Sat, Feb 21, 2026
[Archive]
Volume 23, Issue 11 (November 2025)
IJRM 2025, 23(11): 927-936 |
Back to browse issues page
Ethics code: IR.MUI.PHANUT.REC.1402.098
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shirani M, Bagherniya M, Sadeghi O, Ghasemi Tehrani H, Eskandari M H, Sharma M et al . Effects of supplementation with two probiotic strains on metabolic profile, hormonal status, oxidative stress, and quality of life in women with polycystic ovary syndrome: A study protocol for a randomized clinical trial. IJRM 2025; 23 (11) :927-936
URL: http://ijrm.ir/article-1-3685-en.html
URL: http://ijrm.ir/article-1-3685-en.html
Mahsa Shirani1 

 , Mohammad Bagherniya1
, Mohammad Bagherniya1 

 , Omid Sadeghi2
, Omid Sadeghi2 

 , Hatav Ghasemi Tehrani3
, Hatav Ghasemi Tehrani3 

 , Mohammad Hadi Eskandari4
, Mohammad Hadi Eskandari4 

 , Manoj Sharma5
, Manoj Sharma5 

 , Gholamreza Askari *6
, Gholamreza Askari *6 




 , Mohammad Bagherniya1
, Mohammad Bagherniya1 

 , Omid Sadeghi2
, Omid Sadeghi2 

 , Hatav Ghasemi Tehrani3
, Hatav Ghasemi Tehrani3 

 , Mohammad Hadi Eskandari4
, Mohammad Hadi Eskandari4 

 , Manoj Sharma5
, Manoj Sharma5 

 , Gholamreza Askari *6
, Gholamreza Askari *6 


1- Nutrition and Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. & Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Isfahan Medical Obstetrics and Gynecology Group, Reproductive Sciences and Sexual Health Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Food Science and Technology, School of Agriculture, Shiraz University, Shiraz, Iran.
5- Department of Social and Behavioral Health, School of Public Health, University of Nevada, Las Vegas, Nevada, USA. & Department of Internal Medicine, Kirk Kerkorian School of Medicine, University of Nevada, Las Vegas, Nevada, USA.
6- Nutrition and Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. & Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran. ,askari@mui.ac.ir
2- Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. & Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Isfahan Medical Obstetrics and Gynecology Group, Reproductive Sciences and Sexual Health Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Food Science and Technology, School of Agriculture, Shiraz University, Shiraz, Iran.
5- Department of Social and Behavioral Health, School of Public Health, University of Nevada, Las Vegas, Nevada, USA. & Department of Internal Medicine, Kirk Kerkorian School of Medicine, University of Nevada, Las Vegas, Nevada, USA.
6- Nutrition and Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. & Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran. ,
Keywords: Probiotics, Polycystic ovary syndrome, Testosterone, Sex hormone-binding globulin, Randomized clinical trial.
Full-Text [PDF 434 kb]
(408 Downloads)
| Abstract (HTML) (232 Views)
= 100 × ( total testosterone ) / SHBG
Insulin sensitivity index: to calculate quick index of insulin sensitivity, the following formula is used (23):
= 1/[ log ( I 0) + log ( G 0)] h omeostasis model assessment of insulin resistance index = [ ( fasting insulin ) µ U / ml × ( fasting glucose ) mmol / l ] /22.5
2.9. Interventions
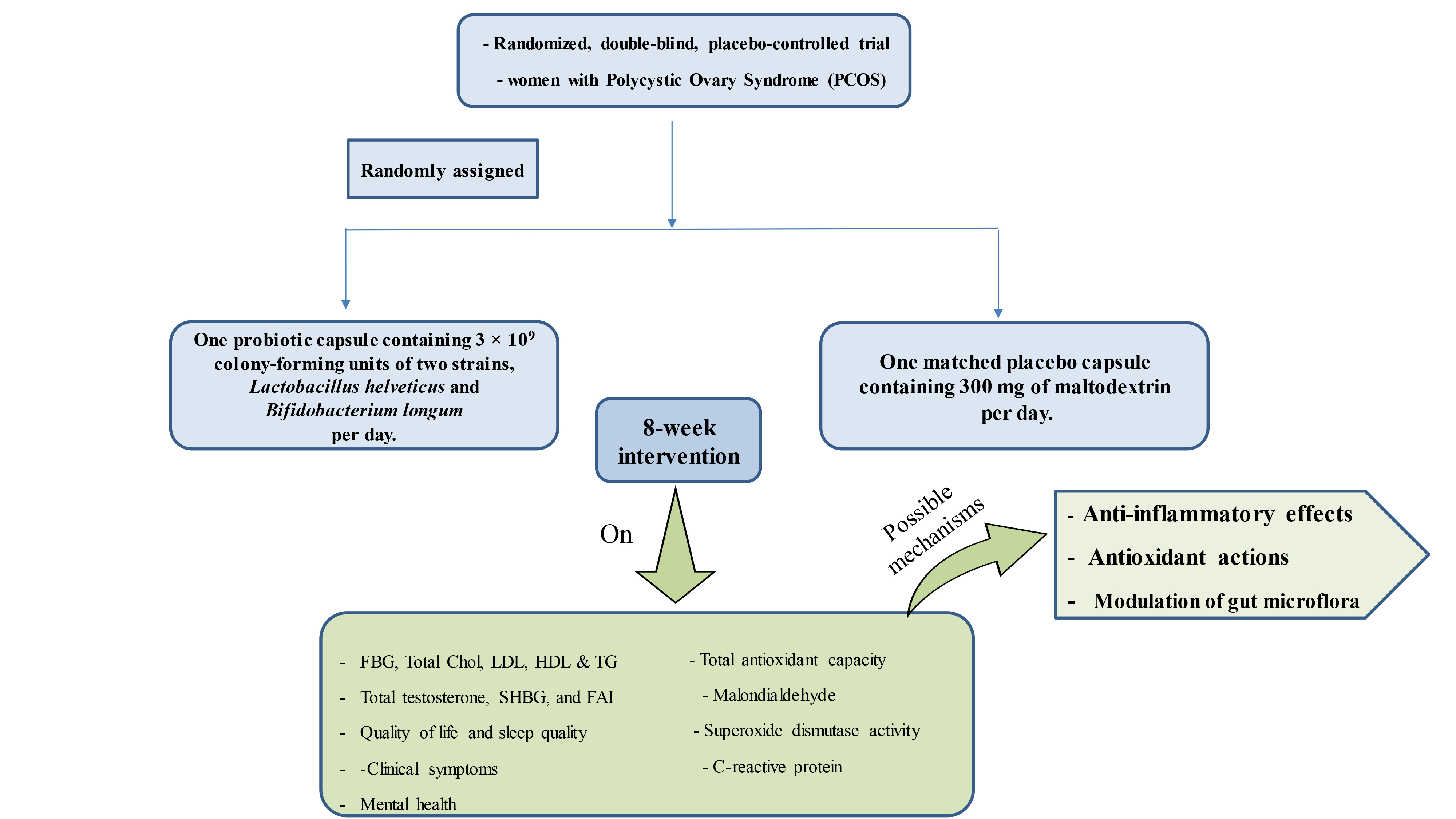
Alongside standard care, participants will be divided into 2 groups: the probiotic group, who will receive one probiotic capsule per day containing 3 × 109 colony-forming units of 2 specific strains, L. helveticus R0052 and B. longum R0175, and the placebo group, who will receive one capsule per day containing 300 mg of maltodextrin. The intervention will last for 8 wk. Dietary recommendations for PCOS will be given to both groups, and participants are allowed to follow their usual care routines throughout the trial. Once the study is over, participants will resume their regular medical treatments.
2.9.1. Intervention description
Participants in the treatment group will take a (Psychobiotic, PersiLife Co., Iran) capsule daily before meal, for 8 wk, while the control group receives a placebo containing 300 mg of maltodextrin. New supplies are given every 4 wk, and adherence and side effects are monitored through calls and visits to the clinic. Participants may withdraw at any given point, and researchers can also remove participants if needed.
2.9.2. Strategies to improve adherence to interventions and follow-up
Participants will receive supplements or placebo packs at weeks 1 and 4 and return any unused capsules at weeks 4 and 8. Weekly phone calls monitored compliance, and returned packs will be counted at the end of the study. Compliance is calculated as (packs taken/packs prescribed) × 100. Women with < 80% compliance will be excluded from the final analysis (24). The benefits of probiotics in preventing PCOS complications will be explained, and participants will receive reminders via calls and texts.
2.9.3. Data management
An expert will manage data encryption, storage, and verification. Reported side effects will be assessed to decide participant’s exclusion. The conditions were set by the Medical Ethics Commission under which probiotic assignment could be disclosed.
2.9.4. Oversight and monitoring
The Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran will oversee the entire trial to ensure ethical compliance and participant safety. The principal investigator and a gynecologist will manage participant inclusion, treatment, and follow-up. Based on previous studies, the probiotic doses used are considered safe, with no expected toxicity or side effects (9, 10). Any adverse effects will be reported to Isfahan University of Medical Sciences, which will supervise the study. Inspectors will conduct at least 2 monitoring visits, and the project team will hold monthly meetings to address issues and ensure protocol adherence.
2.10. Ethical Considerations
The study protocol received approval from the Research Ethics Committee of the School of Pharmacy and Nutrition, Isfahan University of Medical Sciences, Isfahan, Iran onMarch 19, 2024 (Code: IR.MUI.PHANUT.REC.1402.098). The trial protocol was registered in the Iranian Registry of Clinical Trials on 30 March 2024 (ID: IRCT20121216011763N62). A distinct identification code will be allocated to every participant, which will be used to manage research data. According to the research guidelines, upon completion of the study, the responsible researcher will collect and keep the code list. Furthermore, the private information of the study participants will remain confidential.
2.11. Statistical Analysis
Statistical analysis will be performed using SPSS version 26.0, with a significance level set at p < 0.05. Data normality will be assessed by the one-sample Kolmogorov-Smirnov test. Categorical variables will be reported as frequencies and percentages, while continuous variables will be expressed as means ± standard deviations. Baseline comparisons between intervention groups will use Chi-square tests for categorical data and independent t tests for continuous data. The intervention effects on primary and secondary outcomes will be evaluated using one-way ANCOVA. Missing data will be addressed via an intention-to-treat approach combined with multiple imputations.
3. Discussion
PCOS is a multifaceted endocrine disorder marked by ovulatory dysfunction, hyperandrogenism, and various metabolic issues (25). Elevated insulin levels, along with imbalances in gut microbiota, can significantly take part in the development of PCOS (26). Recently, lifestyle changes, dietary adjustments, and alterations in gut microbiota have been promoted as beneficial strategies for improving the comorbidities in PCOS cases (4). Women with PCOS may experience significant changes in their gut microbiota composition (27). The relationship between dysbiosis and obesity-related diseases have been demonstrated in various animal and human models. Recent advances in metagenomic sequencing have shown that dysbiosis is associated with many systemic disorders, including obesity, cancer, diabetes, metabolic syndrome, and insulin resistance. Additionally, some evidence suggests that gut dysbiosis is linked to sex hormone levels and ovarian morphological changes (4).
Probiotics can maintain the integrity of the intestinal barrier through the production of short-chain fatty acids, which are related to a reduction in insulin resistance and inflammation (28). Also, probiotics may have a reducing effect on blood lipid levels through the production of these metabolites (8). According to recent evidence, treatment with prebiotics and probiotics may inhibit many metabolic side effects, such as hyperinsulinemia in obese people, affecting gut microflora (29).
Furthermore, probiotics in the gut can increase the production of tryptophan as an enhancer of serotonin signaling and subsequently improve social behavior, stress, anxiety, and depression (30). Additionally, a recent trial has shown that supplementation with L. helveticus and B. longum has a beneficial effect on CRP levels, depression, anxiety, stress indicators, and sleep quality (9). In another randomized clinical trial, Rode et al. performed the same probiotic supplementation for 4 wk on healthy people and found a beneficial effect on stress-related disorders (10).
This study hypothesizes that probiotic supplementation will improve metabolic, hormonal, inflammatory, and antioxidant markers compared to placebo. However, several limitations should be noted. First, the study did not measure any specific marker to definitively assess the diversity of the gut microbiota population before and after intervention. Additionally, the role of probiotic foods, such as probiotic yogurt, is not considered. Furthermore, for ethical reasons, the effects of probiotics as a standalone treatment cannot be assessed, as it would be unethical to instruct participants to discontinue their standard medications. Lastly, the study is confined to a particular dose and ratio of supplements, which prevents the determination of the optimal dosage.
To the best of our knowledge, no study has evaluated the effect of L. helveticus and B. longum combination on the clinical outcomes of PCOS subjects. Therefore, our study focused on evaluating the effects of these 2 probiotic strain supplementations on metabolic status, inflammation, oxidative stress, QOL, and clinical symptoms in women with PCOS. If successful, nutritionists in clinical environments may recommend a new strain of probiotic supplements for women with PCOS to treat the comorbidities of this common disorder.
Data Availability
Data availability is managed by the first and corresponding authors.
Author Contributions
Study design: M. Shirani, M. Bagherniya, O. Sadeghi, Gh. Askari. Study conception: M. Shirani, M. Bagherniya, H. Ghasemi Tehrani, MH. Eskandari, M. Sharma, and Gh. Askari. Interpretation of data for the work: M. Shirani and O. Sadeghi and Gh. Askari. Drafting the manuscript of the protocol: M. Shirani, M. Bagherniya, O. Sadeghi, Gh. Askari, MH. Eskandari, and H. Ghasemi Tehrani. Revising the manuscript of the protocol: M. Shirani and M. Sharma. Everyone read and approved the final draft of the manuscript. All the authors have agreed to be accountable for all aspects of the work, ensuring that questions regarding the accuracy or completeness of any part of the work are appropriately investigated and resolved.
Acknowledgments
We are extremely grateful for the assistance provided by the research vice-chancellor of Isfahan University of Medical Sciences, Isfahan, Iran. This study will be implemented with the financial support of Isfahan University of Medical Sciences, Isfahan, Iran (grant number: 3402794). However, the investor was not involved in any of the study design and data dissemination processes. The article has undergone grammatical review utilizing artificial intelligence tools (Grammarly).
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (29 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is a leading cause of female infertility and among the most common endocrine disorders in women. It affects roughly 10% of women of reproductive age and is frequently associated with obesity (30-60%), insulin resistance, and elevated insulin levels (1). According to the Rotterdam criteria, diagnosis is based on at least 2 of the following: hyperandrogenism, ovulatory dysfunction, or polycystic ovarian morphology (2). Additional hormonal features include increased levels of testosterone and luteinizing hormone, with decreased follicle-stimulating hormone levels. PCOS affects multiple body systems, causing symptoms such as menstrual irregularities, alopecia, acne, hirsutism, and infertility. It also increases the risk of cardiovascular disease and type 2 diabetes (3). Lifestyle factors, including dietary patterns, socioeconomic status, and metabolic conditions, play a key role in the development and management of PCOS (1). Recently, dietary interventions have gained attention, and international guidelines recommend diet and exercise as first-line treatments (3). The gut microbiome has been proposed as a key factor in the pathogenesis of PCOS. Dysbiosis, or imbalance in gut microbiota, is linked to hormonal disturbances, chronic inflammation, insulin resistance, and obesity, all of which contribute to PCOS symptoms. Moreover, gut dysbiosis is associated with neurological disorders like Alzheimer’s disease, anxiety, and depression (4, 5). Probiotics may help manage PCOS by modulating gut microbiota and improving antioxidant, inflammatory, and hormonal profiles (4). Certain strains of Lactobacillus and Bifidobacterium have shown significant potential for regulating hormonal balance and metabolic processes, making them particularly promising for improving overall health and wellness (6, 7). Lactobacillus species possess beta-glucuronidase enzymes, which enable them to metabolize estrogens in the gut (6). They also produce short-chain fatty acid metabolites, which can reduce insulin resistance and inflammation by regulating androgen levels (8). Lactobacillus helveticus (L. helveticus) and Bifidobacterium longum (B. longum) have been known to show antioxidant and anti-inflammatory effects (9, 10). Additionally, these strains have been found to enhance mood and alleviate anxiety disorders significantly (9). However, no clinical trial has assessed the effects of a combination of these 2 strains on laboratory parameters and clinical symptoms in women with PCOS. Consequently, this trial focuses on investigating the effects of L. helveticus and B. longum strains on metabolic profile, hormonal status, oxidative stress, quality of life (QOL), and sleep quality in women with PCOS.
2. Materials and Methods
2. Materials and Methods
2.1. Study design
This study is a parallel, double-blind, randomized, placebo-controlled trial that will enroll 90 women with PCOS and will have an intervention duration of 8 wk. Participants will be assigned to 2 groups in a 1:1 ratio using a stratified block randomization technique.
2.2. Participant timeline
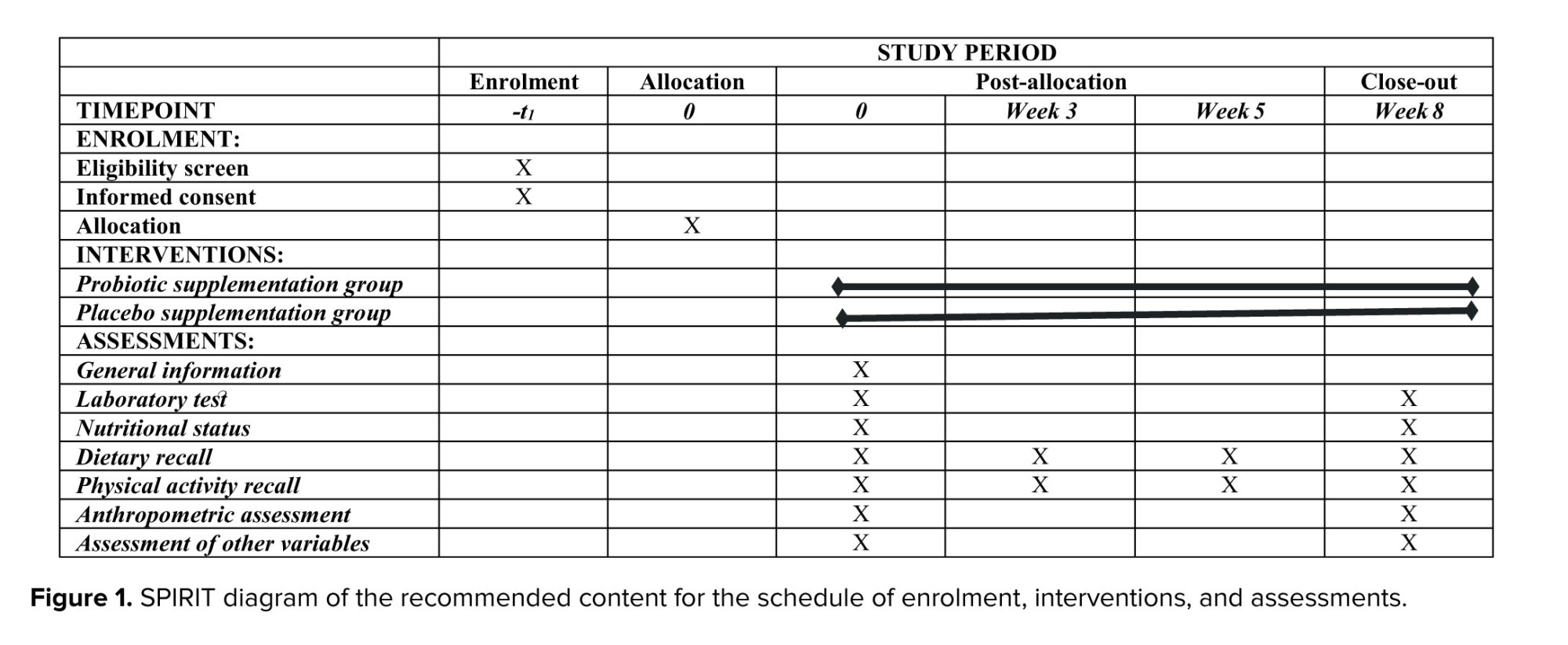
The study protocol adheres to the guidelines for reporting outcomes in trial protocols (11). The protocol schedule is provided in figure 1.
2.3. Eligibility criteria
2.3.1. Inclusion criteria
Participants will be considered eligible for inclusion if they voluntarily agree to participate by providing informed consent and have a confirmed diagnosis of PCOS by a gynecologist (based on the Rotterdam criteria).
2.3.2. Exclusion criteria
Participants will be excluded from the study if they are pregnant or breastfeeding; have used dietary supplements or probiotic products within the past 4 wk; have a current or prior diagnosis of psychiatric disorders, including major depressive disorder, bipolar disorder, or schizophrenia based on self-report, history of psychiatric hospitalization, or use of psychotropic medications; have hyperprolactinemia; are postmenopausal; have uncontrolled diabetes mellitus, defined as an HbA1C level ≥ 8%; have been diagnosed with chronic medical conditions such as cardiovascular, hepatic, renal diseases, or malignancies; or have a history of ovarian laparoscopic surgery or in vitro fertilization.
2.4. Sample size
The sample size for the current study was determined using an appropriate formula based on sex hormone binding globulin (SHBG) as the key variable. The mean and standard deviation of SHBG were extracted from a study by Ji et al. (12). In this calculation, the type 1 error, type 2 error, and study power rate considered were 5%, 20%, and 80%, respectively. Based on these considerations, the estimated sample size was 37 participants per group (total: 74). With a 20% dropout rate, we will include 45 participants per group.
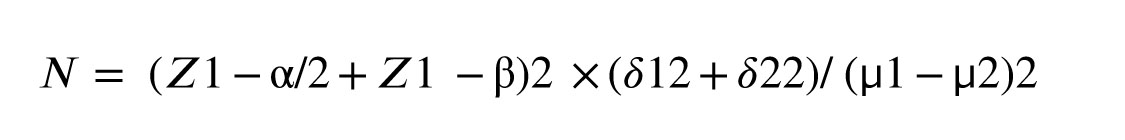
2.5. Recruitment
The study will include 90 individuals diagnosed with PCOS, selected from Shahid Beheshti hospital, Isfahan, Iran. An experienced gynecologist (H.Gh) evaluated the symptoms of PCOS according to the Rotterdam criteria. The Rotterdam criteria diagnose PCOS if at least 2 of the following are present: clinical or biochemical hyperandrogenism, oligo- or anovulation, and polycystic ovaries on ultrasound (defined as > 12 follicles per ovary or ovarian volume over 10 cm³). Additionally, the gynecologist will distinguish PCOS from other endocrine disorders, such as hyperprolactinemia, hypothyroidism, Cushing's syndrome, endometriosis, fibroids, uterine polyps, and various menstrual cycle irregularities. All eligible applicants who agree to be involved in the study will complete the written informed consent.
2.6. Randomization and blinding
Participants will be randomly assigned to 2 intervention groups using stratified block randomization based on body mass index categories (< 25 and ≥ 25). 6 blocks of 4 will be used to allocate individuals evenly (1:1) to either the intervention or placebo group. Randomization will be conducted by an independent person who was not involved in the study. A project collaborator managed group assignment, while a second researcher (M.B.) will oversee the allocation sequence. To maintain blinding, probiotic and placebo packets will be labeled as A or B by a third party. This study is double-blind, meaning participants, laboratory staff, and researchers will remain unaware of group assignments. Additionally, packages of probiotics are packed in the same color and shape as the placebo packets to maintain blinding.
2.7. Outcomes
The primary objectives contain the effect of probiotic supplementation on biochemical markers including: fasting blood glucose, fasting insulin, lipid profile biomarkers including total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, serum C-reactive protein (CRP), total antioxidant capacity, malondialdehyde, superoxide dismutase, hormonal biomarkers including SHBG and total testosterone, mental health (stress, depression, and anxiety), sleep quality, QOL, and hirsutism.
Secondary objectives contain the effect of probiotic supplementation on the following parameters: insulin resistance biomarkers, including quantitative insulin sensitivity check index and homeostasis model assessment insulin resistance, free androgen index, clinical symptoms, including acne and alopecia. The study will assess both primary and secondary issues at the onset and conclusion of the intervention.
2.8. Data collection
This study is a parallel, double-blind, randomized, placebo-controlled trial that will enroll 90 women with PCOS and will have an intervention duration of 8 wk. Participants will be assigned to 2 groups in a 1:1 ratio using a stratified block randomization technique.
2.2. Participant timeline
The study protocol adheres to the guidelines for reporting outcomes in trial protocols (11). The protocol schedule is provided in figure 1.
2.3. Eligibility criteria
2.3.1. Inclusion criteria
Participants will be considered eligible for inclusion if they voluntarily agree to participate by providing informed consent and have a confirmed diagnosis of PCOS by a gynecologist (based on the Rotterdam criteria).
2.3.2. Exclusion criteria
Participants will be excluded from the study if they are pregnant or breastfeeding; have used dietary supplements or probiotic products within the past 4 wk; have a current or prior diagnosis of psychiatric disorders, including major depressive disorder, bipolar disorder, or schizophrenia based on self-report, history of psychiatric hospitalization, or use of psychotropic medications; have hyperprolactinemia; are postmenopausal; have uncontrolled diabetes mellitus, defined as an HbA1C level ≥ 8%; have been diagnosed with chronic medical conditions such as cardiovascular, hepatic, renal diseases, or malignancies; or have a history of ovarian laparoscopic surgery or in vitro fertilization.
2.4. Sample size
The sample size for the current study was determined using an appropriate formula based on sex hormone binding globulin (SHBG) as the key variable. The mean and standard deviation of SHBG were extracted from a study by Ji et al. (12). In this calculation, the type 1 error, type 2 error, and study power rate considered were 5%, 20%, and 80%, respectively. Based on these considerations, the estimated sample size was 37 participants per group (total: 74). With a 20% dropout rate, we will include 45 participants per group.
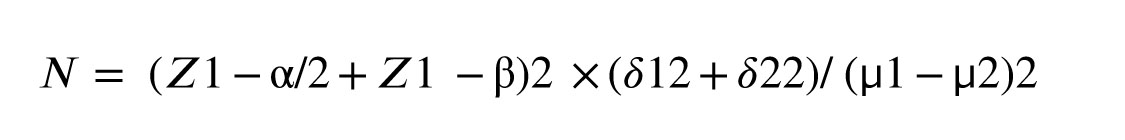
2.5. Recruitment
The study will include 90 individuals diagnosed with PCOS, selected from Shahid Beheshti hospital, Isfahan, Iran. An experienced gynecologist (H.Gh) evaluated the symptoms of PCOS according to the Rotterdam criteria. The Rotterdam criteria diagnose PCOS if at least 2 of the following are present: clinical or biochemical hyperandrogenism, oligo- or anovulation, and polycystic ovaries on ultrasound (defined as > 12 follicles per ovary or ovarian volume over 10 cm³). Additionally, the gynecologist will distinguish PCOS from other endocrine disorders, such as hyperprolactinemia, hypothyroidism, Cushing's syndrome, endometriosis, fibroids, uterine polyps, and various menstrual cycle irregularities. All eligible applicants who agree to be involved in the study will complete the written informed consent.
2.6. Randomization and blinding
Participants will be randomly assigned to 2 intervention groups using stratified block randomization based on body mass index categories (< 25 and ≥ 25). 6 blocks of 4 will be used to allocate individuals evenly (1:1) to either the intervention or placebo group. Randomization will be conducted by an independent person who was not involved in the study. A project collaborator managed group assignment, while a second researcher (M.B.) will oversee the allocation sequence. To maintain blinding, probiotic and placebo packets will be labeled as A or B by a third party. This study is double-blind, meaning participants, laboratory staff, and researchers will remain unaware of group assignments. Additionally, packages of probiotics are packed in the same color and shape as the placebo packets to maintain blinding.
2.7. Outcomes
The primary objectives contain the effect of probiotic supplementation on biochemical markers including: fasting blood glucose, fasting insulin, lipid profile biomarkers including total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, serum C-reactive protein (CRP), total antioxidant capacity, malondialdehyde, superoxide dismutase, hormonal biomarkers including SHBG and total testosterone, mental health (stress, depression, and anxiety), sleep quality, QOL, and hirsutism.
Secondary objectives contain the effect of probiotic supplementation on the following parameters: insulin resistance biomarkers, including quantitative insulin sensitivity check index and homeostasis model assessment insulin resistance, free androgen index, clinical symptoms, including acne and alopecia. The study will assess both primary and secondary issues at the onset and conclusion of the intervention.
2.8. Data collection
2.8.1. Demographic data
Data on demographic variables, including age, education levels, marital status, medical history, medication and supplement use, PCOS onset, and history of infertility, will be collected from each participant at baseline using the validated questionnaire.
2.8.2. Dietary intake assessment
4 one-day food recalls will be completed at baseline at weeks 3, 5, and 8 of the intervention, to assess the dietary intake of individuals. Dietary food recalls will be completed by a dietitian who is not aware of the study protocol. The quantity of each food is expressed in grams per day using Iranian household measurements and subsequently converted into nutrient and calorie values using Nutritionist 4 software (13).
2.8.3. Physical activity assessment
To assess physical activity levels, 4 24-hr recalls will be collected during each dietary assessment. Participants will report time spent sitting, sleeping, and engaging in mild, moderate, and vigorous activities. Physical activity will be expressed as metabolic equivalent of task (MET) hours per day (MET/h/day). Previous studies have confirmed the validity and reliability of this 24-hr physical activity assessment method (14).
2.8.4. Anthropometric assessments
The body weight will be recorded using a Seca scale with an accuracy of 0.1 kg while they wear limited clothing. Also, a portable stadiometer will be used to measure the height with an accuracy of 0.5 cm, ensuring that participants are barefoot.
2.8.5. QOL assessment
The PCOS-specific quality of life questionnaire will be used to assess participants’ QOL over the past month. It includes 26 items across 5 subscales: emotional well-being, excess hair, weight, infertility, and menstrual disorders. Each item is rated on a 7-point scale (1 = weakest performance, 7 = best performance), with subscale scores calculated by summing relevant items. Score ranges are 8-56 for emotional well-being, 5-35 for excess hair and weight, and 4-28 for infertility and menstrual issues. The questionnaire’s validity has been confirmed in the Iranian population (15).
2.8.6. Evaluation of hirsutism
The severity of hirsutism will be assessed using the simplified Ferriman-Gallwey score questionnaire in this study. This tool consists of 3 visual questions that evaluate hair growth intensity in 3 areas: upper lip, lower abdomen, and groin, with scores ranging from 1 (least) to 4 (most). A total score of 4 or higher indicates a predictor of hirsutism. The reliability and validity of this questionnaire have been confirmed in Iran (16).
2.8.7. Evaluation of acne
To evaluate the severity of acne in this study, the global acne grading system will be used (17). The reliability and validity of this method in Iran have been established in accordance with previous studies (18). In this scoring system, 6 areas of the forehead, nose, right cheek, left cheek, chin, chest, and upper back will be evaluated for acne (17).
2.8.8. Evaluation of alopecia
To evaluate the clinical signs of alopecia, the Sinclair scale will be used at the beginning and end of the study (19). This method has been validated by previous studies in Iran (20). This visual questionnaire consists of 5 stages that indicate the severity of alopecia.
2.8.9. Mental health assessment
Mental health status will be evaluated using a modified depression, anxiety and stress scale-21 questionnaire, which includes 21 items divided into 3 subscales: stress, anxiety, and depression, each containing 7 questions. Respondents rate these questions from 0 (not relevant) to 3 (completely relevant). To accurately assess symptom severity, scores for each subscale were multiplied by 2 (21).
2.8.10. Sleep quality assessment
Pittsburgh sleep quality index, which is specifically tailored for the Iranian population, was used to evaluate participants’ sleep quality (22). The pittsburgh sleep quality index includes 9 questions evaluating different aspects of sleep habits and quality, such as bedtime, wake times, and daily sleep length.
2.8.11. Measurement of biochemical factors
Laboratory evaluations were done both at the beginning and at the end of the study. After a 12-hr fast, a 10-ml blood sample is collected from the participants and centrifuged at 2500 rpm for 10 min at room temperature. The serum will be aliquoted into microtubules and immediately frozen at -80°C. Serum levels of fasting blood sugar, total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride will be assessed using the enzymatic colorimetric method, while quantitative CRP will be measured using the turbidometric method. Fasting insulin levels will be determined using a Monobind kit. Total antioxidant capacity will be assessed using the CUPric reducing antioxidant capacity method. Malondialdehyde levels will be quantified using the thiobarbituric acid reactive substances assay. At the same time, superoxide dismutase activity will be measured by evaluating the ability of Mn-superoxide dismutase to prohibit the conversion of resazurin to resorufin. All measurements will follow the instructions from Kiazist Life Sciences (Iran).
Free androgen index: to calculate the free androgen index, the following formula is used:
Data on demographic variables, including age, education levels, marital status, medical history, medication and supplement use, PCOS onset, and history of infertility, will be collected from each participant at baseline using the validated questionnaire.
2.8.2. Dietary intake assessment
4 one-day food recalls will be completed at baseline at weeks 3, 5, and 8 of the intervention, to assess the dietary intake of individuals. Dietary food recalls will be completed by a dietitian who is not aware of the study protocol. The quantity of each food is expressed in grams per day using Iranian household measurements and subsequently converted into nutrient and calorie values using Nutritionist 4 software (13).
2.8.3. Physical activity assessment
To assess physical activity levels, 4 24-hr recalls will be collected during each dietary assessment. Participants will report time spent sitting, sleeping, and engaging in mild, moderate, and vigorous activities. Physical activity will be expressed as metabolic equivalent of task (MET) hours per day (MET/h/day). Previous studies have confirmed the validity and reliability of this 24-hr physical activity assessment method (14).
2.8.4. Anthropometric assessments
The body weight will be recorded using a Seca scale with an accuracy of 0.1 kg while they wear limited clothing. Also, a portable stadiometer will be used to measure the height with an accuracy of 0.5 cm, ensuring that participants are barefoot.
2.8.5. QOL assessment
The PCOS-specific quality of life questionnaire will be used to assess participants’ QOL over the past month. It includes 26 items across 5 subscales: emotional well-being, excess hair, weight, infertility, and menstrual disorders. Each item is rated on a 7-point scale (1 = weakest performance, 7 = best performance), with subscale scores calculated by summing relevant items. Score ranges are 8-56 for emotional well-being, 5-35 for excess hair and weight, and 4-28 for infertility and menstrual issues. The questionnaire’s validity has been confirmed in the Iranian population (15).
2.8.6. Evaluation of hirsutism
The severity of hirsutism will be assessed using the simplified Ferriman-Gallwey score questionnaire in this study. This tool consists of 3 visual questions that evaluate hair growth intensity in 3 areas: upper lip, lower abdomen, and groin, with scores ranging from 1 (least) to 4 (most). A total score of 4 or higher indicates a predictor of hirsutism. The reliability and validity of this questionnaire have been confirmed in Iran (16).
2.8.7. Evaluation of acne
To evaluate the severity of acne in this study, the global acne grading system will be used (17). The reliability and validity of this method in Iran have been established in accordance with previous studies (18). In this scoring system, 6 areas of the forehead, nose, right cheek, left cheek, chin, chest, and upper back will be evaluated for acne (17).
2.8.8. Evaluation of alopecia
To evaluate the clinical signs of alopecia, the Sinclair scale will be used at the beginning and end of the study (19). This method has been validated by previous studies in Iran (20). This visual questionnaire consists of 5 stages that indicate the severity of alopecia.
2.8.9. Mental health assessment
Mental health status will be evaluated using a modified depression, anxiety and stress scale-21 questionnaire, which includes 21 items divided into 3 subscales: stress, anxiety, and depression, each containing 7 questions. Respondents rate these questions from 0 (not relevant) to 3 (completely relevant). To accurately assess symptom severity, scores for each subscale were multiplied by 2 (21).
2.8.10. Sleep quality assessment
Pittsburgh sleep quality index, which is specifically tailored for the Iranian population, was used to evaluate participants’ sleep quality (22). The pittsburgh sleep quality index includes 9 questions evaluating different aspects of sleep habits and quality, such as bedtime, wake times, and daily sleep length.
2.8.11. Measurement of biochemical factors
Laboratory evaluations were done both at the beginning and at the end of the study. After a 12-hr fast, a 10-ml blood sample is collected from the participants and centrifuged at 2500 rpm for 10 min at room temperature. The serum will be aliquoted into microtubules and immediately frozen at -80°C. Serum levels of fasting blood sugar, total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglyceride will be assessed using the enzymatic colorimetric method, while quantitative CRP will be measured using the turbidometric method. Fasting insulin levels will be determined using a Monobind kit. Total antioxidant capacity will be assessed using the CUPric reducing antioxidant capacity method. Malondialdehyde levels will be quantified using the thiobarbituric acid reactive substances assay. At the same time, superoxide dismutase activity will be measured by evaluating the ability of Mn-superoxide dismutase to prohibit the conversion of resazurin to resorufin. All measurements will follow the instructions from Kiazist Life Sciences (Iran).
Free androgen index: to calculate the free androgen index, the following formula is used:
Insulin sensitivity index: to calculate quick index of insulin sensitivity, the following formula is used (23):
I0 = fasting insulin (μU/ml), G0 = fasting glucose (mg/dl)
Insulin resistance index: to calculate homeostasis model assessment of insulin resistance index, the following formula is used (23):
Insulin resistance index: to calculate homeostasis model assessment of insulin resistance index, the following formula is used (23):
2.9. Interventions
Alongside standard care, participants will be divided into 2 groups: the probiotic group, who will receive one probiotic capsule per day containing 3 × 109 colony-forming units of 2 specific strains, L. helveticus R0052 and B. longum R0175, and the placebo group, who will receive one capsule per day containing 300 mg of maltodextrin. The intervention will last for 8 wk. Dietary recommendations for PCOS will be given to both groups, and participants are allowed to follow their usual care routines throughout the trial. Once the study is over, participants will resume their regular medical treatments.
2.9.1. Intervention description
Participants in the treatment group will take a (Psychobiotic, PersiLife Co., Iran) capsule daily before meal, for 8 wk, while the control group receives a placebo containing 300 mg of maltodextrin. New supplies are given every 4 wk, and adherence and side effects are monitored through calls and visits to the clinic. Participants may withdraw at any given point, and researchers can also remove participants if needed.
2.9.2. Strategies to improve adherence to interventions and follow-up
Participants will receive supplements or placebo packs at weeks 1 and 4 and return any unused capsules at weeks 4 and 8. Weekly phone calls monitored compliance, and returned packs will be counted at the end of the study. Compliance is calculated as (packs taken/packs prescribed) × 100. Women with < 80% compliance will be excluded from the final analysis (24). The benefits of probiotics in preventing PCOS complications will be explained, and participants will receive reminders via calls and texts.
2.9.3. Data management
An expert will manage data encryption, storage, and verification. Reported side effects will be assessed to decide participant’s exclusion. The conditions were set by the Medical Ethics Commission under which probiotic assignment could be disclosed.
2.9.4. Oversight and monitoring
The Ethical Committee of Isfahan University of Medical Sciences, Isfahan, Iran will oversee the entire trial to ensure ethical compliance and participant safety. The principal investigator and a gynecologist will manage participant inclusion, treatment, and follow-up. Based on previous studies, the probiotic doses used are considered safe, with no expected toxicity or side effects (9, 10). Any adverse effects will be reported to Isfahan University of Medical Sciences, which will supervise the study. Inspectors will conduct at least 2 monitoring visits, and the project team will hold monthly meetings to address issues and ensure protocol adherence.

2.10. Ethical Considerations
The study protocol received approval from the Research Ethics Committee of the School of Pharmacy and Nutrition, Isfahan University of Medical Sciences, Isfahan, Iran on
2.11. Statistical Analysis
Statistical analysis will be performed using SPSS version 26.0, with a significance level set at p < 0.05. Data normality will be assessed by the one-sample Kolmogorov-Smirnov test. Categorical variables will be reported as frequencies and percentages, while continuous variables will be expressed as means ± standard deviations. Baseline comparisons between intervention groups will use Chi-square tests for categorical data and independent t tests for continuous data. The intervention effects on primary and secondary outcomes will be evaluated using one-way ANCOVA. Missing data will be addressed via an intention-to-treat approach combined with multiple imputations.
3. Discussion
PCOS is a multifaceted endocrine disorder marked by ovulatory dysfunction, hyperandrogenism, and various metabolic issues (25). Elevated insulin levels, along with imbalances in gut microbiota, can significantly take part in the development of PCOS (26). Recently, lifestyle changes, dietary adjustments, and alterations in gut microbiota have been promoted as beneficial strategies for improving the comorbidities in PCOS cases (4). Women with PCOS may experience significant changes in their gut microbiota composition (27). The relationship between dysbiosis and obesity-related diseases have been demonstrated in various animal and human models. Recent advances in metagenomic sequencing have shown that dysbiosis is associated with many systemic disorders, including obesity, cancer, diabetes, metabolic syndrome, and insulin resistance. Additionally, some evidence suggests that gut dysbiosis is linked to sex hormone levels and ovarian morphological changes (4).
Probiotics can maintain the integrity of the intestinal barrier through the production of short-chain fatty acids, which are related to a reduction in insulin resistance and inflammation (28). Also, probiotics may have a reducing effect on blood lipid levels through the production of these metabolites (8). According to recent evidence, treatment with prebiotics and probiotics may inhibit many metabolic side effects, such as hyperinsulinemia in obese people, affecting gut microflora (29).
Furthermore, probiotics in the gut can increase the production of tryptophan as an enhancer of serotonin signaling and subsequently improve social behavior, stress, anxiety, and depression (30). Additionally, a recent trial has shown that supplementation with L. helveticus and B. longum has a beneficial effect on CRP levels, depression, anxiety, stress indicators, and sleep quality (9). In another randomized clinical trial, Rode et al. performed the same probiotic supplementation for 4 wk on healthy people and found a beneficial effect on stress-related disorders (10).
This study hypothesizes that probiotic supplementation will improve metabolic, hormonal, inflammatory, and antioxidant markers compared to placebo. However, several limitations should be noted. First, the study did not measure any specific marker to definitively assess the diversity of the gut microbiota population before and after intervention. Additionally, the role of probiotic foods, such as probiotic yogurt, is not considered. Furthermore, for ethical reasons, the effects of probiotics as a standalone treatment cannot be assessed, as it would be unethical to instruct participants to discontinue their standard medications. Lastly, the study is confined to a particular dose and ratio of supplements, which prevents the determination of the optimal dosage.
To the best of our knowledge, no study has evaluated the effect of L. helveticus and B. longum combination on the clinical outcomes of PCOS subjects. Therefore, our study focused on evaluating the effects of these 2 probiotic strain supplementations on metabolic status, inflammation, oxidative stress, QOL, and clinical symptoms in women with PCOS. If successful, nutritionists in clinical environments may recommend a new strain of probiotic supplements for women with PCOS to treat the comorbidities of this common disorder.
Data Availability
Data availability is managed by the first and corresponding authors.
Author Contributions
Study design: M. Shirani, M. Bagherniya, O. Sadeghi, Gh. Askari. Study conception: M. Shirani, M. Bagherniya, H. Ghasemi Tehrani, MH. Eskandari, M. Sharma, and Gh. Askari. Interpretation of data for the work: M. Shirani and O. Sadeghi and Gh. Askari. Drafting the manuscript of the protocol: M. Shirani, M. Bagherniya, O. Sadeghi, Gh. Askari, MH. Eskandari, and H. Ghasemi Tehrani. Revising the manuscript of the protocol: M. Shirani and M. Sharma. Everyone read and approved the final draft of the manuscript. All the authors have agreed to be accountable for all aspects of the work, ensuring that questions regarding the accuracy or completeness of any part of the work are appropriately investigated and resolved.
Acknowledgments
We are extremely grateful for the assistance provided by the research vice-chancellor of Isfahan University of Medical Sciences, Isfahan, Iran. This study will be implemented with the financial support of Isfahan University of Medical Sciences, Isfahan, Iran (grant number: 3402794). However, the investor was not involved in any of the study design and data dissemination processes. The article has undergone grammatical review utilizing artificial intelligence tools (Grammarly).
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |