Sat, Jan 31, 2026
[Archive]
Volume 3, Issue 2 (7-2005)
IJRM 2005, 3(2): 68-73 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hadinedoushan H, Normann R. The role of ghrelin and ghrelin receptors in polycystic ovary syndrome. IJRM 2005; 3 (2) :68-73
URL: http://ijrm.ir/article-1-38-en.html
URL: http://ijrm.ir/article-1-38-en.html
Full-Text [PDF 125 kb]
(868 Downloads)
| Abstract (HTML) (3576 Views)
Full-Text: (436 Views)
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women of reproductive age with primary manifestations of infertility, menstrual dysfunction and clinical or biochemical hyperandrogenism (hirsutism, acne and elevated androgen). The pathogenesis of PCOS is still controversial (1, 2). PCOS is related to obesity and to major metabolic alterations including both insulin resistance and b-cell dysfunction (3-5). A positive and highly significant correlation of ghrelin concentration to the degree of insulin resistance was found in PCOS subjects.
Furthermore, insulin-resistant PCOS patients treated with metformin, which improves insulin sensivity in PCOS patients, increased serum ghrelin levels (6). Ghrelin was identified as the endogenous ligand for the growth hormone secretagogue (GHS) receptor (7). Two molecular forms of ghrelin are found in the stomach: the 28 amino acid ghrelin having n-octanoylated serin in position 3 and the 27 amino acid des-[Gln14] ghrelin produced by alternative splicing of the ghrelin gene (8). The actions of ghrelin are carried out through interaction with a specific G protein-coupled receptor, named GHS-R .Two GHS-R mRNA isoforms, defined as type 1a and 1b, are encoded by the GHS-R gene and are produced by alternative mRNA processing. Translation of the GHS-R type 1a mRNA isoform produces the
Results
Ghrelin and GHS-Rs mRNA expression in follicular fluid cells
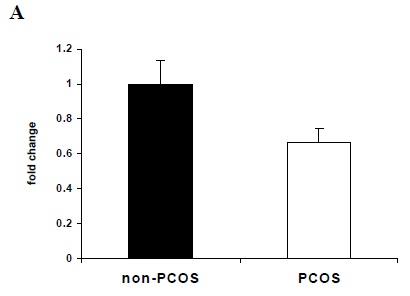
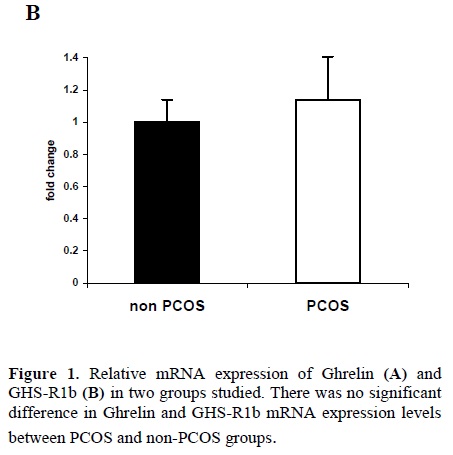
Quantitative real-time RT-PCR showed that mRNA for ghrelin and GHS-R 1b were detectable in follicular fluid cells from all patients. We failed to find mRNA for GHS-R 1a in any of follicular fluid cells. There were no significant differences in ghrelin and GHS-R1b mRNA expression levels between PCOS and non-PCOS groups (Figure1).
Gaytan et al (2003) used an immunohistochemical approach to characterize in detail the expression and pattern of cellular location of ghrelin and GHS-R1a subtype in the cyclic human ovary. They found that both components of the ghrelin signalling system are present in the granulosa and the theca layers of growing follicles. Determining of GHS-R1a in Gaytan’s study is in contrast with our results (19). This might be explained by the use of different techniques, because, we used RT-PCR technique for determining of mRNA expression of GHS-R1a.
In our knowledge, this is the first study to explore ghrelin and ghrelin receptors mRNA expression in follicular fluid cells in PCOS and non-PCOS subjects. Our results indicated that expression of ghrelin and GHS-R1b mRNA was similar between two groups under investigation. Therefore, production of ghrelin and non-functional GHS R1b in follicular fluid were not significantly different between PCOS and control groups and ghrelin may not be considered as a risk factor in pathogenesis of PCOS.
Conclusion
Our finding showed that ghrelin and GHS-R1b mRNA have been expressed in follicular cells of PCOS and non-PCOS patients. There were not significantly differences in ghrelin and GHS R1b mRNA expression between PCOS and non-PCOS groups. Ghrelin and ghrelin receptors may not be considered risk factors for pathogenesis of PCOS.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder in women of reproductive age with primary manifestations of infertility, menstrual dysfunction and clinical or biochemical hyperandrogenism (hirsutism, acne and elevated androgen). The pathogenesis of PCOS is still controversial (1, 2). PCOS is related to obesity and to major metabolic alterations including both insulin resistance and b-cell dysfunction (3-5). A positive and highly significant correlation of ghrelin concentration to the degree of insulin resistance was found in PCOS subjects.
Furthermore, insulin-resistant PCOS patients treated with metformin, which improves insulin sensivity in PCOS patients, increased serum ghrelin levels (6). Ghrelin was identified as the endogenous ligand for the growth hormone secretagogue (GHS) receptor (7). Two molecular forms of ghrelin are found in the stomach: the 28 amino acid ghrelin having n-octanoylated serin in position 3 and the 27 amino acid des-[Gln14] ghrelin produced by alternative splicing of the ghrelin gene (8). The actions of ghrelin are carried out through interaction with a specific G protein-coupled receptor, named GHS-R .Two GHS-R mRNA isoforms, defined as type 1a and 1b, are encoded by the GHS-R gene and are produced by alternative mRNA processing. Translation of the GHS-R type 1a mRNA isoform produces the
biologically active receptor and GHS-R type 1b encodes a C terminally truncated protein (9). The human GHS-R type 1a is a polypeptide of 366 amino acids with seven transmembrane domains and is the functional form of the receptor (10). Ghrelin, apart from its GH-releasing activity, also significantly stimulates prolactin, ACTH and cortisol secretion. Recently, a number of different central and peripheral effects have been described: on appetite and weight regulation, carbohydrate metabolism, heart function, the gonadal axis, exocrine function, and cell proliferation (11-17). mRNA expression of ghrelin and GHS-R1b were reported in many different human tissues including ovary (18). Using immunohistichemistry method Gaytan et al (2003) showed the presence of ghrelin and its cognate functional receptor in cyclic human ovary with distinct but partially overlapping patterns of cellular distribution (19). Plasma ghrelin concentrations have been reported to be correlated to body mass index (BMI) with reduced levels in obesity that increases in response to weight loss (20). Schofl et al (2002) showed that in PCOS women, serum ghrelin were significantly lower than in healthy controls and there was a close correlation of ghrelin to insulin sensitivity (21). No difference in plasma ghrelin concentrations between the PCOS and the control groups were reported (22). It is unknown wether abnormal expression of ghrelin and GHS-Rs in ovary might play a role in the pathogenesis of women with PCOS. This study was performed in order to determine the role of ghrelin and GHS-Rs in pathogenesis of PCOS.
Materials and Methods
Cell isolation from follicular fluid
Samples were centrifuged at 200g for 10 mins at 4°C and interface cells were aspirated and resuspended in Hank’s balanced salt solution (HBSS, GibcoBRL) containing 0.06 % gentamycin (DBL, Mulgrave, Victoria, Australia), 0.01% sodium azide (Sigma Aldrich) and 2mM ethylenediamine tetraacetic acid (EDTA, Sigma Aldrich) (HBSS/gent/EDTA/Az). The cells were then centrifuged over 1:1 v:v PBS:Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) at 400 g for 20 mins at 4°C to remove contaminating red blood cells. Interface cells were removed and resuspended in HBSS/gent/EDTA/ Az, centrifuged at 200g for 10 min. Finally, the cell pellet was resuspended in HBSS/gent/EDTA/Az, filtered through a 70μm diameter cell strainer (Becton Dickinson, Franklin Lakes, NJ, USA) and washed with HBSS/gent/EDTA/Az containing 0.5% BSA. Total cells were counted and viability assessed by trypan blue dye exclusion (Sigma Aldrich).
RNA extraction and quantitation
Total RNA was extracted from isolated follicular fluid cells by Tri-Reagent (Sigma Aldrich), followed by a series of phenol-chloroform extraction and ethanol precipitations as stipulated by Tri-Reagent manufacturers. Contaminating residual DNA was eliminated by treatment with deoxyribonuclease I (DNase I, Promega, Madison, WI). Resulting RNA was dissolved in 20μl ribonuclease-free water and concentration was determined using a Ribogreen® RNA quantitation kit (Molecular Probes, Eugene, OR) according to manufacturer’s instructions.
Quantitative real time RT-PCR
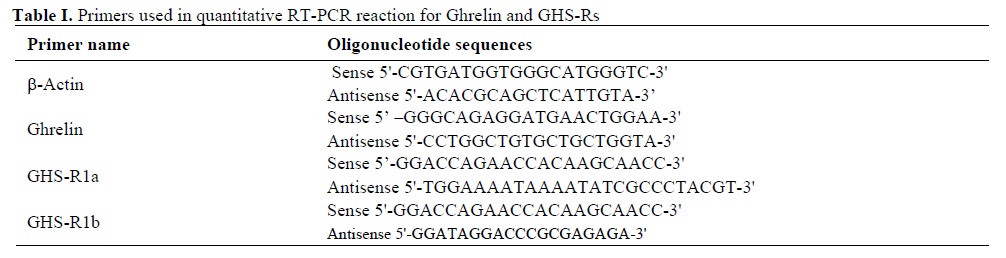
For each sample, 2 mg RNA was diluted in PCR-grade water and was reverse transcribed using random primers (Roche Diagnostics GmbH, Mannheim, Germany) and a Superscript II Reverse Transcription kit (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Negative controls omitting RNA or Superscript II enzyme were included. Specific primers for the ghrelin, GHS-R1a and GHS-R1b were designed on using primer express software (Applied Biosystems, Foster City, CA) and manufactured by Geneworks (Adelaide, Australia) (Table I). Samples were run in triplicate on an ABI Geneamp 5700 sequence detection system (Applied Biosystems). They were subjected to 40 cycles of amplification under condition 92°C-20S and 62°C-1min using 3 µl diluted cDNA (1:7), 10 µl 2× SYBR green buffer (Applied Biosystems) and 5 pmol of each primer. Negative controls omitting template cDNA were included in each run. Following RT-PCR amplification, a dissociation analysis was run on the products to ensure that a single product was amplified during the PCR process. To ensure that β-actin was an acceptable housekeeping or control gene for this study, it was necessary to determine if its mRNA levels were constant for the two treatment groups. β-actin mRNA was assayed and then normalised to total RNA measurements for each sample. For β-actin to be an acceptable control gene, the cycle threshold (CT) value per µg RNA should not vary statistically across treatment groups. It was also necessary to check that the primers for the housekeeping gene, β-actin and the other genes of interest had similar amplification efficiencies. Therefore, each primer set was run with serially diluted cDNA and the resultant CT values plotted against log dilutions. The slopes of each graph were determined and statistically compared. To determine if primer amplification efficiencies were comparable to that of the housekeeping gene, t-tests were performed on the slopes produced by each primer set for log dilutions plotted against CT value, along with the number of data points and the standard error generated for each slope. For all genes of interest, mRNA content was calculated for each sample relative to the housekeeping gene, β-actin. This was performed using the equation 2-ΔΔCt (as described in PE-ABI, Sequence Detector User Bulletin 2), where ΔCT is the difference between the gene of target and the housekeeping gene, and ΔΔCT is the change between the ΔCT for each sample and the control group (e.g. non-PCOS patients). Each target gene is then described as the fold change from the control group. Statistical significance was analyzed by a Students t-test. In all cases, p<0.05 was considered statistically significant.
Materials and Methods
Patient recruitment and follicular fluid collection
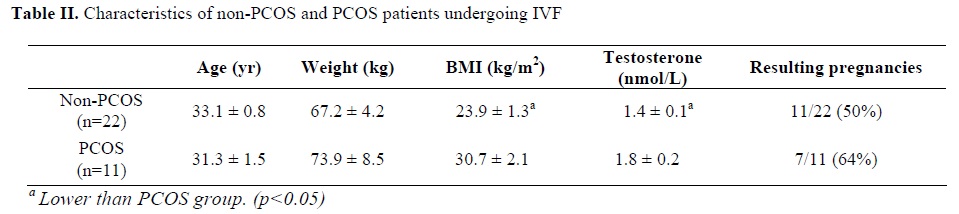
This study was carried out as case-control study on two different groups. Eleven patients were diagnosed as having PCOS according to criteria derived from the 1990 National Institutes of Health (NIH)/National Institute of Child Health and Human Development (NICHHD) conference. Briefly, the criteria include: 1) ovulatory dysfunction, 2) clinical hyperandrogen (hirsutism, acne, androgenic alopecia) 3) exclusion of other related disorders, such as hyperprolactinemia, nonclassic adrenal hyperplasia, or thyroid disease (23). Twenty-two non PCOS women matched for age and weight, undergoing IVF-ET for other causes of infertility, including 6 for tubal disease, 9 for male factor, 4 for endometriosis and 3 for unexplained infertility, were selected as the control group. The Research Ethics Committee of The Queen Elizabeth Hospital and The University of Adelaide approved the study protocols and consent forms. Written informed consent was obtained from each patient before follicular fluid collection. Prior to oocyte collection, a general clinical examination and hormonal evaluations were performed including baseline levels of testosterone (T), luteinizing hormone (LH), sex hormone binding globulin (SHBG), follicle stimulating hormone (FSH), and mid-luteal phase estradiol and progesterone. The average age, weight, body mass index (BMI), testosterone and resulting pregnancies for both groups are shown in Table 1. Samples underwent a standard IVF protocol; with recombinant FSH (N.V. Organon, The Netherlands) -stimulated follicles (15-25mm in diameter) aspirated 32-34 h after hCG (Profasi, Serono, Rockland, MA) administration. After removal of the cumulus-oocyte complexes, follicular fluid was pooled for each sample and placed on ice.Cell isolation from follicular fluid
Samples were centrifuged at 200g for 10 mins at 4°C and interface cells were aspirated and resuspended in Hank’s balanced salt solution (HBSS, GibcoBRL) containing 0.06 % gentamycin (DBL, Mulgrave, Victoria, Australia), 0.01% sodium azide (Sigma Aldrich) and 2mM ethylenediamine tetraacetic acid (EDTA, Sigma Aldrich) (HBSS/gent/EDTA/Az). The cells were then centrifuged over 1:1 v:v PBS:Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) at 400 g for 20 mins at 4°C to remove contaminating red blood cells. Interface cells were removed and resuspended in HBSS/gent/EDTA/ Az, centrifuged at 200g for 10 min. Finally, the cell pellet was resuspended in HBSS/gent/EDTA/Az, filtered through a 70μm diameter cell strainer (Becton Dickinson, Franklin Lakes, NJ, USA) and washed with HBSS/gent/EDTA/Az containing 0.5% BSA. Total cells were counted and viability assessed by trypan blue dye exclusion (Sigma Aldrich).
RNA extraction and quantitation
Total RNA was extracted from isolated follicular fluid cells by Tri-Reagent (Sigma Aldrich), followed by a series of phenol-chloroform extraction and ethanol precipitations as stipulated by Tri-Reagent manufacturers. Contaminating residual DNA was eliminated by treatment with deoxyribonuclease I (DNase I, Promega, Madison, WI). Resulting RNA was dissolved in 20μl ribonuclease-free water and concentration was determined using a Ribogreen® RNA quantitation kit (Molecular Probes, Eugene, OR) according to manufacturer’s instructions.
Quantitative real time RT-PCR
For each sample, 2 mg RNA was diluted in PCR-grade water and was reverse transcribed using random primers (Roche Diagnostics GmbH, Mannheim, Germany) and a Superscript II Reverse Transcription kit (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Negative controls omitting RNA or Superscript II enzyme were included. Specific primers for the ghrelin, GHS-R1a and GHS-R1b were designed on using primer express software (Applied Biosystems, Foster City, CA) and manufactured by Geneworks (Adelaide, Australia) (Table I). Samples were run in triplicate on an ABI Geneamp 5700 sequence detection system (Applied Biosystems). They were subjected to 40 cycles of amplification under condition 92°C-20S and 62°C-1min using 3 µl diluted cDNA (1:7), 10 µl 2× SYBR green buffer (Applied Biosystems) and 5 pmol of each primer. Negative controls omitting template cDNA were included in each run. Following RT-PCR amplification, a dissociation analysis was run on the products to ensure that a single product was amplified during the PCR process. To ensure that β-actin was an acceptable housekeeping or control gene for this study, it was necessary to determine if its mRNA levels were constant for the two treatment groups. β-actin mRNA was assayed and then normalised to total RNA measurements for each sample. For β-actin to be an acceptable control gene, the cycle threshold (CT) value per µg RNA should not vary statistically across treatment groups. It was also necessary to check that the primers for the housekeeping gene, β-actin and the other genes of interest had similar amplification efficiencies. Therefore, each primer set was run with serially diluted cDNA and the resultant CT values plotted against log dilutions. The slopes of each graph were determined and statistically compared. To determine if primer amplification efficiencies were comparable to that of the housekeeping gene, t-tests were performed on the slopes produced by each primer set for log dilutions plotted against CT value, along with the number of data points and the standard error generated for each slope. For all genes of interest, mRNA content was calculated for each sample relative to the housekeeping gene, β-actin. This was performed using the equation 2-ΔΔCt (as described in PE-ABI, Sequence Detector User Bulletin 2), where ΔCT is the difference between the gene of target and the housekeeping gene, and ΔΔCT is the change between the ΔCT for each sample and the control group (e.g. non-PCOS patients). Each target gene is then described as the fold change from the control group. Statistical significance was analyzed by a Students t-test. In all cases, p<0.05 was considered statistically significant.

Results
Characterization of the patients and follicular fluid cells
Age, weight and resulting pregnancies did not vary between PCOS and non-PCOS patients, whereas the BMI and serum testosterone level of PCOS were significantly higher than non-PCOS patients (TableII). The number of cells recovered from the follicular fluid of patients ranged from 5-30×106 cells, and cell viability as determined by trypan blue dye exclusion ranged from 50%-85%.
Ghrelin and GHS-Rs mRNA expression in follicular fluid cells
Quantitative real-time RT-PCR showed that mRNA for ghrelin and GHS-R 1b were detectable in follicular fluid cells from all patients. We failed to find mRNA for GHS-R 1a in any of follicular fluid cells. There were no significant differences in ghrelin and GHS-R1b mRNA expression levels between PCOS and non-PCOS groups (Figure1).


Discussion
Ghrelin is unique gut-derived peptides in its action on pituitary hormone secretion and energy homeostasis. Long-term infusion of ghrelin in animal model via centrally stimulates food intake, increase body fat accumulation and causes adiposity (24, 25). In humans, in states of chronic malnutrition such as anorexia nervosa, serum ghrelin levels are increased where obesity is associated with decreased ghrelin concentration that increases after weigh loss (26). These data support a potential role of ghrelin in the pathogenesis of human obesity. PCOS women are predisposed to obesity and to metabolic changes such as insulin resistance (6). There is paucity of data on ghrelin homeostasis in PCOS. Fasting ghrelin is decreased in subjects with PCOS compared to control in some (21, 27) but not all studies (22). Pagotto et al (2002) observed no change in fasting plasma ghrelin following 7 months of hypocaloric diet for either PCOS or non-PCOS age and weight-match subjects (27). In this study, using RT-PCR, we sought ghrelin and ghrelin receptors mRNA expression in follicular fluid cells in PCOS and non-PCOS subjects. Our observations showed that the ghrelin and GHS-R1b mRNA have been expressed in follicular fluid cells of PCOS and non-PCOS samples. We failed to determine mRNA expression of the functional type 1a GHS-R in any sample. Granulosa cells make up the primary component of intrafollicular cells. Products of these cells within the follicular possibly contribute to the functioning of ovary and may contribute to the abnormal ovarian pathology observed in PCOS. A widespread pattern of expression of the genes encoding ghrelin and its cognate receptor has been reported in human. GHS/ghrelin binding sites have been demonstrated in a variety of peripheral human tissues (28). Using RT-PCR, expression of ghrelin and GHS-R1b in human ovary has been postulated by Gnanapavan et al (2002). Consistent with our study, they did not find GHS-R1a subtype mRNA in human ovary (18). The role of the ovary expression of the non-functional, unspliced receptor mRNA transcript (type 1b) is unknown.Gaytan et al (2003) used an immunohistochemical approach to characterize in detail the expression and pattern of cellular location of ghrelin and GHS-R1a subtype in the cyclic human ovary. They found that both components of the ghrelin signalling system are present in the granulosa and the theca layers of growing follicles. Determining of GHS-R1a in Gaytan’s study is in contrast with our results (19). This might be explained by the use of different techniques, because, we used RT-PCR technique for determining of mRNA expression of GHS-R1a.
In our knowledge, this is the first study to explore ghrelin and ghrelin receptors mRNA expression in follicular fluid cells in PCOS and non-PCOS subjects. Our results indicated that expression of ghrelin and GHS-R1b mRNA was similar between two groups under investigation. Therefore, production of ghrelin and non-functional GHS R1b in follicular fluid were not significantly different between PCOS and control groups and ghrelin may not be considered as a risk factor in pathogenesis of PCOS.
Conclusion
Our finding showed that ghrelin and GHS-R1b mRNA have been expressed in follicular cells of PCOS and non-PCOS patients. There were not significantly differences in ghrelin and GHS R1b mRNA expression between PCOS and non-PCOS groups. Ghrelin and ghrelin receptors may not be considered risk factors for pathogenesis of PCOS.
Type of Study: Original Article |
References
1. Battaglia C, Mancini F, Persico N, Zaccaria V, de Aloysio D. Ultrasound evaluation of PCO, PCOS and OHSS. Reprod Biomed Online 2004 9(6): 614-19. [DOI:10.1016/S1472-6483(10)61770-1]
2. Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinol Metab Clin North Am 1999; 28(2): 295-324. [DOI:10.1016/S0889-8529(05)70071-2]
3. Franks S. Polycystic ovary syndrome. N Engl J Med 1995 28; 333(13): 853-61. [DOI:10.1056/NEJM199509283331307] [PMID]
4. Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997; 18(6): 774-800. [DOI:10.1210/edrv.18.6.0318] [PMID]
5. Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab1996; 81(3): 942-47. [DOI:10.1210/jc.81.3.942]
6. Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles,and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab 2000; 85(1): 139-46. [DOI:10.1210/jcem.85.1.6293] [PMID]
7. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402(6762): 656-60. [DOI:10.1038/45230] [PMID]
8. Hosoda H, Kojima M, Matsuo H, Kangawa K. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem 2000; 275(29): 21995-2000. [DOI:10.1074/jbc.M002784200] [PMID]
9. Barlier A, Zamora AJ, Grino M, Gunz G, Pellegrini-Bouiller I, Morange-Ramos I, et al. Expression of functional growth hormone secretagogue receptors in human pituitary adenomas: polymerase chain reaction, triple in-situ hybridization and cell culture studies. J Neuroendocrinol 1999; 11(7): 491-502. [DOI:10.1046/j.1365-2826.1999.00351.x] [PMID]
10. Smith RG, Feighner S, Prendergast K, Guan X, Howard A. A New Orphan Receptor Involved in Pulsatile Growth Hormone Release. Trends Endocrinol Metab 1999; 10(4): 128-35. [DOI:10.1016/S1043-2760(98)00132-5]
11. Tena-Sempere M, Barreiro ML, Gonzalez LC, Gaytan F, Zhang FP, Caminos JE, et al. Novel expression and functional role of ghrelin in rat testis. Endocrinology 2002; 143(2): 717-25. [DOI:10.1210/endo.143.2.8646] [PMID]
12. Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 2002; 51(1): 124-29. [DOI:10.2337/diabetes.51.1.124] [PMID]
13. Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, et al. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab 2001; 86(12): 5854-59. [DOI:10.1210/jcem.86.12.8115] [PMID]
14. Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86(12): 5992. [DOI:10.1210/jcem.86.12.8111] [PMID]
15. Murata M, Okimura Y, Iida K, Matsumoto M, Sowa H, Kaji H, et al. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem 2002; 277(7): 5667-74. [DOI:10.1074/jbc.M103898200] [PMID]
16. Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 2001; 86(10): 5083-86. [DOI:10.1210/jcem.86.10.8098] [PMID]
17. Lee HM, Wang G, Englander EW, Kojima M, Greeley GH Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002; 143(1): 185-90. [DOI:10.1210/endo.143.1.8602] [PMID]
18. Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002; 87(6): 2988-91. [DOI:10.1210/jcem.87.6.8739] [PMID]
19. Gaytan F, Barreiro ML, Chopin LK, Herington AC, Morales C, Pinilla L, et al. Immunolocalization of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in the cyclic human ovary. J Clin Endocrinol Metab 2003; 88(2): 879-87. [DOI:10.1210/jc.2002-021196] [PMID]
20. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 2002; 87(1): 240-44. [DOI:10.1210/jcem.87.1.8129] [PMID]
21. Schofl C, Horn R, Schill T, Schlosser HW, Muller MJ, Brabant G. Circulating ghrelin levels in patients with polycystic ovary syndrome. J Clin Endocrinol Metab 2002; 87(10): 4607-10 [DOI:10.1210/jc.2002-020505] [PMID]
22. Orio F Jr, Lucidi P, Palomba S, Tauchmanova L, Cascella T, Russo T, et al. Circulating ghrelin concentrations in the polycystic ovary syndrome. J Clin Endocrinol Metab 2003; 88(2): 942-45. [DOI:10.1210/jc.2002-021451] [PMID]
23. Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome.
24. Dunaif A, Givens JR, Haseltine FP, Merriam GM, eds. In: towards a rational approach. Polycystic ovary syndrome. Boston: Blackwell, 1992: 377-84.
25. Tschöp M, Smiley DL, Heiman ML Ghrelin induces adiposity in rodents. Nature 2000; 407: 908-13 [DOI:10.1038/35038090] [PMID]
26. Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA,et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001; 50: 2540-47. [DOI:10.2337/diabetes.50.11.2540] [PMID]
27. Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol 2001; 145: 669-73. [DOI:10.1530/EJE-1450669] [PMID]
28. Pagotto U, Gambineri A, Vicennati V, Heiman ML, Tschop M, Pasquali R. Plasma ghrelin, obesity, and the polycystic ovary syndrome: correlation with insulin resistance and androgen levels. Clin Endocrinol Metab 2002; 87(12): 5625-29. [DOI:10.1210/jc.2002-020776] [PMID]
29. Papotti M, Ghé C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, et al. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab 2000; 85: 3803-07. [DOI:10.1210/jc.85.10.3803] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




