Sun, Feb 22, 2026
[Archive]
Volume 23, Issue 7 (July 2025)
IJRM 2025, 23(7): 559-568 |
Back to browse issues page
Ethics code: IR.IAU.SARI.REC.1400.050
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sedaghatizadeh K, Nemati F, Gholamitabar Tabari M, Peyravii Ghadikolaii F, Bishehkolaei R. Protective effects of Spirulina platensis and phycocyanin on sperm quality, DNA integrity, and testicular tissue following oxidative stress in rats: An experimental study. IJRM 2025; 23 (7) :559-568
URL: http://ijrm.ir/article-1-3533-en.html
URL: http://ijrm.ir/article-1-3533-en.html
Katayoun Sedaghatizadeh1 

 , Farkhondeh Nemati *2
, Farkhondeh Nemati *2 

 , Maryam Gholamitabar Tabari3
, Maryam Gholamitabar Tabari3 

 , Fatemeh Peyravii Ghadikolaii1
, Fatemeh Peyravii Ghadikolaii1 

 , Roya Bishehkolaei1
, Roya Bishehkolaei1 




 , Farkhondeh Nemati *2
, Farkhondeh Nemati *2 

 , Maryam Gholamitabar Tabari3
, Maryam Gholamitabar Tabari3 

 , Fatemeh Peyravii Ghadikolaii1
, Fatemeh Peyravii Ghadikolaii1 

 , Roya Bishehkolaei1
, Roya Bishehkolaei1 


1- Department of Biology, Faculty of Basic Sciences, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran.
2- Department of Biology, Faculty of Basic Sciences, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran. ,2060306833@iau.ir; far.nemati12@gmail.com
3- Health Reproductive Research Center, Sari Branch, Islamic Azad University, Sari, Iran.
2- Department of Biology, Faculty of Basic Sciences, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran. ,
3- Health Reproductive Research Center, Sari Branch, Islamic Azad University, Sari, Iran.
Full-Text [PDF 2467 kb]
(979 Downloads)
| Abstract (HTML) (668 Views)
Full-Text: (134 Views)
1. Introduction
A significant contributor in male infertility is oxidative stress, which arises from an imbalance between reactive oxygen species (ROS) and the body's antioxidant defenses (1). When the production of ROS surpasses antioxidant capacity, it leads to cellular damage that can contribute to chronic illnesses such as cardiovascular diseases, diabetes, and cancer (2). Oxidative stress arises from an imbalance between ROS production and antioxidant defenses. This state can lead to cellular damage, inflammation, and apoptosis (3).
Hydrogen peroxide (HP, or H2O2) causes significant DNA damage in various cell types; however, DNA repair mechanisms can mitigate this damage. Inhibiting these repair mechanisms exacerbates oxidative damage, highlighting their crucial role in cellular responses to oxidative stress (4).
Spermatozoa are particularly vulnerable to ROS-induced damage due to limited antioxidant defenses and high levels of polyunsaturated fatty acids, which are prone to oxidation (5). Additionally, sperm cells generate ROS themselves, further exacerbating oxidative stress. As these cells depend on external antioxidants for protection, they are more susceptible to oxidative damage (6). Natural antioxidants found in Spirulina platensis (Sp), a nutrient-rich blue-green microalga, may provide therapeutic benefits against male infertility (7). Sp is rich in essential nutrients and antioxidants, particularly phycocyanin (PC), a protein believed to drive its biological effects (8).
Furthermore, a recent study has highlighted the potential benefits of Sp and its component C-PC on male reproductive health. C-PC has been shown to protect against reproductive system damage in male mice, improving spermatogenesis, sperm quality, and fertility (9). Similarly, Sp demonstrated protective effects against arsenic-induced testicular oxidative stress in rats, enhancing sperm parameters and hormonal levels (10). These findings suggest that Sp and its derivatives could serve as promising reproductive system protective agents. However, research gaps remain, particularly regarding the effects of microcystins on reproductive toxicity across various species (11). Additionally, further investigation is needed to optimize the delivery methods of Sp for enhanced therapeutic outcomes, including improved bioavailability and targeted delivery (12).
This study investigated the effects of PC and Sp on testicular redox balance (oxidant/antioxidant status), morphology, and sperm parameters (motility, viability, DNA integrity) in male Wistar rats.
2. Materials and Methods
2.1. Animals
This experimental study was conducted in 2022 at Islamic Azad University, Qaemshahr Branch, Qaemshahr, Iran, using 36 male Wistar rats (7-8 wk, 180-220 gr). The rats were sourced from the North Research Center of Institut Pasteur, Amol, Iran, and housed in standard cages under controlled conditions (22 ± 2°C, 50 ± 5% humidity, 12-hr light/dark cycle) with unrestricted access to water and a standard diet.
A power analysis was conducted using G*Power software (version 3.1) to ensure adequate statistical power. Assuming an effect size of 0.5 (medium effect), a significance level (α) of 0.05, and a power (1-β) of 0.80, the minimum required sample size per group was calculated to be 6 rats. The sample size aligns with the results of a similar study in the field (13).
2.2. Experimental design
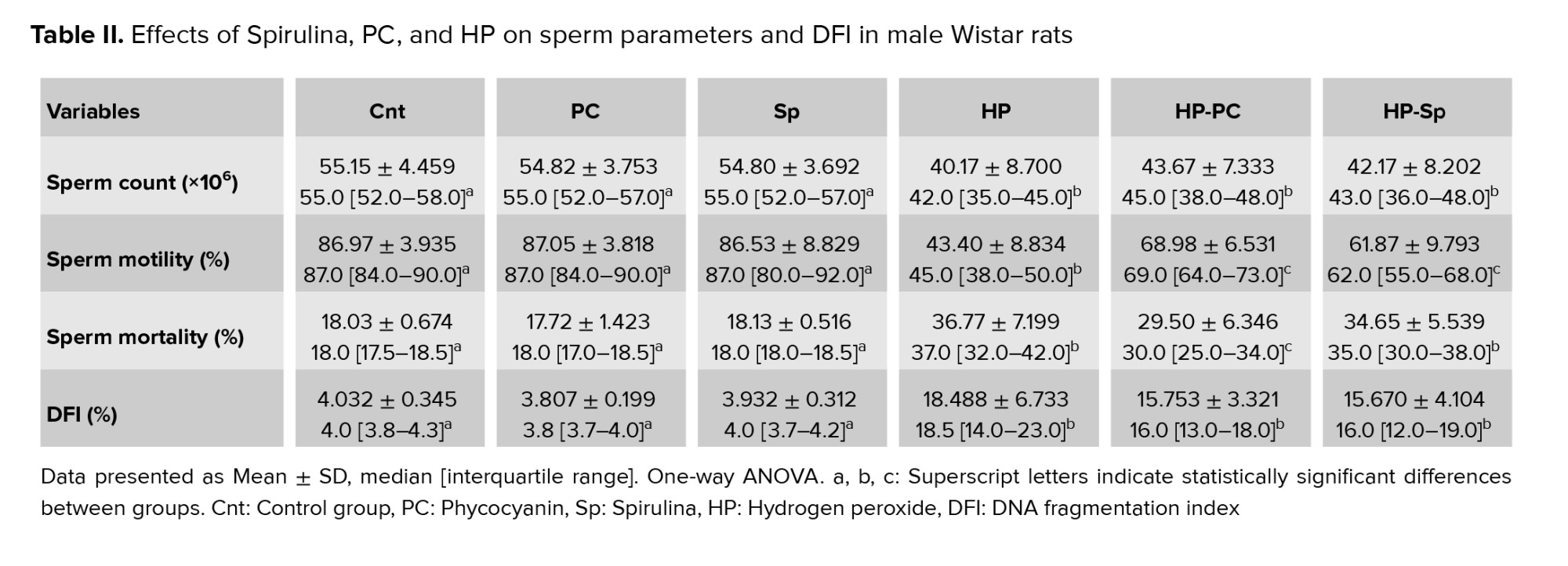
Rats were randomly assigned to 6 groups using a computer-generated randomization table to ensure unbiased allocation. Each rat was assigned a unique identification number, and the randomization process was performed by an independent researcher not involved in the experimental procedures (single-blinding). Only the first researcher was aware of the group assignments. The treatment period lasted for 60 days (Table I). Additionally, rats were acclimatized to laboratory conditions for at least 7 days before the experiment.
C-PC powder (Merck, Germany, product code 11016-15-2) and Spirulina powder (Merck, Germany, product code 724424-92-4) were freshly prepared as suspensions for oral administration. The outcome measures, included 1) sperm parameters (count, motility, and viability); 2) oxidative stress markers (superoxide dismutase [SOD] activity, malondialdehyde [MDA] concentration, total thiol concentration [TTC], total antioxidant capacity [TAC], total oxidant status [TOS], and carbonyl protein [CP] content); 3) histopathological indices (tubule differentiation index [TDI], repopulation index [RI], and spermiogenesis index [SPI]); and 4) DNA integrity-DNA fragmentation index [DFI].
2.3. Experimental procedures
After treatment, rats were anesthetized and euthanized. Blood was collected via Microtainer® serum separator tubes, allowed to clot for 10 min, and then centrifuged at 16,000 g for 5 min. Serum was stored at -70oC for analysis. Testes and epididymides were dissected; the epididymides were incubated in T6 medium with BSA for sperm capacitation. One testis was frozen for oxidative stress testing; the other was fixed in 10% neutral buffered formalin for histological analysis (14).
2.3.1. Chemicals
All reagents were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). The CP kit was obtained from Kiazist Life Science (KCAR-96). In contrast, various biochemical assay kits were procured from Navand Salamat Co. PC extraction followed standard procedures with a salting-out process. A solution of PC was prepared at a concentration of 100 mg/mL in distilled water for gavage administration (15).
2.3.2. Sperm analysis
A 15 µL sperm sample was placed in a Neubauer chamber for counting under a light microscope at x400 magnification, with results reported in million/mL. Sperm motility was categorized as progressive (P), non-progressive (NP), or immotile (IM). The motility percentage was calculated as: motility = (mean of P NP200 sperm) ×100. Motility = (200 sperm mean of P NP) ×100. Sperm viability was assessed using eosin Y staining. A mixture of 40 µL sperm sample and 10 µL of 0.5% eosin Y stained dead sperm pink due to damaged plasma membranes. Viability was determined as the mean percentage of dead sperm relative to the total count, averaged over 3 replicates (16).
2.3.3. Oxidative stress markers
2.3.3.1. SOD activity
SOD activity was measured using an nitroblue tetrazolium reduction assay with a commercial ELISA kit (ZellBio GmbH, Germany) following the manufacturer's instructions (17).
2.3.3.2. MDA concentration
MDA levels were assessed using a colorimetric assay kit (ZellBio GmbH, Ulm, Germany) that measures MDA-thiobarbituric acid complex formation at high temperatures. The concentration of MDA was determined by measuring the color intensity at 532 nm, with detection limits as low as 0.1 μM (18).
2.3.3.3. TTC
TTC levels were measured using a colorimetric method involving 5′-dithiobis-(2-nitrobenzoic acid) (DTNB), which produces a yellow complex upon reaction with thiol groups. A serum sample (25 µL) was mixed with DTNB and methanol, incubated, centrifuged, and the absorbance of the supernatant was measured at 412 nm. Thiol content was reported in nanomoles per milligram of protein (nM/mg protein) using an extinction coefficient for DTNB of 13.6 mM (19).
2.3.3.4. TAC
TAC in tissue samples was evaluated using a commercial kit (Kiazist, Iran) based on the ferric reducing antioxidant power method. The reduction of ferric reducing antioxidant power reagent results in a color change measurable at 405 nm, correlating with antioxidant capacity, with detection limits down to 0.1 mM (17).
2.3.3.5. TOS
Serum TOS levels were measured using an automated method developed by Erel, which assesses color change due to oxidant molecules in the sample (μmol H2O2 Eq/L) (18).
2.3.3.6. CP content
CP content, indicating protein oxidation, was measured using a commercial kit from Cayman Chemical. This method involves the reaction between 2,4-dinitrophenylhydrazine and carbonyl groups to form a hydrazone compound, analyzed spectrophotometrically at 360-385 nm. Results were standardized based on total protein concentration measured at 280 nm to account for potential loss during preparation (17).
2.3.4. Histopathological examination
Testicular tissue samples were preserved in formalin, dehydrated using a progressively increasing ethanol concentration series, and then embedded in paraffin. Standard hematoxylin and eosin staining was performed for microscopic examination. Stained sections were analyzed under a light microscope (Olympus Co., Germany) with a 20X lens, and image analysis was conducted using Leica DMLB and Leica Qwin software. Seminiferous tubules were categorized as either differentiating, which included those containing stage B spermatogonia or later stages, or nondifferentiating. The TDI was determined by calculating the proportion of differentiating tubules. The RI measured the rate of tubules populated with germ cells at least at the intermediate spermatogonial stage. The SPI was calculated as the ratio of seminiferous tubules filled with spermatozoa to empty tubules (20).
2.3.5. DNA integrity evaluation
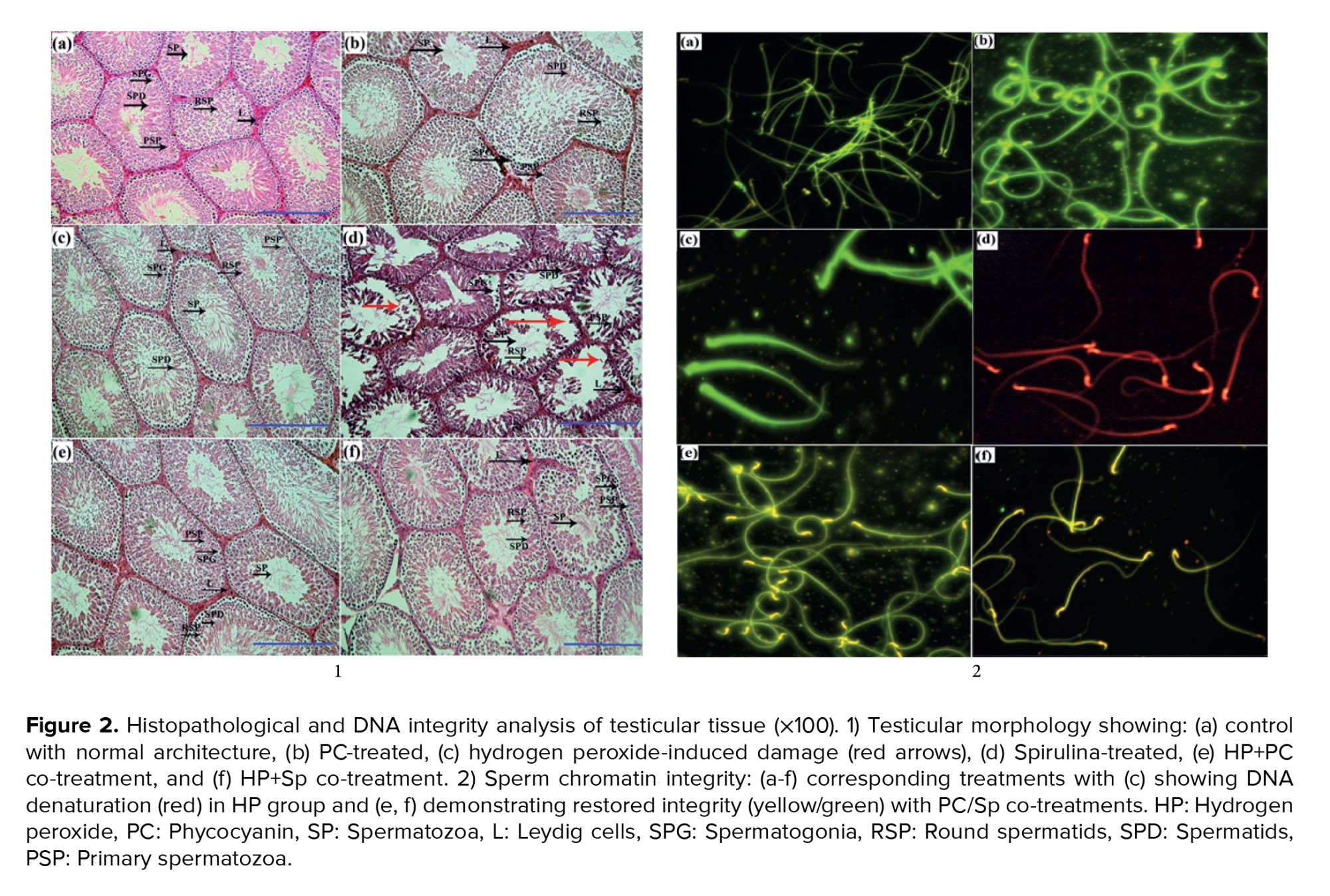
Sperm DNA integrity was assessed using the acridine orange (AO) fluorescence staining method. This method is fast, easy to use, ideal for routine analysis, and highly sensitive in detecting sperm DNA damage. A 15 µL sperm sample was smeared, fixed in a 1:1 ethanol-acetone solution for 30 min at 4°C, and stained with AO for 5 min in the dark at room temperature. After rinsing with distilled water, slides were examined under a fluorescence microscope with a 470 nm beam. AO fluoresces green when binding to double-stranded DNA, indicating intact chromatin and normal spermatozoa. Conversely, red, orange, or yellow fluorescence indicates denatured single-stranded DNA and chromatin abnormalities. Approximately 300 sperm cells were counted and classified as green (intact DNA and fertility) or non-green (denatured DNA and lower fertility). The DFI was calculated by dividing the number of sperm with denatured DNA by the total sperm count, expressed as a percentage. A sperm count of at least 50 million/mL was deemed indicative of fertility (21).
2.4. Ethical Considerations
This study was approved by the Ethics Committee of Azad University of Medical Sciences in Sari, Iran (Code: IR.IAU.SARI.REC.1400.050), in accordance with ethical guidelines and standards for the use of animals in scientific research. Rats were housed in standard cages (22 ± 2°C, 50 ± 5% humidity, 12-hr light/dark cycle) with ad libitum access to food/water after 7-day acclimatization. All procedures minimized distress: euthanasia followed anesthesia, and humane endpoints (weight loss > 20%, lethargy, pain, or mobility/grooming deficits) were monitored twice daily. No adverse events occurred beyond expected oxidative stress from HP exposure.
2.5. Statistical Analysis
Data normality was assessed via the Kolmogorov-Smirnov test. Descriptive statistics were calculated for each group. One-way ANOVA compared factors across groups; results presented as mean ± SD or SEM. Tukey's multiple-range test identified significant differences at p < 0.05 using SPSS version 24 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Sperm analysis
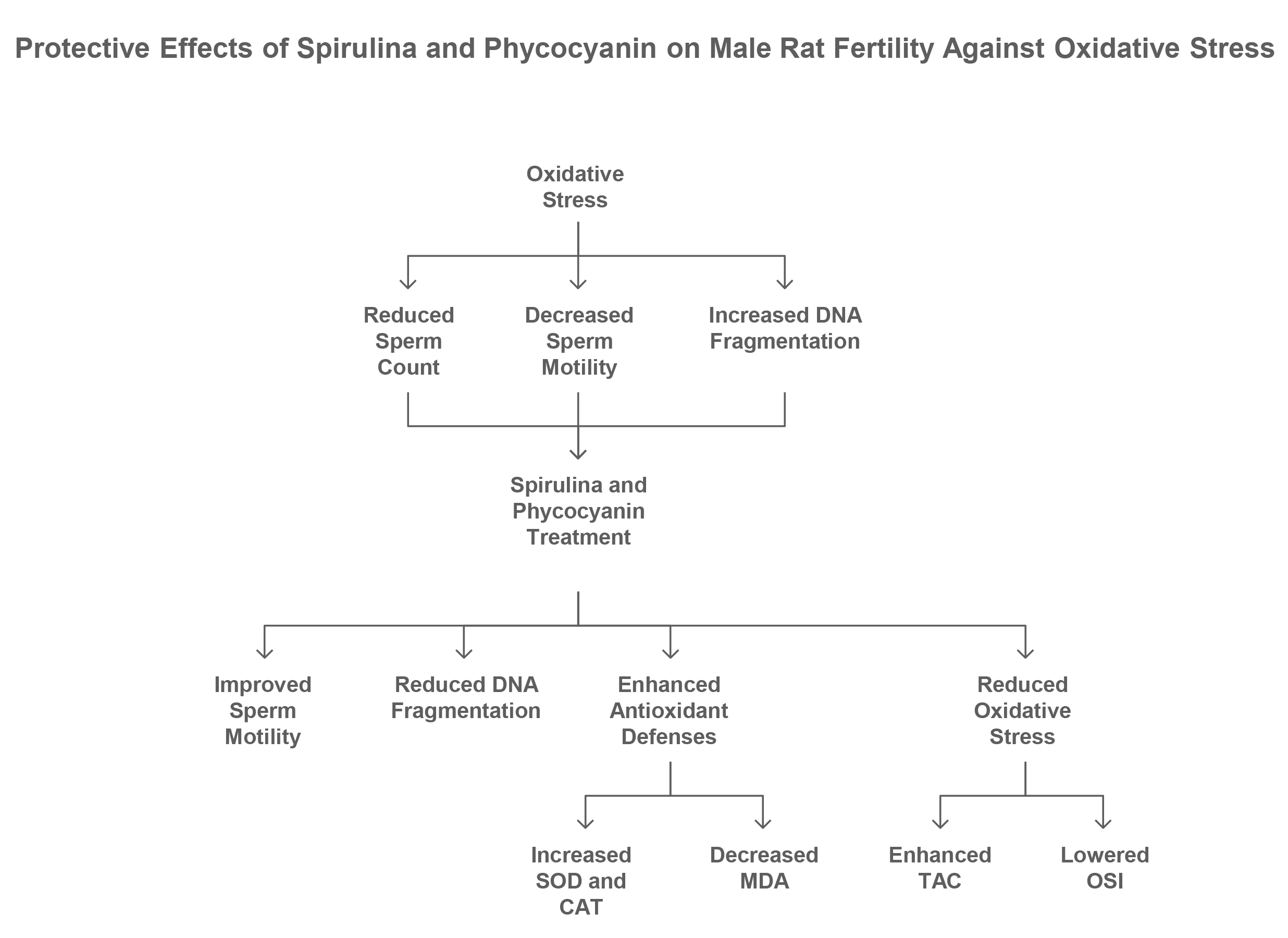
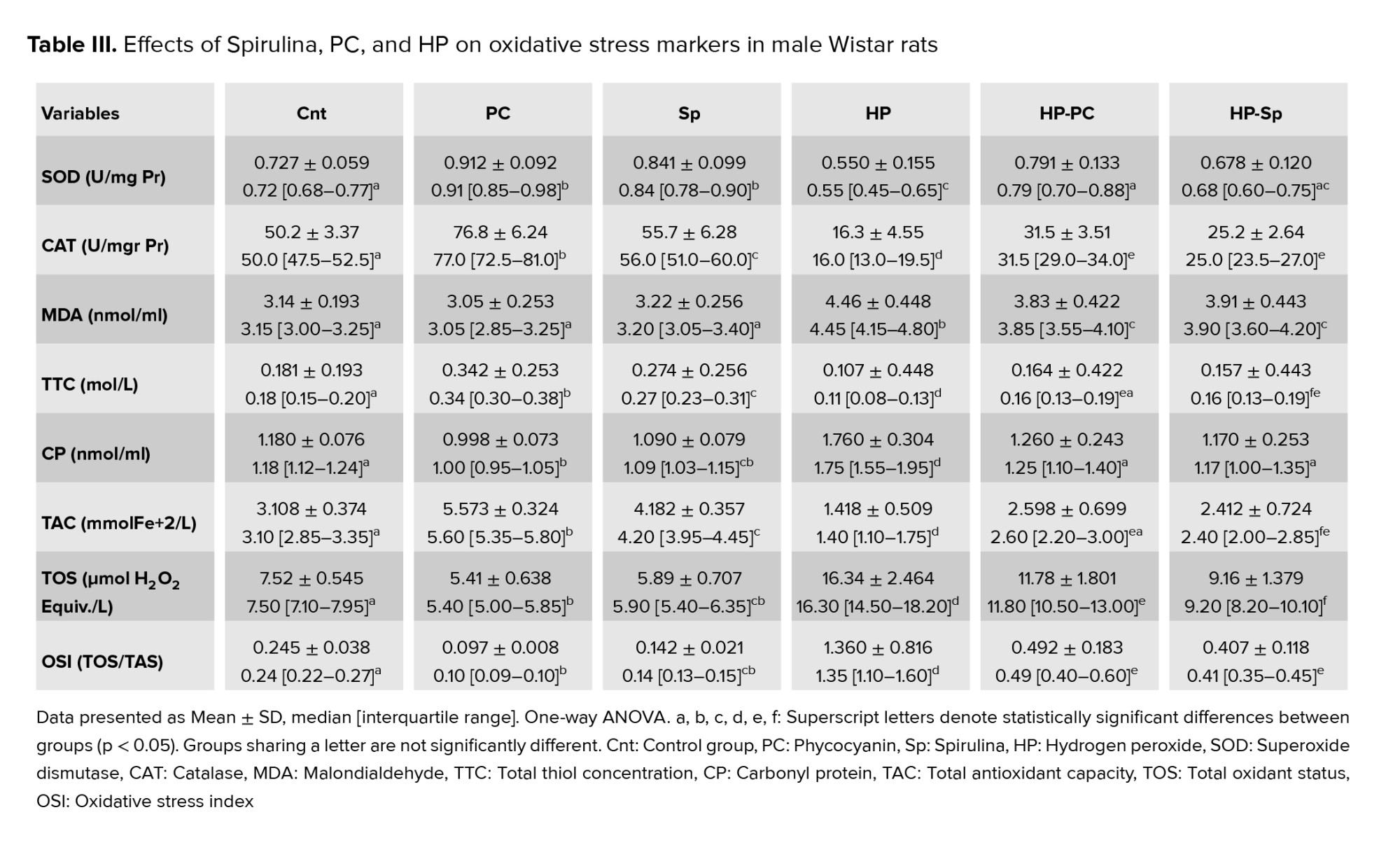
Sperm count, motility, and viability were significantly lower in the HP-exposed group compared to controls (p = 0.001). Treatment with PC or Spirulina improved these parameters in HP-exposed rats (p = 0.001). Although both HP-PC and HP-Sp groups showed increased sperm count, this increase was not statistically significant (p = 0.24). Notably, both groups exhibited significant improvements in sperm motility and reduced sperm mortality, particularly in the HP-PC group (p = 0.001) (Table II).
3.2. DNA integrity
The DFI was significantly higher in the HP group than in controls (p < 0.05). Treatment with PC or Spirulina significantly reduced DFI in the HP-treated groups (p = 0.001). Both HP-Sp and HP-PC groups showed a notable decrease in DFI compared to the HP-only group (p = 0.001) (Figure 1).
3.3. Serum oxidant/antioxidant parameters
In the HP group, activities of SOD, catalase (CAT), TTC, and TAC were significantly lower compared to controls (p = 0.001). Conversely, MDA and TOS levels were higher in the HP group (p = 0.01). Treatment with PC or Spirulina significantly increased SOD, CAT, TTC, and TAC activities while reducing MDA and TOS levels in the HP-treated groups (p = 0.001) (Table III).
3.4. Histological parameters
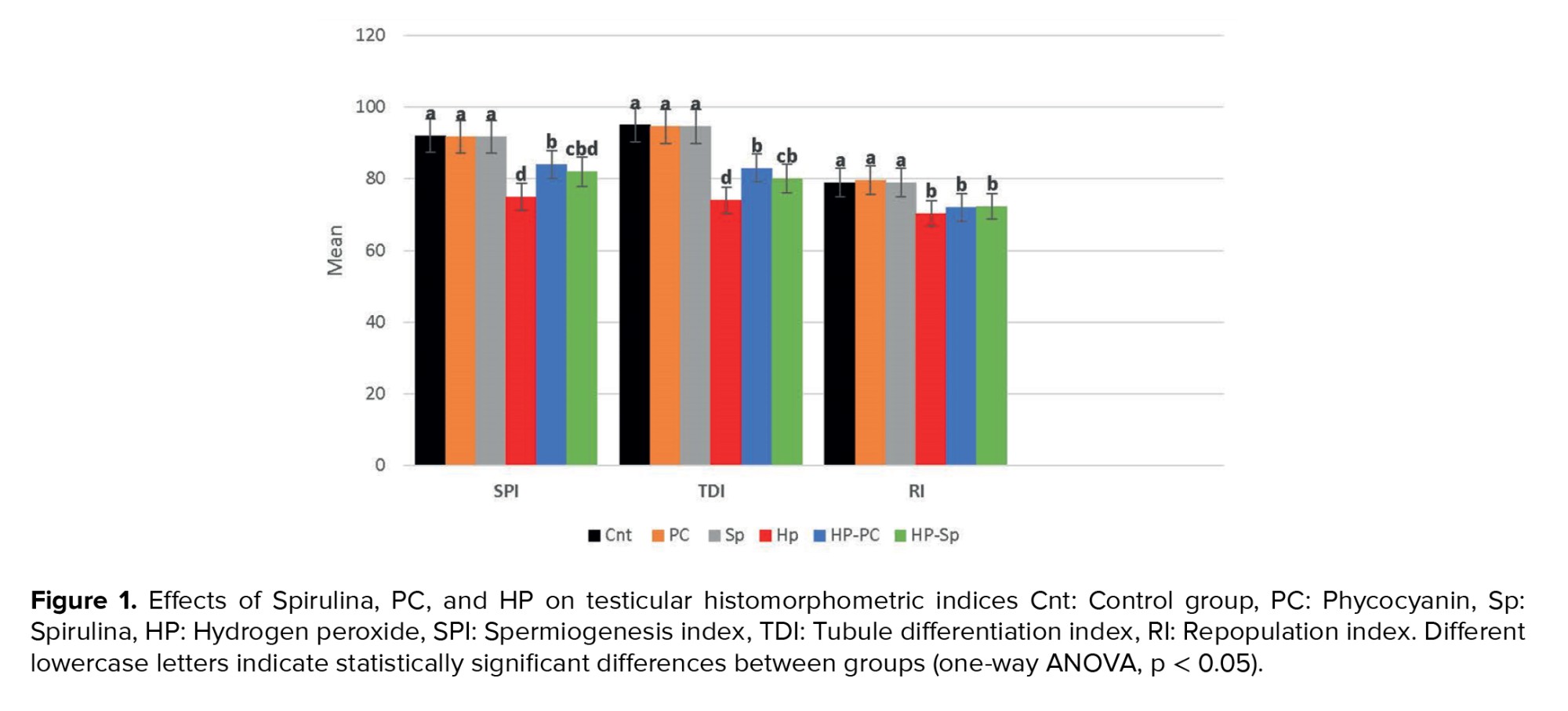
HP exposure significantly decreased the SPI, TDI, and RI compared to other groups (p = 0.01). However, SPI and TDI improved significantly in the HP-Sp and HP-PC groups compared to the HP-only group (p = 0.01) (Figure 1).
Histopathological analysis revealed testicular damage, including atrophy and degeneration due to HP exposure (Figure 2).
4. Discussion
This study investigated the antioxidant protective effects of Spirulina and PC on sperm quality, DNA integrity, and testicular tissue in rats subjected to HP-induced oxidative stress. Both compounds demonstrated beneficial impacts on male reproductive health, with PC showing superior efficacy across most assessed parameters. Sp and PC significantly improved sperm count, motility, and viability in HP-exposed rats, with PC being particularly effective. These findings align with prior studies noting Sp’s protective role against oxidative stress in male rats (10). However, conflicting reports indicate no significant improvements in sperm quality with Sp supplementation in cooled semen, highlighting the need for further research in varied contexts (22). The improvement in sperm count observed in the HP-PC and HP-Sp groups was not statistically significant. This non-significant increase can be attributed to the persistent effects of oxidative stress, possibly insufficient dosages of the antioxidants, and the complexity of the body's response to oxidative damage (23).
The significant reduction in TTC and TAC in the HP group, which was reversed by PC and Sp treatments, likely reflects the strong antioxidant activity of PC- a key component of Sp. PC enhances antioxidant enzymes like SOD and CAT, aiding in free radical scavenging. The restoration of TTC and TAC in the HP-PC and HP-Sp groups suggests that these treatments effectively counteract oxidative stress by ROS levels and replenishing thiol content (24). The significant increase in TOS and oxidative stress index in the HP group, which was reduced by PC and Sp treatments, reflects the oxidative stress induced by HP. As a known oxidizing agent, HP generates ROS, leading to elevated TOS levels and cellular damage. The reduction in TOS and OSI following treatment suggests that PC and Spirulina effectively mitigate oxidative stress (25).
The reduction in RI following HP exposure, and its improvement with treatment, can be attributed to HP-induced germ cell apoptosis. This leads to fewer viable sperm and spermatogenic cells, directly lowering RI due to reduced spermatogonial activity (26).
PC, a biliprotein from Sp, has demonstrated protective effects on reproductive health in both male and female models. In aging females, PC prevents oocyte fragmentation and aneuploidy by maintaining cytoskeletal integrity and reducing ROS production (27). Similarly, it improves fertility in obese females by enhancing ovarian quality (28) and protects male reproductive function by improving spermatogenesis and antioxidant capacity (9). A notable reduction in the DFI in Sp- and PC-treated groups indicates their potential to safeguard sperm DNA from oxidative damage. Both treatments increased antioxidant enzyme activities (SOD, CAT) while reducing lipid peroxidation (MDA), with PC showing a more pronounced effect. These results are supported by studies highlighting PC’s potent antioxidant properties (29). However, caution is warranted, as high doses of Sp may act as pro-oxidants under certain conditions (30).
4.1. Strengths and limitations
This study was conducted on rats, limiting the generalizability of findings to humans. Future research should explore these effects in human subjects for a more comprehensive understanding. Additionally, our investigation focused on specific aspects of male reproductive health; future studies could expand to include other reproductive outcomes and fertility measures.
5. Conclusion
This study demonstrates that oxidative stress induced by HP significantly impairs sperm quality, DNA integrity, and antioxidant defenses in male Wistar rats. Both Sp and PC exhibit potent protective effects by enhancing antioxidant enzyme activities, reducing lipid and protein oxidation, and improving sperm parameters and DNA fragmentation. While PC showed a slightly stronger antioxidant and protective capacity, Spirulina also effectively mitigated oxidative damage and restored redox balance.
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
K. Sedaghatizadeh, F. Nemati, and M. Gholamitabar Tabari conceived and designed the study. K. Sedaghatizadeh performed experiments, collected data, and conducted data processing. K. Sedaghatizadeh, F. Nemati, M. Gholamitabar Tabari, F. Peyravii Ghadikolaii, and R. Bishehkolaei analyzed results and developed research strategy. F. Nemati and M. Gholamitabar Tabari supervised the project and provided research direction. All authors contributed to manuscript drafting, critically revised the content, and approved the final version for publication.
Acknowledgments
This study was conducted without institutional grants and was solely supported by the student's personal financial resources. The authors employed Perplexity, based on the GPT-4 architecture, as an artificial intelligence for language editing and grammar checking during the preparation of this manuscript.
Conflict of Interest
The authors declare that they have no known competing financial or non-financial interests that could have influenced the work reported in this study.
A significant contributor in male infertility is oxidative stress, which arises from an imbalance between reactive oxygen species (ROS) and the body's antioxidant defenses (1). When the production of ROS surpasses antioxidant capacity, it leads to cellular damage that can contribute to chronic illnesses such as cardiovascular diseases, diabetes, and cancer (2). Oxidative stress arises from an imbalance between ROS production and antioxidant defenses. This state can lead to cellular damage, inflammation, and apoptosis (3).
Hydrogen peroxide (HP, or H2O2) causes significant DNA damage in various cell types; however, DNA repair mechanisms can mitigate this damage. Inhibiting these repair mechanisms exacerbates oxidative damage, highlighting their crucial role in cellular responses to oxidative stress (4).
Spermatozoa are particularly vulnerable to ROS-induced damage due to limited antioxidant defenses and high levels of polyunsaturated fatty acids, which are prone to oxidation (5). Additionally, sperm cells generate ROS themselves, further exacerbating oxidative stress. As these cells depend on external antioxidants for protection, they are more susceptible to oxidative damage (6). Natural antioxidants found in Spirulina platensis (Sp), a nutrient-rich blue-green microalga, may provide therapeutic benefits against male infertility (7). Sp is rich in essential nutrients and antioxidants, particularly phycocyanin (PC), a protein believed to drive its biological effects (8).
Furthermore, a recent study has highlighted the potential benefits of Sp and its component C-PC on male reproductive health. C-PC has been shown to protect against reproductive system damage in male mice, improving spermatogenesis, sperm quality, and fertility (9). Similarly, Sp demonstrated protective effects against arsenic-induced testicular oxidative stress in rats, enhancing sperm parameters and hormonal levels (10). These findings suggest that Sp and its derivatives could serve as promising reproductive system protective agents. However, research gaps remain, particularly regarding the effects of microcystins on reproductive toxicity across various species (11). Additionally, further investigation is needed to optimize the delivery methods of Sp for enhanced therapeutic outcomes, including improved bioavailability and targeted delivery (12).
This study investigated the effects of PC and Sp on testicular redox balance (oxidant/antioxidant status), morphology, and sperm parameters (motility, viability, DNA integrity) in male Wistar rats.
2. Materials and Methods
2.1. Animals
This experimental study was conducted in 2022 at Islamic Azad University, Qaemshahr Branch, Qaemshahr, Iran, using 36 male Wistar rats (7-8 wk, 180-220 gr). The rats were sourced from the North Research Center of Institut Pasteur, Amol, Iran, and housed in standard cages under controlled conditions (22 ± 2°C, 50 ± 5% humidity, 12-hr light/dark cycle) with unrestricted access to water and a standard diet.
A power analysis was conducted using G*Power software (version 3.1) to ensure adequate statistical power. Assuming an effect size of 0.5 (medium effect), a significance level (α) of 0.05, and a power (1-β) of 0.80, the minimum required sample size per group was calculated to be 6 rats. The sample size aligns with the results of a similar study in the field (13).
2.2. Experimental design
Rats were randomly assigned to 6 groups using a computer-generated randomization table to ensure unbiased allocation. Each rat was assigned a unique identification number, and the randomization process was performed by an independent researcher not involved in the experimental procedures (single-blinding). Only the first researcher was aware of the group assignments. The treatment period lasted for 60 days (Table I). Additionally, rats were acclimatized to laboratory conditions for at least 7 days before the experiment.
C-PC powder (Merck, Germany, product code 11016-15-2) and Spirulina powder (Merck, Germany, product code 724424-92-4) were freshly prepared as suspensions for oral administration. The outcome measures, included 1) sperm parameters (count, motility, and viability); 2) oxidative stress markers (superoxide dismutase [SOD] activity, malondialdehyde [MDA] concentration, total thiol concentration [TTC], total antioxidant capacity [TAC], total oxidant status [TOS], and carbonyl protein [CP] content); 3) histopathological indices (tubule differentiation index [TDI], repopulation index [RI], and spermiogenesis index [SPI]); and 4) DNA integrity-DNA fragmentation index [DFI].
2.3. Experimental procedures
After treatment, rats were anesthetized and euthanized. Blood was collected via Microtainer® serum separator tubes, allowed to clot for 10 min, and then centrifuged at 16,000 g for 5 min. Serum was stored at -70oC for analysis. Testes and epididymides were dissected; the epididymides were incubated in T6 medium with BSA for sperm capacitation. One testis was frozen for oxidative stress testing; the other was fixed in 10% neutral buffered formalin for histological analysis (14).
2.3.1. Chemicals
All reagents were purchased from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). The CP kit was obtained from Kiazist Life Science (KCAR-96). In contrast, various biochemical assay kits were procured from Navand Salamat Co. PC extraction followed standard procedures with a salting-out process. A solution of PC was prepared at a concentration of 100 mg/mL in distilled water for gavage administration (15).
2.3.2. Sperm analysis
A 15 µL sperm sample was placed in a Neubauer chamber for counting under a light microscope at x400 magnification, with results reported in million/mL. Sperm motility was categorized as progressive (P), non-progressive (NP), or immotile (IM). The motility percentage was calculated as: motility = (mean of P NP200 sperm) ×100. Motility = (200 sperm mean of P NP) ×100. Sperm viability was assessed using eosin Y staining. A mixture of 40 µL sperm sample and 10 µL of 0.5% eosin Y stained dead sperm pink due to damaged plasma membranes. Viability was determined as the mean percentage of dead sperm relative to the total count, averaged over 3 replicates (16).
2.3.3. Oxidative stress markers
2.3.3.1. SOD activity
SOD activity was measured using an nitroblue tetrazolium reduction assay with a commercial ELISA kit (ZellBio GmbH, Germany) following the manufacturer's instructions (17).
2.3.3.2. MDA concentration
MDA levels were assessed using a colorimetric assay kit (ZellBio GmbH, Ulm, Germany) that measures MDA-thiobarbituric acid complex formation at high temperatures. The concentration of MDA was determined by measuring the color intensity at 532 nm, with detection limits as low as 0.1 μM (18).
2.3.3.3. TTC
TTC levels were measured using a colorimetric method involving 5′-dithiobis-(2-nitrobenzoic acid) (DTNB), which produces a yellow complex upon reaction with thiol groups. A serum sample (25 µL) was mixed with DTNB and methanol, incubated, centrifuged, and the absorbance of the supernatant was measured at 412 nm. Thiol content was reported in nanomoles per milligram of protein (nM/mg protein) using an extinction coefficient for DTNB of 13.6 mM (19).
2.3.3.4. TAC
TAC in tissue samples was evaluated using a commercial kit (Kiazist, Iran) based on the ferric reducing antioxidant power method. The reduction of ferric reducing antioxidant power reagent results in a color change measurable at 405 nm, correlating with antioxidant capacity, with detection limits down to 0.1 mM (17).
2.3.3.5. TOS
Serum TOS levels were measured using an automated method developed by Erel, which assesses color change due to oxidant molecules in the sample (μmol H2O2 Eq/L) (18).
2.3.3.6. CP content
CP content, indicating protein oxidation, was measured using a commercial kit from Cayman Chemical. This method involves the reaction between 2,4-dinitrophenylhydrazine and carbonyl groups to form a hydrazone compound, analyzed spectrophotometrically at 360-385 nm. Results were standardized based on total protein concentration measured at 280 nm to account for potential loss during preparation (17).
2.3.4. Histopathological examination
Testicular tissue samples were preserved in formalin, dehydrated using a progressively increasing ethanol concentration series, and then embedded in paraffin. Standard hematoxylin and eosin staining was performed for microscopic examination. Stained sections were analyzed under a light microscope (Olympus Co., Germany) with a 20X lens, and image analysis was conducted using Leica DMLB and Leica Qwin software. Seminiferous tubules were categorized as either differentiating, which included those containing stage B spermatogonia or later stages, or nondifferentiating. The TDI was determined by calculating the proportion of differentiating tubules. The RI measured the rate of tubules populated with germ cells at least at the intermediate spermatogonial stage. The SPI was calculated as the ratio of seminiferous tubules filled with spermatozoa to empty tubules (20).
2.3.5. DNA integrity evaluation
Sperm DNA integrity was assessed using the acridine orange (AO) fluorescence staining method. This method is fast, easy to use, ideal for routine analysis, and highly sensitive in detecting sperm DNA damage. A 15 µL sperm sample was smeared, fixed in a 1:1 ethanol-acetone solution for 30 min at 4°C, and stained with AO for 5 min in the dark at room temperature. After rinsing with distilled water, slides were examined under a fluorescence microscope with a 470 nm beam. AO fluoresces green when binding to double-stranded DNA, indicating intact chromatin and normal spermatozoa. Conversely, red, orange, or yellow fluorescence indicates denatured single-stranded DNA and chromatin abnormalities. Approximately 300 sperm cells were counted and classified as green (intact DNA and fertility) or non-green (denatured DNA and lower fertility). The DFI was calculated by dividing the number of sperm with denatured DNA by the total sperm count, expressed as a percentage. A sperm count of at least 50 million/mL was deemed indicative of fertility (21).

2.4. Ethical Considerations
This study was approved by the Ethics Committee of Azad University of Medical Sciences in Sari, Iran (Code: IR.IAU.SARI.REC.1400.050), in accordance with ethical guidelines and standards for the use of animals in scientific research. Rats were housed in standard cages (22 ± 2°C, 50 ± 5% humidity, 12-hr light/dark cycle) with ad libitum access to food/water after 7-day acclimatization. All procedures minimized distress: euthanasia followed anesthesia, and humane endpoints (weight loss > 20%, lethargy, pain, or mobility/grooming deficits) were monitored twice daily. No adverse events occurred beyond expected oxidative stress from HP exposure.
2.5. Statistical Analysis
Data normality was assessed via the Kolmogorov-Smirnov test. Descriptive statistics were calculated for each group. One-way ANOVA compared factors across groups; results presented as mean ± SD or SEM. Tukey's multiple-range test identified significant differences at p < 0.05 using SPSS version 24 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Sperm analysis
Sperm count, motility, and viability were significantly lower in the HP-exposed group compared to controls (p = 0.001). Treatment with PC or Spirulina improved these parameters in HP-exposed rats (p = 0.001). Although both HP-PC and HP-Sp groups showed increased sperm count, this increase was not statistically significant (p = 0.24). Notably, both groups exhibited significant improvements in sperm motility and reduced sperm mortality, particularly in the HP-PC group (p = 0.001) (Table II).
3.2. DNA integrity
The DFI was significantly higher in the HP group than in controls (p < 0.05). Treatment with PC or Spirulina significantly reduced DFI in the HP-treated groups (p = 0.001). Both HP-Sp and HP-PC groups showed a notable decrease in DFI compared to the HP-only group (p = 0.001) (Figure 1).
3.3. Serum oxidant/antioxidant parameters
In the HP group, activities of SOD, catalase (CAT), TTC, and TAC were significantly lower compared to controls (p = 0.001). Conversely, MDA and TOS levels were higher in the HP group (p = 0.01). Treatment with PC or Spirulina significantly increased SOD, CAT, TTC, and TAC activities while reducing MDA and TOS levels in the HP-treated groups (p = 0.001) (Table III).
3.4. Histological parameters
HP exposure significantly decreased the SPI, TDI, and RI compared to other groups (p = 0.01). However, SPI and TDI improved significantly in the HP-Sp and HP-PC groups compared to the HP-only group (p = 0.01) (Figure 1).
Histopathological analysis revealed testicular damage, including atrophy and degeneration due to HP exposure (Figure 2).




4. Discussion
This study investigated the antioxidant protective effects of Spirulina and PC on sperm quality, DNA integrity, and testicular tissue in rats subjected to HP-induced oxidative stress. Both compounds demonstrated beneficial impacts on male reproductive health, with PC showing superior efficacy across most assessed parameters. Sp and PC significantly improved sperm count, motility, and viability in HP-exposed rats, with PC being particularly effective. These findings align with prior studies noting Sp’s protective role against oxidative stress in male rats (10). However, conflicting reports indicate no significant improvements in sperm quality with Sp supplementation in cooled semen, highlighting the need for further research in varied contexts (22). The improvement in sperm count observed in the HP-PC and HP-Sp groups was not statistically significant. This non-significant increase can be attributed to the persistent effects of oxidative stress, possibly insufficient dosages of the antioxidants, and the complexity of the body's response to oxidative damage (23).
The significant reduction in TTC and TAC in the HP group, which was reversed by PC and Sp treatments, likely reflects the strong antioxidant activity of PC- a key component of Sp. PC enhances antioxidant enzymes like SOD and CAT, aiding in free radical scavenging. The restoration of TTC and TAC in the HP-PC and HP-Sp groups suggests that these treatments effectively counteract oxidative stress by ROS levels and replenishing thiol content (24). The significant increase in TOS and oxidative stress index in the HP group, which was reduced by PC and Sp treatments, reflects the oxidative stress induced by HP. As a known oxidizing agent, HP generates ROS, leading to elevated TOS levels and cellular damage. The reduction in TOS and OSI following treatment suggests that PC and Spirulina effectively mitigate oxidative stress (25).
The reduction in RI following HP exposure, and its improvement with treatment, can be attributed to HP-induced germ cell apoptosis. This leads to fewer viable sperm and spermatogenic cells, directly lowering RI due to reduced spermatogonial activity (26).
PC, a biliprotein from Sp, has demonstrated protective effects on reproductive health in both male and female models. In aging females, PC prevents oocyte fragmentation and aneuploidy by maintaining cytoskeletal integrity and reducing ROS production (27). Similarly, it improves fertility in obese females by enhancing ovarian quality (28) and protects male reproductive function by improving spermatogenesis and antioxidant capacity (9). A notable reduction in the DFI in Sp- and PC-treated groups indicates their potential to safeguard sperm DNA from oxidative damage. Both treatments increased antioxidant enzyme activities (SOD, CAT) while reducing lipid peroxidation (MDA), with PC showing a more pronounced effect. These results are supported by studies highlighting PC’s potent antioxidant properties (29). However, caution is warranted, as high doses of Sp may act as pro-oxidants under certain conditions (30).
4.1. Strengths and limitations
This study was conducted on rats, limiting the generalizability of findings to humans. Future research should explore these effects in human subjects for a more comprehensive understanding. Additionally, our investigation focused on specific aspects of male reproductive health; future studies could expand to include other reproductive outcomes and fertility measures.
5. Conclusion
This study demonstrates that oxidative stress induced by HP significantly impairs sperm quality, DNA integrity, and antioxidant defenses in male Wistar rats. Both Sp and PC exhibit potent protective effects by enhancing antioxidant enzyme activities, reducing lipid and protein oxidation, and improving sperm parameters and DNA fragmentation. While PC showed a slightly stronger antioxidant and protective capacity, Spirulina also effectively mitigated oxidative damage and restored redox balance.
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
K. Sedaghatizadeh, F. Nemati, and M. Gholamitabar Tabari conceived and designed the study. K. Sedaghatizadeh performed experiments, collected data, and conducted data processing. K. Sedaghatizadeh, F. Nemati, M. Gholamitabar Tabari, F. Peyravii Ghadikolaii, and R. Bishehkolaei analyzed results and developed research strategy. F. Nemati and M. Gholamitabar Tabari supervised the project and provided research direction. All authors contributed to manuscript drafting, critically revised the content, and approved the final version for publication.
Acknowledgments
This study was conducted without institutional grants and was solely supported by the student's personal financial resources. The authors employed Perplexity, based on the GPT-4 architecture, as an artificial intelligence for language editing and grammar checking during the preparation of this manuscript.
Conflict of Interest
The authors declare that they have no known competing financial or non-financial interests that could have influenced the work reported in this study.
Type of Study: Original Article |
Subject:
Reproductive Biology
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |