Tue, Apr 16, 2024
[Archive]
Volume 3, Issue 2 (7-2005)
IJRM 2005, 3(2): 62-67 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Babaei H, Derakhshanfar A, Nematollahi-Mahani S N, Nabipour F, Zeraatpisheh A. Morphologic changes in fresh and vitrified mouse ovaries after retinol palmitate administration. IJRM 2005; 3 (2) :62-67
URL: http://ijrm.ir/article-1-41-en.html
URL: http://ijrm.ir/article-1-41-en.html
Homayoon Babaei * 
 , Amin Derakhshanfar
, Amin Derakhshanfar 
 , Seyed Noureddin Nematollahi-Mahani
, Seyed Noureddin Nematollahi-Mahani 
 , Fathemeh Nabipour
, Fathemeh Nabipour 
 , Akram Zeraatpisheh
, Akram Zeraatpisheh 


 , Amin Derakhshanfar
, Amin Derakhshanfar 
 , Seyed Noureddin Nematollahi-Mahani
, Seyed Noureddin Nematollahi-Mahani 
 , Fathemeh Nabipour
, Fathemeh Nabipour 
 , Akram Zeraatpisheh
, Akram Zeraatpisheh 

Full-Text [PDF 698 kb]
(369 Downloads)
| Abstract (HTML) (2337 Views)
Full-Text: (278 Views)
Introduction
Retinol and its cellular metabolites, all-trans retinoic acid and 9-cis retinoic acid are known as retinoids. These compounds are recognized as important regulators of vertebrate development, cellular differentiation and tissue function. In addition, retinoids are essential for normal reproductive processes in both males and females (1-3). Several studies indicate a positive effect of retinol supplementation when diets are inadequate in vitamin A. In litter-bearing species, administration of retinol or β-carotene has been reported to increase embryo survival in mice (4), rabbits (5) and swine (6,7). Retinoids may function as important regulators of oogenesis and oocyte survival in the mouse fetal ovary and positively impact events associated with oocyte maturation in adult farm species leading to improved embryonic survival (8). Thus, embryonal survival may be associated with differences in oocyte development before ovulation (9).
Transplantation of cryopreserved ovaries is a strategy for improving the reproductive efficiency of young female patients, suffering from infertility,
Discussion
The results of the present study show clearly that retinol palmitate administered mouse produce more antral follicles in response to superovulation. In addition, we did not observe any positive effect of retinol palmitate on follicle quality after vitrification of ovaries.
Cryopreservation of oocytes and embryos by vitrification has been widely conducted because of its simplicity and high survival rate of oocytes and
embryos as well as cryopreservation of ovarian tissues (17). Sugimoto et al. (2000) observed that rat ovarian follicles survived after vitrification and transplantation with a decrease in the number of healthy antral follicles (approximately 30%) (16), and according to another report by Baird et al. (1999), about 28% of primordial follicles survived after transplantation of frozen/thawed ovarian tissue(22). In contrast, in the present investigation, the percentage of normal follicles including 'small', 'growing' and 'antral' in both vitrified groups (Table II) was lower than the above mentioned reports. Higher rate of damaged follicles in both vitrified groups shows that vitrification may induce some short-term deleterious effects on ovarian tissues. Transplantation of ovaries following vitrification may decrease such harmful effects of cryopreservation through change in the milieu of follicles (23), and autorepair of follicles under the control of various tropic factors (24). This finding is in agreement with the studies of Sugimoto et al. (16), and Schmidt et al. (25) which reported that continuous follicle maturation occurred in cryopreserved ovarian autografts following transplantation.
Antioxidant agents such as alpha-tocopherol as well as vitamin A improve the survival rate of follicles (8, 26, 27). However, the effect of retinoids on follicles quality following cryopreservation stress has not been yet investigated. Schweigert & Zucker (1988) suggested that concentrations of vitamin A in follicular fluid might serve as an indicator of follicular quality in cattle (28). Vitamin A concentrations were observed to be highest in large non-atretic follicles and lowest in small atretic follicles. Some reports from in vivo and in vitro studies in domestic livestock species lend supported that retinoids may target the oocyte and influence the subsequent development of embryos (7, 29-31). Improvement of follicular quality that is gained through retinol palmitate administration may in turn lead to better tolerance against deleterious effects of vitrification following transplantation. However, we did not observe any useful effect of retinol palmitate on follicle quality after vitrification of ovaries.
There are some evidences that vitamin A may influence oocyte and embryo development. The results obtained by Lima et al. (2004) demonstrated that the addition of retinol to the embryo culture media had a significant positive effect on bovine early embryonic development (32). Rabbits that had higher blood levels of vitamin A produced more oocytes and embryos in response to superovulation than those that had lower levels (33), and mouse treated with vitamin A before superovulation had higher ovulation rate and produced more zygotes (19). As our results show, the percentage of antral follicles in retinol palmitate administered mice was statistically higher than the control group following superovulation. This may be the probable mechanism of the positive effect of retinol palmitate on folliculogenesis after superovulation, which describes the results of our study and the reports mentioned above. In contrast, Shaw et al. (29) and Brown et al. (34) did not show any effect of vitamin A on the response of ovaries to superovulation in cows.
Transport and metabolism of retinoids are mediated and regulated by specific binding proteins (35, 36). Retinol is transported systemically and intercellularly by retinol-binding protein (RBP). Within target cells, retinol binds cellular retinol-binding protein (CRBP) which functions in retinol accumulation and presentation to dehydrogenases for conversion to retinal and retinoic acid (37). Brown et al. (2003) reported that within the ovarian follicles, strongest CRBP was observed in granulosa cells of preantral follicles (38). This evidence demonstrates that exogenous retinoids may have better effect on growing and antral than small follicles. Furthermore, retinoids have been shown to stimulate steroidogenesis by granulosa cells in vitro and synergistically enhance the ability of FSH to induce LH receptors (39). Antrum formation and final growth are entirely FSH/LH dependent (40). Together, these reports suggest that retinoids play a role in moving normal preantral toward antral follicles and may explain increasing the proportion of antral follicles in response to superovulation that is shown in the present study as well.
Conclusion
In conclusion, the results of the present study demonstrate that when retinol palmitate is administered to the mice, growing follicles developed to antral follicles in response to superovulation. However, short term harmful effects of vitrification on ovaries may not be overcome by retinol palmitate administration. Further in vitro investigations are needed to evaluate long term effects of vitrification in retinoids treated animals.
Acknowledgements
Special thanks to Mr. Ali Haddad Narafshan for revising the English of the manuscript.
Retinol and its cellular metabolites, all-trans retinoic acid and 9-cis retinoic acid are known as retinoids. These compounds are recognized as important regulators of vertebrate development, cellular differentiation and tissue function. In addition, retinoids are essential for normal reproductive processes in both males and females (1-3). Several studies indicate a positive effect of retinol supplementation when diets are inadequate in vitamin A. In litter-bearing species, administration of retinol or β-carotene has been reported to increase embryo survival in mice (4), rabbits (5) and swine (6,7). Retinoids may function as important regulators of oogenesis and oocyte survival in the mouse fetal ovary and positively impact events associated with oocyte maturation in adult farm species leading to improved embryonic survival (8). Thus, embryonal survival may be associated with differences in oocyte development before ovulation (9).
Transplantation of cryopreserved ovaries is a strategy for improving the reproductive efficiency of young female patients, suffering from infertility,
due to iatrogenic loss of ovarian function, resulting from chemotherapy and/or radiation therapy (10). In an early study, Deansely (1957) reported the successful cryopreservation of ovarian tissue (11) and then, Parrot (1960) reported the birth of live offspring after orthopic transplantation of a slice of cryopreserved mouse ovary (12). Ovarian cryopreservation is mostly carried out by slow freezing (13) but many attempts have been made to improve cryopreservation conditions by using simple and efficient procedures. For this purpose, the vitrification method was applied to the field of biology as a method of cryopreservation and its recent development has greatly simplified the cryopreservation procedures. This approach was first applied for oocyte cryopreservation by Critser et al (14) and recently, Nematallahi-Mahani, et al. (15), Sugimoto et al. (16) and Salehnia et al. (17) showed that vitrification is a useful strategy for the preservation of some mammalian ovaries.
Despite many reports on improvement of vitrification procedure, the rate of success is still lower than non-vitrified materials (18). To the best of our knowledge, there are not any reports which evaluate the effect of retinoids on ovaries following vitrification. The current experiment, therefore, was designed to study 1) the effects of retinol palmitate administration on changes in proportion of different follicles in response to superovulation. 2) the effects of retinol palmitate administration on quality of follicles after vitrification-warming of ovaries.
Materials and Methods
Chemicals were purchased from Sigma Company (St. Louis, MO, USA) unless otherwise indicated.
Animals
All animals were cared for and used in accordance with the International Guiding Principle for Biomedical Research Involving Animals at Kerman University of Medical Science. The BALB/c Mice were housed under a lighting regimen of 12 hours light to 12 hours dark and temperature-controlled conditions (22 ± 2°C). Food and water were freely available at all times. Ten, 4 week old female mice were used in this study.
Experimental Design
To determine whether retinol palmitate is capable of increasing follicles quality and lowering follicular damage following vitrification of ovaries, the mice (n=10) were randomly assigned to either paraffin (n=5) or vitamin A (n=5) administered groups. Vitamin A administered animals were injected 250 IU of retinol palmitate (Osvah, Iran), dissolved in 0.1 ml of paraffin oil intraperitoneally on days one and ten. Paraffin administered mice only received 0.1 ml of the vehicle (paraffin oil). The animals were superovulated with 10 IU PMSG (Folligon®, Intervet) simultaneously with the second injection of paraffin or retinol palmitate (19). Forty eight hours after PMSG injection, the mice were killed by cervical dislocation and ovaries were collected from both groups. The collected left ovaries from both paraffin and vitamin A administered mice were considered as non-vitrified (NOP, non-vitrified ovaries from paraffin administered mice; NOV, non-vitrified ovaries from vitamin A administered mice) and the effects of retinol palmitate administration on changes in follicles differential counts were evaluated. In addition, the collected right ovaries from both paraffin and vitamin A administered mice underwent vitrification (VOP, vitrified ovaries from paraffin administered mice; VOV, vitrified ovaries from vitamin A administered mice) and the effects of retinol palmitate administration on quality of follicles after vitrification-warming were evaluated.
Cryopreservation of Ovaries by Vitrification
We adopted the method of Vajta et al. (1998) with some modification (20). Briefly, the vitrification solution consisted of VS1 {10% ethylene glycol (EG) and 10% DMSO in holding medium (TCM-199 + 20% FBS: HM)} and VS2 {20% EG and 20% DMSO in HM}. The ovaries were equilibrated in VS1 for 15 minutes at room temperature, and then in VS2 for 2 minutes. Each ovary was loaded into plastic straw (outer diameter: 5mm) with the least volume of the vitrification solution at room temperature, followed by plunge directly into liquid nitrogen.
Warming and Cryoprotectant dilution
The vitrified samples were thawed rapidly by immersing the end of the tubes in a thawing solution composed of 1.0 M sucrose in HM for 10 minutes. The temperature of the media used for warming was held at 37°C.
Histopathological Evaluation
The fresh (non-vitrified) and the recovered vitrified ovaries were fixed in 10% buffered formalin, embedded in paraffin wax, serially sectioned at 6μm, stained with hematoxylin & eosin and analyzed under light microscope. Each ovary yielded approximately 600 sections, and differential follicle counts were obtained from every 10th section to provide a 10% sample selection (approximately 60 sections per ovary).
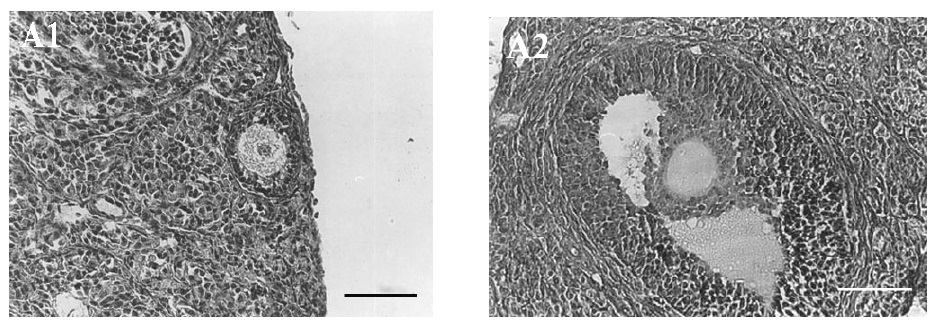
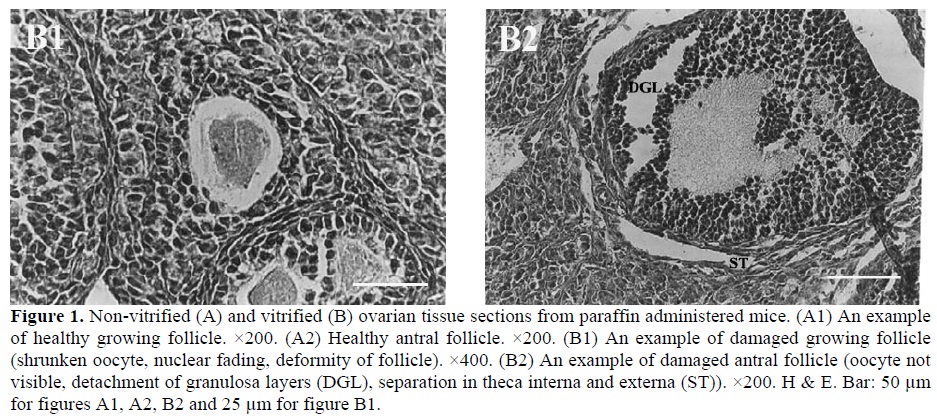
Three ovarian follicle classes (small, growing, antral) were identified in the tissue sections according to Thomas et al. (1997), (21). Briefly, 'small' follicles consisted of an oocyte surrounded by one unbroken monolayer of granulosa cells. 'Growing' follicles had an oocyte surrounded by a multilayered, solid mantle of granulosa cells. 'Antral' follicles were characterized by a central oocyte encircled by a fluid filled space and bordered by several layers of granulosa cells. The number of normal and damaged follicles at each follicular class was also recorded. In addition, structural normality (follicle and stromal cell morphology, even distribution of granulosa cells, intact theca and appearance of oocytes) was evaluated.
Statistical Analysis
The SPSS ® software for windows (standard version, 10.0.1) was used for data evaluation (SPSS Inc, Chicago, IL, USA). Data were analysed by Pearson's Chi-square test.
Results
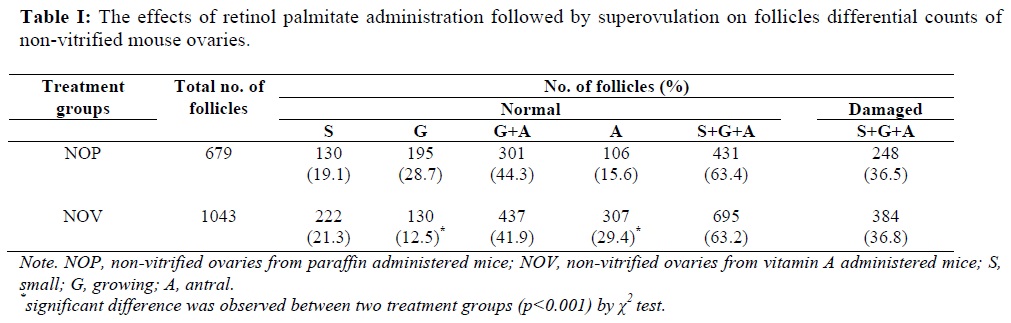
The number of morphologically normal follicles as well as changes in different follicular classes was calculated for the non-vitrified ovaries after retinol palmitate versus paraffin administration followed by superovulation (Table I). No statistical difference due to retinol palmitate injection was observed for the percentage of small follicles between the two non-vitrified groups (19.1% vs. 21.3%). The rate of growing and antral follicles was nearly identical in the two groups (44.3% vs. 41.9%). While the proportion of the antral follicles in the NOV group was statistically higher in comparison to the NOP group (29.4% vs. 15.6%; p<0.001). There were not any statistical differences in the rate of damaged follicles between the two non-vitrified groups.
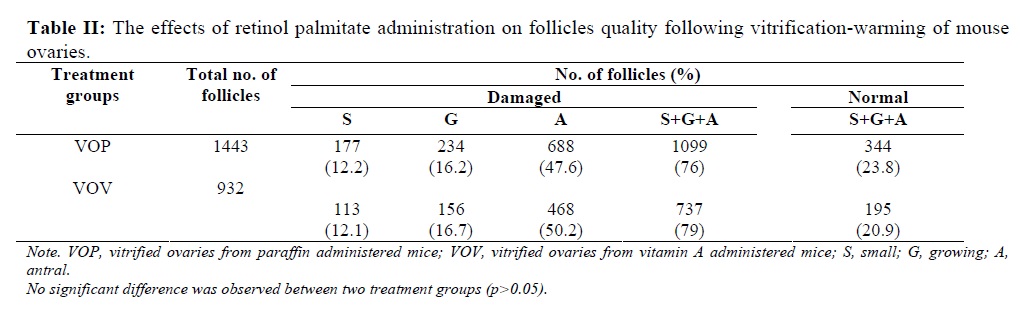
When the retinol palmitate group was compared with the control after vitrification-warming, no positive effect was found due to retinol palmitate administration. As shown in Table II, the proportion of the damaged follicles did not show any significant difference between two vitrified groups (VOP: 76% vs. VOV: 79%).
Most histopathological features of both non-vitrified groups were normal. The cytoplasm of the oocytes was clear and normal and all the granulosa layers and theca interna and externa were intact and firmly attached to the related basement membranes. The stromal cells were normal with distinct boundaries, prominent nucleus and nucleolus and pinkish cytoplasm. In contrast, in both vitrified groups the stromal cells had distinct margins with foamy cytoplasm. The detachment of the granulosa layers from the basement membrane and the deformity of the oocytes was also seen (Figure 1).
Despite many reports on improvement of vitrification procedure, the rate of success is still lower than non-vitrified materials (18). To the best of our knowledge, there are not any reports which evaluate the effect of retinoids on ovaries following vitrification. The current experiment, therefore, was designed to study 1) the effects of retinol palmitate administration on changes in proportion of different follicles in response to superovulation. 2) the effects of retinol palmitate administration on quality of follicles after vitrification-warming of ovaries.
Materials and Methods
Chemicals were purchased from Sigma Company (St. Louis, MO, USA) unless otherwise indicated.
Animals
All animals were cared for and used in accordance with the International Guiding Principle for Biomedical Research Involving Animals at Kerman University of Medical Science. The BALB/c Mice were housed under a lighting regimen of 12 hours light to 12 hours dark and temperature-controlled conditions (22 ± 2°C). Food and water were freely available at all times. Ten, 4 week old female mice were used in this study.
Experimental Design
To determine whether retinol palmitate is capable of increasing follicles quality and lowering follicular damage following vitrification of ovaries, the mice (n=10) were randomly assigned to either paraffin (n=5) or vitamin A (n=5) administered groups. Vitamin A administered animals were injected 250 IU of retinol palmitate (Osvah, Iran), dissolved in 0.1 ml of paraffin oil intraperitoneally on days one and ten. Paraffin administered mice only received 0.1 ml of the vehicle (paraffin oil). The animals were superovulated with 10 IU PMSG (Folligon®, Intervet) simultaneously with the second injection of paraffin or retinol palmitate (19). Forty eight hours after PMSG injection, the mice were killed by cervical dislocation and ovaries were collected from both groups. The collected left ovaries from both paraffin and vitamin A administered mice were considered as non-vitrified (NOP, non-vitrified ovaries from paraffin administered mice; NOV, non-vitrified ovaries from vitamin A administered mice) and the effects of retinol palmitate administration on changes in follicles differential counts were evaluated. In addition, the collected right ovaries from both paraffin and vitamin A administered mice underwent vitrification (VOP, vitrified ovaries from paraffin administered mice; VOV, vitrified ovaries from vitamin A administered mice) and the effects of retinol palmitate administration on quality of follicles after vitrification-warming were evaluated.
Cryopreservation of Ovaries by Vitrification
We adopted the method of Vajta et al. (1998) with some modification (20). Briefly, the vitrification solution consisted of VS1 {10% ethylene glycol (EG) and 10% DMSO in holding medium (TCM-199 + 20% FBS: HM)} and VS2 {20% EG and 20% DMSO in HM}. The ovaries were equilibrated in VS1 for 15 minutes at room temperature, and then in VS2 for 2 minutes. Each ovary was loaded into plastic straw (outer diameter: 5mm) with the least volume of the vitrification solution at room temperature, followed by plunge directly into liquid nitrogen.
Warming and Cryoprotectant dilution
The vitrified samples were thawed rapidly by immersing the end of the tubes in a thawing solution composed of 1.0 M sucrose in HM for 10 minutes. The temperature of the media used for warming was held at 37°C.
Histopathological Evaluation
The fresh (non-vitrified) and the recovered vitrified ovaries were fixed in 10% buffered formalin, embedded in paraffin wax, serially sectioned at 6μm, stained with hematoxylin & eosin and analyzed under light microscope. Each ovary yielded approximately 600 sections, and differential follicle counts were obtained from every 10th section to provide a 10% sample selection (approximately 60 sections per ovary).
Three ovarian follicle classes (small, growing, antral) were identified in the tissue sections according to Thomas et al. (1997), (21). Briefly, 'small' follicles consisted of an oocyte surrounded by one unbroken monolayer of granulosa cells. 'Growing' follicles had an oocyte surrounded by a multilayered, solid mantle of granulosa cells. 'Antral' follicles were characterized by a central oocyte encircled by a fluid filled space and bordered by several layers of granulosa cells. The number of normal and damaged follicles at each follicular class was also recorded. In addition, structural normality (follicle and stromal cell morphology, even distribution of granulosa cells, intact theca and appearance of oocytes) was evaluated.
Statistical Analysis
The SPSS ® software for windows (standard version, 10.0.1) was used for data evaluation (SPSS Inc, Chicago, IL, USA). Data were analysed by Pearson's Chi-square test.
Results
The number of morphologically normal follicles as well as changes in different follicular classes was calculated for the non-vitrified ovaries after retinol palmitate versus paraffin administration followed by superovulation (Table I). No statistical difference due to retinol palmitate injection was observed for the percentage of small follicles between the two non-vitrified groups (19.1% vs. 21.3%). The rate of growing and antral follicles was nearly identical in the two groups (44.3% vs. 41.9%). While the proportion of the antral follicles in the NOV group was statistically higher in comparison to the NOP group (29.4% vs. 15.6%; p<0.001). There were not any statistical differences in the rate of damaged follicles between the two non-vitrified groups.
When the retinol palmitate group was compared with the control after vitrification-warming, no positive effect was found due to retinol palmitate administration. As shown in Table II, the proportion of the damaged follicles did not show any significant difference between two vitrified groups (VOP: 76% vs. VOV: 79%).
Most histopathological features of both non-vitrified groups were normal. The cytoplasm of the oocytes was clear and normal and all the granulosa layers and theca interna and externa were intact and firmly attached to the related basement membranes. The stromal cells were normal with distinct boundaries, prominent nucleus and nucleolus and pinkish cytoplasm. In contrast, in both vitrified groups the stromal cells had distinct margins with foamy cytoplasm. The detachment of the granulosa layers from the basement membrane and the deformity of the oocytes was also seen (Figure 1).




Discussion
The results of the present study show clearly that retinol palmitate administered mouse produce more antral follicles in response to superovulation. In addition, we did not observe any positive effect of retinol palmitate on follicle quality after vitrification of ovaries.
Cryopreservation of oocytes and embryos by vitrification has been widely conducted because of its simplicity and high survival rate of oocytes and
embryos as well as cryopreservation of ovarian tissues (17). Sugimoto et al. (2000) observed that rat ovarian follicles survived after vitrification and transplantation with a decrease in the number of healthy antral follicles (approximately 30%) (16), and according to another report by Baird et al. (1999), about 28% of primordial follicles survived after transplantation of frozen/thawed ovarian tissue(22). In contrast, in the present investigation, the percentage of normal follicles including 'small', 'growing' and 'antral' in both vitrified groups (Table II) was lower than the above mentioned reports. Higher rate of damaged follicles in both vitrified groups shows that vitrification may induce some short-term deleterious effects on ovarian tissues. Transplantation of ovaries following vitrification may decrease such harmful effects of cryopreservation through change in the milieu of follicles (23), and autorepair of follicles under the control of various tropic factors (24). This finding is in agreement with the studies of Sugimoto et al. (16), and Schmidt et al. (25) which reported that continuous follicle maturation occurred in cryopreserved ovarian autografts following transplantation.
Antioxidant agents such as alpha-tocopherol as well as vitamin A improve the survival rate of follicles (8, 26, 27). However, the effect of retinoids on follicles quality following cryopreservation stress has not been yet investigated. Schweigert & Zucker (1988) suggested that concentrations of vitamin A in follicular fluid might serve as an indicator of follicular quality in cattle (28). Vitamin A concentrations were observed to be highest in large non-atretic follicles and lowest in small atretic follicles. Some reports from in vivo and in vitro studies in domestic livestock species lend supported that retinoids may target the oocyte and influence the subsequent development of embryos (7, 29-31). Improvement of follicular quality that is gained through retinol palmitate administration may in turn lead to better tolerance against deleterious effects of vitrification following transplantation. However, we did not observe any useful effect of retinol palmitate on follicle quality after vitrification of ovaries.
There are some evidences that vitamin A may influence oocyte and embryo development. The results obtained by Lima et al. (2004) demonstrated that the addition of retinol to the embryo culture media had a significant positive effect on bovine early embryonic development (32). Rabbits that had higher blood levels of vitamin A produced more oocytes and embryos in response to superovulation than those that had lower levels (33), and mouse treated with vitamin A before superovulation had higher ovulation rate and produced more zygotes (19). As our results show, the percentage of antral follicles in retinol palmitate administered mice was statistically higher than the control group following superovulation. This may be the probable mechanism of the positive effect of retinol palmitate on folliculogenesis after superovulation, which describes the results of our study and the reports mentioned above. In contrast, Shaw et al. (29) and Brown et al. (34) did not show any effect of vitamin A on the response of ovaries to superovulation in cows.
Transport and metabolism of retinoids are mediated and regulated by specific binding proteins (35, 36). Retinol is transported systemically and intercellularly by retinol-binding protein (RBP). Within target cells, retinol binds cellular retinol-binding protein (CRBP) which functions in retinol accumulation and presentation to dehydrogenases for conversion to retinal and retinoic acid (37). Brown et al. (2003) reported that within the ovarian follicles, strongest CRBP was observed in granulosa cells of preantral follicles (38). This evidence demonstrates that exogenous retinoids may have better effect on growing and antral than small follicles. Furthermore, retinoids have been shown to stimulate steroidogenesis by granulosa cells in vitro and synergistically enhance the ability of FSH to induce LH receptors (39). Antrum formation and final growth are entirely FSH/LH dependent (40). Together, these reports suggest that retinoids play a role in moving normal preantral toward antral follicles and may explain increasing the proportion of antral follicles in response to superovulation that is shown in the present study as well.
Conclusion
In conclusion, the results of the present study demonstrate that when retinol palmitate is administered to the mice, growing follicles developed to antral follicles in response to superovulation. However, short term harmful effects of vitrification on ovaries may not be overcome by retinol palmitate administration. Further in vitro investigations are needed to evaluate long term effects of vitrification in retinoids treated animals.
Acknowledgements
Special thanks to Mr. Ali Haddad Narafshan for revising the English of the manuscript.
Type of Study: Original Article |
References
1. Blomhoff R. Overview of vitamin A metabolism and function. In: Blomhoff R. Vitamin A in health and disease, 1st Ed. New York: Marcel Dekker; 1994; 1-35. [DOI:10.1201/9781482277562] [PMCID]
2. Cudas LJ. Retinoids and vertebrate development. J Biol Chem 1994; 269: 15399-15402.
3. Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev 2000; 80: 1021-1054. [DOI:10.1152/physrev.2000.80.3.1021] [PMID]
4. Chew BP, Archer RG. Comparative role of vitamin A and β-carotene on reproductive and neonatal survival in rats. Therio 1983; 20: 459 (abstract). [DOI:10.1016/0093-691X(83)90205-4]
5. Besenfelder UL, Solti J, Seregi M, Brem G. Influence of β-carotene on fertility in rabbits when using embryo transfer programs. Therio 1993; 39: 1093-1109. [DOI:10.1016/0093-691X(93)90009-T]
6. Britt JH, Whaley SL, Hedgpeth VS. Improvement of embryo survival by injection of vitamin A in gilts fed normal or high energy diets before and after mating. J Anim Sci 1992; 641(suppl. 1): 271.
7. Coffey MT, Britt JH. Enhancement of sow reproductive performance by β-carotene or vitamin A. J Anim Sci 1993; 71: 1198-1202. [DOI:10.2527/1993.7151198x] [PMID]
8. Livera G, Rouiller-Fabre V, Valla J, Habert R. Effects of retinoids on meiosis in the fetal rat ovary in culture. Mol Cell Endocrinol 2000; 165: 225-231. [DOI:10.1016/S0303-7207(00)00271-9]
9. Hidalgo CO, Diez C, Duque P, Facal N, Gomez E. Pregnancies and improved early embryonic development with bovine oocytes matured in vitro with 9-cis-retinoic acid. Reproduction 2003; 125: 409-416. [DOI:10.1530/rep.0.1250409] [PMID]
10. Migishima F, Suzuki-Migishima R, Song SY, Kuramochi T, Azuma S, Nishijima M, Yokoyama M. Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod 2003; 68: 881-887. [DOI:10.1095/biolreprod.102.007948] [PMID]
11. Deansely R. Immature rat ovaries grafted after freezing and thawing. Endocrinol 1957; 11: 197-200. [DOI:10.1677/joe.0.0110197] [PMID]
12. Parrot D.M. The fertility of mouse with orthopic ovarian grafts derived from frozen tissue. J Reprod Fertil 1960; 1: 230-241. [DOI:10.1530/jrf.0.0010230]
13. Candy CJ, Wood MJ, Whittingham DG. Effect of cryoprotectants on the survival of follicles in frozen mouse ovaries. J Reprod Fertil 1997; 110: 11-19. [DOI:10.1530/jrf.0.1100011] [PMID]
14. Critser JK, Arneson BW, Aaker DW, Ball GD. Cryopreservation of hamster oocytes: effects of vitrification or freezing on human sperm penetration of zona-free hamster oocytes. Fertil Steril 1986; 46: 277-284. [DOI:10.1016/S0015-0282(16)49526-9]
15. Nematallahi-Mahani SN, Saito H, Hiroi M. Vitrification of mouse ovarian tissue in ethylene glycol based solution, thawing, in vitro maturation of follicles and fertilization of oocytes. Fertil Steril 1999; 72 (suppl. 1): 200.
16. Sugimoto M, Maeda S, Manabe N, Miyamoto H. Development of infantile rat ovaries autotransplanted after cryopreservation by vitrification. Therio 2000; 53: 1093-1103. [DOI:10.1016/S0093-691X(00)00255-7]
17. Salehnia M, Abbasian Moghadam E, Rezazadeh Velojerdi M. Ultrastructural of follicles after vitrification of mouse ovarian tissue. Fertil Steril 2002; 78: 644-645. [DOI:10.1016/S0015-0282(02)03287-9]
18. Bos-Mikich A, Wood MJ, Candy CJ, Whittingham DG. Cytogenetical analysis and developmental potential of vitrified mouse oocytes. Biol Reprod 1995; 53: 780-785. [DOI:10.1095/biolreprod53.4.780] [PMID]
19. Elmarimi AA, Holdas JS, Ven E, Imrik P. Effect of vitamin A supplementation on mice embryo production and viability. Reprod Dom Anim 1990; 25: 247-248. [DOI:10.1111/j.1439-0531.1990.tb00468.x]
20. Vajta G, Holm F, Kuwayama M, Booth PJ, Jacobsen H, Greve T, Callesen H. Open pulled straw (OPS) vitrification: a new way to reduce cryoinjuries of bovine ova and embryos. Mol Reprod Dev 1998; 51: 53-58.
https://doi.org/10.1002/(SICI)1098-2795(199809)51:1<53::AID-MRD6>3.0.CO;2-V [DOI:10.1002/(SICI)1098-2795(199809)51:13.0.CO;2-V]
21. Thomas JB, Brad B, Alen RW, James JC, Jerrold LH. Influence of sampling on the reproducibility of ovarian follicle counts in mouse toxicity studies. Reprod Toxicol 1997; 11: 689-696. [DOI:10.1016/S0890-6238(97)00034-8]
22. Baird DT, Webb BK, Campbell LM, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196C. Endocrinol 1999; 140: 462-471. [DOI:10.1210/endo.140.1.6453] [PMID]
23. Waterhouse T, Cox SL, Snow M, Jenkin G, Shaw J. Offspring produced from heterotopic ovarian allografts in male and female recipient mice. Reproduction 2004; 127: 689-694. [DOI:10.1530/rep.1.00081] [PMID]
24. Okamura H, Katabuchi H, Ohba T. What we have learned from isolated cells from human ovary?. Mol Cell Endocrinol 2003; 202: 37-45. [DOI:10.1016/S0303-7207(03)00060-1]
25. Schmidt KL, Yding Andersen C, Loft A, Byskov AG, Ernst E, Nyboe Andersen A. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod 2005; 20: 3539-3546. [DOI:10.1093/humrep/dei250] [PMID]
26. Nugent D, Newton H, Gallivan L, Godsen R. Protective effect of vitamin E on ischaemia reperfusion injury in ovarian grafts. J Reprod Fertil 1998; 114: 341-346. [DOI:10.1530/jrf.0.1140341] [PMID]
27. Newton H, Illingworth P. In vitro growth of murine preantral follicles after isolation from cryopreserved ovarian tissue. Hum Reprod 2001; 16: 423-429. [DOI:10.1093/humrep/16.3.423] [PMID]
28. Schweigert FJ, Zucker H. Concentration of vitamin A injection before mating on oocyte development, follicular hormones and ovulation in gilts fed high-energy diets. J Anim Sci 1988; 78: 1598-1607. [DOI:10.2527/2000.7861598x] [PMID]
29. Shaw DW, Farin PW, Washburn SP, Britt JH. Effect of retinol palmitate on ovulation rate and embryo quality in superovulated cattle. Therio 1995; 44: 51-58. [DOI:10.1016/0093-691X(95)00147-Z]
30. Eberhardt DM, Will WA, Godsen GD. Retinol administration to superovulated ewes improves in vitro embryonic viability. Biol Reprod 1999; 60: 1483-1487. [DOI:10.1095/biolreprod60.6.1483] [PMID]
31. Whaley SL, Hedgpeth VS, Farin CE, Martus NS, Jayes FCL, Britt JH. Influence of vitamin A injection before mating on oocyte development, follicular hormones, and ovulation in gilts fed high energy diets. J Anim Sci 2000; 78: 1598-1607. [DOI:10.2527/2000.7861598x] [PMID]
32. Lima PF, Oliveira MAL, Goncalves PBD, Montagner MM, Reichenbach HD, et al. Effects of retinol on the in vitro development of Bos Indicus embryos to blastocysts in two different culture systems. Reprod Dom Anim 2004; 39: 356-360. [DOI:10.1111/j.1439-0531.2004.00528.x] [PMID]
33. Besenfelder UL, Solti J, Seregi M, Muller M, Brem G. Different roles for β-carotene and vitamin A in the reproduction of rabbits. Therio 1996; 45: 1583-1591. [DOI:10.1016/0093-691X(96)00127-6]
34. Brown BS, Whisnant CS, Reed BK. Effect of vitamin A injection on the response to superovulation in beef cattle. J Anim Sci 1997; 75(suppl. 1): 211.
35. Napoli JL. Retinoic acid biosynthesis and metabolism. FASEB J 1996; 10: 993-1001. [DOI:10.1096/fasebj.10.9.8801182] [PMID]
36. Noy N. Retinoid-binding proteins mediators of retinoid action. Biochem J 2000; 348: 481-495. [DOI:10.1042/bj3480481] [PMID] [PMCID]
37. Mangelsdrof D, Umesono K, Evans RM. The retinoid receptors. In: Sporn MB, Roberts AB, Goodman DS. Retinoids: Biology, chemistry and medicine, 2nd Ed. New York: Raven Press; 1994; 319-350.
38. Brown JA, Eberhardt DM, Shrick FN, Roberts MP, Godkin JD. Expression of retinol-binding protein and cellular retinol-binding protein in the bovine ovary. Mol Reprod Dev 2003; 64: 261-269. [DOI:10.1002/mrd.10225] [PMID]
39. Bagavandoss P, Midgley AR. Biphasic action of retinoids on gonadotropin receptor induction in rat granulosa cells in vitro. Life Sci 1988; 43: 1607-1614. [DOI:10.1016/0024-3205(88)90532-2]
40. Hafez ESE, Hafez B. Folliculogenesis, Egg maturation and Ovulation. In: Hafez ESE, Hafez B. Reproduction in Farm Animals, 7th Ed. Philadelphia: Lippincott Williams & Wilkins; 2000; 68-81. [DOI:10.1002/9781119265306.ch5]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


