Thu, Feb 19, 2026
[Archive]
Volume 5, Issue 2 (7-2007)
IJRM 2007, 5(2): 17-22 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khalili M A, Anvari M. The effect of in vitro culture on cleavage rates and morphology of the in vivo- developed embryos in mice. IJRM 2007; 5 (2) :17-22
URL: http://ijrm.ir/article-1-63-en.html
URL: http://ijrm.ir/article-1-63-en.html
1- Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran , Khalili59@hotmail.com
2- Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2- Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Full-Text [PDF 82 kb]
(766 Downloads)
| Abstract (HTML) (3628 Views)
Full-Text: (518 Views)
Introduction
The clinical advancements in the field of ART have been related to the commitments of the reproductive biologists with their works on experimental animals. In this regards, the continuous work of the father of human IVF, Professor Robert Edwards, is well recognized by scientists all around the world. He was the first one to successfully develop IVF in mice during 1964 (1). Following ovarian puncture, the fertilization and embryo development is generally performed in the laboratory with application of suitable culture media (37oC/ 5%CO2) (1). Finally, the best looking embryos are transferred to the recipient (2). It is now well-believed that both number and quality/ cleavage rate of embryo is directly related to the implantation rates (1). Therefore, it is essential to prepare the most suitable environment for the embryo development outside the body to achieve high rates of implantation (2-5).
Although, there have been extensive advancement in assisted reproductive conception, but only 12% of developed embryos from human IVF-ET will implant successfully (5). In addition, the implantation rates of the in-vitro formed embryos in mammals such as bovine, swine, and rat is 42%, 8%, and 28%, respectively (6-8). The data show that the implantation rates vary from one species to others. Reproductive specialists pinpoint several factors being involved in implantation process. Some of the factors include the quality of the gametes, in-vitro culture condition, and the quality of pre-implantation embryos (2, 3, 8). Therefore, experimental research studies in the field of reproductive biology can elucidate the mechanisms involved in the process of fertilization, embryo development, and embryo implantation. Since, reproductive processes like embryo formation and implantation in laboratory mice is very similar to human being, extensive studies have been conducted in this mammal. In addition, mice are inexpensive animals, accessible, and easy to handle (3,4). Therefore, evaluation and comparing between morphology and cleavage potential of embryos which have been developed in uterine environment with in-vitro development can present the factors involved in growth and development of embryos before implantation (3). In addition, the important role of in-vitro condition, especially culture media, for embryo survival and functioning in ART program will be denounced. Therefore, the main goal of this controlled experimental study was to compare the morphology as well as cleavage rates of 2, 4, 8, cell embryos, and blastocysts that were formed in reproductive tract (in vivo) of NMRI mice with in-vitro culture of them after 24 h. This will elucidate the effect of in-vitro environment on embryo development at different cleaving stages.
Materials and methods
Animals
A total of 30 female NMRI mice (6-8 weeks) were randomly divided into 4 groups. For super-ovulation, each mouse received one injection of 8IU PMSG (i.p) around 5 p.m. Approximately 46 h later, 8IU HCG was injected (i.p) for ovulation to take place. Then, 2 female mice were transferred to a cage with one male for copulation (9). The morning of the next day, presence of vaginal plague (sign of mating) was observed; otherwise, the animal was discarded from the study. The animals in group I were killed 35 h post HCG injection with cervical dislocation for collection of 50 two-cell embryos. Also, for retrieval of 150 embryos at 4, 8 cells and blastocysts stages, the animals were killed 46 h (group II), 58 h (group III), and 75 h (group IV) post HCG injections, respectively (10).
Retrieval and grading of embryos
Sterile surgical instruments and gloves were used for removal of uterine horns and uterine tubes from each animal. The specimens were immediately transferred into culture dish with PBS buffer (PH 7.3). Next, both uterine horns were dissected out with the aid of stereo microscope (Zeiss Co., Germany). The technique of flushing and dissection was applied for removal of embryos from uterine tubes or horns (9). For flushing of embryos, the uterine tubes were first dissected out under aseptic condition, and then small amount of T6 medium was injected from infundibulum of uterine tube to release all the embryos. The collected embryos were immediately transferred into pre-incubated 5 microliter T6 droplets overlaid with washed mineral oils. The fresh culture media T6 was already prepared (260-265 mOsmol, PH 7.2-7.4) and pre-warmed in incubator (Memmert Co., Germany) for 8-10 h before use. A total of 200 embryos (50 per each group) were evaluated morphologically using an invert microscope (Olympus Co., Japan). The morphology of 2 to 8 blastomer embryos were divided into 4 grades of A, B, C, and D. Grade A with equal blastomers, round, with no fragmentation, smooth cytoplasm, and bright yellow zona; grade B with slightly different blastomers in size, up to 10% fragmentation with granules in cytoplasm; grade C with unequal blastomers, up to 50% fragmentations and large granules and vacuels in cytoplasm; grade D with blastomers of unequal size, extreme fragmentation, with dark and large granules and presence of vacuels in cytoplasm. In addition, the grading of blastocysts was done according to the criteria of Rubio et al (2000) as follows; normal grade: equal size of blstomeres and presence of a small blastocele; expanded grade: presence of a large blastocele cavity and thin zona pellucida; hatching grade: extrusion of blastocyst is evident from the zona pellucida (11).
In vitro culture of embryos
Following morphological evaluation of in vivo developed embryos, the in vitro culture of all the embryos was done in incubator (37oC, 5% CO2) for 24 h. The culture media that were used for embryo development was freshly made Whittingham's T6. This culture media is primarily composed of salts, glucose, antibiotics, and serum albumin. The embryos were then re-evaluated with the aid of invert microscope for both morphological features and cleavage stage. Therefore, all embryos were evaluated twice: 1. Following retrieval from reproductive tract (control group), and 2. Following in-vitro culture for 24 h in T6 medium (experimental group).
Statistical analysis
For statistical evaluation, chi-square and fisher exact tests were applied. P-value<0.05 was considered significant in this study. This experimental study was approved by the university research committee.
Results
In-vivo developed embryos
The results showed that the best quality embryos developed in vivo were 2 cell embryos. However, the quality of embryos was reduced at more advanced cleaving stages. For example, 88% of 2-cell embryos were graded as "A" (Figure 1); while only 40% of 4-cell embryos were considered as grade "A". At higher stage of embryo development (8-cells) this rate was increased to 52% (Table I). The findings also showed that all embryos were in grades A, B, or C; and grade "D" was not observed in any of the embryos that were formed in reproductive tracts of mice.


In-vitro culture of embryos for 24 h
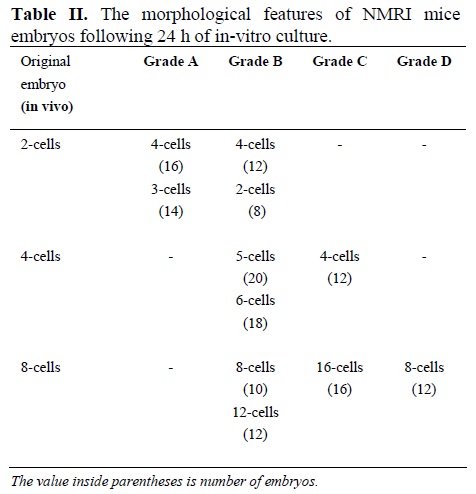
The results showed that in-vitro culture reduced the quality of embryos that were already formed in-vivo (Figure 2). In this regards, 16%, 24%, 24%, and 40% of 2, 4, 8, and blastocysts were respectively arrested following 24 h culture in T6 medium. Therefore, more embryo arrests were noticed at higher cleavage stage- blastocyst (Table II). In addition, only 2 blastocysts reached hatching stage after 24 h in vitro culture. The findings also declared that embryos at 4 and 8 cleaving stages were more sensitive to in vitro culture that 2-cell embryos. The observation showed that grade "A" was not achieved in any of the 4 or 8 cell embryos. A total of 28 eight-cell embryos were regarded as grades "C" and "D".

Blastocysts (in-vivo and in-vitro)
Table III presents the results generated from blastocysts developed in uterine horns as well as in-vitro culture for 24 h. The result showed that 72%, 28%, and 0% of the blastocysts were in stages of normal, expanded, and hatching following their retrieval from reproductive tracts (Figure 3). However, the aforementioned grading stages were changed to 60%, 32%, and 8% during in-vitro culture. This was, however, insignificant when compared with the in-vivo results (p>0.05).


Discussion
One of the basic research studies in the field of reproductive sciences is to evaluate the effect of in-vitro condition, such as culture media, on the development and cleavage speed of the embryos. In both IVF and microinjection techniques, the embryos are cultured for at least one day following fertilization sign. Finally, a limited number of embryos with good morphology are transferred to the uterine cavity (2). In one study, the quality of embryos that were formed in reproductive tracts of mice following superovulation were compared with other group without superovulation (natural cycles). The embryos were retrieved at different time intervals in both groups of mice. The results showed that in contrast to the embryos resulted from superovulation cycles, the quality of embryos generated from natural cycles were superior. Also, the quality and quantity of embryos were gradually reduced as the cleaving stages proceeded to more advanced stages. Therefore, it was suggested that the embryos should be cultured up to blastocyst, in order to avoid the uterine transfer of non-viable or arrested embryos in ART cycles (12).
In our study, the morphological features of in-vivo formed 2-cell embryos were superior to other cleaving stages. It is also emphasized that embryos usually develop better in natural environment of the reproductive systems than in-vitro system. For example, a total of 44, 20, and 26 embryos at 2, 4, and 8 cell stages from in-vivo formed were grade "A". This was, however, reduced to 30, 0, and 0 after in-vitro culture in standard medium such as T6. In addition, gene expression takes place in embryos with 4 blastomeres in mouse. Therefore, the embryos may become more susceptible to some factors in culture media (13). The aforementioned factors may indicate that embryos need to be transferred into uterine environment at early rather than more advanced cleavage stage.
Recently, the implantation rates of embryos generated from in-vivo were compared with the ones developed in-vitro (8). The results showed that the implantation rates of in-vitro developed embryos were significantly reduced when compared with in-vivo embryos (from 76.4% to 28.2%). Importantly, Gardner et al (1993) suggested that cellular damage or even arrest may have been related to amino acid toxicity inside the culture media. Also, replacement of human serum albumin (HSA) with bovine serum albumin (BSA) will improve the quality of the culture environment (14). In addition, some activating factors such as Plasminogen activators (PAs), which is secreted by trophoblast cells, are directly involved with implantation process. Alflalo and associates (2004) realized that the rates of PAs generated by 2 and 4-cell embryos as well as morulla and blastocysts that were formed in-vitro were significantly reduced when compared with embryos formed in-vivo condition in rat (8). This might be associated with the reduced metabolic activities of embryos developed in in-vitro situation. It is also important to note that the concentrations of PAs in 8 cell embryos were increased by two-folds, which was also highly stable up to the blastocysts stage. This may have been related with the metabolic activity of blastocysts for implantation in endometrial layer of uterus (8). Carrol and colleagues (1993) showed that uterine tubes are enriched with PAs which are able to attach to the embryo membrane, and enhance the growth and development of the embryo (15). In another study, the lower rate of embryo development during in-vitro culture was related to some factors such as light, culture metallic ions, oxidative stresses, and PH fluctuation; all of which enhance the production of reactive oxygen species (ROS). Goto et al (1993) detected higher quantities of ROS in early cleavage-stage mouse embryos produced in-vitro compared with their in-vivo counterparts (16). They suggested the involvement of ROS in the impairment of mammalian embryo developed in-vitro. Concurrent with this, the in-vitro formed embryos have lower anti-oxidants mechanism when compared with in-vivo generated embryos (17). As a consequence, adding antioxidants during embryo culture may have a beneficial effect on embryo development and quality.
Although, the gross microscopical evaluation of embryo is an non-invasive technique for the study of morphological features of blastomeres, but normal phenotype is not always representative of genotype condition. In this regards, Munn et al (1995) recognized that abnormal chromosomes were present in over 60% of embryos. Also, 15% of abnormal sex chromosomes were present in embryos with normal morphological features (18). In our study, the genotypes of embryos were not evaluated due to lack of necessary equipments in our center. However, we should draw our attention to evaluate and compare the genotypes of mice embryos formed in-vivo with in-vitro ones. This will not only show us the effect of in-vitro environment on the quality and cleaving stages of embryos, but will help us to find the embryos with the least chromosomal anomalies.
Conclusion
In conclusion, the results showed that the cleavage as well as quality of 2-cell embryos to blastocysts were gradually reduced, although sterile and suitable conditions were considered during the course of the study. Also, some become degenerated or arrested during in-vitro culture of embryos. It is, therefore, necessary to set an in-vitro condition to mimic the in-vivo environment for the development of pre-implantation embryos. In addition, embryo transfer at early stage will assure us that the selected embryos have the chance to grow in natural environment of uterine cavity.
Acknowledgement
The authors would like to thank their medical students, Fatima Azimi, Najmeh Zare-Zadeh, and Fatima Aghai-Maybodi for their help during the course of this study.
The clinical advancements in the field of ART have been related to the commitments of the reproductive biologists with their works on experimental animals. In this regards, the continuous work of the father of human IVF, Professor Robert Edwards, is well recognized by scientists all around the world. He was the first one to successfully develop IVF in mice during 1964 (1). Following ovarian puncture, the fertilization and embryo development is generally performed in the laboratory with application of suitable culture media (37oC/ 5%CO2) (1). Finally, the best looking embryos are transferred to the recipient (2). It is now well-believed that both number and quality/ cleavage rate of embryo is directly related to the implantation rates (1). Therefore, it is essential to prepare the most suitable environment for the embryo development outside the body to achieve high rates of implantation (2-5).
Although, there have been extensive advancement in assisted reproductive conception, but only 12% of developed embryos from human IVF-ET will implant successfully (5). In addition, the implantation rates of the in-vitro formed embryos in mammals such as bovine, swine, and rat is 42%, 8%, and 28%, respectively (6-8). The data show that the implantation rates vary from one species to others. Reproductive specialists pinpoint several factors being involved in implantation process. Some of the factors include the quality of the gametes, in-vitro culture condition, and the quality of pre-implantation embryos (2, 3, 8). Therefore, experimental research studies in the field of reproductive biology can elucidate the mechanisms involved in the process of fertilization, embryo development, and embryo implantation. Since, reproductive processes like embryo formation and implantation in laboratory mice is very similar to human being, extensive studies have been conducted in this mammal. In addition, mice are inexpensive animals, accessible, and easy to handle (3,4). Therefore, evaluation and comparing between morphology and cleavage potential of embryos which have been developed in uterine environment with in-vitro development can present the factors involved in growth and development of embryos before implantation (3). In addition, the important role of in-vitro condition, especially culture media, for embryo survival and functioning in ART program will be denounced. Therefore, the main goal of this controlled experimental study was to compare the morphology as well as cleavage rates of 2, 4, 8, cell embryos, and blastocysts that were formed in reproductive tract (in vivo) of NMRI mice with in-vitro culture of them after 24 h. This will elucidate the effect of in-vitro environment on embryo development at different cleaving stages.
Materials and methods
Animals
A total of 30 female NMRI mice (6-8 weeks) were randomly divided into 4 groups. For super-ovulation, each mouse received one injection of 8IU PMSG (i.p) around 5 p.m. Approximately 46 h later, 8IU HCG was injected (i.p) for ovulation to take place. Then, 2 female mice were transferred to a cage with one male for copulation (9). The morning of the next day, presence of vaginal plague (sign of mating) was observed; otherwise, the animal was discarded from the study. The animals in group I were killed 35 h post HCG injection with cervical dislocation for collection of 50 two-cell embryos. Also, for retrieval of 150 embryos at 4, 8 cells and blastocysts stages, the animals were killed 46 h (group II), 58 h (group III), and 75 h (group IV) post HCG injections, respectively (10).
Retrieval and grading of embryos
Sterile surgical instruments and gloves were used for removal of uterine horns and uterine tubes from each animal. The specimens were immediately transferred into culture dish with PBS buffer (PH 7.3). Next, both uterine horns were dissected out with the aid of stereo microscope (Zeiss Co., Germany). The technique of flushing and dissection was applied for removal of embryos from uterine tubes or horns (9). For flushing of embryos, the uterine tubes were first dissected out under aseptic condition, and then small amount of T6 medium was injected from infundibulum of uterine tube to release all the embryos. The collected embryos were immediately transferred into pre-incubated 5 microliter T6 droplets overlaid with washed mineral oils. The fresh culture media T6 was already prepared (260-265 mOsmol, PH 7.2-7.4) and pre-warmed in incubator (Memmert Co., Germany) for 8-10 h before use. A total of 200 embryos (50 per each group) were evaluated morphologically using an invert microscope (Olympus Co., Japan). The morphology of 2 to 8 blastomer embryos were divided into 4 grades of A, B, C, and D. Grade A with equal blastomers, round, with no fragmentation, smooth cytoplasm, and bright yellow zona; grade B with slightly different blastomers in size, up to 10% fragmentation with granules in cytoplasm; grade C with unequal blastomers, up to 50% fragmentations and large granules and vacuels in cytoplasm; grade D with blastomers of unequal size, extreme fragmentation, with dark and large granules and presence of vacuels in cytoplasm. In addition, the grading of blastocysts was done according to the criteria of Rubio et al (2000) as follows; normal grade: equal size of blstomeres and presence of a small blastocele; expanded grade: presence of a large blastocele cavity and thin zona pellucida; hatching grade: extrusion of blastocyst is evident from the zona pellucida (11).
In vitro culture of embryos
Following morphological evaluation of in vivo developed embryos, the in vitro culture of all the embryos was done in incubator (37oC, 5% CO2) for 24 h. The culture media that were used for embryo development was freshly made Whittingham's T6. This culture media is primarily composed of salts, glucose, antibiotics, and serum albumin. The embryos were then re-evaluated with the aid of invert microscope for both morphological features and cleavage stage. Therefore, all embryos were evaluated twice: 1. Following retrieval from reproductive tract (control group), and 2. Following in-vitro culture for 24 h in T6 medium (experimental group).
Statistical analysis
For statistical evaluation, chi-square and fisher exact tests were applied. P-value<0.05 was considered significant in this study. This experimental study was approved by the university research committee.
Results
In-vivo developed embryos
The results showed that the best quality embryos developed in vivo were 2 cell embryos. However, the quality of embryos was reduced at more advanced cleaving stages. For example, 88% of 2-cell embryos were graded as "A" (Figure 1); while only 40% of 4-cell embryos were considered as grade "A". At higher stage of embryo development (8-cells) this rate was increased to 52% (Table I). The findings also showed that all embryos were in grades A, B, or C; and grade "D" was not observed in any of the embryos that were formed in reproductive tracts of mice.


In-vitro culture of embryos for 24 h
The results showed that in-vitro culture reduced the quality of embryos that were already formed in-vivo (Figure 2). In this regards, 16%, 24%, 24%, and 40% of 2, 4, 8, and blastocysts were respectively arrested following 24 h culture in T6 medium. Therefore, more embryo arrests were noticed at higher cleavage stage- blastocyst (Table II). In addition, only 2 blastocysts reached hatching stage after 24 h in vitro culture. The findings also declared that embryos at 4 and 8 cleaving stages were more sensitive to in vitro culture that 2-cell embryos. The observation showed that grade "A" was not achieved in any of the 4 or 8 cell embryos. A total of 28 eight-cell embryos were regarded as grades "C" and "D".


Blastocysts (in-vivo and in-vitro)
Table III presents the results generated from blastocysts developed in uterine horns as well as in-vitro culture for 24 h. The result showed that 72%, 28%, and 0% of the blastocysts were in stages of normal, expanded, and hatching following their retrieval from reproductive tracts (Figure 3). However, the aforementioned grading stages were changed to 60%, 32%, and 8% during in-vitro culture. This was, however, insignificant when compared with the in-vivo results (p>0.05).


Discussion
One of the basic research studies in the field of reproductive sciences is to evaluate the effect of in-vitro condition, such as culture media, on the development and cleavage speed of the embryos. In both IVF and microinjection techniques, the embryos are cultured for at least one day following fertilization sign. Finally, a limited number of embryos with good morphology are transferred to the uterine cavity (2). In one study, the quality of embryos that were formed in reproductive tracts of mice following superovulation were compared with other group without superovulation (natural cycles). The embryos were retrieved at different time intervals in both groups of mice. The results showed that in contrast to the embryos resulted from superovulation cycles, the quality of embryos generated from natural cycles were superior. Also, the quality and quantity of embryos were gradually reduced as the cleaving stages proceeded to more advanced stages. Therefore, it was suggested that the embryos should be cultured up to blastocyst, in order to avoid the uterine transfer of non-viable or arrested embryos in ART cycles (12).
In our study, the morphological features of in-vivo formed 2-cell embryos were superior to other cleaving stages. It is also emphasized that embryos usually develop better in natural environment of the reproductive systems than in-vitro system. For example, a total of 44, 20, and 26 embryos at 2, 4, and 8 cell stages from in-vivo formed were grade "A". This was, however, reduced to 30, 0, and 0 after in-vitro culture in standard medium such as T6. In addition, gene expression takes place in embryos with 4 blastomeres in mouse. Therefore, the embryos may become more susceptible to some factors in culture media (13). The aforementioned factors may indicate that embryos need to be transferred into uterine environment at early rather than more advanced cleavage stage.
Recently, the implantation rates of embryos generated from in-vivo were compared with the ones developed in-vitro (8). The results showed that the implantation rates of in-vitro developed embryos were significantly reduced when compared with in-vivo embryos (from 76.4% to 28.2%). Importantly, Gardner et al (1993) suggested that cellular damage or even arrest may have been related to amino acid toxicity inside the culture media. Also, replacement of human serum albumin (HSA) with bovine serum albumin (BSA) will improve the quality of the culture environment (14). In addition, some activating factors such as Plasminogen activators (PAs), which is secreted by trophoblast cells, are directly involved with implantation process. Alflalo and associates (2004) realized that the rates of PAs generated by 2 and 4-cell embryos as well as morulla and blastocysts that were formed in-vitro were significantly reduced when compared with embryos formed in-vivo condition in rat (8). This might be associated with the reduced metabolic activities of embryos developed in in-vitro situation. It is also important to note that the concentrations of PAs in 8 cell embryos were increased by two-folds, which was also highly stable up to the blastocysts stage. This may have been related with the metabolic activity of blastocysts for implantation in endometrial layer of uterus (8). Carrol and colleagues (1993) showed that uterine tubes are enriched with PAs which are able to attach to the embryo membrane, and enhance the growth and development of the embryo (15). In another study, the lower rate of embryo development during in-vitro culture was related to some factors such as light, culture metallic ions, oxidative stresses, and PH fluctuation; all of which enhance the production of reactive oxygen species (ROS). Goto et al (1993) detected higher quantities of ROS in early cleavage-stage mouse embryos produced in-vitro compared with their in-vivo counterparts (16). They suggested the involvement of ROS in the impairment of mammalian embryo developed in-vitro. Concurrent with this, the in-vitro formed embryos have lower anti-oxidants mechanism when compared with in-vivo generated embryos (17). As a consequence, adding antioxidants during embryo culture may have a beneficial effect on embryo development and quality.
Although, the gross microscopical evaluation of embryo is an non-invasive technique for the study of morphological features of blastomeres, but normal phenotype is not always representative of genotype condition. In this regards, Munn et al (1995) recognized that abnormal chromosomes were present in over 60% of embryos. Also, 15% of abnormal sex chromosomes were present in embryos with normal morphological features (18). In our study, the genotypes of embryos were not evaluated due to lack of necessary equipments in our center. However, we should draw our attention to evaluate and compare the genotypes of mice embryos formed in-vivo with in-vitro ones. This will not only show us the effect of in-vitro environment on the quality and cleaving stages of embryos, but will help us to find the embryos with the least chromosomal anomalies.
Conclusion
In conclusion, the results showed that the cleavage as well as quality of 2-cell embryos to blastocysts were gradually reduced, although sterile and suitable conditions were considered during the course of the study. Also, some become degenerated or arrested during in-vitro culture of embryos. It is, therefore, necessary to set an in-vitro condition to mimic the in-vivo environment for the development of pre-implantation embryos. In addition, embryo transfer at early stage will assure us that the selected embryos have the chance to grow in natural environment of uterine cavity.
Acknowledgement
The authors would like to thank their medical students, Fatima Azimi, Najmeh Zare-Zadeh, and Fatima Aghai-Maybodi for their help during the course of this study.
Type of Study: Original Article |
References
1. Edwards RG. An introduction to Bourn Hall: The biomedical background of Bourn Hall Clinic. In: Textbook of in vitro fertilization and assisted reproduction; ed. Brinsden PR. Taylor & Francis Group; London 2005; 26: 1-8. [DOI:10.1201/b14680-2]
2. Khalili MA, Moinia F. Role of embryo morphology and cumulative embryo score in pregnancy outcome from in-vitro fertilization and intracytoplasmic sperm injection cycles. Mid East Fert Soc J 2002; 7: 231-236.
3. Barsiter BD. Mouse embryo cleavage, metabolism and viability: Role of medium composition. Hum Reprod 1993; 8: 288-295. [DOI:10.1093/oxfordjournals.humrep.a138039]
4. Ebner T, Moser M, Sommeryruber M, Gaiswinkler U. Presence, but not type of degree of extension of a cytoplasmic halo has a significant influence on preimplantation development and implantation behavior. Hum Reprod 2003; 18: 2406-2412. [DOI:10.1093/humrep/deg452]
5. Schleve LA, Wilcox LS. Use of assisted reproductive technology in United States, 1996 and 1998. JAMA 2002; 287: 1521-1522. [DOI:10.1001/jama.287.12.1521]
6. Olson SE, Seidel GE. Culture of in-vitro produced bovine embryos with vitamin E improves development in vitro and after transfer to recipients. Bio Reprod 2000; 62: 248-252. [DOI:10.1095/biolreprod62.2.248]
7. Kikuchi K, Kashiwazaki N, Noguchi J, Shimada A, Takahashi R, Hirabayashi M. Developmental competence, after transfer to recipients, of porcine oocyte matured, fertilized and cultured in vitro. Bio Reprod 1999; 60: 336-340. [DOI:10.1095/biolreprod60.2.336]
8. Aflalo ED, Sod-Moriah UA, Potashnik G, Har-Vardi I. Differences in the implantation rates of rat embryos developed in-vivo and in-vitro: possible role for plasminogen activators. Fert Steril 2004; 81: 780-785. [DOI:10.1016/j.fertnstert.2003.10.014]
9. Hogan B, Beddington R, Costantini F, Lacey E. Manipulating the mouse embryo: a laboratory manual. 2nd ed NY: Cold spring harbor laboratory, 1995.
10. Wolf DP. In vitro fertilization and embryo transfer. A manual of basic techniques. Penum Press; Phil, USA, 1988.
11. Rubio C, Simon C, Mercader A, Garcia-Velasco J, Remohi J, Pellicer A. Clinical experience employing co-culture of embryos with autologous human endometrial epithelial cells. Hum Reprod 2000; 15: 31-38.
12. Vander-Auwera I. Mouse embryo development in vivo. Embryo Mail News, 2000.
13. Braude P, Bolton V, Moore S. Human gene expression first occurs between the four and eight cell stage of pre-implantation development. Nature 1988; 332: 459-461. [DOI:10.1038/332459a0]
14. Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Bio Rep 1993; 48: 377-385. [DOI:10.1095/biolreprod48.2.377]
15. Carroll PM, Richards WG, Darrow AL, Wells JM, Strickland S. Pre-implantation mouse embryos express a cell surface receptor for tissue-plasminogen activator. Develop 1993; 119: 191-198.
16. Goto Y, Noda Y, Mori T, Nakano M. Increased generation of reactive oxygen species in embryo cultured in vitro. Free Rad Biol Med 1993; 15: 69-75. [DOI:10.1016/0891-5849(93)90126-F]
17. Feugang JM, Langendonckt AV, Sayoud H, Rees JF, Pampfer S, Moens A, et al. Effect of prooxidant agents added at the morula/blastocyst stage on bovine embryo development, cell death, and glutathione content. Zygote 2003; 11: 107-118. [DOI:10.1017/S0967199403002144]
18. Munne G. Embryo morphology, developmental rate and maternal age are correlated with chromosome abnormalities. Fert Steril 1995; 64: 382-390.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




