though they provide lower immune responses than adults (40). Fetuses are more enriched with ILCs than NK cells compared with infants (41). Fetus-derived IFN-I can activate signal transducer and activator of transcription 1 (STAT1) and STAT2 along with JAK1 and TYK2 signaling, activating numerous IFN-stimulated-related genes and promoting the response to Zika virus infection (42).
3. Th17 cells
Following exposure to a microbial pathogen or foreign antigen, the innate immune system stimulates the CD4+ T cells to become Th cells that are basic managers of adaptive (acquired) immunity. Based on certain transcription factors and cytokine expression, naive CD4 T cells distinguish one of the Th subsets and induce Treg, as defined by the cytokines they secrete and/or their specific surface markers (43). The significant cytokine moderators of Th17 are TGFβ along with IL-1β, IL-6, IL-21, and/or IL-23 (44). TGFβ, in combination with IL-1β and IL-6, activates retinoid orphan receptor gamma t (RORγt) transcription factor via STAT3 signaling (45, 46). In a recent study, Fang et al. revealed that TGFβ/SMAD and IL-6/STAT3 signaling induce RORγt expression through Rorc-CNS-1.5 and Rorc-CNS+6.5 elements, during Th17 cell differentiation (47). IL-1β and TNF-α may also synergistically elevate IL-6 expression and, consequently, RORγt expression (48). TNF-α promotes the expansion of T cell subsets, producing IL-17 through both receptors TNFR1 or TNFR2 (49). Following Th17 cell differentiation, RORγt stimulates IL-17 and IL-23 receptor expressions (50). STAT3 can also upregulate the expression of IL-17 genes via its promoter binding, although this upregulation requires RORγt existence (51).
IL-17, in turn, motivates the expression of TNF-α, IL-6, and IL-1β by human macrophages and monocytes. Expressed IL-23 on the antigen-presenting cells binds to IL-23R, which maintains and expan7ds Th17 cells. Meanwhile, a positive feedback loop upregulates IL-17 and RORγt expression. Robust phosphorylation and dimerization of STAT3 following JAK-STAT axis activation by IL-6 and IL-23 enhances functions of RORγt (52). Since IL-23R is only expressed on differentiated Th17 cells, IL-23 alone cannot induce their development (43). Therefore, the role of IL-23 in the survival and maintenance of Th17 cells is more than initiating their differentiation (53). In another positive feedback loop, IL-6-induced STAT3 joins the promoter of IL-21, and then IL-21 induces RORγt and IL-17 expression synergistically with IL-6 and TGFβ (16, 54).
4. The role of Th17 cells in pregnancy
Pregnancy is the process of growth and development of the fetus in the womb. As a requirement for this process, the fetus should be resistant to maternal rejection and external environmental challenges. Since the uterine cavity is not fully sterile, Th17 cells expand within pregnancy to protect the maternal-fetal interface from microbial infections. In support of this idea, altered numbers of Th17 cells or Th17/Treg cells ratio in the maternal circulation or uterus can be a reason for pregnancy complications such as preterm birth, spontaneous abortion, and preeclampsia (55). Research has shown the correlation of Th17-cell activities, recruitment of neutrophils and monocytes with the maternal-fetal interface for clearance of pathogens, producing cytokines that promote antimicrobial peptide production, and B-cell differentiation into plasma cells, which produce antibodies against extracellular pathogens (56, 57). Th17 cells support implantation and provoke a defensive immune response against bacteria passing through inflammation induction (55). In addition, IL-17 is a regulatory cytokine that is vital for the preservation of prolonged periods of gestation (58). Conversely, to the high levels of peripheral IL-17 at late stages of healthy pregnancy, low levels were revealed immediately after natural abortion (59). In a study of 13 healthy pregnant women, IL-17 meaningfully increased from the 1st-3rd trimester, possibly as a member of the cytokines network due to implantation, embryo development, and the birth process itself (60).
On the one hand, the IL-17-granulocyte colony-stimulating factor axis promotes neutrophil development, while the IL-17-CXCR2 axis facilitates the recruitment of neutrophils. In contrast, IL-17 increases progesterone secretion, which is essential for maintaining neutrophils during pregnancy (61). Neutrophils drive maternal tolerance in progesterone hormone exposure by inducing a distinct sub-population of CD4+ T cells, generating IL-10, IL-17, and vascular endothelial growth factor (VEGF), promoting vessel development (62). Neutrophil reduction during mouse pregnancy leads to abnormal development of the fetal-maternal unit and reduced fetal growth (46, 62, 63). Neutrophil-induced induction of T cells and their regulatory function occurs through the transfer of forkhead box protein 1 (FOXO1) to T cells. The lack of FOXO1 transfer from neutrophils to T cells prevents the activation of induced T cells, as shown by the blood and placental neutrophils of women with preeclampsia. Furthermore, reducing FOXO1 expression only in neutrophils causes poor placentation and impaired fetal growth, mimicking the effects of neutrophil depletion (62). It has been reported that neutrophil depletion led to an impairment of the junctional zone development and reduction in the number of placenta cells expressing VEGF, intercellular cell adhesion molecule, and platelet-endothelial cell adhesion molecule, which are essential for adhesiveness and angiogenesis (64).
Nevertheless, Th17 cells may play a dual role in pregnancy: they can be protective or harmful depending on the context. Several studies link Th17 cells to allograft rejection and state that they contribute to adverse pregnancy outcomes, such as RSA and preeclampsia (10, 65, 66). In animal models, exogenous IL-17 can cause increased rates of abortion (67). In human studies, women with RSA have higher levels of Th17 cells and their related cytokines (e.g., IL-17 and IL-23) in both their blood and decidual tissue (68). Similarly, in patients with preeclampsia, Th17 cells and IL-6 levels are elevated, disrupting the balance between decidual Th17 and Tregs, and creating a pro-inflammatory environment (10, 65, 66, 69). The underlying mechanisms of Th17 activation in pregnancy may involve defective IL-27 signaling. IL-27 expression is reduced in the decidua of women with unexplained recurrent miscarriage compared to women with spontaneous miscarriage or normal pregnancy (70). Another possible factor is the high mobility group protein B1, a cytokine that promotes inflammation and is released by innate immune cells. High mobility group protein B1 may signal excessive Th17 responses, leading to excessive neutrophil infiltration at the maternal-fetal interface and potentially harmful pregnancy outcomes (55, 71). Additionally, the number of Th17 cells in the chorioamniotic membrane of cases with preterm labor was elevated. Amniotic fluid IL-17 upregulates TNF-α-induced IL-8 secretion from amniotic mesenchymal cells through IKK and MAPK signaling pathways (72, 73). The results of a study on mice demonstrated that Th17 and IL-17a are associated with an abnormal cortical phenotype in the fetal brain (74). Conversely, Th17, through the production of cytokines, causes the differentiation of macrophages, dendritic cells, monocytes, and other immune cells. The differentiation of these cells during inflammation can lead to spontaneous miscarriage, whereas cytokines can cause the secretion of progesterone. Progesterone stimulates Th17 and increase the differentiation of cells. Th17 also controls immune system modulator cells, including Treg. The balance between Treg cells and other T cells can play an important role in spontaneous miscarriage. This balance is maintained by cytokines produced by Th17 (75). These results indicate that Th17 cells can have a photogenic role at the feto-maternal interface in preterm delivery (Figure 1).
5. The role of Th17 cells in decidua
Decidua is the modified mucosal lining of the uterus that forms the maternal part of the placenta under the influence of progesterone and remains for the duration of the pregnancy. First, the ovary’s corpus luteum and, later, the placenta produce progesterone-inducing vascular alterations and cellular modifications within endometrial stromal cells, called the decidual reaction (76). The cellular composition of the decidua includes fibroblast-transformed stromal cells, glands, and immune cells, mostly ILCs, NKs, macrophages, Tregs, and DCs (77-79). Besides, it has already been revealed that decidual stromal cells drive Th17 cells from peripheral blood into the decidua while secreting high levels of CCL2 (80), known as monocytic chemotactic protein 1 (MCP-1), which binds to CCR2 expressed on Th17 cells (81, 82). Th17 cells in decidua express αβTCR, CD45RO, CCR7, and CD161 with effector memory phenotype (80). Th17 cells are attracted via the C-type lectin domain family (83), and CD161 is a C-type lectin-like receptor (84). Therefore, this molecule also has a role in the maternal/fetal interface by facilitating the movement of Th17 cells across the endothelium and influencing their selection in the decidua. These recruited Th17 cells can prevent human trophoblast cell apoptosis by producing IL-17 in the primary trimester of pregnancy (80). IL-17 activates the Wnt pathway through the peroxisome proliferator-activated receptor (PPAR)-γ/retinoid X receptor (RXR)-α heterodimer, thereby inhibiting the apoptosis of trophoblast cells (85).
As described earlier, IL-17 enhances hormone progesterone secretion (61), which functions fundamentally in decidua development and maintenance (86). It helps prepare the uterus for implanting the fertilized egg. It also promotes the growth of blood vessels in the endometrium, which helps supply nutrients to the developing embryo (87). Progesterone also inhibits the contraction of the uterus, contributing to the prevention of premature labor (88). It also plays a role in thickening the cervical mucus and creating a barrier to prevent bacteria from entering the uterus (89).
Another population of decidual T cells are named Th17/Th2/Th22 cells, producing IL-4, IL-22, and IL-17 during successful pregnancies. The embryo implantation site is the exclusive location of these cells and Th17/Th0/IL-22+ cells, where the mRNA expression levels of IL-4, GATA-3, IL-17A, ROR-C, IL-22, and AHR are amplified (90, 91). In patients with recurrent abortion, IFN-γ producing CD4+ T cells are predominant. These cells do not secrete IL-4 and are recognized as Th17/Th1/Th22+ cells (91). However, some studies reported an elevated frequency of Th17 cells in the decidua of pregnant women with spontaneous abortion or preeclampsia relative to their non-pregnant or healthy pregnant counterparts. This information suggests that excessive Th17 cells in decidua might adversely affect gestation (11, 92, 93). Chronic infusion of Th17 can lead to embryo resorption (94) because of the impaired vascular reactivity of uterine arteries following the increased number and activation of dNK cells (95). Th17-expressed macrophage inflammatory protein-3 alpha (MIP‐3α), a chemoattractant for NKs, could describe the expanded attendance of NK cells in the placentas (96). The main secretors of cytokines that attract angiogenesis and trophoblasts (e.g., IL-8, VEGF, and IL-10) are dNK cells with slight cytolytic activity (97). They are important in increasing tolerance to cytotrophoblasts and maternal vascular regeneration in myometrium and endometrium (98-100). The remodeling of maternal vessels by cytotrophoblasts is essential for establishing a functional uteroplacental circulation, which is necessary to deliver nutrients and oxygen to the developing fetus (101). As NKs express the IL‐17 RC receptor, IL‐17 can stimulate both their expansion and secretion of cytolytic enzymes, such as granzymes A, B, and K. These enzymes are responsible for the increased placental reactive oxygen species (ROS) in women with pre-eclampsia (95, 102). As previously mentioned, IL-17 directly recruits neutrophils and increases their activity, boosting oxidative stress (103, 104). In addition, it increases cytolytic granzymes, resulting in significant ROS production when it degrades mitochondrial proteins (105-108). Notably, dNK cells produce less VEGF following increased IL-17 levels in the placenta, which may lead to endothelial-dependent impaired uterine vasorelaxation (95).
Regarding the mentioned points, a dynamic balance of Th17 cells is required during pregnancy, and Treg cells play a central part in maintaining this balance (109). Compared to controls, women with unexplained recurrent pregnancy loss exhibited elevated levels of Th17 cells and IL-17A in peripheral blood throughout the ovulatory cycle, especially during the proliferative and secretory phases. Conversely, Treg cells and their related cytokines (i.e., IL-10 and TGFβ1) were reduced in the proliferative phase in women with unexplained recurrent pregnancy loss (11). Although the Th17 population is normal in women with preeclampsia, the Treg population is decreased, leading to an altered Th17/Treg ratio (110).
It has been reported that the population of CD4 + CD25 + and CD4 + CD25bright cells augments during pregnancy (111, 112). Also, the sustained, not transient, expression of Foxp3 in Treg cells enables them to suppress the immune response (113). Treg can participate in the regulation of Th17 cells via suppressing T cell differentiation, activation, and moderating molecular secretion patterns through the programmed death 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) axis (114). PD-1 and PD-L1 are the 2 leading immune checkpoint pathways that modulate immune responses by inhibiting PI3K/AKT and Ras-MEK-ERK signaling pathways (115, 116). In mice, blocking the PD-1/PD-L1 axis decreased Treg, leading to pregnancy loss by inducing Th17 and Th1 cell differentiation (117-119). PD-L1 expressions on Th17 cells were significantly downregulated in women with recurrent pregnancy loss (RPL), although they had similar proportions of Tregs to those of controls (120). Based on these findings, a possible cause of RPL is the reduced expression of PD-1, which can lead to an imbalance between Th17 and Treg. These cells are involved in Th17 immunity, which may affect pregnancy outcomes depending on how well they are balanced.
Moreover, progesterone administration via the vaginal route is one of the accepted strategies to lower the risk of preterm birth (121). According to Areia et al. the membrane progesterone receptor α is expressed by Treg in healthy pregnant women (122). This data suggests that progesterone’s protective effect on preterm birth conditions may be due to the activation of Treg cell proliferation; besides, progesterone considerably augments the PD-1 expression in Treg (123). Therefore, it seems that Th17-produced IL-17 creates a progesterone-rich environment that prevents them from over-activating in a negative feedback mechanism while activating Treg cell proliferation (Figure 1).
6. The role of Th17 cells in trophoblast cell invasion
Trophoblast cells are specialized cells that form the outer layer of the blastocyst, which later develops into the placenta (124). During pregnancy, trophoblast cells migrate and invade the decidua (i.e., the thickened endometrium of the uterus) to establish a connection between the fetal and maternal circulatory systems (125). The placenta needs this process to connect to the mother physically and change the uterine blood vessels to supply enough blood to the developing fetus during pregnancy (126). The exact mechanisms of trophoblast migration and invasion are not fully understood. However, studies suggest that this mechanism involves interacting with various cellular and molecular players, including decorin, a small leucine-rich proteoglycan produced by stromal cells (127).
During normal placenta development, cytokines and adhesion molecules are some factors that regulate trophoblasts’ growth and spread. By producing IL-17, Th17 cells contribute to trophoblasts’ survival, invasion, and expansion with IL-17 receptors in the first trimester of human pregnancy (80). Moreover, IL-17 increased the production of MMP-13 via the NF-kB pathway and led to the degradation of the extracellular matrix. Placental trophoblast also secretes matrix metalloproteinases (MMPs), which may play a role in trophoblast invasion mediated by IL-17. However, abnormal MMP expression can cause pregnancy complications, such as fetal intrauterine growth restriction and pre-eclampsia (128, 129).
Furthermore, crosstalk between STAT3 and the progesterone receptor in the uterus has boosted trophoblast cell proliferation, invasion, and/or differentiation (130). Since progesterone hormone secretion can increase following IL-17 expression, it is also involved in all processes mentioned for trophoblast cells. Pongcharoen and Supalap demonstrated that IL-17-increased progesterone induces invasive capacity in cells (61), which is associated with increased human leukocyte antigen G (HLA-G) expression in human trophoblast cells (131). Researchers have extensively investigated how HLA-G affects the invasion of trophoblast cells in both normal and abnormal human pregnancy and in trophoblast cell lines (132-134). This molecule increases the production of MMPs and urokinase, which are known to remodel the endometrial extracellular matrix (135, 136). The interaction between HLA-G on trophoblasts and dNKs also leads to CXCL8 and CXCL10 secretion. These cytokines elevate trophoblasts invasiveness by motivation of CXCR1 and CXCR3 expression (97).
According to a study, producing IL-17 is the main way Th17 cells prevent trophoblast cell death (80). Nevertheless, the exact processes involved are unclear. In this respect, overexpression of IL-17 can lead to excessive trophoblast cell proliferation, migration, and invasion (85), resulting in complications during pregnancy (137). Over-activation of PPAR-γ/RXR-α/Wnt signaling promotes trophoblast cell invasion due to the overexpression of IL-17 in women with preeclampsia (85). Moreover, retinoic acids (i.e., active products of vitamin A) act as a main part of cell differentiation, migration, and invasion when binding to RXR-α (i.e., a retinoic acid receptor) and improve trophoblast cell differentiation. Thus, high levels of RXR-α are related to preeclampsia (138). As IL-17 increases the expression of RXR-α, its overexpression can also participate in the pathogenesis of preeclampsia. Besides, specific DNA elements can be bound by hetero- or homodimers formed by RXRs. Research has also shown the role of PPAR-γ /RXR-α heterodimer signaling in enhancing trophoblast cell invasion capacity by IL-17 activation (85). In addition, the imbalance of Th17, Treg, and the associated cytokines induces premature ovarian failure (139). Thus, a balanced Th17/Treg cell immunity is also necessary for a successful pregnancy, as these findings suggest.
7. The role of Th17 cells in placenta formation
The placenta is a special organ that transfers substances between the mother and the fetus. It originates from tissues outside the embryo and constantly adapts its shape and activity (140). The placenta not only meets the fetus’s needs for immune tolerance, nutrition, and embryonic support but also significantly alters the mother’s metabolism by releasing various hormones into her bloodstream (141). Therefore, pregnancy is a complicated process that demands strict coordination between the fetus and the mother. As an indicator of healthy pregnancy, the placental formation involves the conversion of the uterine spiral arteries into vessels with high flow and low resistance, which enhances the blood delivery to the fetoplacental unit. The structural changes during the spiral artery remodeling resemble those in the initial phases of angiogenesis (142). It is believed that Th17 cells play a role in regulating spiral artery modification by stimulating the formation of new blood vessels and the secretion of proangiogenic factors (143, 144). IL-17 stimulates angiogenesis while regulating the production of VEGF (143, 145). The formation of new blood vessels, either by expanding existing vessels (angiogenesis) or creating new ones (vasculogenesis), depends on VEGF, a crucial factor for embryonic development and vessel repair (146). When VEGF binds to its receptors, it activates them and triggers several signaling pathways downstream. These pathways include Ras/MAPK, focal adhesion kinase/paxillin, PI3K/AKT, RhoA/ROCK, and the PLCγ/REF. These pathways regulate cell proliferation and gene expression, cytoskeleton rearrangement, cell survival, new capillary formation, and vascular permeability, respectively (147, 148).
Studies show that pregnancies with problems in the placenta, have fewer Tregs. Consequently, Th17 cells become more active and cause inflammation where the mother and the fetus connect (149, 150). Meanwhile, the levels of IL-17 positively correlated with systolic blood pressure in patients with placental insufficiency. Hypertension during pregnancy can be fatal, as it can result in fetal growth restriction, early placental detachment, and low oxygen levels, which often lead to death (151). A remarkable finding is that oxidative stress and autoantibodies that target angiotensin II receptor type 1 cause blood pressure to rise. Meanwhile, the blood pressure is reduced by either giving superoxide dismutase or blocking angiotensin II receptor type 1 (152). Therefore, it is no wonder that IL‐17 increases the placental ROS in women with preeclampsia (95, 102).
Although some studies reported elevated levels of TGFβ1 in pregnant women with hypertension or miscarriages and premature labor, this result contradicts to the results of Santner-Nanan et al. and Darmochwal-Kolarz et al. (93, 153). However, Treg activation and Th17 reduction are necessary for ensuring pregnancy success (60, 154). Through proangiogenic effects, TGFβ also helps to transform maternal T cells into T CD4+FoxP3+ Tregs. Therefore, it can restore the balance between Th17 and Treg and promote the proper development of placental blood vessels (29, 155).

8. The treatment of abortion by Th17
The treatment of patients at risk of abortion is determined based on their clinical conditions. The imbalance of Th17 and Treg cells that regulate immune responses can produce cytokines and inflammation, and eventually miscarriage. Accordingly, the balance of Th17/Treg and preventing the increase in Th17 levels in the peripheral blood is among the therapeutic strategies to prevent abortion (Tables I and II). Based on this data, intravenous immunoglobulin (IVIG) is injected into patients who are at risk of abortion to balance Th17/Treg (156). It has also been found that using IVIG along with human chorionic gonadotropin (hCG) can effectively balance Th17/Treg (157). Another strategy is immunotherapy to balance Th17/Treg. In other words, mononuclear cells are received from the spouse or a third person. This method can decrease the Th17 level and increase the Treg level in patients (158). Also, using hydroxychloroquine and adiponectin can be effective in balancing Th17/Treg. Adiponectin balances Th17/Treg through the P38/MAPK/STAT5 pathway (159).
Overall, it can be stated that the goal of therapeutic strategies is to balance Th17/Treg. This balance regulates immune responses and prevents miscarriage in patients.


9. Conclusion
In general, it can be said that Th17 plays an important role in modulating the immune system. Th17 regulates the inflammatory response and immune cell function through the secretion of cytokines. These functions interfere with the physiological processes of the placenta and can affect RPL in patients. In future studies, it is better to evaluate the main mechanisms of Th17 and the upstream and downstream factors that are affected so that an appropriate therapeutic strategy can be designed based on them.
Author Contributions
V. Hazari and A. Etezadi conducted the research and wrote the manuscript. P. Shirkosh designed the figure. Further, A. Aleyasin and Z. Heidar reviewed the article. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
The authors appreciate and thank the efforts of the Center for the Development of Clinical Researches of the Educational and Therapeutic Research Complex of Birjand University of Medical Science, Birjand, Iran. No artificial intelligence was used for search, grammar checks, or other applications.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Biology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







