Thu, Feb 19, 2026
[Archive]
Volume 23, Issue 7 (July 2025)
IJRM 2025, 23(7): 545-558 |
Back to browse issues page
Ethics code: IR.KUMS.AEC.1401.004
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Moradi M, Ghanbari E, Rashidi Z, Moradi S, Shakeri A, Faramarzi A. Carob extract restores testicular function and sperm quality in cadmium-exposed mice: An experimental study. IJRM 2025; 23 (7) :545-558
URL: http://ijrm.ir/article-1-3488-en.html
URL: http://ijrm.ir/article-1-3488-en.html
Mojtaba Moradi1 

 , Elham Ghanbari2
, Elham Ghanbari2 

 , Zahra Rashidi3
, Zahra Rashidi3 

 , Saman Moradi4
, Saman Moradi4 

 , Ali Shakeri5
, Ali Shakeri5 

 , Azita Faramarzi *6
, Azita Faramarzi *6 




 , Elham Ghanbari2
, Elham Ghanbari2 

 , Zahra Rashidi3
, Zahra Rashidi3 

 , Saman Moradi4
, Saman Moradi4 

 , Ali Shakeri5
, Ali Shakeri5 

 , Azita Faramarzi *6
, Azita Faramarzi *6 


1- Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran.
2- Department of Tissue Engineering, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
3- Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. & Department of Anatomical Sciences, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
4- Tissue Engineering and Applied Cell Sciences Division, Department of Anatomical Sciences, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
5- Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran.
6- Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. & Department of Anatomical Sciences, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran. ,azita.faramarzi@kums.ac.ir; a.faramarzi90@gmail.com
2- Department of Tissue Engineering, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
3- Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. & Department of Anatomical Sciences, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran.
4- Tissue Engineering and Applied Cell Sciences Division, Department of Anatomical Sciences, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
5- Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran.
6- Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. & Department of Anatomical Sciences, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran. ,
Full-Text [PDF 2537 kb]
(640 Downloads)
| Abstract (HTML) (663 Views)
Full-Text: (194 Views)
1. Introduction
Infertility is a significant global health concern, with male factors contributing to about half of all cases. Environmental exposures, lifestyle choices, and oxidative stress are key contributors to reduced sperm quality, which is strongly linked to reduced fertility potential and adverse reproductive outcomes. Men are particularly susceptible to fertility impairment from environmental pollutants and occupational risk factors (1). Cadmium (Cd) poses a significant threat to both the environment and human health, whether through occupational exposure or environmental contamination (2). Individuals working directly with Cd in industrial processes, such as in anti-corrosive agents, nickel-cadmium batteries, and phosphate fertilizers, are knowingly exposed (2, 3). However, tobacco leaves absorb Cd from the soil, making smoking a major non-occupational source of exposure for both active and passive smokers (4). Reproductive issues are commonly reported in men exposed to Cd, with testicular damage emerging as a prominent and concerning consequence of such exposure (5). Cd can disrupt sperm production and maturation through various pathways, including damage to the spermatogenic endothelium, disruption of the blood-testis barrier, and abnormal histone modification in the sperm nucleus during spermiogenesis (5, 6).
A recent study has specifically targeted plant-derived products with antioxidant properties to protect cells and tissues from Cd toxicity in both animals and humans (7). These bioactive compounds, readily available in plants at low cost and considered safe, can effectively neutralize reactive oxygen species or bind to metals, serving as natural antagonists to Cd (8). Ceratonia siliqua L., also known as carob or locust, belongs to the Fabaceae family and is an evergreen tree originating from the Mediterranean and Middle East regions (9). The fruits of this tree are highly nutritious and boast numerous medicinal applications, including serving as diuretics, purgatives, antidiabetic agents, laxatives, antimicrobials, anti-inflammatories, anti-diarrheals, antiulcer remedies, antioxidants, gastroprotective agents, anti-constipation aids, and hepatoprotective agents (10, 11). In recent years, carob has garnered attention due to its abundant polyphenolic content and remarkable antioxidant capabilities (12). These bioactive compounds, which include proanthocyanidin, ellagitannin, and gallotannin, as well as tannins, flavonoids, anthocyanins, glycosides, alkaloids, and vitamins C, D, E, pyridoxine, niacin, and folic acid, along with numerous minerals such as calcium, magnesium, selenium, potassium, sodium, phosphorus, and iron, have been utilized in disease treatment and have demonstrated scavenging properties against reactive oxygen species (12, 13). Thus far, however, there have been no reports of toxicity associated with the use of carob (11). In traditional medicine of several Middle Eastern countries, carob fruit has been used as an aphrodisiac and supplement to enhance sexual desire, potency, and pleasure, as well as to increase human semen volume for the treatment of male infertility (14). Previous research has indicated that carob extract improves semen quality and elevates testosterone levels by enhancing the function of Leydig cells and the biosynthesis of testosterone, consequently leading to enhancements in the spermatogenesis process (15).
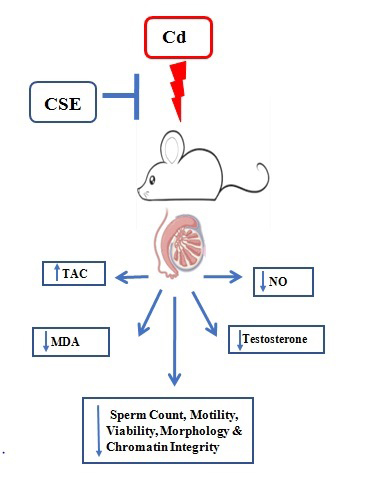
Hence, this study aims to evaluate the effects of the hydroalcoholic extract of Ceratonia siliqua extract (CSE) on sperm count, motility, viability, and morphology; sperm DNA integrity; blood testosterone levels; antioxidant capacity and nitro-oxidative markers in the testes; as well as the histological structure of testicular tissue in mice with Cd-induced reproductive toxicity, mimicking real-life human Cd exposure.
2. Materials and Methods
2.1. Animals
The experimental study was done in the animal house of the Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran, from May 2022-February 2023. 40 adult male BALB/c mice (8-12 wk, 30 ± 5 gr) were obtained from the Pasteur Institute (Tehran, Iran). Following a 1 wk acclimatization period, the mice were assigned to either the control or experimental groups. The animals were housed under standardized environmental conditions, including a 12-hr light/dark cycle, a temperature of 23 ± 1°C, and 50% relative humidity. They had access to food (standard diet) and water ad libitum throughout the study.
2.2. Preparation of plant extract
The plant fruits employed in this investigation were harvested from Ilam Province, Iran. Following verification of the genus and species (Herbarium No. 7123 confirmed at the Natural Resources Center) and verified by a botanist, Amir Mohammad Mahdavi, PhD in Plant Genetics, a portion of the collected fruits was utilized to produce the extract. The hydro-alcoholic extract was prepared by first breaking the fruit into crumbs and separating and crushing the seeds to get a fine powder. 1 kg of this resulting powder was then mixed with 96% ethanol alcohol in a 50/50 ratio and soaked in water for 72 hr. Subsequently, the solution was put in an oven at 40°C until both the alcohol and water had evaporated, leaving behind a concentrated brown extract. The intended concentrations were attained by dissolving precise quantities of the extract in distilled water.
2.3. Experimental design
The animals were divided into 4 groups, each consisting of 10 mice (16):
Infertility is a significant global health concern, with male factors contributing to about half of all cases. Environmental exposures, lifestyle choices, and oxidative stress are key contributors to reduced sperm quality, which is strongly linked to reduced fertility potential and adverse reproductive outcomes. Men are particularly susceptible to fertility impairment from environmental pollutants and occupational risk factors (1). Cadmium (Cd) poses a significant threat to both the environment and human health, whether through occupational exposure or environmental contamination (2). Individuals working directly with Cd in industrial processes, such as in anti-corrosive agents, nickel-cadmium batteries, and phosphate fertilizers, are knowingly exposed (2, 3). However, tobacco leaves absorb Cd from the soil, making smoking a major non-occupational source of exposure for both active and passive smokers (4). Reproductive issues are commonly reported in men exposed to Cd, with testicular damage emerging as a prominent and concerning consequence of such exposure (5). Cd can disrupt sperm production and maturation through various pathways, including damage to the spermatogenic endothelium, disruption of the blood-testis barrier, and abnormal histone modification in the sperm nucleus during spermiogenesis (5, 6).
A recent study has specifically targeted plant-derived products with antioxidant properties to protect cells and tissues from Cd toxicity in both animals and humans (7). These bioactive compounds, readily available in plants at low cost and considered safe, can effectively neutralize reactive oxygen species or bind to metals, serving as natural antagonists to Cd (8). Ceratonia siliqua L., also known as carob or locust, belongs to the Fabaceae family and is an evergreen tree originating from the Mediterranean and Middle East regions (9). The fruits of this tree are highly nutritious and boast numerous medicinal applications, including serving as diuretics, purgatives, antidiabetic agents, laxatives, antimicrobials, anti-inflammatories, anti-diarrheals, antiulcer remedies, antioxidants, gastroprotective agents, anti-constipation aids, and hepatoprotective agents (10, 11). In recent years, carob has garnered attention due to its abundant polyphenolic content and remarkable antioxidant capabilities (12). These bioactive compounds, which include proanthocyanidin, ellagitannin, and gallotannin, as well as tannins, flavonoids, anthocyanins, glycosides, alkaloids, and vitamins C, D, E, pyridoxine, niacin, and folic acid, along with numerous minerals such as calcium, magnesium, selenium, potassium, sodium, phosphorus, and iron, have been utilized in disease treatment and have demonstrated scavenging properties against reactive oxygen species (12, 13). Thus far, however, there have been no reports of toxicity associated with the use of carob (11). In traditional medicine of several Middle Eastern countries, carob fruit has been used as an aphrodisiac and supplement to enhance sexual desire, potency, and pleasure, as well as to increase human semen volume for the treatment of male infertility (14). Previous research has indicated that carob extract improves semen quality and elevates testosterone levels by enhancing the function of Leydig cells and the biosynthesis of testosterone, consequently leading to enhancements in the spermatogenesis process (15).
Hence, this study aims to evaluate the effects of the hydroalcoholic extract of Ceratonia siliqua extract (CSE) on sperm count, motility, viability, and morphology; sperm DNA integrity; blood testosterone levels; antioxidant capacity and nitro-oxidative markers in the testes; as well as the histological structure of testicular tissue in mice with Cd-induced reproductive toxicity, mimicking real-life human Cd exposure.
2. Materials and Methods
2.1. Animals
The experimental study was done in the animal house of the Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran, from May 2022-February 2023. 40 adult male BALB/c mice (8-12 wk, 30 ± 5 gr) were obtained from the Pasteur Institute (Tehran, Iran). Following a 1 wk acclimatization period, the mice were assigned to either the control or experimental groups. The animals were housed under standardized environmental conditions, including a 12-hr light/dark cycle, a temperature of 23 ± 1°C, and 50% relative humidity. They had access to food (standard diet) and water ad libitum throughout the study.
2.2. Preparation of plant extract
The plant fruits employed in this investigation were harvested from Ilam Province, Iran. Following verification of the genus and species (Herbarium No. 7123 confirmed at the Natural Resources Center) and verified by a botanist, Amir Mohammad Mahdavi, PhD in Plant Genetics, a portion of the collected fruits was utilized to produce the extract. The hydro-alcoholic extract was prepared by first breaking the fruit into crumbs and separating and crushing the seeds to get a fine powder. 1 kg of this resulting powder was then mixed with 96% ethanol alcohol in a 50/50 ratio and soaked in water for 72 hr. Subsequently, the solution was put in an oven at 40°C until both the alcohol and water had evaporated, leaving behind a concentrated brown extract. The intended concentrations were attained by dissolving precise quantities of the extract in distilled water.
2.3. Experimental design
The animals were divided into 4 groups, each consisting of 10 mice (16):
- Group 1 (normal control group): the negative control, which was injected with a vehicle.
- Group 2 (Cd group): as the positive control group, mice were administered with Cd chloride (CdCl2) 0.35 mg/kg/day (17) at 10 am by intraperitoneal (ip) injections.
- Group 3 (100 CSE): mice were treated with 100 mg/kg/day of CSE (16) at 9 am by ip injections.
- Group 4 (Cd+CSE): mice received 0.35 mg/kg/day of Cd chloride and received 100 mg/kg/day of CSE.
CdCl2 was administered for 35 days, CSE administration commenced 7 days prior to the onset of Cd chloride injection and continued throughout the 35-day period of Cd chloride administration (18).
The doses were selected based on previous research and a pilot study, which determined the optimal dose for CSE (19). Upon completion of the treatment period, the animals were weighed and subsequently euthanized via cervical dislocation following ip administration of ketamine (50 mg/kg) and xylazine (7 mg/kg). Testes and epididymides were collected through laparotomy for sperm analysis. One testis was fixed for histopathological examination, while the contralateral testis was immediately fresh-frozen for assessment of oxidative and antioxidative parameters. Unless otherwise noted, all reagents were obtained from Sigma-Aldrich (USA).
2.4. Evaluation of sperm parameters
The epididymis was carefully separated from the testis, and the cauda epididymis was dissected into 1 ml of pre-warmed Ham's F10 medium supplemented with 5% human serum albumin. The sample was then incubated in a CO2 incubator for 15 min to allow spermatozoa to swim out from it. Sperm count was determined using a Neubauer chamber and light microscopy (Olympus Co., Tokyo, Japan), with the results expressed as 106 cells/ml. Total sperm motility was assessed by counting motile sperm, including progressive and non-progressive motility. For assessing viability, smears were prepared by mixing 20 µL of eosin and nigrosine stain with an equal volume of sperm solution. Then, unstained live sperm and stained dead sperm were counted on dried slides at room temperature, counting 200 sperm per mouse.
Additionally, morphological abnormalities were examined using the Papanicolaou stain and light microscopy at a magnification of ×1000. Sperm with head and tail defects were identified and recorded as a percentage of the total sperm count. Additionally, aniline blue and toluidine blue staining, along with their evaluations, were performed as described in our previous study (17).
2.5. Histopathological examination
For histopathological analysis, a single tissue section (thickness = 0.05 µm) was obtained from each testis and examined for any potential alterations in the structure of the germinal epithelium and interstitial connective tissue following the administration of Cd and CSE (17).
2.6. Assessment of testosterone level in serum
Serum testosterone levels were quantified using mice testosterone enzyme-linked immunosorbent assay kits (Moonblind Inc., USA) through the enzyme-linked immunosorbent assay method, following the manufacturer's protocol (17).
2.7. Assessment of nitro-oxidative stress in testicular tissue
2.7.1. Evaluation of total antioxidant capacity (TAC) levels in testicular tissue
TAC in testicular tissue was evaluated by spectrophotometric analysis using a colorimetric assay kit (Naxifer™, Navand Salamat Co., Iran), which quantifies ferric-reducing antioxidant power. This assay operates on the principle of reducing ferric ions (Fe³⁺) to ferrous ions (Fe²⁺) in the presence of 2,4,6-Tripyridyl-S-triazine, forming a blue-colored complex with a peak absorbance at 593 nm. The ferric-reducing antioxidant power values were indicated as nanomoles of Fe²⁺ equivalents per mg of wet tissue weight (nmol/mg protein) (17).
2.7.2. Assessment of lipid peroxidation (LPO) levels in testicular tissue
LPO levels in testicular tissue samples were assessed by measuring malondialdehyde (MDA) using the thiobarbituric acid reaction with a Nalondi™ assay kit (Navand Salamat Co., Iran). Briefly, 0.2 ml of tissue homogenate was mixed with 0.8 ml of working solution containing phosphoric acid and thiobarbituric acid and then added to the samples. The mixture was heated at 90°C for 45 min and subsequently cooled on ice. The thiobarbituric acid-reactive substances were quantified by measuring absorbance at 532 nm based on an MDA standard curve. Thiobarbituric acid-reactive substances levels were reported as MDA equivalents per milligram of wet tissue weight (nmol/mg protein) (20).
2.7.3. Assessment of nitric oxide (NO) levels in testicular tissue
The total NO content in homogenized testicular tissue was measured using the Griess reaction, following the manufacturer's instructions (Natrix™ assay kit; Navand Salamat Co., Iran). In this reaction, NO is rapidly converted to nitrite in an acidic environment, then to HNO2. Sulfanilamide is added, enabling HNO2 to form a diazonium salt, which subsequently reacts with N-(1-Naphthyl) ethylenediamine dihydrochloride to produce an azo dye measurable at 570 nm. NO levels in the samples were reported as micromoles per milligram of protein (µmol/mg protein) (17).
2.8. Ethical Considerations
All experimental procedures were done in accordance with international guidelines for the care and use of laboratory animals and were approved by Kermanshah University of Medical Sciences, Kermanshah, Iran (Code: IR.KUMS.AEC.1401.004).
2.9. Statistical Analysis
Data analyses were performed using GraphPad Prism (version 9.0, San Diego, CA, USA). Data normality and homogeneity were verified with the Kolmogorov-Smirnov test. Quantitative data were presented as mean ± standard deviation and 95% confidence intervals (CI). Differences between experimental groups were evaluated using one-way ANOVA, followed by Tukey's post hoc test for comparisons. Statistical significance was defined as p < 0.05.
3. Results
3.1. Carob attenuates the decrease in testes weight induced by Cd
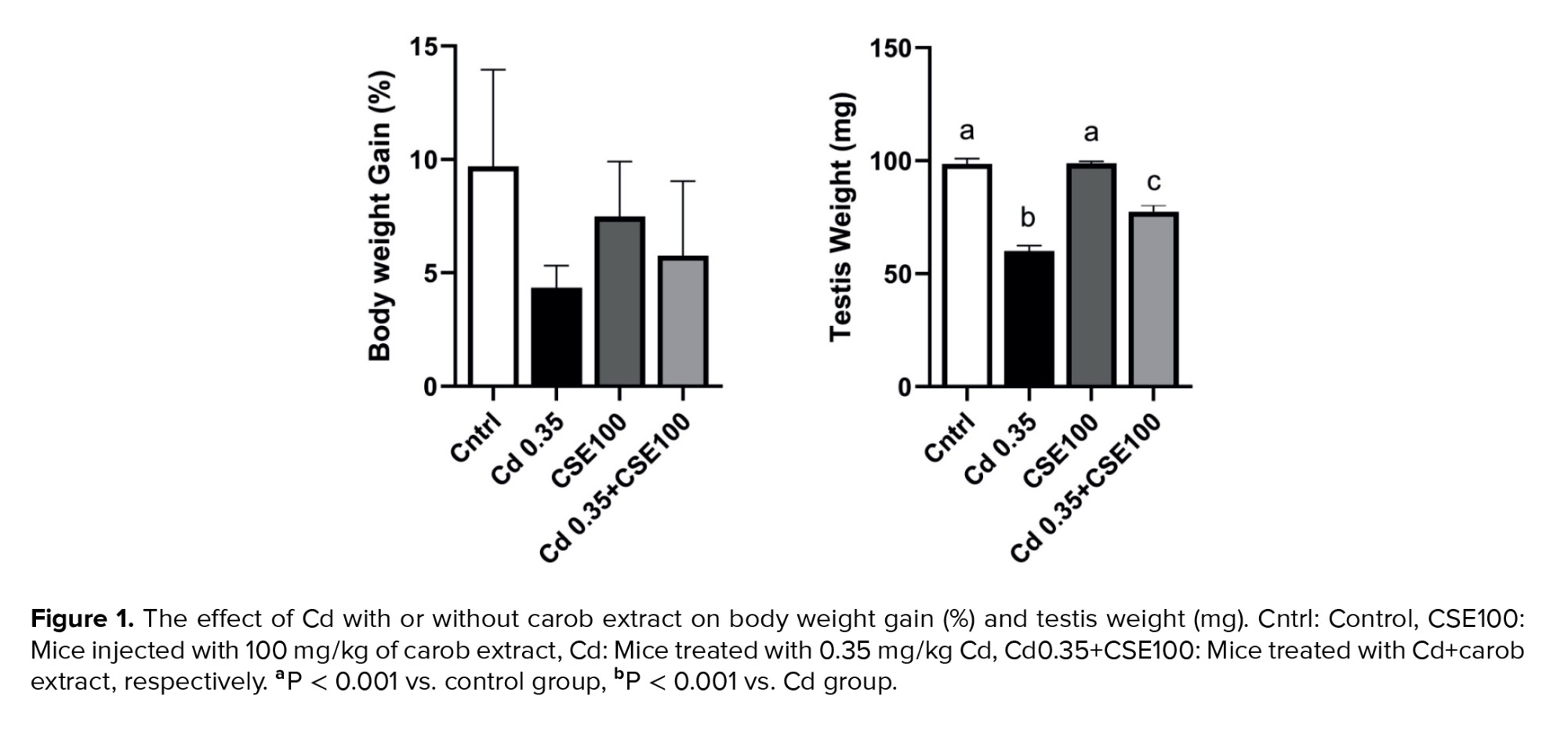
The increase in body weight observed in the Cd-injected group was lower compared to the control group, yet this difference did not reach statistical significance (p > 0.05). Additionally, no significant difference was observed between the CSE-treated groups and the control group (p > 0.05). Testis weight decreased significantly in both the Cd and Cd+CSE groups compared to the control group (p < 0.001), suggesting potential Cd-induced testis toxicity in these animals. Although CSE appeared to maintain testis weight to some extent, the notable difference between the Cd+CSE group and the control group persisted (p < 0.001) (Figure 1).
3.2. Carob reverses the adverse effects of Cd on spermatogenesis
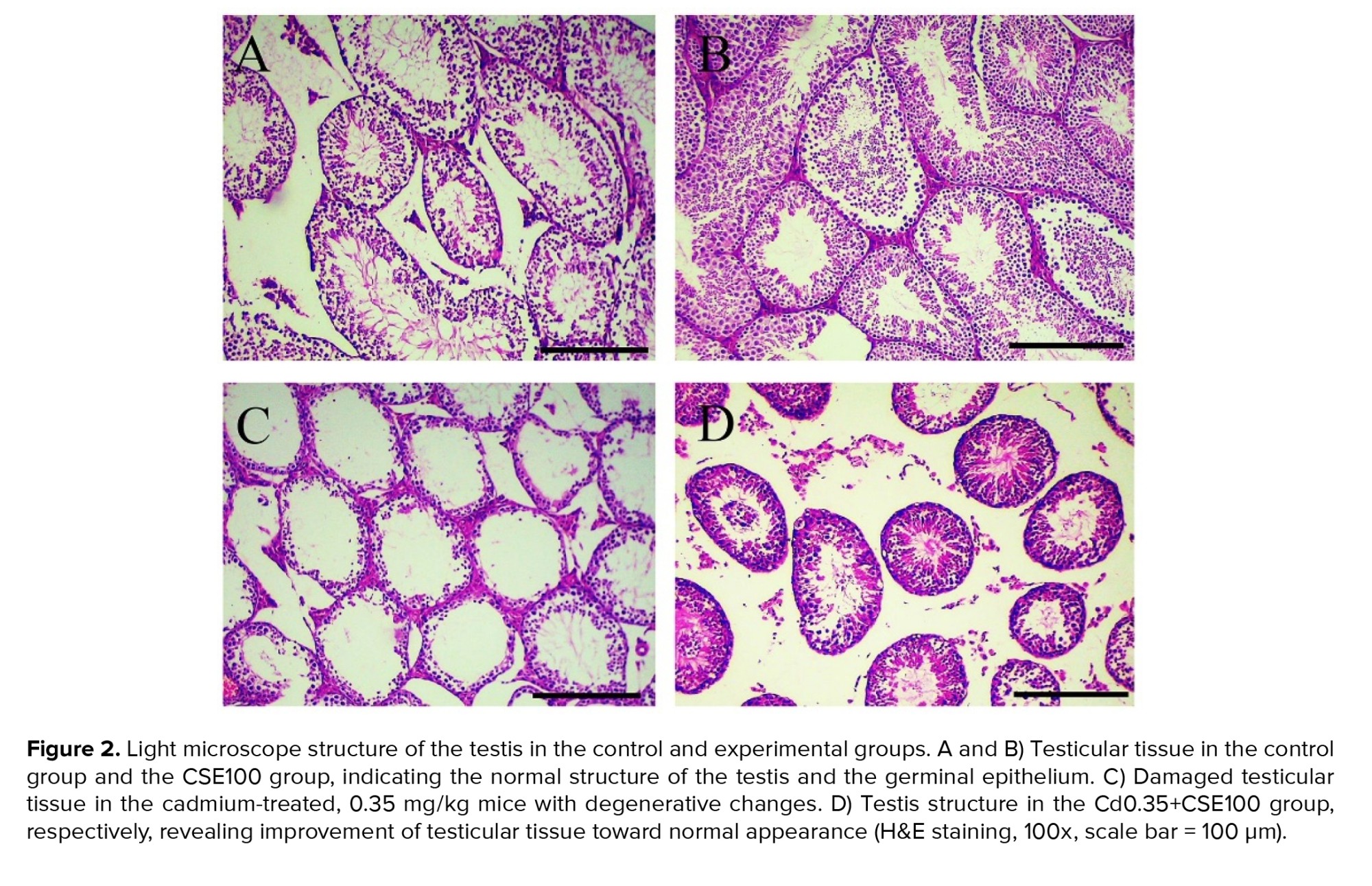
Histopathological examinations of the control groups revealed normal histoarchitecture within the seminiferous tubules, featuring a sequential arrangement of germ cells at various stages of development and filled lumens containing spermatozoa (Figures 2A, B). In contrast, the group injected with Cd exhibited heterogeneous degenerative changes in the seminiferous tubule epithelium, including the presence of vacuoles, dissociation of the germinal epithelium, necrosis, interstitial edema, and compromised lumens, indicating adverse effects of Cd on testicular structure (Figure 2C). Conversely, the histological alterations induced by Cd in the testes were notably mitigated by CSE therapy (Figure 2D). To conclude, the reduced histological parameters suggest that Cd therapy triggered apoptosis in the spermatogenic cell line, consequently halting the process of spermatogenesis, which was significantly restored by CSE administration (Figure 2).
3.3. Carob alleviates the changes in testosterone synthesis induced by Cd
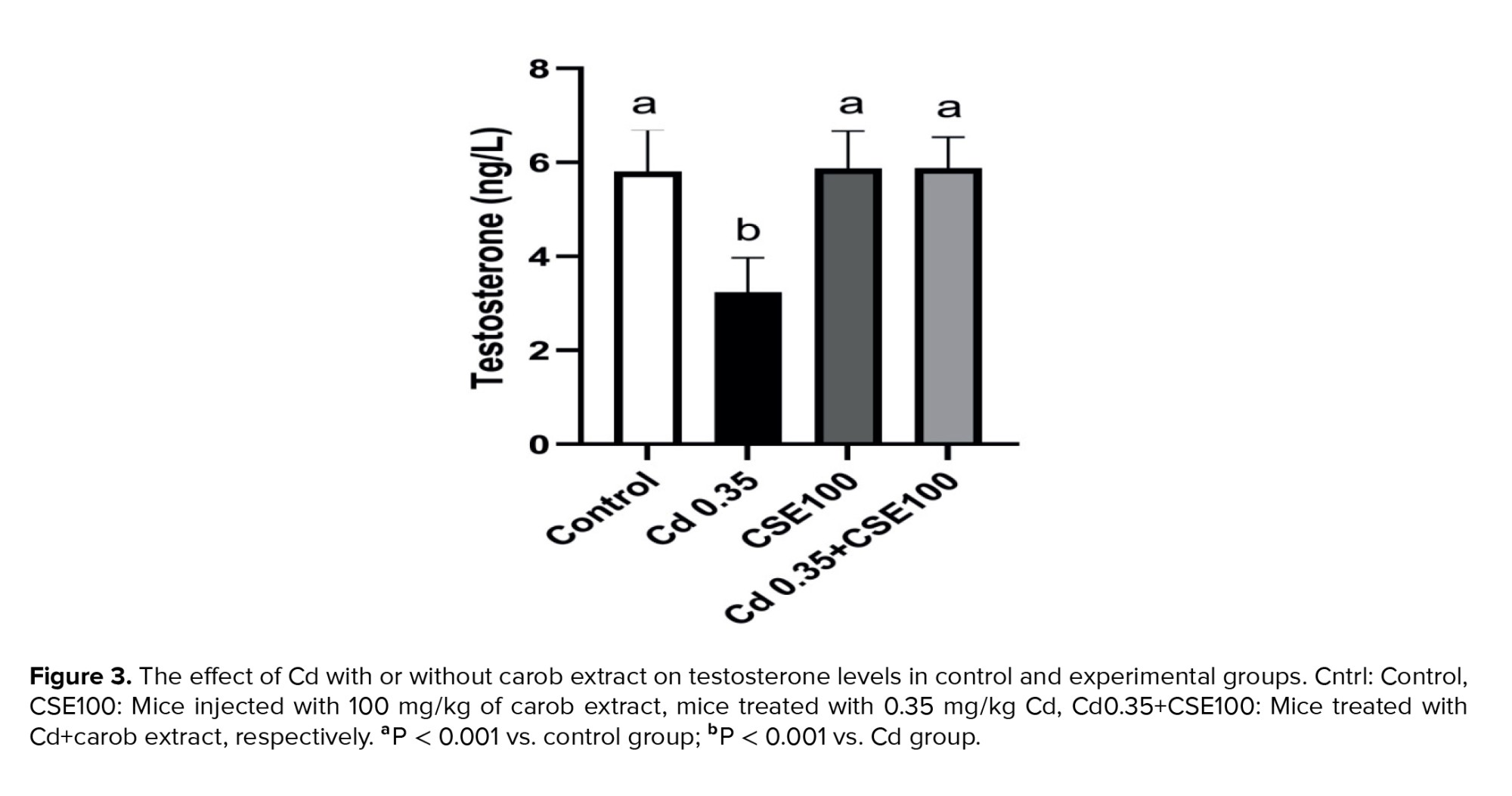
The testosterone level in the group treated with Cd showed a significant reduction compared to the control group (p < 0.001). Yet, administration of 100 mg/kg of CSE effectively restored the serum testosterone level significantly (p < 0.001), indicating a potential protective effect of CSE on testicular structure and function (Figure 3).
3.4. Carob rectifies the disruptions in sperm parameters caused by Cd
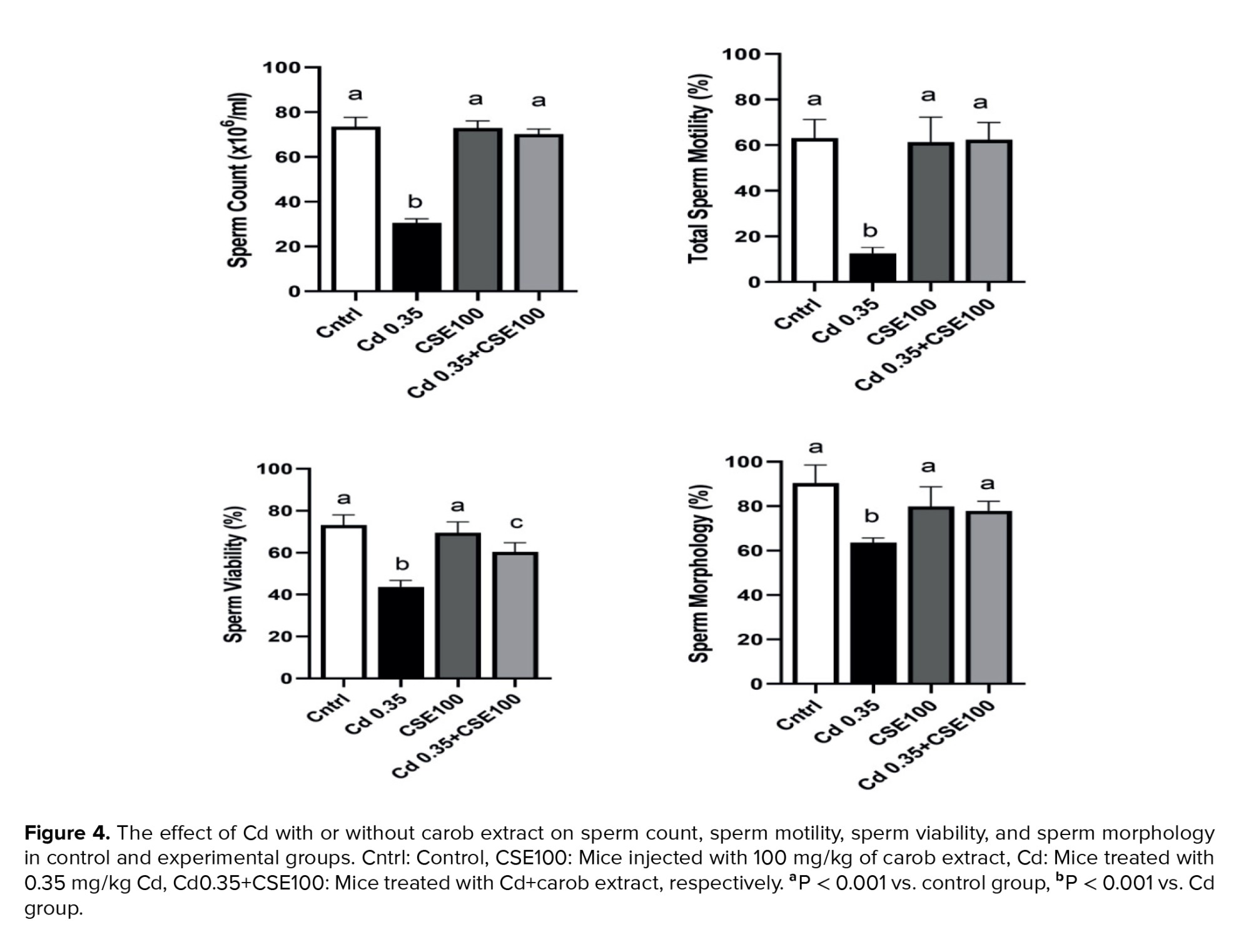
Figure 4 illustrates a significant decrease in sperm count observed in the Cd-administered group compared to the control group (p < 0.001), yet CSE therapy demonstrated potential for promoting recovery in Cd-injected mice (p < 0.001). Sperm motility decreased in the Cd group compared to the control group (p < 0.001), which was significantly mitigated by CSE (p < 0.001). Besides, the Cd-exposed group exhibited a significant increase in the number of dead sperm compared to the control (p < 0.001). Yet, CSE administration notably improved sperm survival compared to the Cd group (p < 0.001). Our findings also revealed that Cd treatment significantly induced abnormal sperm morphology compared to the control group (p < 0.001). Notably, CSE therapy alleviated this abnormal morphology, although the difference between the Cd+CSE group and the control group remained significant (p = 0.02) (Figure 4).
3.5. Carob alleviates Cd-induced impairment in sperm DNA integrity
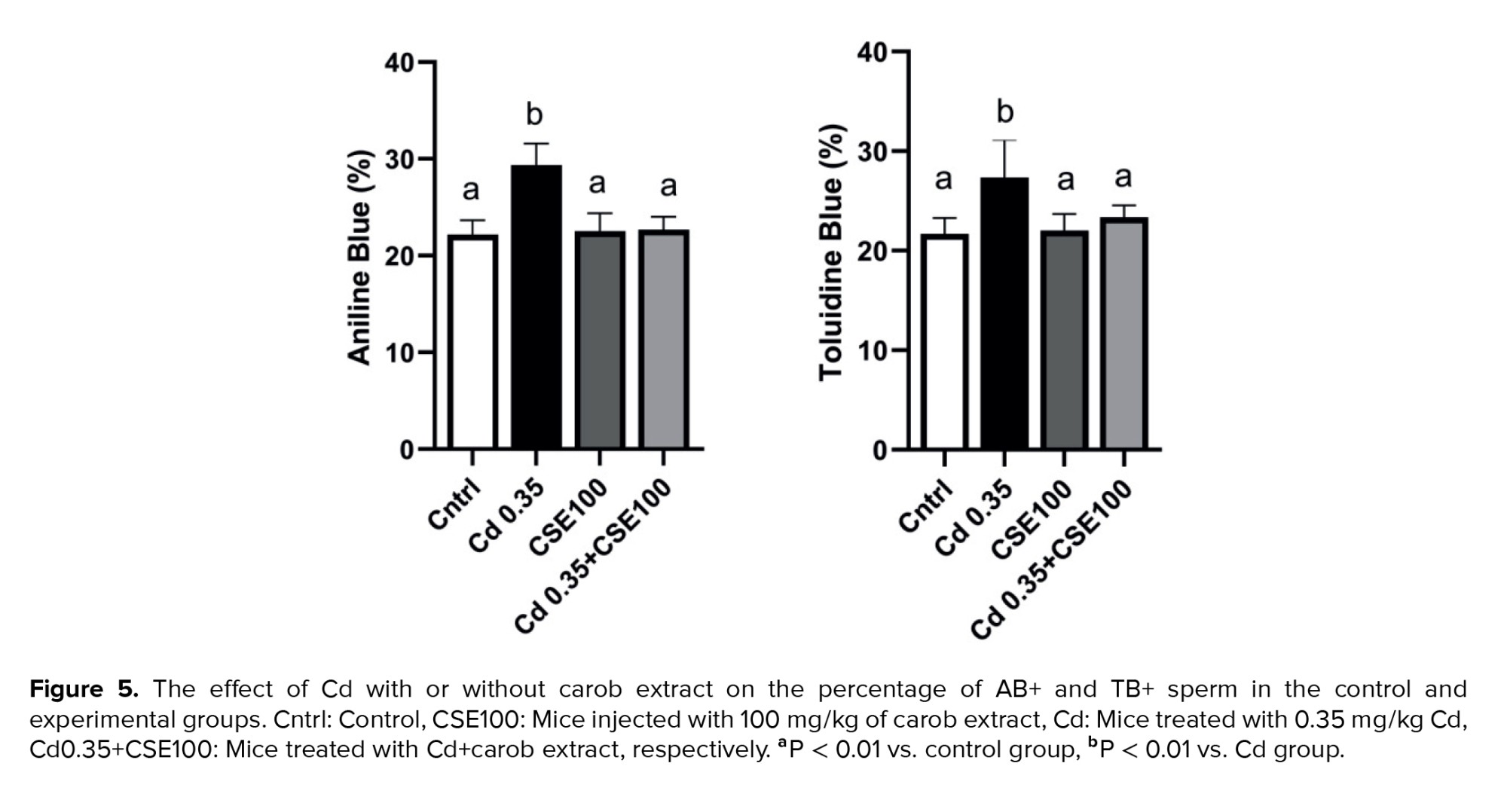
Assessment of chromatin quality revealed a significant increase in both acridine orange (AB) and toluidine blue (TB) indices in the Cd-treated group compared to controls (p < 0.001 and p = 0.002, respectively). Conversely, treatment with CSE markedly decreased the proportion of AB-positive spermatozoa relative to the Cd-exposed group (p < 0.001) and significantly reduced the percentage of TB-positive sperm compared to the Cd-injected group (p = 0.004) (Figure 5).
3.6. Carob modulates testicular nitro-oxidative status after Cd exposure
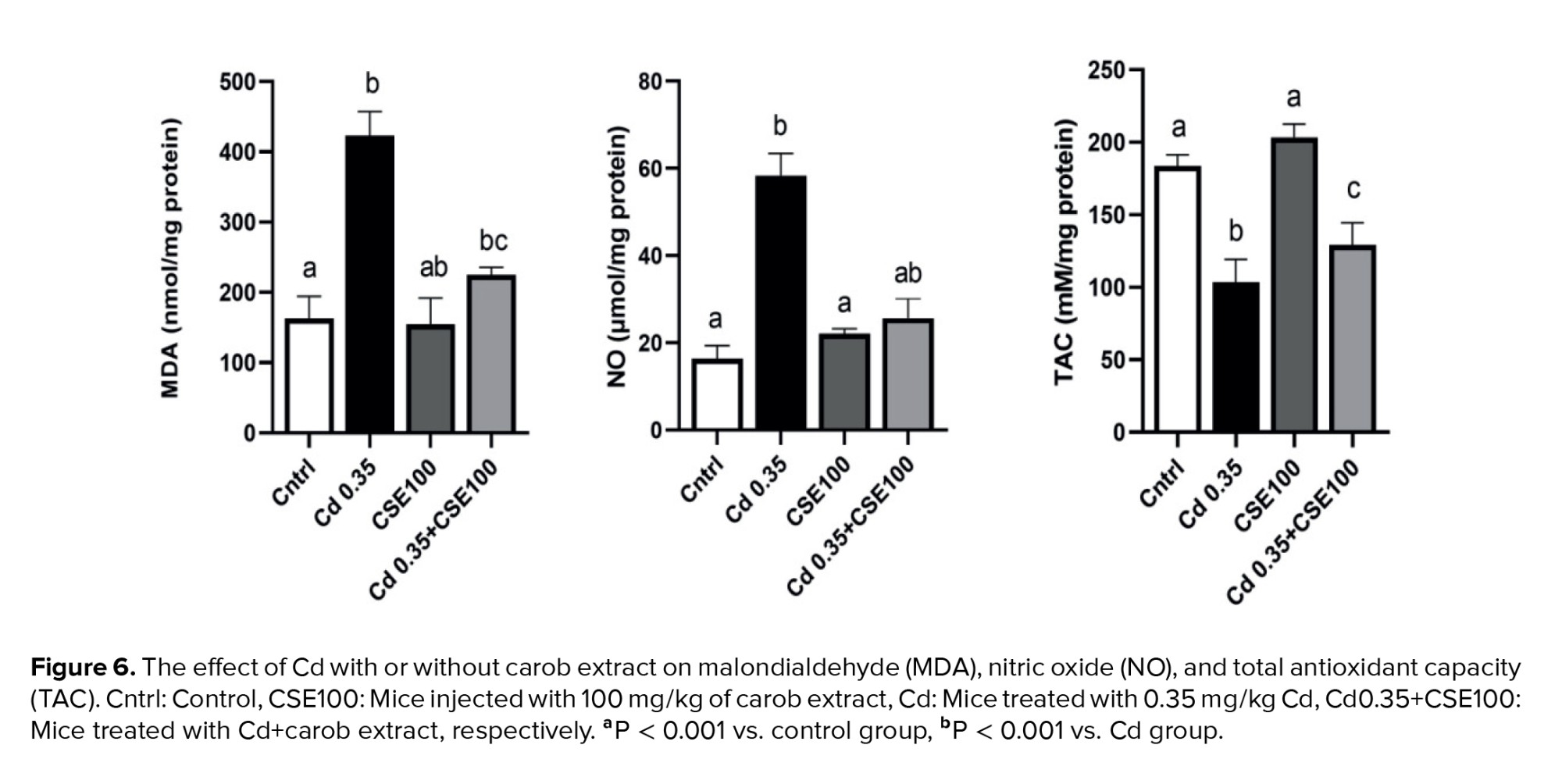
Cd therapy led to a notable reduction in the level of TAC compared to the control group (p < 0.001); however, CSE therapy demonstrated potential for promoting recovery in Cd-injected mice (p < 0.001). It is imperative to note that the difference between the Cd+CSE group and the control group remained significant (p < 0.001). Further, the level of MDA significantly increased in the testes of the Cd-administered animals compared to the control (p < 0.001), indicating Cd exposure induced oxidative stress in the testes. Conversely, treatment with 100 mg/kg of CSE notably decreased the level of MDA in testicular tissue (p < 0.001). With respect to NO levels, a significant difference was observed between the Cd-treated and control groups (p < 0.001), indicating that Cd exposure induced nitrosative stress in testicular tissue. Even so, treatment with CSE led to a significant reduction in the NO level (p < 0.001), indicating that CSE was able to mitigate reactive nitrogen species in the testicular tissue (Figure 6).
4. Discussion
Recently, the decrease in male fertility has become a significant issue in public health, with one of the major contributing factors being the widespread presence of environmental toxins worldwide. Previous studies indicate that Cd poses a significant risk to both the environment and human health, and exposure to this toxic pollutant, whether through environmental factors or occupation, has harmful effects on male fertility (19, 21). Carob is highly nutritious and boasts numerous promising antioxidant and anti-inflammatory properties (12). To the best of our knowledge, for the first time, we deciphered that CSE protects against Cd-induced male reproductive toxicity by improving testosterone secretion and anti-oxidative defense in the testis.
Histopathological examinations revealed that CSE treatment preserved normal morphological and histopathological structures in the testes of mice injected with Cd. Cd administration disrupted Leydig and Sertoli cells, most likely due to inducing oxidative stress (22). Studies have indicated that exposure to Cd leads to the deterioration of Sertoli and Leydig cells, impacting testosterone levels and resulting in testicular necrosis and atrophy (8, 17). These effects may be explained by Cd's ability to disrupt the integrity of the blood-testis barrier, trigger oxidative stress and the release of inflammatory cytokines such as tumor necrosis factor α, induce DNA damage, and initiate apoptosis in germ cells. Spermatogenesis is a complex process affected by genetic, hormonal, and environmental elements, and any disruption in these areas leads to decreased sperm quality, ultimately causing infertility (3). CSE administration, however, restored the structure of seminiferous tubules and various stages of spermatogenesis, likely thanks to the modulation of nitro-oxidative stress by its antioxidant components, such as flavonoids, vitamins C, D, and E, and numerous minerals found in this herbal remedy (12, 13, 15).
A significant reduction in testosterone levels was observed in the Cd-treated group, supporting previous reports that Cd exposure interferes with steroid biosynthesis and lowers circulating testosterone levels. Cd disrupts hormone production through direct binding to androgenic and estrogenic receptors, altering the expression of steroidogenesis enzymes and inhibiting luteinizing hormone receptor expression (23). Additionally, Cd indirectly affects hormone secretion by hindering noradrenaline release, altering gonadotropin-releasing hormone release, and impacting luteinizing hormone and prolactin secretion (6). Thus, the impaired sperm parameters observed in our study may be attributed to reduced testosterone levels in the testes, which are essential for normal sperm production (24). Administration of 100 mg/kg of CSE could significantly improve the Cd-evoked disturbances in testosterone levels. This effect can be attributed to the ability of the potent antioxidant properties of carob extract components to modulate oxidative stress in the testis (14). In other words, the extract's antioxidant properties probably protect Leydig cells from damage caused by free radicals and inhibit steroidogenesis in the testes (10, 14, 15). In this regard, animal studies revealed that the utilization of carob extract positively affected cyclic adenosine monophosphate production and the functioning of enzymes engaged in rat testicular steroidogenesis (15).
Cd treatment resulted in a significant reduction in sperm count, motility, and viability, along with a noticeable rise in abnormal morphology. These findings are consistent with previous research showing that Cd exposure leads to reductions in sperm concentration, motility, and viability, as well as an increase in abnormal sperm morphology (8, 24). These findings could be attributed to Cd-induced oxidative stress and apoptosis factors, which contribute to a subsequent decline in sperm quality (8). Further, these results are in accordance with prior research suggesting that even at low levels of Cd accumulation in semen, it could potentially contribute to poor sperm parameters by causing oxidative damage to sperm, ultimately leading to male subfertility and infertility (25). More notably, Cd treatment markedly increased the aniline blue and toluidine blue indexes in sperm, indicating chromatin fragmentation in sperm cells. Likewise, research has shown that men with unexplained infertility often have elevated Cd levels in their semen, which correlates with a notable increase in sperm DNA fragmentation and seminal reactive oxygen species levels (26). It was also demonstrated that Cd plays a role in causing abnormal modifications to histones in the sperm nucleus during spermiogenesis and leads to damage in the sperm's nuclear DNA (17, 26). Yet, CSE therapy protected sperm characteristics and chromatin quality against Cd-evoked detrimental effects. We attributed these beneficial effects to the CSE's ability to quench free radicals by preventing the production of new radicals or scavenging and eliminating reactive species. With key constituents such as vitamins C, E, D, and folic acid, along with essential minerals like magnesium and selenium, which are crucial for spermatogenesis, CSE could enhance sperm parameters (27). Another explanation for the improvement in sperm parameters is testosterone-related behaviors, since it was reported that carob extract improves testosterone levels significantly. In this context, it was indicated that treatment of infertile men with carob for 90 days significantly improved semen parameters, including sperm concentration and motility, leading to increased pregnancy rates (15).
Our results showed a marked reduction in TAC with a noteworthy rise in NO and MDA levels, suggesting that Cd disturbs the testis redox system, resulting in considerable nitro-oxidative stress. The study has shown that Cd can provoke oxidative stress and LPO in several organs, such as the liver and testes (8). Oxidative stress plays a pivotal role in the etiology and pathogenicity of testis dysfunction and male infertility induced by Cd (8, 24). Cd-induced oxidative stress in the testes happens due to several mechanisms, including the reactive oxygen species production, glutathione reduction, elevation in LPO, inhibition of antioxidant enzyme expression and activity like catalase and superoxide dismutase, and interaction with proteins through sulfhydryl groups in cellular membrane proteins and other cellular proteins (17, 28, 29). Here, CSE played a crucial role in modulating nitro-oxidative stress induced by Cd via inhibition of NO and MDA generation and enhancing the antioxidant defense mechanisms in the testes. Our observations align with previous findings, which reported that treating infertile men with carob for 90 days increased TAC and reduced MDA levels in serum (15). Recent studies have revealed that CSE contains various compounds, including proanthocyanidin, ellagitannin, and gallotannin, as well as tannins, flavonoids, anthocyanins, glycosides, alkaloids, and vitamins such as C, D, E, pyridoxine, niacin, and folic acid. Additionally, it contains numerous minerals such as calcium, magnesium, selenium, potassium, phosphorus, and iron, most of which possess antioxidant properties (12, 13). Studies also have shown that carob’s protective effects against oxidative stress stem from its phenolic compounds, which act as hydrogen donors. This action reduces inflammation and apoptosis in the testicular tissue, improving testicular function (30).
5. Conclusion
Carob extract demonstrates protective effects on testicular function and spermatogenesis against Cd-induced reproductive toxicity. These beneficial findings can be largely attributed to the potent antioxidant properties of carob extract, as it contains phenolic and flavonoid compounds that function as free radical scavengers. These findings underscore the therapeutic potential of carob extract as a natural supplement for managing Cd-related male infertility, warranting further research to characterize its bioactive compounds and confirm its efficacy.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
M. Moradi: Methodology, conceptualization, investigation, writing, review, and editing. E. Ghanbari: Methodology, formal analysis, and investigation. Z. Rashidi: Visualization, validation, formal analysis, and supervision. S. Moradi: Methodology and investigation. A. Shakeri: Methodology, investigation, and conceptualization. A. Faramarzi: Conceptualization, visualization, validation, writing, review, editing, supervision, project administration. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
This work was performed in partial fulfillment of the requirements for the medical degree of Ali Shakeri in the School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran. This work was supported by the Kermanshah University of Medical Sciences, Kermanshah, Iran. The authors are grateful to Prof. Dr. Mitra Bakhtiari, Dr. Mohsen Shayestehyekta, and Bahareh Moradi for their technical assistance. Additionally, we would like to express our gratitude to the staff of the Fertility and Infertility Research Center, Kermanshah, Iran. Artificial intelligence has not been used in any way, including translation, revision, and grammar check in this work.
Conflict of Interest
The authors declare that there is no conflict of interest.
The doses were selected based on previous research and a pilot study, which determined the optimal dose for CSE (19). Upon completion of the treatment period, the animals were weighed and subsequently euthanized via cervical dislocation following ip administration of ketamine (50 mg/kg) and xylazine (7 mg/kg). Testes and epididymides were collected through laparotomy for sperm analysis. One testis was fixed for histopathological examination, while the contralateral testis was immediately fresh-frozen for assessment of oxidative and antioxidative parameters. Unless otherwise noted, all reagents were obtained from Sigma-Aldrich (USA).
2.4. Evaluation of sperm parameters
The epididymis was carefully separated from the testis, and the cauda epididymis was dissected into 1 ml of pre-warmed Ham's F10 medium supplemented with 5% human serum albumin. The sample was then incubated in a CO2 incubator for 15 min to allow spermatozoa to swim out from it. Sperm count was determined using a Neubauer chamber and light microscopy (Olympus Co., Tokyo, Japan), with the results expressed as 106 cells/ml. Total sperm motility was assessed by counting motile sperm, including progressive and non-progressive motility. For assessing viability, smears were prepared by mixing 20 µL of eosin and nigrosine stain with an equal volume of sperm solution. Then, unstained live sperm and stained dead sperm were counted on dried slides at room temperature, counting 200 sperm per mouse.
Additionally, morphological abnormalities were examined using the Papanicolaou stain and light microscopy at a magnification of ×1000. Sperm with head and tail defects were identified and recorded as a percentage of the total sperm count. Additionally, aniline blue and toluidine blue staining, along with their evaluations, were performed as described in our previous study (17).
2.5. Histopathological examination
For histopathological analysis, a single tissue section (thickness = 0.05 µm) was obtained from each testis and examined for any potential alterations in the structure of the germinal epithelium and interstitial connective tissue following the administration of Cd and CSE (17).
2.6. Assessment of testosterone level in serum
Serum testosterone levels were quantified using mice testosterone enzyme-linked immunosorbent assay kits (Moonblind Inc., USA) through the enzyme-linked immunosorbent assay method, following the manufacturer's protocol (17).
2.7. Assessment of nitro-oxidative stress in testicular tissue
2.7.1. Evaluation of total antioxidant capacity (TAC) levels in testicular tissue
TAC in testicular tissue was evaluated by spectrophotometric analysis using a colorimetric assay kit (Naxifer™, Navand Salamat Co., Iran), which quantifies ferric-reducing antioxidant power. This assay operates on the principle of reducing ferric ions (Fe³⁺) to ferrous ions (Fe²⁺) in the presence of 2,4,6-Tripyridyl-S-triazine, forming a blue-colored complex with a peak absorbance at 593 nm. The ferric-reducing antioxidant power values were indicated as nanomoles of Fe²⁺ equivalents per mg of wet tissue weight (nmol/mg protein) (17).
2.7.2. Assessment of lipid peroxidation (LPO) levels in testicular tissue
LPO levels in testicular tissue samples were assessed by measuring malondialdehyde (MDA) using the thiobarbituric acid reaction with a Nalondi™ assay kit (Navand Salamat Co., Iran). Briefly, 0.2 ml of tissue homogenate was mixed with 0.8 ml of working solution containing phosphoric acid and thiobarbituric acid and then added to the samples. The mixture was heated at 90°C for 45 min and subsequently cooled on ice. The thiobarbituric acid-reactive substances were quantified by measuring absorbance at 532 nm based on an MDA standard curve. Thiobarbituric acid-reactive substances levels were reported as MDA equivalents per milligram of wet tissue weight (nmol/mg protein) (20).
2.7.3. Assessment of nitric oxide (NO) levels in testicular tissue
The total NO content in homogenized testicular tissue was measured using the Griess reaction, following the manufacturer's instructions (Natrix™ assay kit; Navand Salamat Co., Iran). In this reaction, NO is rapidly converted to nitrite in an acidic environment, then to HNO2. Sulfanilamide is added, enabling HNO2 to form a diazonium salt, which subsequently reacts with N-(1-Naphthyl) ethylenediamine dihydrochloride to produce an azo dye measurable at 570 nm. NO levels in the samples were reported as micromoles per milligram of protein (µmol/mg protein) (17).
2.8. Ethical Considerations
All experimental procedures were done in accordance with international guidelines for the care and use of laboratory animals and were approved by Kermanshah University of Medical Sciences, Kermanshah, Iran (Code: IR.KUMS.AEC.1401.004).
2.9. Statistical Analysis
Data analyses were performed using GraphPad Prism (version 9.0, San Diego, CA, USA). Data normality and homogeneity were verified with the Kolmogorov-Smirnov test. Quantitative data were presented as mean ± standard deviation and 95% confidence intervals (CI). Differences between experimental groups were evaluated using one-way ANOVA, followed by Tukey's post hoc test for comparisons. Statistical significance was defined as p < 0.05.
3. Results
3.1. Carob attenuates the decrease in testes weight induced by Cd
The increase in body weight observed in the Cd-injected group was lower compared to the control group, yet this difference did not reach statistical significance (p > 0.05). Additionally, no significant difference was observed between the CSE-treated groups and the control group (p > 0.05). Testis weight decreased significantly in both the Cd and Cd+CSE groups compared to the control group (p < 0.001), suggesting potential Cd-induced testis toxicity in these animals. Although CSE appeared to maintain testis weight to some extent, the notable difference between the Cd+CSE group and the control group persisted (p < 0.001) (Figure 1).
3.2. Carob reverses the adverse effects of Cd on spermatogenesis
Histopathological examinations of the control groups revealed normal histoarchitecture within the seminiferous tubules, featuring a sequential arrangement of germ cells at various stages of development and filled lumens containing spermatozoa (Figures 2A, B). In contrast, the group injected with Cd exhibited heterogeneous degenerative changes in the seminiferous tubule epithelium, including the presence of vacuoles, dissociation of the germinal epithelium, necrosis, interstitial edema, and compromised lumens, indicating adverse effects of Cd on testicular structure (Figure 2C). Conversely, the histological alterations induced by Cd in the testes were notably mitigated by CSE therapy (Figure 2D). To conclude, the reduced histological parameters suggest that Cd therapy triggered apoptosis in the spermatogenic cell line, consequently halting the process of spermatogenesis, which was significantly restored by CSE administration (Figure 2).
3.3. Carob alleviates the changes in testosterone synthesis induced by Cd
The testosterone level in the group treated with Cd showed a significant reduction compared to the control group (p < 0.001). Yet, administration of 100 mg/kg of CSE effectively restored the serum testosterone level significantly (p < 0.001), indicating a potential protective effect of CSE on testicular structure and function (Figure 3).
3.4. Carob rectifies the disruptions in sperm parameters caused by Cd
Figure 4 illustrates a significant decrease in sperm count observed in the Cd-administered group compared to the control group (p < 0.001), yet CSE therapy demonstrated potential for promoting recovery in Cd-injected mice (p < 0.001). Sperm motility decreased in the Cd group compared to the control group (p < 0.001), which was significantly mitigated by CSE (p < 0.001). Besides, the Cd-exposed group exhibited a significant increase in the number of dead sperm compared to the control (p < 0.001). Yet, CSE administration notably improved sperm survival compared to the Cd group (p < 0.001). Our findings also revealed that Cd treatment significantly induced abnormal sperm morphology compared to the control group (p < 0.001). Notably, CSE therapy alleviated this abnormal morphology, although the difference between the Cd+CSE group and the control group remained significant (p = 0.02) (Figure 4).
3.5. Carob alleviates Cd-induced impairment in sperm DNA integrity
Assessment of chromatin quality revealed a significant increase in both acridine orange (AB) and toluidine blue (TB) indices in the Cd-treated group compared to controls (p < 0.001 and p = 0.002, respectively). Conversely, treatment with CSE markedly decreased the proportion of AB-positive spermatozoa relative to the Cd-exposed group (p < 0.001) and significantly reduced the percentage of TB-positive sperm compared to the Cd-injected group (p = 0.004) (Figure 5).
3.6. Carob modulates testicular nitro-oxidative status after Cd exposure
Cd therapy led to a notable reduction in the level of TAC compared to the control group (p < 0.001); however, CSE therapy demonstrated potential for promoting recovery in Cd-injected mice (p < 0.001). It is imperative to note that the difference between the Cd+CSE group and the control group remained significant (p < 0.001). Further, the level of MDA significantly increased in the testes of the Cd-administered animals compared to the control (p < 0.001), indicating Cd exposure induced oxidative stress in the testes. Conversely, treatment with 100 mg/kg of CSE notably decreased the level of MDA in testicular tissue (p < 0.001). With respect to NO levels, a significant difference was observed between the Cd-treated and control groups (p < 0.001), indicating that Cd exposure induced nitrosative stress in testicular tissue. Even so, treatment with CSE led to a significant reduction in the NO level (p < 0.001), indicating that CSE was able to mitigate reactive nitrogen species in the testicular tissue (Figure 6).






4. Discussion
Recently, the decrease in male fertility has become a significant issue in public health, with one of the major contributing factors being the widespread presence of environmental toxins worldwide. Previous studies indicate that Cd poses a significant risk to both the environment and human health, and exposure to this toxic pollutant, whether through environmental factors or occupation, has harmful effects on male fertility (19, 21). Carob is highly nutritious and boasts numerous promising antioxidant and anti-inflammatory properties (12). To the best of our knowledge, for the first time, we deciphered that CSE protects against Cd-induced male reproductive toxicity by improving testosterone secretion and anti-oxidative defense in the testis.
Histopathological examinations revealed that CSE treatment preserved normal morphological and histopathological structures in the testes of mice injected with Cd. Cd administration disrupted Leydig and Sertoli cells, most likely due to inducing oxidative stress (22). Studies have indicated that exposure to Cd leads to the deterioration of Sertoli and Leydig cells, impacting testosterone levels and resulting in testicular necrosis and atrophy (8, 17). These effects may be explained by Cd's ability to disrupt the integrity of the blood-testis barrier, trigger oxidative stress and the release of inflammatory cytokines such as tumor necrosis factor α, induce DNA damage, and initiate apoptosis in germ cells. Spermatogenesis is a complex process affected by genetic, hormonal, and environmental elements, and any disruption in these areas leads to decreased sperm quality, ultimately causing infertility (3). CSE administration, however, restored the structure of seminiferous tubules and various stages of spermatogenesis, likely thanks to the modulation of nitro-oxidative stress by its antioxidant components, such as flavonoids, vitamins C, D, and E, and numerous minerals found in this herbal remedy (12, 13, 15).
A significant reduction in testosterone levels was observed in the Cd-treated group, supporting previous reports that Cd exposure interferes with steroid biosynthesis and lowers circulating testosterone levels. Cd disrupts hormone production through direct binding to androgenic and estrogenic receptors, altering the expression of steroidogenesis enzymes and inhibiting luteinizing hormone receptor expression (23). Additionally, Cd indirectly affects hormone secretion by hindering noradrenaline release, altering gonadotropin-releasing hormone release, and impacting luteinizing hormone and prolactin secretion (6). Thus, the impaired sperm parameters observed in our study may be attributed to reduced testosterone levels in the testes, which are essential for normal sperm production (24). Administration of 100 mg/kg of CSE could significantly improve the Cd-evoked disturbances in testosterone levels. This effect can be attributed to the ability of the potent antioxidant properties of carob extract components to modulate oxidative stress in the testis (14). In other words, the extract's antioxidant properties probably protect Leydig cells from damage caused by free radicals and inhibit steroidogenesis in the testes (10, 14, 15). In this regard, animal studies revealed that the utilization of carob extract positively affected cyclic adenosine monophosphate production and the functioning of enzymes engaged in rat testicular steroidogenesis (15).
Cd treatment resulted in a significant reduction in sperm count, motility, and viability, along with a noticeable rise in abnormal morphology. These findings are consistent with previous research showing that Cd exposure leads to reductions in sperm concentration, motility, and viability, as well as an increase in abnormal sperm morphology (8, 24). These findings could be attributed to Cd-induced oxidative stress and apoptosis factors, which contribute to a subsequent decline in sperm quality (8). Further, these results are in accordance with prior research suggesting that even at low levels of Cd accumulation in semen, it could potentially contribute to poor sperm parameters by causing oxidative damage to sperm, ultimately leading to male subfertility and infertility (25). More notably, Cd treatment markedly increased the aniline blue and toluidine blue indexes in sperm, indicating chromatin fragmentation in sperm cells. Likewise, research has shown that men with unexplained infertility often have elevated Cd levels in their semen, which correlates with a notable increase in sperm DNA fragmentation and seminal reactive oxygen species levels (26). It was also demonstrated that Cd plays a role in causing abnormal modifications to histones in the sperm nucleus during spermiogenesis and leads to damage in the sperm's nuclear DNA (17, 26). Yet, CSE therapy protected sperm characteristics and chromatin quality against Cd-evoked detrimental effects. We attributed these beneficial effects to the CSE's ability to quench free radicals by preventing the production of new radicals or scavenging and eliminating reactive species. With key constituents such as vitamins C, E, D, and folic acid, along with essential minerals like magnesium and selenium, which are crucial for spermatogenesis, CSE could enhance sperm parameters (27). Another explanation for the improvement in sperm parameters is testosterone-related behaviors, since it was reported that carob extract improves testosterone levels significantly. In this context, it was indicated that treatment of infertile men with carob for 90 days significantly improved semen parameters, including sperm concentration and motility, leading to increased pregnancy rates (15).
Our results showed a marked reduction in TAC with a noteworthy rise in NO and MDA levels, suggesting that Cd disturbs the testis redox system, resulting in considerable nitro-oxidative stress. The study has shown that Cd can provoke oxidative stress and LPO in several organs, such as the liver and testes (8). Oxidative stress plays a pivotal role in the etiology and pathogenicity of testis dysfunction and male infertility induced by Cd (8, 24). Cd-induced oxidative stress in the testes happens due to several mechanisms, including the reactive oxygen species production, glutathione reduction, elevation in LPO, inhibition of antioxidant enzyme expression and activity like catalase and superoxide dismutase, and interaction with proteins through sulfhydryl groups in cellular membrane proteins and other cellular proteins (17, 28, 29). Here, CSE played a crucial role in modulating nitro-oxidative stress induced by Cd via inhibition of NO and MDA generation and enhancing the antioxidant defense mechanisms in the testes. Our observations align with previous findings, which reported that treating infertile men with carob for 90 days increased TAC and reduced MDA levels in serum (15). Recent studies have revealed that CSE contains various compounds, including proanthocyanidin, ellagitannin, and gallotannin, as well as tannins, flavonoids, anthocyanins, glycosides, alkaloids, and vitamins such as C, D, E, pyridoxine, niacin, and folic acid. Additionally, it contains numerous minerals such as calcium, magnesium, selenium, potassium, phosphorus, and iron, most of which possess antioxidant properties (12, 13). Studies also have shown that carob’s protective effects against oxidative stress stem from its phenolic compounds, which act as hydrogen donors. This action reduces inflammation and apoptosis in the testicular tissue, improving testicular function (30).
5. Conclusion
Carob extract demonstrates protective effects on testicular function and spermatogenesis against Cd-induced reproductive toxicity. These beneficial findings can be largely attributed to the potent antioxidant properties of carob extract, as it contains phenolic and flavonoid compounds that function as free radical scavengers. These findings underscore the therapeutic potential of carob extract as a natural supplement for managing Cd-related male infertility, warranting further research to characterize its bioactive compounds and confirm its efficacy.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
M. Moradi: Methodology, conceptualization, investigation, writing, review, and editing. E. Ghanbari: Methodology, formal analysis, and investigation. Z. Rashidi: Visualization, validation, formal analysis, and supervision. S. Moradi: Methodology and investigation. A. Shakeri: Methodology, investigation, and conceptualization. A. Faramarzi: Conceptualization, visualization, validation, writing, review, editing, supervision, project administration. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
This work was performed in partial fulfillment of the requirements for the medical degree of Ali Shakeri in the School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran. This work was supported by the Kermanshah University of Medical Sciences, Kermanshah, Iran. The authors are grateful to Prof. Dr. Mitra Bakhtiari, Dr. Mohsen Shayestehyekta, and Bahareh Moradi for their technical assistance. Additionally, we would like to express our gratitude to the staff of the Fertility and Infertility Research Center, Kermanshah, Iran. Artificial intelligence has not been used in any way, including translation, revision, and grammar check in this work.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |