Fri, Apr 26, 2024
[Archive]
Volume 1, Issue 1 (1-2003)
IJRM 2003, 1(1): 1-6 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Asimakopoulos B, Nikolettos N, Al-Hasani S. Is There a Place for Round and Elongated Spermatids Injection in Assisted Reproduction?. IJRM 2003; 1 (1) :1-6
URL: http://ijrm.ir/article-1-7-en.html
URL: http://ijrm.ir/article-1-7-en.html
Keywords: -
Full-Text [PDF 51 kb]
(658 Downloads)
| Abstract (HTML) (2580 Views)
Spermatids are the youngest male germ cells with a single set of haploid chromosomes (complete meiosis). Once, they have completed meiosis, they undergo a complex cellular differentiation and maturation process known as spermiogenesis. Spermiogenesis starts at puberty and continues throughout the reproductive life of males. During spermiogenesis, round spermatids (Sa) have approximately 7μm size, which will transform into mature spermatozoa. One of the most important changes that takes place in this process is nuclear DNA packaging. DNA condensation is associated with biochemical alterations such as the replacement of lysine-rich histones, first by transition proteins and later by arginine-rich protamines, as well as with the formation of disulphide bonds that stabilize the formation of disulphide bonds that stabilize the chromatin structure (Nikolettos et al., 1999; de Kretser et al., 1998; de Kretser and Kerr, 1969;). As a result of DNA condensation, the cell size is reduced; thereby less energy is required to support its mobility and the cell is better protected against mechanical and chemical damage (Nikolettos et al., 1999). Other important changes during spermiogenesis include the process of genomic imprinting, the disappearance of the distal centriole and the formation of the acrosome.
reported that from 1418 testicular biopsies (766 subfertile men), only in 26 samples spermatogenesis arrest was in the round spermatid stage. There are also other investigators supporting that maturation arrest is extremely rare in the round spermatid stage (Silber and Johnson, 1998; Silber et al., 1997; 2000).
The assessment of spermatid viability is a hard task, particularly of the round ones (Schoysman et al., 1999). Aslam et al. (1998), using the Trypan blue exclusion test, found that 97% of the collected round spermatids were viable. However, distinguishing the viable round spermatids from the non-viable ones, without staining or destroying the cells, is not an easy task. Usually, the viability of round spermatids is estimated during aspiration, according to their ability to undergo a reversible deformation. The dead round spermatids are usually subjected to lysis upon spiration (Vanderzwalmen et al., 1998; Tesarik and Mendoza, 1996).
Recently, results for the development of blastocysts after testicular round spermatid injection were published. Balaban et al. (2000) reported that 34% of the embryos derived from round spermatids injections reached the blastocyst stage, but none of them hatched. Urman et al. (2002) also presented results from transfer of blastocysts derived from injection with testicular round spermatids: with a fertilization rate of 19.7%, the blastocyst stage reached by only a few embryos (7.6%), which failed to implant.
It is clear that the reproductive potency of round spermatids is inferior to that of elongated spermatids. So far, the fertilization rate with round spermatids appears to be low ranging from 20% to 25% in most of the reported cycles, while the fertilisation rate with elongated spermatids is significantly higher, being between 40% to 60%. Correspondingly, the implantation rate is extremely low with round spermatids, while it is higher with the elongated ones.
It is possible that the immaturity of spermatids may be the most important factor to impair fertilization capacity in various. This can be due to an incomplete histone/protamine transition, since it can lead to chromatin instability and sensitivity, making spermatids more vulnerable to denaturing stress. This can further lead to DNA fragmentation and apoptosis. In addition, a lack of protamines may enhance the cell cycle imbalance between spermatid and oocyte resulting in premature chromatin condensation, with a consequent failure of the transformation of spermatid nucleus into male pronucleus (Aslam and Fishel, 1999; Sousa et al., 1998). Aneuploidy is also a major consideration in spermatid injection. Oppedisano et al. (2002) studied the rate of aneuploidy/diploidy in spermatids from three different sterile mice strains showing pathological and histological similarities to human idiopathic non-obstructive males. They found that round spermatids had elevated level of numerical chromosomal abnormalities in two out of three different sterile strains. Their results support the hypothesis that abnormal testicular environment can adversely affect meiosis (Oppedisano et al., 2002).
Nevertheless, one of the most important considerations in DNA which is developed essentially during gametogenesis.spermatid injection is the incomplete or abnormal genomic imprinting. This process is an allele-specific modification of Genomic imprinting results in the expression or repression of maternal or paternal alleles of certain genes (Sleutels et al., 2000; Brannan and Bartolomei, 1999; Reik and Walter, 1998). Disruption of the imprinting mechanism is associated with disordered growth and development, especially prenatal, as well as with certain clinical syndromes (Preece and Moore, 2000). The status of genomic imprinting has been studied in mouse embryos derived by round spermatid injection (Shamanski et al., 1999). In this study, the expression of imprinted genes did not differ significantly from controls, indicating that paternal genes underwent proper imprinting by the round spermatids (Shamanski et al., 1999). However, the problem still exists since there are no studies from primates or human embryos available. It is worth to note that defects on genomic imprinting process may be manifested relatively late in postnatal life (stavik et al., 2003; Cox et al., 2002; Preece and Moore, 2000).
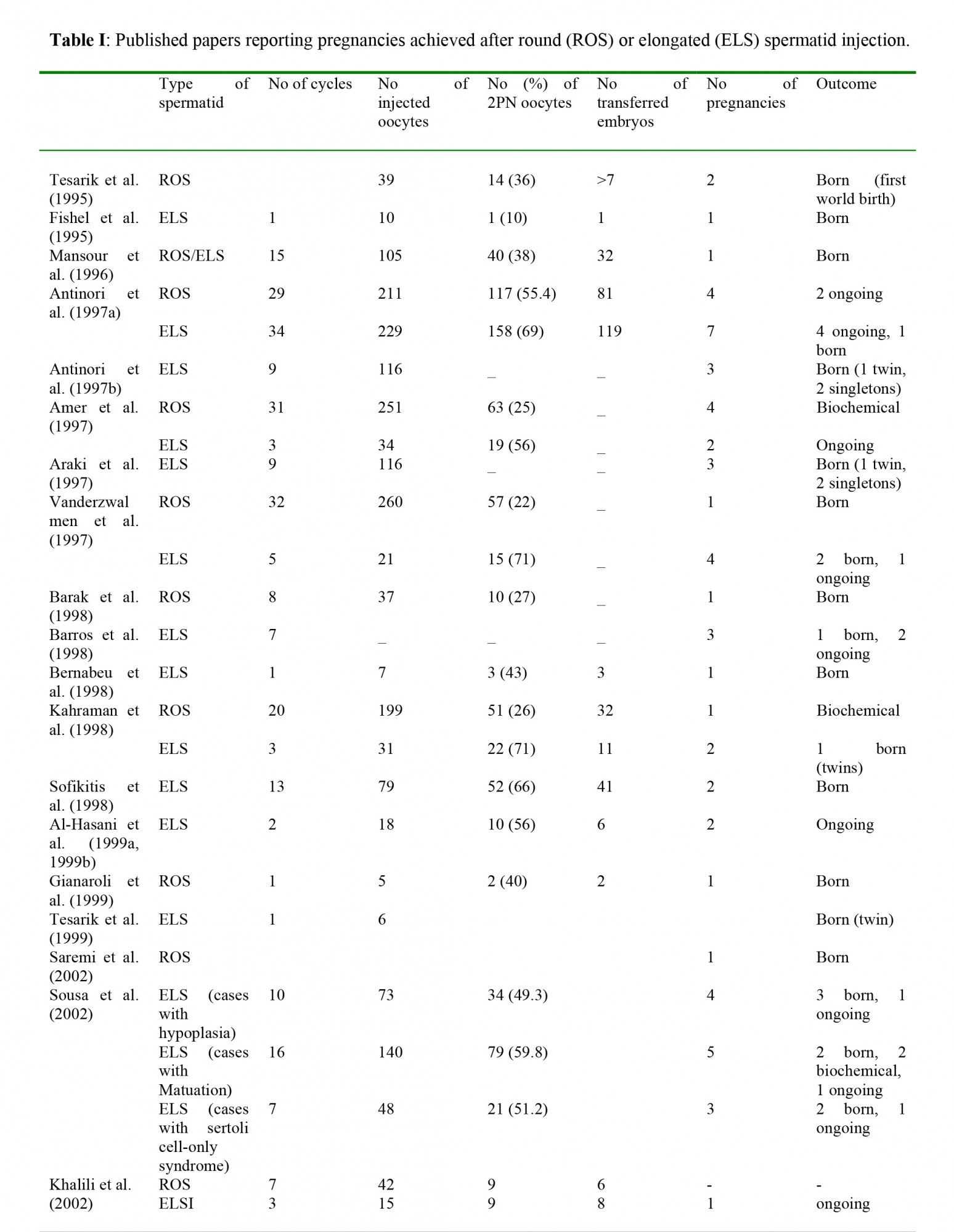
At the present time, there is not enough data on the rate of malformation of the children born after spermatid injection. In fact, an extremely small number of such births have been reported (Saremi et al., 2002; Sousa et al., 2002; Zech et al., 2002; Al-Hasani et al., 1999a, 1999b; Gianaroli et al., 1999; Tesarik et al., 1999; Barak et al., 1998; Barros et al., 1998; Bernabeu et al., 1998; Kahraman et al., 1998; Sofikitis et al., 1998; Amer et al., 1997; Antinori et al., 1997a, 1997b; Mansour et al., 1996; Fishel et al., 1995; Tesarik et al., 1995). A recent report described two out of four cases of pregnancies obtained through injection with elongated spermatids, in which congenital malformations were observed (Zech et al., 2002). The authors decided to postpone injection with spermatids due to unpromising results and to potentially high rates of malformation (Zech et al., 2002).
Last but not least, the methodology for isolation, identification and assessment of viability, especially of the round ones, are also important factors affecting the outcome of spermatid injection (Silber et al., 2000; Vanderzwalmen et al., 1998).
Taking these facts into consideration, it is a question whether spermatids injection should be considered even as a treatment of last choice. According to our opinion, for the time being, round spermatid injection should be faced as an interesting technique for animal experiments. In that way, the methodology will be developed, the fertilization, implantation and pregnancy rates will be improved, and there will be a better estimation of the possible genetic risks. The injections with elongated spermatids seem to be more efficient, theoretically more secure and consequently could be considered as a treatment of last choice in cases of azoospermia; however, after the couples have received appropriate counselling for the possible risks of the procedure.
Full-Text: (317 Views)
Introduction
Forty years have passed since the first intracytoplasmic sperm injection (ISCI). It was 1962 when sea urchin eggs were successfully fertilized by microinjection of live spermatozoa (Hiramoto, 1962). Thirty years later this technique was introduced for the treatment of human infertility (Palermo et al., 1992). Today, ICSI is “the state of the art” in assisted reproduction technologies. It gives reliable solutions in cases of severe male factor infertility there are still cases of azoospermia where ICSI is not possible even after MESA or TESE. In such cases, spermatid injection is considered as a promising alternative.Spermatids are the youngest male germ cells with a single set of haploid chromosomes (complete meiosis). Once, they have completed meiosis, they undergo a complex cellular differentiation and maturation process known as spermiogenesis. Spermiogenesis starts at puberty and continues throughout the reproductive life of males. During spermiogenesis, round spermatids (Sa) have approximately 7μm size, which will transform into mature spermatozoa. One of the most important changes that takes place in this process is nuclear DNA packaging. DNA condensation is associated with biochemical alterations such as the replacement of lysine-rich histones, first by transition proteins and later by arginine-rich protamines, as well as with the formation of disulphide bonds that stabilize the formation of disulphide bonds that stabilize the chromatin structure (Nikolettos et al., 1999; de Kretser et al., 1998; de Kretser and Kerr, 1969;). As a result of DNA condensation, the cell size is reduced; thereby less energy is required to support its mobility and the cell is better protected against mechanical and chemical damage (Nikolettos et al., 1999). Other important changes during spermiogenesis include the process of genomic imprinting, the disappearance of the distal centriole and the formation of the acrosome.
Spermatids as the only finding of TESE
It is known that various pathological conditions can lead in spermatogenetic abnormalities with resultant subfertility or infertility (Martin-du Pan and Campana, 1993). In cases of non-obstractive azoospermia with a lack of spermatozoa in the ejaculate, TESE is considered as the next step of the treatment. For most of these cases, TESE results in retrieval of enough spermatozoa to proceed in ICSI cycles, while in other cases spermatids are completely absent. In the latter cases, round and elongated spermatids may be present, indicating spermatogenesis arrest after the meiosis stage. In a large series of TESE (N=364) performed at the Medical University of Lübeck, the incidence of such cases was 3.9%:2.2% elongated spermatids were found, whereas only 1.7% round spermatids were observed (Al-Hasani, unpublished data). Although it is still controversial, it seems that in most of the cases the spermatogenesis arrest happens in elongated spermatid stage. According to Cremades et al. (1999) the recovery of only round spermatids is a frequent finding in non-obstructive azoospermic patients with complete absence of spermatozoa. On the other hand, Schulze et al. (1999)reported that from 1418 testicular biopsies (766 subfertile men), only in 26 samples spermatogenesis arrest was in the round spermatid stage. There are also other investigators supporting that maturation arrest is extremely rare in the round spermatid stage (Silber and Johnson, 1998; Silber et al., 1997; 2000).
Isolation and identification
In wet preparation, the identification of round spermatids has many difficulties mainly due to their morphological similarities with small lymphocytes (Vanderzwalmen et al., 1998; Silber et al, 2000). A considerable experience is necessary for reliable identification of round spermatids and avoidance of mistakes. Under the inverted microscope, without using any specific staining methods, four different stages of spermatids can be distinguished, according to their morphology: round spermatids (Sa, Sb1), elongating spermatids (Sb2), elongated spermatids (Sc, Sd1) and late elongated spermatids (Sd2) (Vanderzwalmen et al., 1998). Round spermatids appear as round cells with a diameter of 7μmwhich are characterized by a dense, smooth and dark nucleus positioned centrally or inclining towards the cell membrane. The nucleus is surrounded by a continuous rim of cytoplasm. In some cells, the early acrosomal vesicle or acrosomal cap is visible as a bright white spot or sickle-shaped adjacent to the nucleus (Sousa et al., 1998; Verheyen et al., 1998; Tesarik and Mendoza, 1996;). The distinction of elongating and elongated spermatids is made on the basis of their shape and the size of the tail. The mature spermatids (late elongated) appear to be similar to the ultimate sperm morphology (Vanderzwalmen et al., 1998). The appearance of abnormal spermatid forms in the preparation makes the identification procedure more difficult.The assessment of spermatid viability is a hard task, particularly of the round ones (Schoysman et al., 1999). Aslam et al. (1998), using the Trypan blue exclusion test, found that 97% of the collected round spermatids were viable. However, distinguishing the viable round spermatids from the non-viable ones, without staining or destroying the cells, is not an easy task. Usually, the viability of round spermatids is estimated during aspiration, according to their ability to undergo a reversible deformation. The dead round spermatids are usually subjected to lysis upon spiration (Vanderzwalmen et al., 1998; Tesarik and Mendoza, 1996).
Spermatids injection in assisted reproduction
The concept of using round spermatids in ICSI cycles was born in 1993, when Ogura and his collaborators reported that the spermatids nuclei were able to duplicate their DNA and participate in syngamy when incorporated into hamster or mouse oocyte either by microsurgery or by electro fusion (Ogura and Yanagimachi, 1993; Ogura et al., 1993). A year later, Ogura et al (1994) reported the normal birth of four young mice after electro fusion of oocytes with round spermatids; while, Sofikitis et al. (1994) reported successful pregnancy after injection of round spermatids nuclei into rabbit oocytes. In 1995, the same research center reported that mouse oocytes developed into normal offspring after injection with testicular round spermatids (Kimura and Yanagimachi, 1995). Based on these animal tudies, Edwards et al (1994) put forward the question: “are spermatid injections into human oocytes now mandatory?” That suggestion along with the success of these animal experiments was the (1995) reported the successful fertilization of an oocyte after injection with late stage testicular spermatid and during the next years several papers presented pregnancies achieved after spermatid injection (Table I).Recently, results for the development of blastocysts after testicular round spermatid injection were published. Balaban et al. (2000) reported that 34% of the embryos derived from round spermatids injections reached the blastocyst stage, but none of them hatched. Urman et al. (2002) also presented results from transfer of blastocysts derived from injection with testicular round spermatids: with a fertilization rate of 19.7%, the blastocyst stage reached by only a few embryos (7.6%), which failed to implant.
It is clear that the reproductive potency of round spermatids is inferior to that of elongated spermatids. So far, the fertilization rate with round spermatids appears to be low ranging from 20% to 25% in most of the reported cycles, while the fertilisation rate with elongated spermatids is significantly higher, being between 40% to 60%. Correspondingly, the implantation rate is extremely low with round spermatids, while it is higher with the elongated ones.
In-vitro maturation attempts
Obviously, the aforementioned poor results reduced the previous enthusiasm for the use of spermatids in assisted reproduction procedures. However it is true that while the outcome of round spermatid-ICSI cycles is totally disappointing, the use of elongated spermatids resulted in better fertilization and pregnancy rates. Consequently, attempts for the in-vitro maturation of spermatids were made in order to solve the problem. Tesarik et al. (2000,1999, 1998) presented some encouraging results on in-vitro maturation of primary spermatocytes and round spermatids, especially using media supplemented with rFSH. Cremades et al. (1999) managed to obtain elongating spermatids and a few mature spermatozoa, after a prolonged co-culture on Vero cells, in four cases. Aslam and Fishel (1999) found out that although short term in-vitro culture of the spermatogenetic cells has a positive effect; it does not improve the incidence of fertilization significantly. They pointed out that spermatid maturation, which takes about 16 days to complete in-vivo, can not be accelerated in- vitro, especially within 48 z(Aslam and Fishel, 1999).Problems and Concerns
Which are the main problems responsible for the poor outcome of spermatid injection, especially the round one?
It is possible that the immaturity of spermatids may be the most important factor to impair fertilization capacity in various. This can be due to an incomplete histone/protamine transition, since it can lead to chromatin instability and sensitivity, making spermatids more vulnerable to denaturing stress. This can further lead to DNA fragmentation and apoptosis. In addition, a lack of protamines may enhance the cell cycle imbalance between spermatid and oocyte resulting in premature chromatin condensation, with a consequent failure of the transformation of spermatid nucleus into male pronucleus (Aslam and Fishel, 1999; Sousa et al., 1998). Aneuploidy is also a major consideration in spermatid injection. Oppedisano et al. (2002) studied the rate of aneuploidy/diploidy in spermatids from three different sterile mice strains showing pathological and histological similarities to human idiopathic non-obstructive males. They found that round spermatids had elevated level of numerical chromosomal abnormalities in two out of three different sterile strains. Their results support the hypothesis that abnormal testicular environment can adversely affect meiosis (Oppedisano et al., 2002).
Nevertheless, one of the most important considerations in DNA which is developed essentially during gametogenesis.spermatid injection is the incomplete or abnormal genomic imprinting. This process is an allele-specific modification of Genomic imprinting results in the expression or repression of maternal or paternal alleles of certain genes (Sleutels et al., 2000; Brannan and Bartolomei, 1999; Reik and Walter, 1998). Disruption of the imprinting mechanism is associated with disordered growth and development, especially prenatal, as well as with certain clinical syndromes (Preece and Moore, 2000). The status of genomic imprinting has been studied in mouse embryos derived by round spermatid injection (Shamanski et al., 1999). In this study, the expression of imprinted genes did not differ significantly from controls, indicating that paternal genes underwent proper imprinting by the round spermatids (Shamanski et al., 1999). However, the problem still exists since there are no studies from primates or human embryos available. It is worth to note that defects on genomic imprinting process may be manifested relatively late in postnatal life (stavik et al., 2003; Cox et al., 2002; Preece and Moore, 2000).
At the present time, there is not enough data on the rate of malformation of the children born after spermatid injection. In fact, an extremely small number of such births have been reported (Saremi et al., 2002; Sousa et al., 2002; Zech et al., 2002; Al-Hasani et al., 1999a, 1999b; Gianaroli et al., 1999; Tesarik et al., 1999; Barak et al., 1998; Barros et al., 1998; Bernabeu et al., 1998; Kahraman et al., 1998; Sofikitis et al., 1998; Amer et al., 1997; Antinori et al., 1997a, 1997b; Mansour et al., 1996; Fishel et al., 1995; Tesarik et al., 1995). A recent report described two out of four cases of pregnancies obtained through injection with elongated spermatids, in which congenital malformations were observed (Zech et al., 2002). The authors decided to postpone injection with spermatids due to unpromising results and to potentially high rates of malformation (Zech et al., 2002).
Last but not least, the methodology for isolation, identification and assessment of viability, especially of the round ones, are also important factors affecting the outcome of spermatid injection (Silber et al., 2000; Vanderzwalmen et al., 1998).
Conclusion
Spermatid injection, as an assisted reproduction technique, concerns a small number of the cases of male infertility. It presents significant difficulties in methodology, regarding the isolation, identification and assessment of the viability of spermatids, especially the round ones. The fertilization and the implantation rates are extremely low with round spermatids, while they are higher with elongated spermatids. Until today, only a few pregnancies have been achieved. The risk for genetic abnormalities of the offspring has not been estimated yet which could be high, mainly because of incomplete biochemical transitions in the nucleus and of possible high rates of aneuploidy. Unfortunately, there is a complete lack of experimental studies in primates and there are no large clinical studies available.Taking these facts into consideration, it is a question whether spermatids injection should be considered even as a treatment of last choice. According to our opinion, for the time being, round spermatid injection should be faced as an interesting technique for animal experiments. In that way, the methodology will be developed, the fertilization, implantation and pregnancy rates will be improved, and there will be a better estimation of the possible genetic risks. The injections with elongated spermatids seem to be more efficient, theoretically more secure and consequently could be considered as a treatment of last choice in cases of azoospermia; however, after the couples have received appropriate counselling for the possible risks of the procedure.
Type of Study: Original Article |
References
1. Al-Hasani S, Ludwig M, Palermo I, Küpker W, Sandmann J, Johannisson R, Fornara P, Sturm R, Bals-Pratsch M, Bauer O, Diedrich K. (1999a) Intracytoplasmic injection of round and elongated spermatids from azoospermic patients: results and review. Hum Reprod 14: 97-107. [DOI:10.1093/humrep/14.suppl_1.97] [PMID]
2. Al-Hasani S, Schِpper B, Küpker W, Sandmann J, Johannisson R, Fornara P, Sturm R, Bals-Pratsch M, Bauer O, Diedrich K. (1999b) Die intrazytoplasmatische Injektion von runden und elongierten Spermatiden bei Patienten mit Reifungsarrest der Spermatogenese. Geburtsh. Frauenheilk 59: 220-4. [DOI:10.1055/s-1999-14192]
3. Amer M, Soliman E, El-Sadek M, Mendoza C, Tesarik J. (1997) Is complete spermiogenesis failure a good
4. indication for spermatid conception? Lancet 350: 116. [DOI:10.1016/S0140-6736(05)61819-3]
5. Antinori S, Versaci C, Dani G, Antinori M, Selman HA. (1997a) The use of round or elongated spermatids for ICSI: which is more effective? In: Gomel V & Leung PCK "In vitro fertilization and assisted reproduction", Proc. 10th World Congr. In-Vitro Fertil.Ass. Reprod Vancouver (Canada), 547-51.
6. Antinori S, Versaci C, Dani G, Antinori M, Selman HA. (1997b) Successful fertilization and pregnancy using frozen-thawed round spermatids. In: Gomel V & Leung PCK "In vitro fertilization and assisted reproduction", Proc. 10th World Congr. In-Vitro Fertil Ass Reprod Vancouver (Canada), 699-703.
7. Araki Y, Motoyama M, Yoshida A, Kim S-Y, Sung H, Araki Sh. (1997) Intracytoplasmic injection with late spermatids: a successful procedure in achieving childbirth for couples in which the male partner suffers from azoospermia due to deficient spermatogenesis. Fertil Steril 67: 559-61. [DOI:10.1016/S0015-0282(97)80086-6]
8. Aslam L, Robins A, Dowell K, Fishel S. (1998) Isolation, purification and assessment of viability of spermatogenic cells from testicular biopsies of azoospermic men. Hum Reprod 13: 639-45. [DOI:10.1093/humrep/13.3.639] [PMID]
9. Aslam I, Fishel S. (1999) Evaluation of the fertilization potential of freshly isolated, in-vitro cultured and cryopreserved human spermatids by injection into hamster oocytes. Hum Reprod 14: 1528-33. [DOI:10.1093/humrep/14.6.1528] [PMID]
10. Balaban B, Urman B, Isiklar A, Alatas C, Aksoy S, Mercan R, Nuhoglu A. (2000) Progression to the blastocyst stage of embryos derived from testicular round spermatids. Hum Reprod 15: 1377-82. [DOI:10.1093/humrep/15.6.1377] [PMID]
11. Barak Y, Kogosowski A, Goldman S, Soffer Y, Gonen Y, Tesarik J. (1998) Pregnancy and birth after transfer of embryos that developed from single-nucleated zygotes obtained by injection of round spermatids into oocytes. Fertil Steril 70: 67-70. [DOI:10.1016/S0015-0282(98)00106-X]
12. Barros A, Bernabeu R, Takahashi K, Oliveira C, Cremades N, Silva J, Sousa M, Tesarik J. (1998) Intracytoplasmic injection of ejaculate and testicle spermatids: report on 35 cycles. Hum Reprod 13 (s1): 154-5.
13. Bernabeu R, Cremades N, Takahashi K, Sousa M. (1998) Successful pregnancy after spermatid injection. Hum Reprod 13: 1898-1900. [DOI:10.1093/humrep/13.7.1898] [PMID]
14. Brannan CI, Bartolomei MS. (1999) Mechanisms of genomic imprinting. Curr. Opin Genet Dev 164-170. [DOI:10.1016/S0959-437X(99)80025-2]
15. Cox FG, Bürger J, Lip V, Mau UA, Sperling K, Wu B-L, Horsthemke B. (2002) Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet 71: 162-4. [DOI:10.1086/341096] [PMID] [PMCID]
16. Cremades N, Bernabeu R, Barros A, Sousa M. (1999) In-vitro maturation of round spermatids using co-culture on Vero cells. Hum Reprod 14: 1287-93. [DOI:10.1093/humrep/14.5.1287] [PMID]
17. De Kretser M, Kerr JB. (1969) The cytology of the testis. In Knobil E and Neill JD (eds), "The Physiology of Reproduction", Raven Press, New York, USA.
18. De Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N. (1998) Spermatogenesis. Hum Reprod 13: S1-8. [DOI:10.1093/humrep/13.suppl_1.1] [PMID]
19. Edwards RG, Tarin JJ, Dean N, Hirsch A, Tan SL. (1994) Are spermatid injections into human oocytes now mandatory? Hum Reprod 9: 2217-9. [DOI:10.1093/oxfordjournals.humrep.a138426] [PMID]
20. Fishel S, Green S, Bishop M. (1995) Pregnancy after intracytoplasmic injection of spermatid Lancet 345: 1641-2. [DOI:10.1016/S0140-6736(95)90149-3]
21. Gianaroli L, Selman HA, Magli MC, Colpi G, Fortini D, Ferraretti AP. (1999) Birth of a healthy infant after conception with round spermatids isolated from cryopreserved testicular tissue. Fertil Steril 72: 539-41. [DOI:10.1016/S0015-0282(99)00285-X]
22. Hiramoto Y. (1962) Microinjection of the live spermatozoa into sea urchin eggs. Exp Cell Res 27: 416-26. [DOI:10.1016/0014-4827(62)90006-X]
23. Kahraman S, Polat G, Samli M, Sِzen E, ozgün OD, Dirican K, Ozbicer T. (1998) Multiple pregnancies obtained by testicular spermatid injection in combination with intracytoplasmic sperm injection. Hum Reprod 13: 104-10. [DOI:10.1093/humrep/13.1.104] [PMID]
24. Khalili MA, Aflatoonian A, Zavos PM. (2002) Intracytolasmic Injection Using Spermatids and Subsequent Preganancies: Round Versus Elongated Spermatids. Journal of Assisted Reproduction and Genetics 19: 84-86. [DOI:10.1023/A:1014447731630] [PMID] [PMCID]
25. Kimura Y, Yanagimachi Y. (1995) Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development 121: 2397-2405.
26. Mansour RT, Aboulghar MA, Serour GI, Kamal A, Tawab NA, Fahmy I, Amin YM. (1996) Pregnancy and delivery after intracytoplasmic injection of spermatids into human oocytes. Middle East Fertil Soc J 1: 223-5.
27. Martin-du Pan R, Campana A. (1993) Physiopathology of spermatogenic arrest. Fertile Steril 60: 937-49. [DOI:10.1016/S0015-0282(16)56388-2]
28. Nikolettos N, Küpker W, Demirel LC, Schِpper B, Blasig C, Sturm R, Felberbaum R, Bauer O, Diedrich K, Al-Hasani S. (1999) Fertilization potentials of spermatozoa with abnormal morphology. Hum Reprod 14: 47-70. [DOI:10.1093/humrep/14.suppl_1.47] [PMID]
29. Ogura A, Yanagimachi R. (1993) Round spermatid nuclei injected into hamster oocytes from pronuclei and participate in syngamy. Boil Reprod 48: 219-25. [DOI:10.1095/biolreprod48.2.219] [PMID]
30. Ogura A, Yanagimachi R, Usui N. (1993) Behaviour of hamster and mouse round spermatid nuclei incorporated into mature oocytes by electrofusion. Zygote 1, 1-18. [DOI:10.1017/S0967199400001234] [PMID]
31. Ogura A, Matsuda J, Yanagimachi R. (1994) Birth of normal young after electrofusion of mouse oocytes with round spermatids. Proc Natl Acad Sci U.S.A 91, 7460-2. [DOI:10.1073/pnas.91.16.7460] [PMID] [PMCID]
32. Oppedisano L, Haines G, Hrabchak C, Fimia G, Elliott R, Sassone-Corsi P, Varmuza S. (2002) The rate of aneuploidy is altered in spermatids from infertile mice. Hum Reprod 17: 710-17. [DOI:10.1093/humrep/17.3.710] [PMID]
33. Orstavik KH, Eiklid K, van der Hagen CB, Spetalen S, Kierulf K, Skjeldal O, Buiting K. (2003) Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic sperm injection. Am. J Hum Genet 72: 218-9,. [DOI:10.1086/346030] [PMID] [PMCID]
34. Palermo G, Joris H, Devroey P, Van Steirteghem AC. (1992) Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340, 17-8. [DOI:10.1016/0140-6736(92)92425-F]
35. Preece MA, Moore GE. (2000) Genomic imprinting, uniparental disomy and foetal growth. TEM 11: 270-75. [DOI:10.1016/S1043-2760(00)00277-0]
36. Reik W, Walter J. (1998) Imprinting mechanisms in mammals. Curr Opin Genet Dev 8: 154-164. [DOI:10.1016/S0959-437X(98)80136-6]
37. Saremi A, Esfandiari N, Salehi N, Saremi MR. (2002) The first successful pregnancy following injection of testicular round spermatid in Iran. Arch Androl 48: 315-9. [DOI:10.1080/01485010290031637] [PMID]
38. Schoysman R, Vanderzwalmen P, Bertin G, Nijs M , van Damme B. (1999) Oocyte insemination with spermatozoa precursors. Curr. Opin. Urology 9: 541-5. [DOI:10.1097/00042307-199911000-00009] [PMID]
39. Schulze W, Thoms F, Knuth UA. (1999) Testicular sperm extraction: comprehensive analysis with simultaneously performed histology in 1418 biopsies from 766 subfertile men. Hum Reprod 14 (s1): 82-96. [DOI:10.1093/humrep/14.suppl_1.82] [PMID]
40. Shamanski FL, Kimura Y, Lavoir M-C, Pedersen RA, Yanagimachi R. (1999) Status of genomic imprinting in mouse spermatids. Hum Reprod 14: 1050-6. [DOI:10.1093/humrep/14.4.1050] [PMID]
41. Silber SJ, Johnson L. (1998) Are spermatid injections of any clinical value? Hum Reprod 13: 509-23. [DOI:10.1093/humrep/13.3.509] [PMID]
42. Silber SJ, Nagy Z, Devroey P, Tournaye H, Van Steirteghem AC. (1997) Distribution of spermatogenesis in the testicles of azoospermic men: the presence or absence of spermatids in the testes of men with germinal failure. Hum Reprod 12: 2422-28. [DOI:10.1093/humrep/12.11.2422] [PMID]
43. Silber SJ, Johnson L, Verheyen G, Van Steirteghem AV. (2000) Round spermatid injection. Fertil Steril 73: 897-900. [DOI:10.1016/S0015-0282(00)00488-X]
44. Sleutels F, Barlow DP, Lyle R. (2000) The uniqueness of the imprinting mechanism. Curr. Opin. Genet Dev 10: 229-33. [DOI:10.1016/S0959-437X(00)00062-9]
45. Sofikitis NV, Miyagawa I, Agapitos E, Pasyianos P, Toda T, Hellstrom WJ, Kawamura H. (1994) Reproductive capacity of the nucleus of the male gamete after completion of meiosis. J Assist Reprod Genet 11: 335-41. [DOI:10.1007/BF02214138] [PMID]
46. Sofikitis NV, Yamamoto Y, Miyagawa I, Mekras G, Mio Y, Toda T, Antypas S, Kawamura H, Kanakas N, Antoniou N, Loutradis D, Mantzavinos T, Kalianidis K, Agapitos E. (1998) Ooplasmic. injection of elongating spermatids for the treatment of non-obstructive azoospermia. Hum Reprod 13: 709-14. [DOI:10.1093/humrep/13.3.709] [PMID]
47. Sousa M, Barros A, Tesarik J. (1998) Current problems with spermatid conception. Hum Reprod 13: 255-8. [DOI:10.1093/humrep/13.2.255] [PMID]
48. Sousa M, Cremades N, Silva J, Oliveira C, Ferraz L, Texeira da Silva J, Viana P, Barros A. (2002) Predictive value of testicular histology in secretory azoospermic subgroups and clinical outcome after microinjection of fresh and frozen-thawed sperm and spermatids. Hum Reprod 17: 1800-10. [DOI:10.1093/humrep/17.7.1800] [PMID]
49. Tesarik J, Mendoza C, Testart J. (1995) Viable embryos from injection of round spermatids into oocytes. N Engl J Med 333: 525. [DOI:10.1056/NEJM199508243330819] [PMID]
50. Tesarik J, Mendoza C. (1996) Spermatid injection into human oocytes. I. Laboratory techniques and special features of zygote development. Hum Reprod 11: 772-9. [DOI:10.1093/oxfordjournals.humrep.a019253] [PMID]
51. Tesarik J, Greco E, Rienzi L, Ubaldi F, Guido M, Cohen-Bacrie P, Mendoza C. (1998) Differentiation of spermatogenic cells during in-vitro culture of testicular biopsy samples from patients with obstructive azoospermia: effect of recombinant follicle stimulating hormone. Hum Reprod 13: 2772-81. [DOI:10.1093/humrep/13.10.2772] [PMID]
52. Tesarik J, Bahceci M, Ozcan C, Greco E, Mendoza C. (1999) Restoration of fertility by in-vitro spermatogenesis Lancet 353: 555-6. [DOI:10.1016/S0140-6736(98)04784-9]
53. Tesarik J, Balaban B, Isiklar A, Alatas C, Urman B, Aksoy S, Mendoza C, Greco E. (2000) In-vitro spermatogenesis resumption in men with maturation arrest: relationship with in-vivo blocking stage and serum FSH. Hum Reprod 15: 1350-4. [DOI:10.1093/humrep/15.6.1350] [PMID]
54. Urman B, Alatas C, Aksoy S, Mercan R, Nuhoglu A, Mumcu A, Isiklar A, Balaban B. (2002) Transfer at the blastocyst stage of embryos derived from testicular round spermatid injection. Hum Reprod 17: 741-3. [DOI:10.1093/humrep/17.3.741] [PMID]
55. Vanderzwalmen P, Lejeune B, Nijs M, Segal-Bertin G, Vandamme B, Schoysman R. (1995) Fertilization of an oocyte microinseminated with a spermatid in an in-vitro fertilization programme. Hum Reprod 10: 502-3. [DOI:10.1093/oxfordjournals.humrep.a135976] [PMID]
56. Vanderzwalmen P, Zech H, Birkenfeld A, Yemini M, Bertin G, Lejeune B, Nijs M, Segal L, Stecher A, Vandamme B, van Roosendaal E, Schoysman R. (1997) Intracytoplasmic injection of spermatids retrieved from testicular tissue: influence of testicular pathology, type of selected spermatids and oocyte activation. Hum Reprod 12: 1203-13. [DOI:10.1093/humrep/12.6.1203] [PMID]
57. Vanderzwalmen P, Nijs M, Schoysman R, Bertin G, Lejeune B, Vandamme B, Kahraman S, Zech H. (1998) The problems of spermatid microinjection in the human: the need for an accurate morphological approach and selective methods for viable and normal cells. Hum Reprod 13: 515-9. [DOI:10.1093/oxfordjournals.humrep.a019693] [PMID]
58. Vanderzwalmen P, Nijs M, Stecher A, Zech H, Bertin G, Lejeune B, Vandamme B, Chatziparasidou A, Prapas Y, Schoysman R. (1998) Is there a future for spermatid injections? Hum Reprod 13: S71-84. [DOI:10.1093/humrep/13.suppl_4.71] [PMID]
59. Verheyen G, Crabbe E, Joris H, Van Steirteghem A. (1998) Simple and reliable identification of the human round spermatid by inverted phase contrast microscopy. Hum Reprod 13: 1570-7. [DOI:10.1093/humrep/13.6.1570] [PMID]
60. Zech H, Vanderzwalmen P, Prapas Y, Lejeune B, Duba E, Schoysman R. (2000) Congenital malformations after intr acytoplasmic injection of spermatids. Hum Reprod 15: 969-71. [DOI:10.1093/humrep/15.4.969] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




