Sat, Jan 31, 2026
[Archive]
Volume 23, Issue 6 (June 2025)
IJRM 2025, 23(6): 507-516 |
Back to browse issues page
Ethics code: 93.12.20.180
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Dolatshahi B, Dorostghoal M, Galehdari H, Hemadi M. Association of sperm protamine1 and protamine2 transcript content with paternal age: A cross-sectional study. IJRM 2025; 23 (6) :507-516
URL: http://ijrm.ir/article-1-3502-en.html
URL: http://ijrm.ir/article-1-3502-en.html
1- Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
2- Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran. & Biotechnology and Bioscience Research Center, Shahid Chamran University of Ahvaz, Ahvaz, Iran. ,dorostghoal@gmail.com
3- Fertility, Infertility and Perinatology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
2- Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran. & Biotechnology and Bioscience Research Center, Shahid Chamran University of Ahvaz, Ahvaz, Iran. ,
3- Fertility, Infertility and Perinatology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Full-Text [PDF 724 kb]
(396 Downloads)
| Abstract (HTML) (434 Views)
4.1. Strengths and limitations
Full-Text: (64 Views)
- Introduction
Globally, infertility affects 15% of couples at reproductive age, with approximately half of the cases attributed by male factors (1). In developed countries, the problem of delayed parenthood brought on by socioeconomic circumstances is growing more common. In recent decades, there has been a notable increase in the average age at which couples first have children (2). There is growing evidence that, regardless of maternal age, declining fertility and offspring health are linked to prolonged paternal age (3, 4). Age-related progressive decreases in semen volume, sperm motility, and the percentage of morphologically normal sperm have been documented in a number of studies.
In contrast, other research has found no correlations or, less frequently, improvements in semen characteristics with age. Additionally, there is mounting evidence that certain genetic and chromosomal abnormalities in spermatozoa are more common as paternal age increases (5). There have been reports of an age-dependent rise in sperm DNA fragmentation (SDF) (6). The integrity of sperm DNA is important for embryonic development and pregnancy, and high levels of SDF have been found in infertile men and association with risk factors (lifestyle, pollutants, and drugs) for infertility (7). "Maintaining the integrity of sperm DNA permits the transfer of the very tightly packaged genetic information to the egg. It ensures that the DNA will be delivered in a physical and chemical form that allows the developing embryo to access genetic information". In this regard, protamines (PRMs) are needed to ensure tight compaction of DNA in sperm and replace about 85% of histones during spermatogenesis. Human sperm contains 2 types of PRM: PRM1 and PRM2 (8).
Several studies have shown that male infertility is linked to aberrant PRM1-PRM2 and histone-to-PRM ratios at the mRNA level in testicular spermatids and the protein level in ejaculated spermatozoa (9). Male fertility can be efficiently assessed using the DNA fragmentation index and the sperm PRM-1/2 mRNA ratio (10). Increased vulnerability to DNA damage has been associated with abnormalities in the PRM level of sperm nuclei (8). Male infertility has also been linked to inadequate PRM packing (11) and poor results from assisted reproduction, which impact the rate of fertilization and the early stages of embryo development (12).
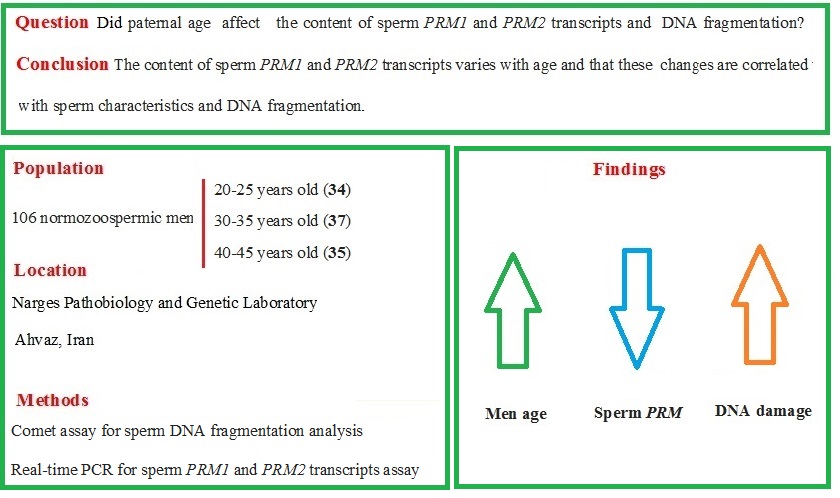
The purpose of the current study was to examine how male age affected the amount of PRM1 and PRM2 transcripts and DNA damage in normozoospermic men.
2. Materials and Methods
2.1. Subjects
In this cross-sectional study, semen samples were collected from normozoospermic men who were referred to the Narges Pathobiology and Genetic Laboratory, Ahvaz, Iran between 2014 and 2016. Our inclusion criteria were men with normal semen parameters (13). Our exclusion criteria were smoking, alcohol consumption, drug use, certain dietary habits, antioxidant supplementation use, exposure to possible poisons that affect reproduction, azoospermia, varicocele, cryptorchidism, prostatitis, urinary tract infections, genital trauma, testicular torsion, history of inguinal or genital surgery, sexually transmitted diseases, diabetes, cardiovascular disease, and kidney or liver disorders. All semen samples were delivered within 2 hr of collection. A single laboratory technician, unaware of the participants' ages and identities, conducted all the analyses. The semen analysis was performed immediately upon receipt, typically around 45 min after production (5-120 min). If a sample had a volume of < 1 ml, zero motility, abnormal numbers of red or white blood cells, or if the donor reported potential sample loss, duplicate samples were requested.
2.2. Sample size
Semen specimens were collected randomly from a cohort of 106 normozoospermic men and were analyzed in 3 age groups: 20-25, 30-35, and 40-45 yr. Samples were chosen at the beginning of 3 different decades of human life. The sample size in the present study was determined based on previous studies (14).
2.3. Semen analysis
Following 3 days of sexual abstinence, sperm samples were collected through masturbation in wide-mouth sterile plastic containers. Semen specimens were allowed to liquefy at room temperature for 30 min before being macroscopically and microscopically analyzed in accordance with World Health Organization guidelines (13). Sperm parameters were judged to be normal when the concentration was ≥ 15 × 106 ml, motility was ≥ 40%, and normal sperm forms were ≥ 4%. Semen samples having > 1 × 106 white blood cells/ml were eliminated.
2.4. Single-cell gel electrophoresis (comet) assay
The modified neutral comet assay was used to assess sperm DNA integrity, as described by Singh, Muller, and Berger (15). In summary, a sperm cell solution was combined with low-melting point agarose (Sigma Co., USA), layered onto a slide pre-coated with low-melting point agarose, allowed to solidify, and then covered with a third layer of low-melting point agarose. The slides were then immersed in an electrophoresis unit (Bio-Rad, USA) filled with freshly prepared neutral electrophoresis solution (100 mm Tris HCl, 500 mm NaCl, and 500 mm).
2.5. RNA extraction, cDNA synthesis and quantitative polymerase chain reaction
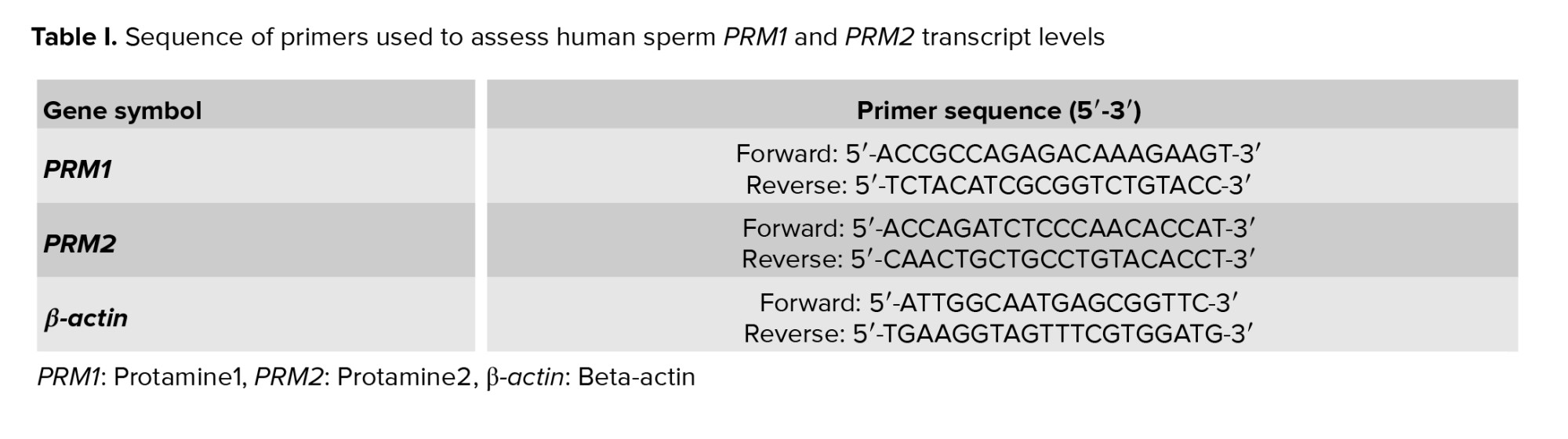
Following 2 rounds of washing semen samples in phosphate buffer saline (Ca2+Mg2+-free, pH 7.4, 0.1 mM), the TRIzol reagent (Invitrogen Corporation, Carlsbad, CA) was used to extract total RNA. Using agarose electrophoresis and a NanoDrop spectrophotometer, the isolated RNA's integrity was assessed (Thermo-Scientific, Wilmington, USA). Total RNA was treated with DNase I (Promega, Madison, USA) and reverse-transcribed into cDNA using PrimeScript® RT reagent kit (TaKaRa, Dalian, China). Version 7.0 of the oligo-dt software was used to design the primers (Molecular Biology Insights, Cascade, CO., USA) and the BLAST website to confirm specificity (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table I). Using the Light Cycler 96 System device, quantitative real-time analysis was conducted (Roche Applied Science, Indianapolis, IN, USA) using SYBR green I Lightcycler FastStart DNA Master Plus (Roche). Every cDNA product was examined twice, and the relative expression of target genes was evaluated using the ΔΔCt method. B-actin was used as a control gene to standardize the PRM1 and PRM2 mRNA levels, and a ΔCt value was computed for every sample.
2.6. Ethical Considerations
The Ethics Committee of the Department of Biology, Shahid Chamran University of Ahvaz, Ahvaz, Iran approved the current study (Code: 93.12.20.180). The written informed consent was obtained from all subjects enrolled in this study.
2.7. Statistical Analysis
SPSS software version 16.0 (SPSS Corp., Chicago, IL, USA) was used to analyze the data. The Mann-Whitney test was used to assess the differences in sperm parameters and ΔCt of PRM1 and PRM2 across groups. The correlations between sperm characteristics and the ΔCt of PRM1 and PRM2 were evaluated using the Spearman test. A significance level of p < 0.05 was deemed statistically significant.
3. Results
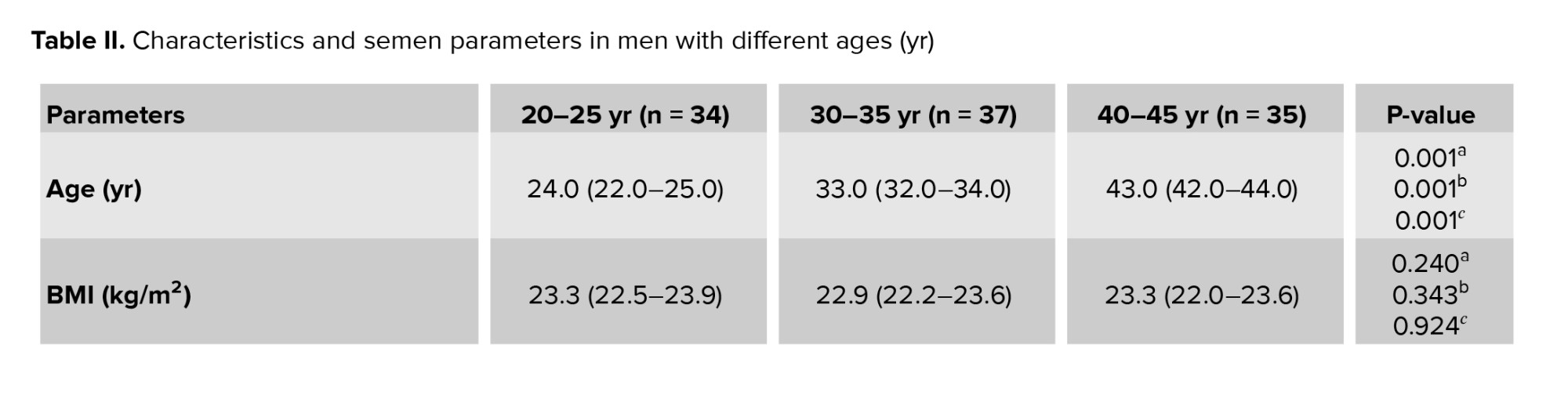
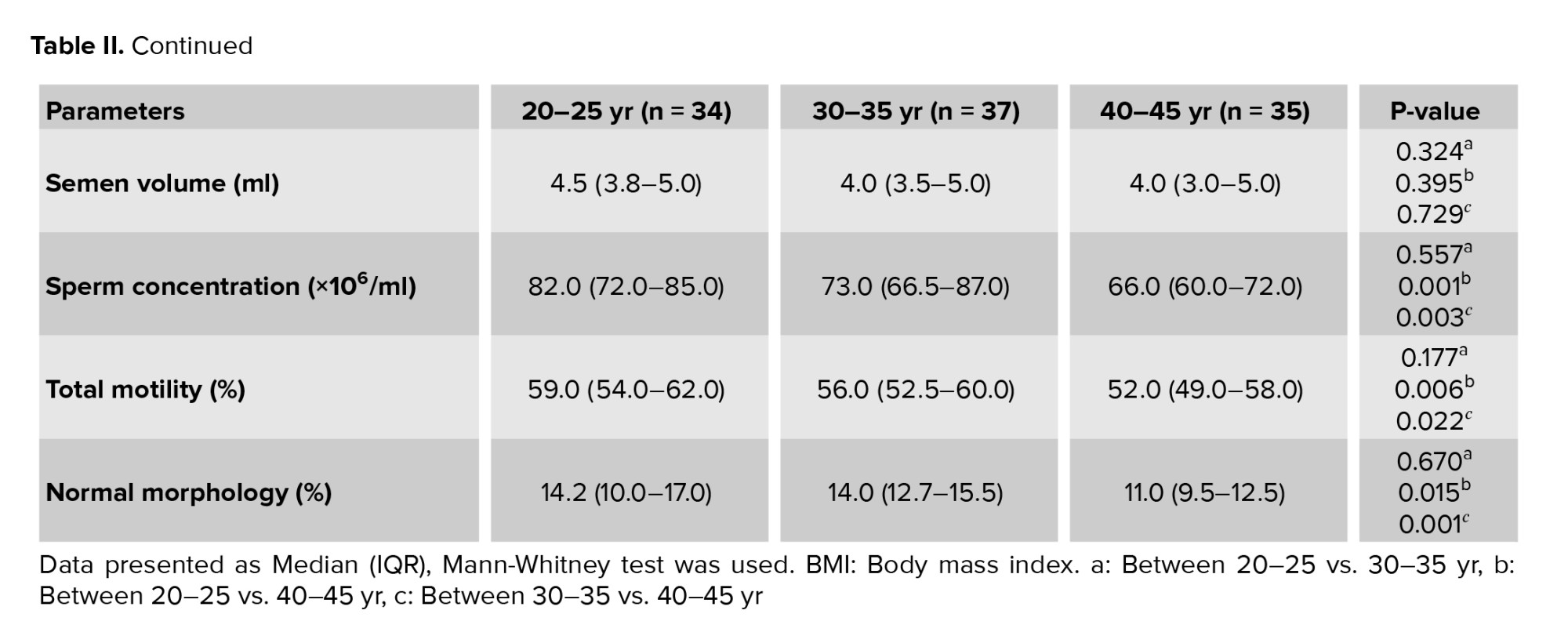
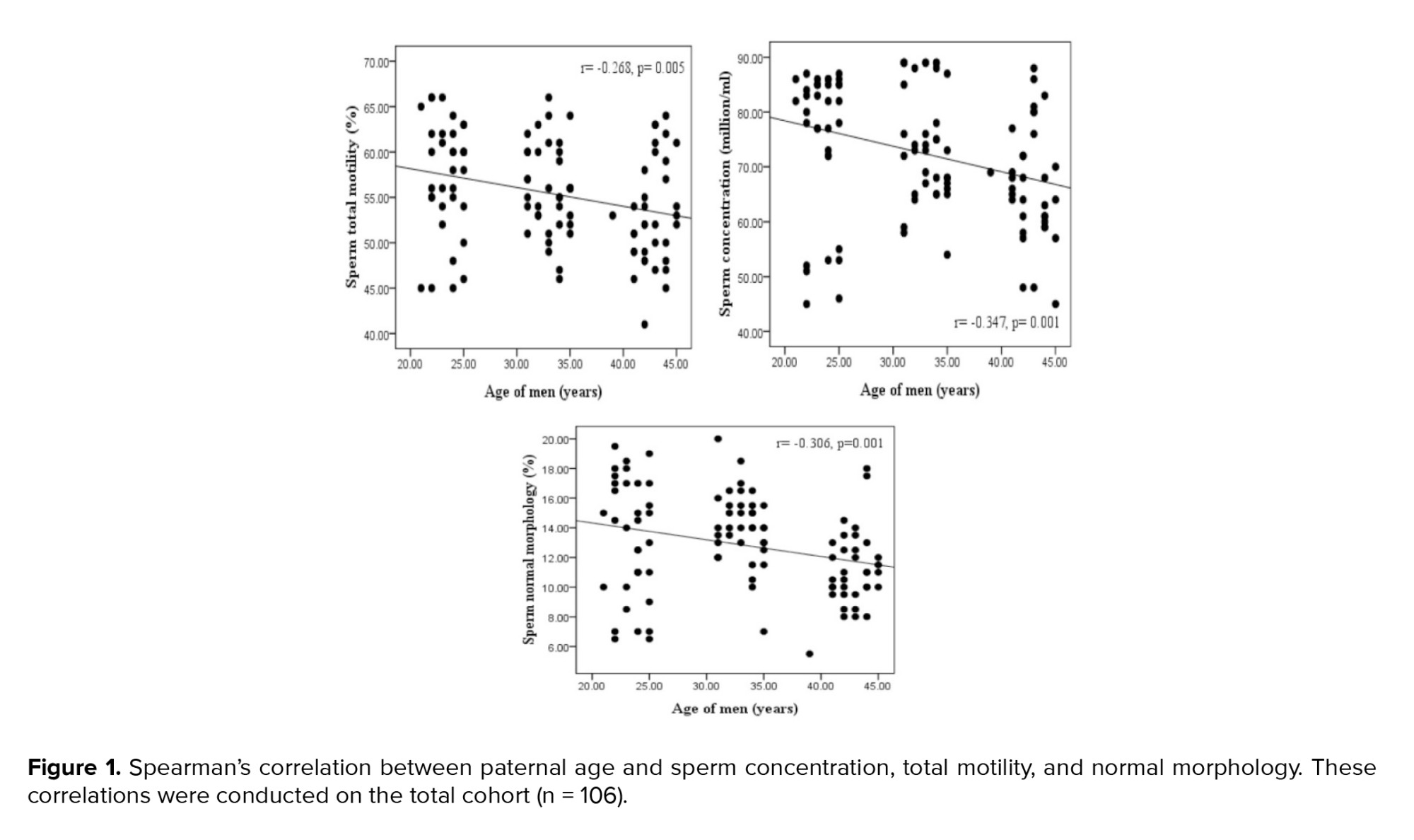
The median and interquartile ranges for sperm parameters are displayed in men of various ages (Table II). No significant difference was observed in the semen volume among the men in different age groups. There were significant differences in the sperm parameters (concentration, motility, and morphology) among different age groups. The age groups of 20-25 and 40-45 yr showed the significantly greatest and lowest levels of sperm concentration, total motility, and normal morphology, respectively. Significant negative correlations were found between sperm concentration and age of men. Additionally, sperm motility and paternal age showed a significant negative correlation (Figure 1).
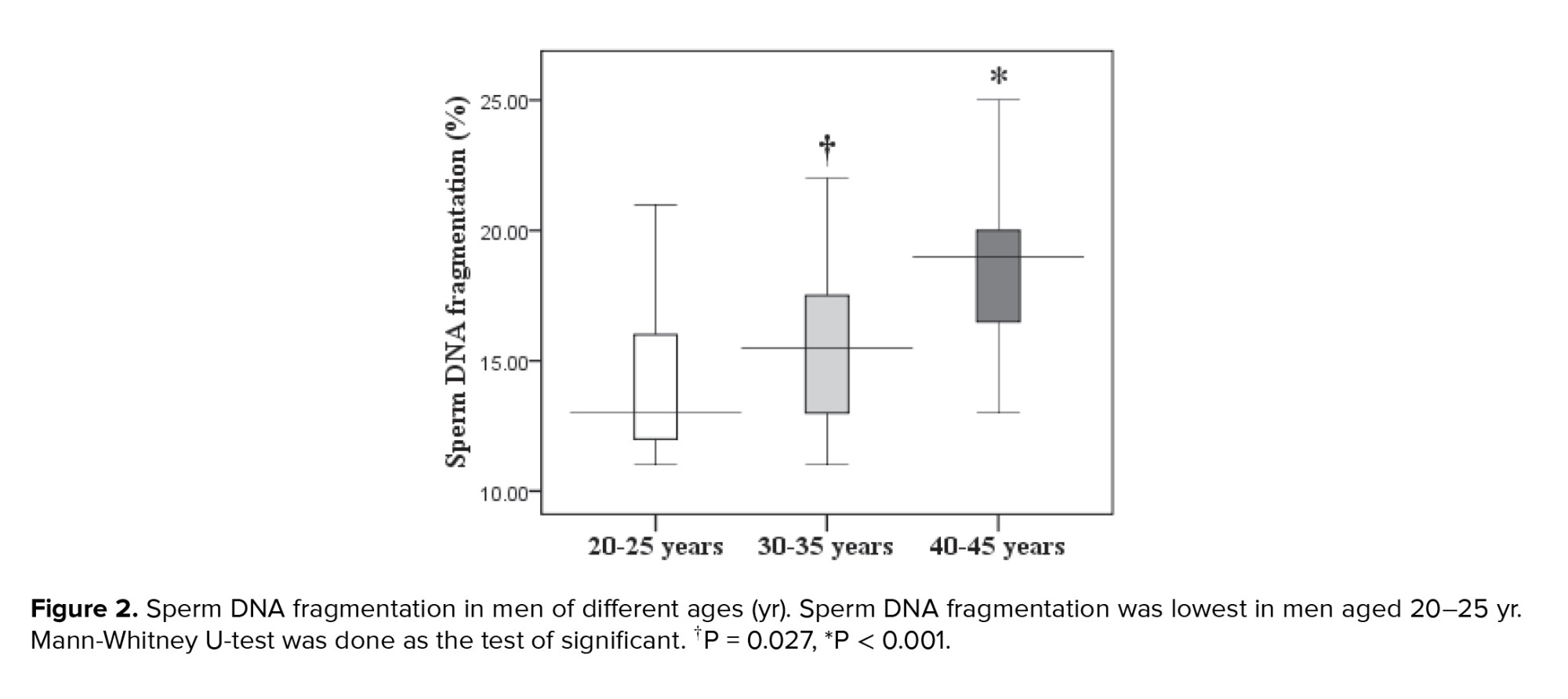
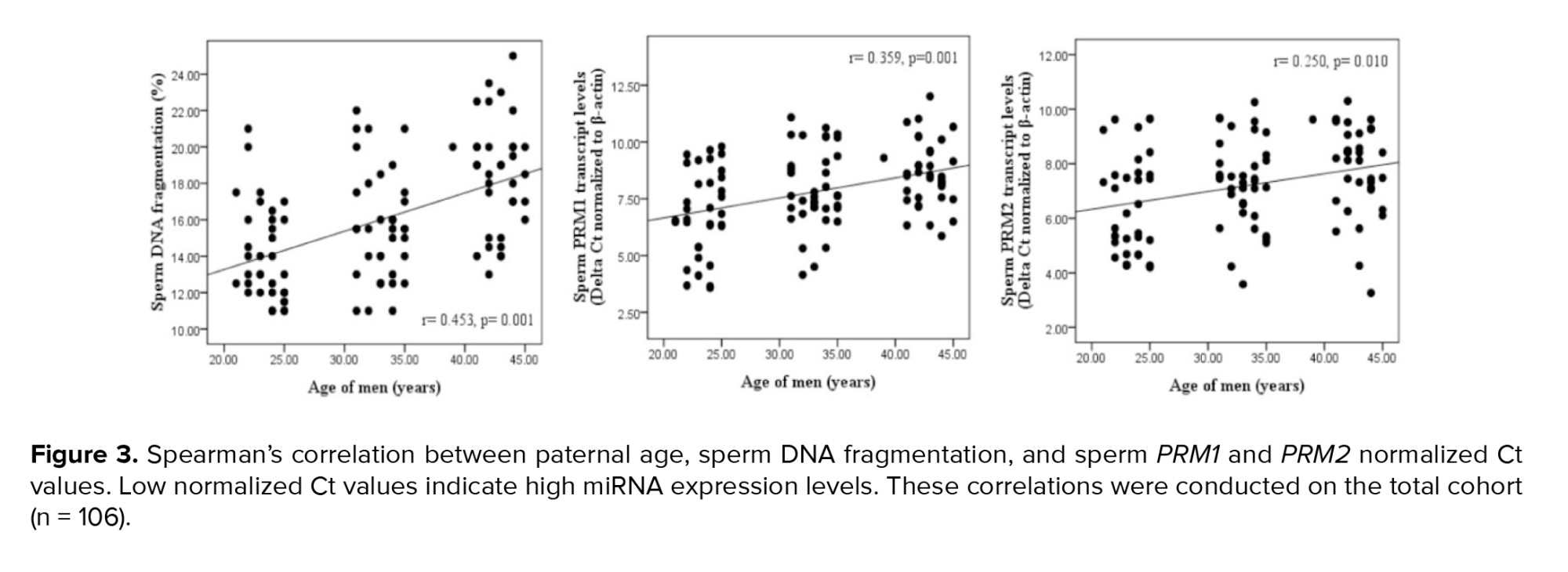
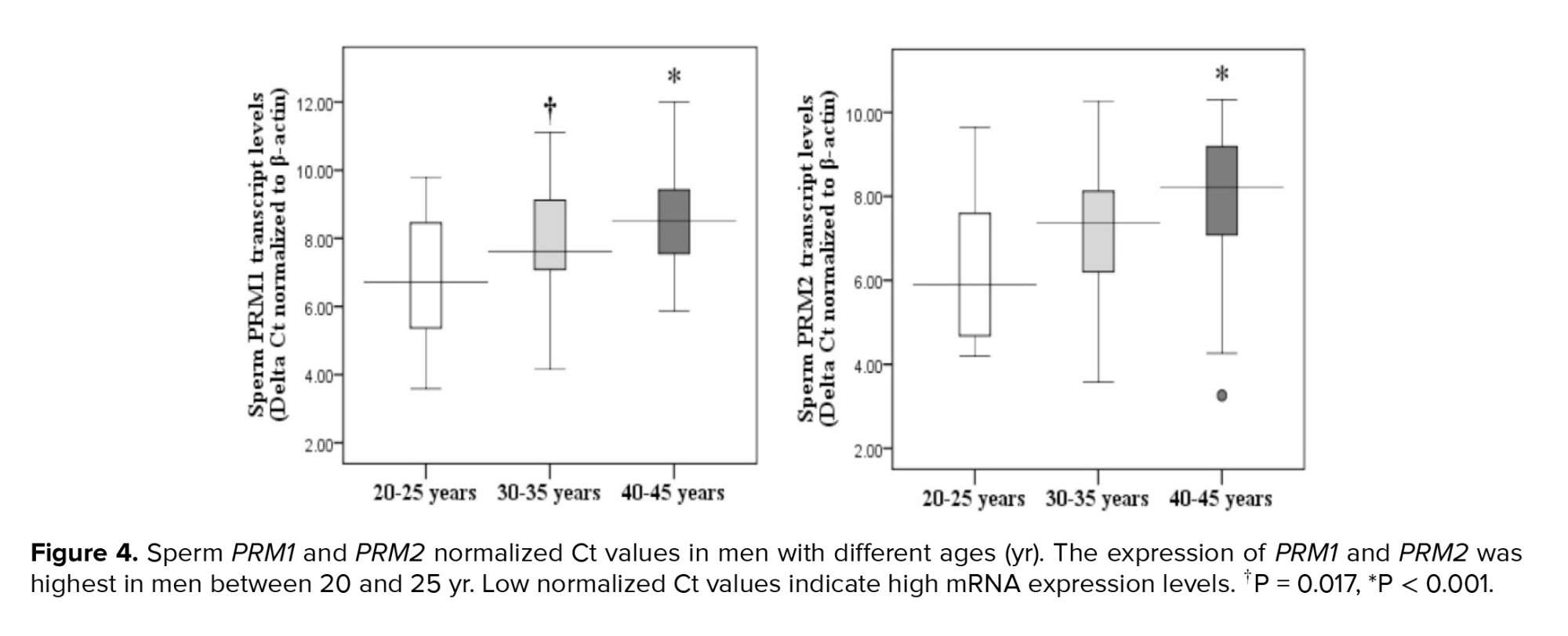
Paternal age and sperm normal morphology showed a significant negative correlation (Figure 1). The fragmentation of sperm DNA varied significantly among men of different ages. SDF levels were significantly lower in the 20-25 yr age group and higher in the 40-45 yr age group (Figure 2). SDF and paternal age were significantly correlated negatively (Figure 3). The content of sperm PRM1 and PRM2 transcripts varied significantly between different age groups. Sperm PRM1 and PRM2 transcript levels were significantly lower in men older than > 40 yr and significantly higher in the 20-25 yr age group (Figure 4).
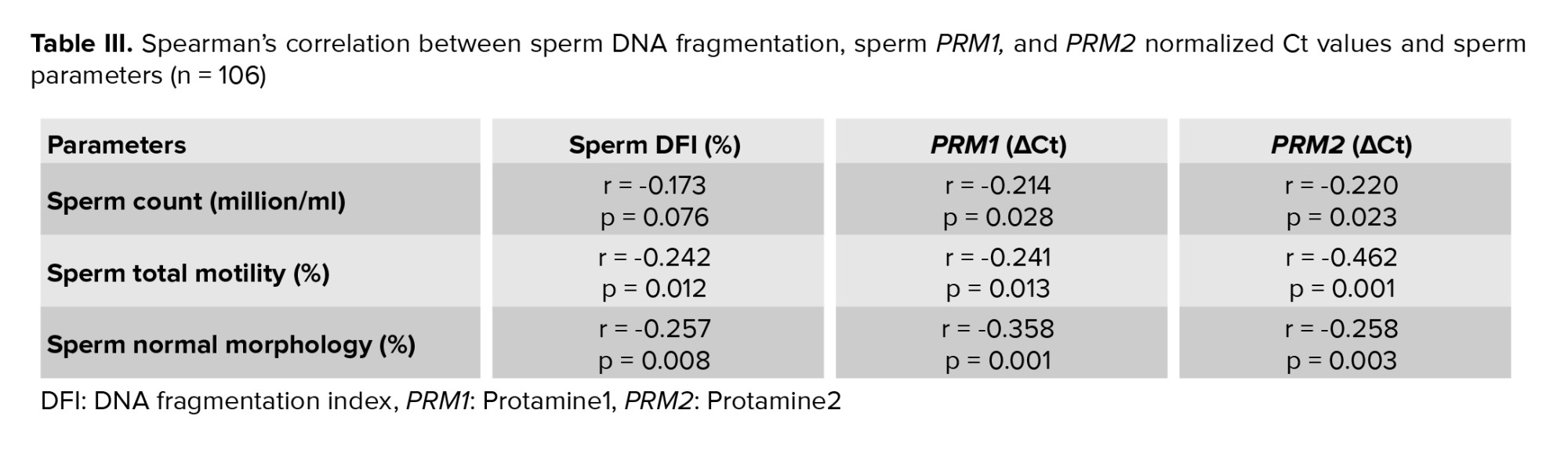
Paternal age and sperm PRM1 showed a significant negative correlation (Figure 3). The content of sperm PRM2 transcripts and paternal age showed a significant negative correlation (Figure 3). A significant correlation was found between sperm concentration and sperm PRM1 and PRM2 transcript content (Table III). Reduced sperm motility and normal morphology were associated with low PRM1 and PRM2 transcript levels in ejaculated spermatozoa.
4. Discussion
In contrast, other research has found no correlations or, less frequently, improvements in semen characteristics with age. Additionally, there is mounting evidence that certain genetic and chromosomal abnormalities in spermatozoa are more common as paternal age increases (5). There have been reports of an age-dependent rise in sperm DNA fragmentation (SDF) (6). The integrity of sperm DNA is important for embryonic development and pregnancy, and high levels of SDF have been found in infertile men and association with risk factors (lifestyle, pollutants, and drugs) for infertility (7). "Maintaining the integrity of sperm DNA permits the transfer of the very tightly packaged genetic information to the egg. It ensures that the DNA will be delivered in a physical and chemical form that allows the developing embryo to access genetic information". In this regard, protamines (PRMs) are needed to ensure tight compaction of DNA in sperm and replace about 85% of histones during spermatogenesis. Human sperm contains 2 types of PRM: PRM1 and PRM2 (8).
Several studies have shown that male infertility is linked to aberrant PRM1-PRM2 and histone-to-PRM ratios at the mRNA level in testicular spermatids and the protein level in ejaculated spermatozoa (9). Male fertility can be efficiently assessed using the DNA fragmentation index and the sperm PRM-1/2 mRNA ratio (10). Increased vulnerability to DNA damage has been associated with abnormalities in the PRM level of sperm nuclei (8). Male infertility has also been linked to inadequate PRM packing (11) and poor results from assisted reproduction, which impact the rate of fertilization and the early stages of embryo development (12).
The purpose of the current study was to examine how male age affected the amount of PRM1 and PRM2 transcripts and DNA damage in normozoospermic men.
2. Materials and Methods
2.1. Subjects
In this cross-sectional study, semen samples were collected from normozoospermic men who were referred to the Narges Pathobiology and Genetic Laboratory, Ahvaz, Iran between 2014 and 2016. Our inclusion criteria were men with normal semen parameters (13). Our exclusion criteria were smoking, alcohol consumption, drug use, certain dietary habits, antioxidant supplementation use, exposure to possible poisons that affect reproduction, azoospermia, varicocele, cryptorchidism, prostatitis, urinary tract infections, genital trauma, testicular torsion, history of inguinal or genital surgery, sexually transmitted diseases, diabetes, cardiovascular disease, and kidney or liver disorders. All semen samples were delivered within 2 hr of collection. A single laboratory technician, unaware of the participants' ages and identities, conducted all the analyses. The semen analysis was performed immediately upon receipt, typically around 45 min after production (5-120 min). If a sample had a volume of < 1 ml, zero motility, abnormal numbers of red or white blood cells, or if the donor reported potential sample loss, duplicate samples were requested.
2.2. Sample size
Semen specimens were collected randomly from a cohort of 106 normozoospermic men and were analyzed in 3 age groups: 20-25, 30-35, and 40-45 yr. Samples were chosen at the beginning of 3 different decades of human life. The sample size in the present study was determined based on previous studies (14).
2.3. Semen analysis
Following 3 days of sexual abstinence, sperm samples were collected through masturbation in wide-mouth sterile plastic containers. Semen specimens were allowed to liquefy at room temperature for 30 min before being macroscopically and microscopically analyzed in accordance with World Health Organization guidelines (13). Sperm parameters were judged to be normal when the concentration was ≥ 15 × 106 ml, motility was ≥ 40%, and normal sperm forms were ≥ 4%. Semen samples having > 1 × 106 white blood cells/ml were eliminated.
2.4. Single-cell gel electrophoresis (comet) assay
The modified neutral comet assay was used to assess sperm DNA integrity, as described by Singh, Muller, and Berger (15). In summary, a sperm cell solution was combined with low-melting point agarose (Sigma Co., USA), layered onto a slide pre-coated with low-melting point agarose, allowed to solidify, and then covered with a third layer of low-melting point agarose. The slides were then immersed in an electrophoresis unit (Bio-Rad, USA) filled with freshly prepared neutral electrophoresis solution (100 mm Tris HCl, 500 mm NaCl, and 500 mm).
2.5. RNA extraction, cDNA synthesis and quantitative polymerase chain reaction
Following 2 rounds of washing semen samples in phosphate buffer saline (Ca2+Mg2+-free, pH 7.4, 0.1 mM), the TRIzol reagent (Invitrogen Corporation, Carlsbad, CA) was used to extract total RNA. Using agarose electrophoresis and a NanoDrop spectrophotometer, the isolated RNA's integrity was assessed (Thermo-Scientific, Wilmington, USA). Total RNA was treated with DNase I (Promega, Madison, USA) and reverse-transcribed into cDNA using PrimeScript® RT reagent kit (TaKaRa, Dalian, China). Version 7.0 of the oligo-dt software was used to design the primers (Molecular Biology Insights, Cascade, CO., USA) and the BLAST website to confirm specificity (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table I). Using the Light Cycler 96 System device, quantitative real-time analysis was conducted (Roche Applied Science, Indianapolis, IN, USA) using SYBR green I Lightcycler FastStart DNA Master Plus (Roche). Every cDNA product was examined twice, and the relative expression of target genes was evaluated using the ΔΔCt method. B-actin was used as a control gene to standardize the PRM1 and PRM2 mRNA levels, and a ΔCt value was computed for every sample.
2.6. Ethical Considerations
The Ethics Committee of the Department of Biology, Shahid Chamran University of Ahvaz, Ahvaz, Iran approved the current study (Code: 93.12.20.180). The written informed consent was obtained from all subjects enrolled in this study.
2.7. Statistical Analysis
SPSS software version 16.0 (SPSS Corp., Chicago, IL, USA) was used to analyze the data. The Mann-Whitney test was used to assess the differences in sperm parameters and ΔCt of PRM1 and PRM2 across groups. The correlations between sperm characteristics and the ΔCt of PRM1 and PRM2 were evaluated using the Spearman test. A significance level of p < 0.05 was deemed statistically significant.
3. Results
The median and interquartile ranges for sperm parameters are displayed in men of various ages (Table II). No significant difference was observed in the semen volume among the men in different age groups. There were significant differences in the sperm parameters (concentration, motility, and morphology) among different age groups. The age groups of 20-25 and 40-45 yr showed the significantly greatest and lowest levels of sperm concentration, total motility, and normal morphology, respectively. Significant negative correlations were found between sperm concentration and age of men. Additionally, sperm motility and paternal age showed a significant negative correlation (Figure 1).
Paternal age and sperm normal morphology showed a significant negative correlation (Figure 1). The fragmentation of sperm DNA varied significantly among men of different ages. SDF levels were significantly lower in the 20-25 yr age group and higher in the 40-45 yr age group (Figure 2). SDF and paternal age were significantly correlated negatively (Figure 3). The content of sperm PRM1 and PRM2 transcripts varied significantly between different age groups. Sperm PRM1 and PRM2 transcript levels were significantly lower in men older than > 40 yr and significantly higher in the 20-25 yr age group (Figure 4).
Paternal age and sperm PRM1 showed a significant negative correlation (Figure 3). The content of sperm PRM2 transcripts and paternal age showed a significant negative correlation (Figure 3). A significant correlation was found between sperm concentration and sperm PRM1 and PRM2 transcript content (Table III). Reduced sperm motility and normal morphology were associated with low PRM1 and PRM2 transcript levels in ejaculated spermatozoa.








4. Discussion
Our study looked at the association of advanced paternal age with semen quality and PRM content. Our findings demonstrated that there are notable differences in sperm parameters among age groups of men. The age group of 20-25 yr exhibited significantly greatest levels of sperm concentration, motility, and normal morphology. The age group 40-45 yr showed the lowest levels of sperm concentration, motility, and normal morphology. Additionally, we discovered a strong inverse relationship between sperm quality and paternal age. Our results are consistent with other research demonstrating a correlation between declining sperm quality and increasing paternal age.
Semen parameters were significantly associated with male age (16). A decrease in semen parameters starts at the age of 34 yr and it is postulated to affect the pregnancy status (17). Increase in aberrant sperm morphology and decrease in normal fertilization are linked to increased paternal age (18). Furthermore, it was revealed that after 50 yr of age, the sperm parameters can be greatly changed, specifically the volume and the concentration (19). Reduced gamete production might be one of the physiological mechanisms underlying the negative effect of increasing paternal age (20).
The results of our study also demonstrated that SDF is positively correlated with paternal age, with men aged 40-45 yr having significantly higher DNA fragmentation than younger normozoosperm men. Additionally, our study revealed that DNA fragmentation was significantly higher in men aged 40-45 yr compared to younger men (21). It has been reported that men > 45 yr old had the largest percentage of DNA fragmentation index when compared to younger age individuals (6), and increasing paternal age was linked to higher SDF (22). Male reproductive potential can be greatly impacted by the detrimental effects of aging on sperm DNA integrity. In fact, alterations in sperm DNA have been connected to decreased implantation rates, a higher risk of miscarriage, altered embryo and blastocyst development, and reduced fertilization (23).
Furthermore, our findings showed that there are significant differences in sperm PRM1 and PRM2 transcript content between different age groups of men. Age groups 20-25 and 40-45 yr had the highest and lowest amounts of sperm PRM1 and PRM2 transcript content, respectively. Our findings also demonstrated a strong relationship between the transcript content of sperm PRM1 and PRM2 and the father's age. The PRM1 and PRM2 genes may be helpful indicators in predicting male infertility (24). Kaarouch et al. also found that expression levels of PRM1 and PRM2 dropped to 31% in aged patients (25).
Additionally, our results demonstrated a strong relationship between sperm parameters and PRM1 and PRM2 mRNA levels. Numerous studies have demonstrated a relationship between PRM transcript patterns and seminal characteristics such as sperm motility and morphology (26, 27). A significant association was observed between the quantity of PRM1 and PRM2 transcripts and sperm morphology (28). Lack of PRM2 results in infertility with significant abnormalities in sperm motility and head morphology (29). Changes in PRM mRNA have been linked to sperm motility (30). Men with higher motility had lower PRM1 transcript levels than those with weakly motile sperm (26). PRM insufficiency was more commonly seen in spermatozoa with defective head morphology (30).
Semen parameters were significantly associated with male age (16). A decrease in semen parameters starts at the age of 34 yr and it is postulated to affect the pregnancy status (17). Increase in aberrant sperm morphology and decrease in normal fertilization are linked to increased paternal age (18). Furthermore, it was revealed that after 50 yr of age, the sperm parameters can be greatly changed, specifically the volume and the concentration (19). Reduced gamete production might be one of the physiological mechanisms underlying the negative effect of increasing paternal age (20).
The results of our study also demonstrated that SDF is positively correlated with paternal age, with men aged 40-45 yr having significantly higher DNA fragmentation than younger normozoosperm men. Additionally, our study revealed that DNA fragmentation was significantly higher in men aged 40-45 yr compared to younger men (21). It has been reported that men > 45 yr old had the largest percentage of DNA fragmentation index when compared to younger age individuals (6), and increasing paternal age was linked to higher SDF (22). Male reproductive potential can be greatly impacted by the detrimental effects of aging on sperm DNA integrity. In fact, alterations in sperm DNA have been connected to decreased implantation rates, a higher risk of miscarriage, altered embryo and blastocyst development, and reduced fertilization (23).
Furthermore, our findings showed that there are significant differences in sperm PRM1 and PRM2 transcript content between different age groups of men. Age groups 20-25 and 40-45 yr had the highest and lowest amounts of sperm PRM1 and PRM2 transcript content, respectively. Our findings also demonstrated a strong relationship between the transcript content of sperm PRM1 and PRM2 and the father's age. The PRM1 and PRM2 genes may be helpful indicators in predicting male infertility (24). Kaarouch et al. also found that expression levels of PRM1 and PRM2 dropped to 31% in aged patients (25).
Additionally, our results demonstrated a strong relationship between sperm parameters and PRM1 and PRM2 mRNA levels. Numerous studies have demonstrated a relationship between PRM transcript patterns and seminal characteristics such as sperm motility and morphology (26, 27). A significant association was observed between the quantity of PRM1 and PRM2 transcripts and sperm morphology (28). Lack of PRM2 results in infertility with significant abnormalities in sperm motility and head morphology (29). Changes in PRM mRNA have been linked to sperm motility (30). Men with higher motility had lower PRM1 transcript levels than those with weakly motile sperm (26). PRM insufficiency was more commonly seen in spermatozoa with defective head morphology (30).
Furthermore, it has been observed that the percentage of increasingly motile sperm and the total motile sperm are substantially correlated with normal ratios of PRM transcripts (31). Sperm transcript abundance of PRM1 and PRM2 were higher in bulls with higher sperm motility. Moreover, it has been revealed that there is a higher transcript level of PRM1 in the Frieswal bulls with high-quality sperm than in the group with low-quality semen (32). Individuals with oligozoospermia, asthenozoospermia, and teratozoospermia had significantly varied percentages of spermatozoa with PRM deficiency (11). In addition, the current study demonstrated that abnormally low transcript levels of PRM1 and PRM2 in ejaculated spermatozoa are linked to SDF. Normozoospermic males and azoospermic patients have quite different sperm PRM mRNA ratios. The PRM2/PRM1 mRNA ratio and SDF were shown to be significantly correlated (33).
4.1. Strengths and limitations
Our study, refers to the effects of paternal age on sperm PRM transcripts content. However, other factors such as occupational status and diet can affect our results. This study needs to be investigated in a larger sample size.
5. Conclusion
Our findings showed that PRM transcript concentration and paternal age are related, and aberrant genomic stability is linked to lower sperm quality and decreased fertility in older men. According to this study, the evaluation of sperm PRM mRNA abundance has high predictive values for sperm quality analysis. It could result in the creation of novel treatment strategies for male infertility.
Data Availability
The corresponding author can provide data supporting the study's conclusions upon reasonable request.
Author Contributions
M. Dorostghoal, H. Galehdari, and M. Hemadi contributed to the study conception and design. B. Dolatshahi performed material preparation, data collection, and analysis. All authors contributed to the writing of the original draft, review, and editing. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
The authors would like to thank the Vice Chancellor for Research of Shahid Chamran University of Ahvaz, Iran for the research (grant no: 95.30.02.31400). The authors declare that no artificial intelligence was used in the preparation of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
The corresponding author can provide data supporting the study's conclusions upon reasonable request.
Author Contributions
M. Dorostghoal, H. Galehdari, and M. Hemadi contributed to the study conception and design. B. Dolatshahi performed material preparation, data collection, and analysis. All authors contributed to the writing of the original draft, review, and editing. All authors approved the final manuscript and take responsibility for the integrity of the data.
Acknowledgments
The authors would like to thank the Vice Chancellor for Research of Shahid Chamran University of Ahvaz, Iran for the research (grant no: 95.30.02.31400). The authors declare that no artificial intelligence was used in the preparation of this manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Genetics
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |