Fri, Apr 19, 2024
[Archive]
Volume 5, Issue 5 (7-2007)
IJRM 2007, 5(5): 177-181 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mostajeran F, Naderi M, Adibi S. Effects of melatonin on histopathological changes after experimental ovarian torsion-detorsion in cat. IJRM 2007; 5 (5) :177-181
URL: http://ijrm.ir/article-1-88-en.html
URL: http://ijrm.ir/article-1-88-en.html
1- Department of Obstetric and Gynecology, Isfahan University of Medical Sciences, Isfahan, Iran , mostajeran@med.mui.ac.ir
2- Department of Obstetric and Gynecology, Isfahan University of Medical Sciences, Isfahan, Iran
3- Torabienejad Dental Research Center, Isfahan University
2- Department of Obstetric and Gynecology, Isfahan University of Medical Sciences, Isfahan, Iran
3- Torabienejad Dental Research Center, Isfahan University
Full-Text [PDF 184 kb]
(469 Downloads)
| Abstract (HTML) (1959 Views)
Full-Text: (266 Views)
Introduction
Ovarian torsion is a serious gynecologic problem and is most common in premenarchal girls and women of childbearing age (1-3). Because most of these women desire future fertility, the removal of an ovary could later adversely effect their reproductive life. On the other hand, when future fertility desired, rapid diagnosis is important and conservative management includes detorsion of the Involved segment (3 ,5). During the detorsion process an excess amount of molecular oxygen is supplied to the tissues and abundant amount of ROS (reactive oxygen species) are produced. Accumulation of activated neutrophils and release of Ros such as O20- , O0H, H2O2 which interact with proteins, lipids and nucleic acids resulting in loss of cell membrane integrity and structural or functional changes in proteins (6-10). Melatonin is a biologically relevant indole compound and a free radical scavenger antioxidant which protect cells against the damage induced by several oxidative agents (11-14).
The protective effects of Melatonin on ovarian ischemia-reperfusion has not yet been investigated until Turkoz et al in Malataya (2004) demonstrated that Melatonin administration reduced the morphological changes by inducing Ischemia/ Reperfusion(I/R). In particular,Polymorphonuclear Neutrophilic (PMN) infiltration, edema, hemorrhage and vascular dilatation were much lower but hadn't been totally prevented (15). To determine whether treatment with Melatonin modifies ischemic indices,we examined its effects on an invivo model of adnexial Ischemia/ Reperfusion injury in cats . The aim of the present study was to investigate the effects of Melatonin on histopathological change in I/R injury in cat ovaries.
Materials and methods
To determine whether ischemia followed by subsequent reperfusion induces ovarian damage and the effects of Melatonin on the injured ovaries, we created a model of adnexal I/R using cats. The permission for the animal tests and experiments was given by the center of animal researches of professor Torabynegad Institution of Isfahan University. All surgical procedures were performed while the cats were under general anesthesia. In total twenty cats with almost similar weights were subjected to right unilateral 360° clockwise adnexial torsion by a ring forceps which induced ischemia by occlusion of the tuboovarian vesseles for 3 hr (15-17).
Melatonin (10mg/kg) that is a white lipophil powder was dissolved in 1% ethanol (total volume =10 cc) just before use and injected intra peritoneally 30 min before reperfusion in the Melatonin group while saline was administered (10cc) in the saline group. Reperfusion was achieved by releasing the occlusion and restoring the circulation for 3 hr. After 3 hr, cats in both groups were reanesthesized, laparatomy was performed and right ovaries were surgically removed. The ovaries were preserved in formalin for histopathological examination.
Histopathological examination
At the end of each experiment, the ovaries were removed and fixed in 10% neutral buffered formalin solution and then were embedded in paraffin as usual. Serial sections were cut using the microtome at a thickness of 4 m and were stained with hematoxylin and eosin.The histologic sections were examined for the presence of interstitial edema, vascular congestion, hemorrhage and PMN infiltration, using a microscope. The slides were coded and semiquantitative analysis of the sections was performed without knowledge of the treatment protocol.
m and were stained with hematoxylin and eosin.The histologic sections were examined for the presence of interstitial edema, vascular congestion, hemorrhage and PMN infiltration, using a microscope. The slides were coded and semiquantitative analysis of the sections was performed without knowledge of the treatment protocol.
The changes seen were graded as follows (16):
Grade I: Mild edema / Mild vascular congestion/No hemorrhage /No PMN.
Grade II: Moderate edema / Moderate vasudar congestion / No hemorrhage / No PMN.
Grade III: Severe edema/Severe vascular Congestion /Minimal hemorrhage / Minimal PMN.
Grade IV: Severe edema / Severe vascular congestion / Severe hemorrhage / Severe PMN.
Statistical analysis
The Statistical package for social sciences (SPSS) version 10 was used for statistical analysis. Individual group parameters were assessed with Manwhitney test. The spearman's test determined the correlation between ischemic indices.
Results
Macroscopically, torsioned ovaries had a cherry–red color. Microscopic examination of ovaries in both groups showed: interstitial edema, vascular congestion, hemorrhage and acute infiltration by PMNs.
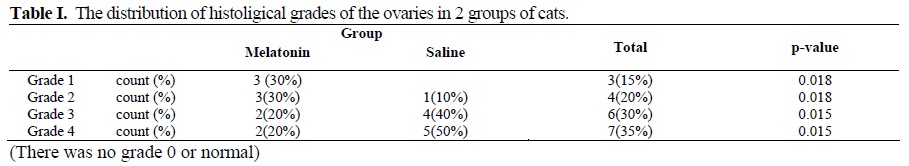
The Manwhitney test demonstrated edema and vascular congestion in saline group were more severe than Melatonin group (p–value=0.009), also hermorrhage and leukocyte infiltration were more obvious in saline group (p–value=0.018). The histological changes, graded as described in the methods, are summarized in Table I. Thehistological grades of the ovaries in melatonin. Group were lower than those of the saline group (p-value=0.015). Further more, there was a correlation between edema and vascular congestion (r =1, p-value =0.001), hemorrhage and PMN infiltration (r=1, p-value=0.001) and the other ischemia indices, for example vascular congestion and hemorrhage (r =0.857, p-value< 0.001).
Discussion
Ovarian injury resulting from torsion and detorsion resembles the phenomenon of Ischemia – Reperfusion injuries in other organs (1-3). It has been demonstrated that oxygen free radical generation is a critical mechanism causing injury in post ischemic cells and tissues , therefore oxygen , despite its vital properties, can be the most toxic elements known to human (6,7,18,19). Detorsion of the ovary, a conservative management that save it for future fertility ,is one of the most important factors in future injury (1,3) Lipid peroxidation by active oxygen radicals can alter both membrane structure and function thus reperfusion injury may increase vascular permeability. Neutrophil infiltration might be regarded as another source of free radicals in the ischemic tissue, because they stimulate inflammatory mediators such as Tumor Necrozing Factor (TNF) and Interluekin-1(IL1) which are involved in the pathogenesis of I/R injury. Edema and increased permeability occurred in vivo after ischemia reperfusion, due to disruption of endothelial cell junction integrity (20, 21). In the present study, Melatonin significantly prevented hemorrhage and greatly reduced the number of PMN and degree of vascular congestion and edema. These effects of Melatonin may occur not only because of its anti oxidant and Ros scavenger properties, but also its stimulatory effect on endogenous anti oxidant enzyme such as: catalase and SOD (super oxide dismutase) which bind to cell membrane receptors and decrease Ca and CAMP concentrations. Thus it is superior to other scavengers (22-26).
The results of the histopathological parameters in our study indicate that administration of Melatonin has beneficial effects in the prevention of reperfusion injury of ovary. Hara et al (1996) demonstrated that melatonin given in advance of severe exercise prevented the reduction in muscle GSH (an antioxidant enzyme in normal tissues) (27).
Another study reported that melatonin preventes the paraquat induced reduction in GSH in both lung and liver (28).Although melatonin scavenging actions have been demonstrated in a number of tissues (29-33), only Turkoz et al demonstrated
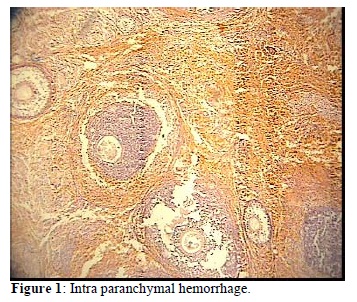

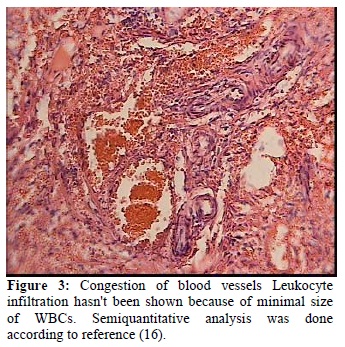
the effects of melatonin on reducing ischemic indices such as xanthine oxidase (p-value <0.001), MDA (Malondialdehyde) (p-value <0.001) , acute PMN infiltration, edema and Vascular dilatation in ovaries . In this study, Melatonin administration reduced the morphological changes by induced I/R; in particular, PMN infiltration, edema and vascular dilatation were much lower but had not been totally prevented by i.p Melatonin administration (15). In addition melatonin has an ameliorating effect on oxidative stress-induced renal tubular damage in diabetic nephropathy via its antioxidant properties (34). High dose of melatonin (50mg/kg), physiologically, biochemically and morphologically can be useful to normalize contractility injured by oxidative stress in intestinal ischemia/reperfusion (35).
Conclusion
We demonstrated that Melatonin reduced tissue damage induced by I/R in cat ovaries. This means that administration of Melatonin could be helpful in protection of ovaries from torsion – detorsion induced damage in humans.
Ovarian torsion is a serious gynecologic problem and is most common in premenarchal girls and women of childbearing age (1-3). Because most of these women desire future fertility, the removal of an ovary could later adversely effect their reproductive life. On the other hand, when future fertility desired, rapid diagnosis is important and conservative management includes detorsion of the Involved segment (3 ,5). During the detorsion process an excess amount of molecular oxygen is supplied to the tissues and abundant amount of ROS (reactive oxygen species) are produced. Accumulation of activated neutrophils and release of Ros such as O20- , O0H, H2O2 which interact with proteins, lipids and nucleic acids resulting in loss of cell membrane integrity and structural or functional changes in proteins (6-10). Melatonin is a biologically relevant indole compound and a free radical scavenger antioxidant which protect cells against the damage induced by several oxidative agents (11-14).
The protective effects of Melatonin on ovarian ischemia-reperfusion has not yet been investigated until Turkoz et al in Malataya (2004) demonstrated that Melatonin administration reduced the morphological changes by inducing Ischemia/ Reperfusion(I/R). In particular,Polymorphonuclear Neutrophilic (PMN) infiltration, edema, hemorrhage and vascular dilatation were much lower but hadn't been totally prevented (15). To determine whether treatment with Melatonin modifies ischemic indices,we examined its effects on an invivo model of adnexial Ischemia/ Reperfusion injury in cats . The aim of the present study was to investigate the effects of Melatonin on histopathological change in I/R injury in cat ovaries.
Materials and methods
To determine whether ischemia followed by subsequent reperfusion induces ovarian damage and the effects of Melatonin on the injured ovaries, we created a model of adnexal I/R using cats. The permission for the animal tests and experiments was given by the center of animal researches of professor Torabynegad Institution of Isfahan University. All surgical procedures were performed while the cats were under general anesthesia. In total twenty cats with almost similar weights were subjected to right unilateral 360° clockwise adnexial torsion by a ring forceps which induced ischemia by occlusion of the tuboovarian vesseles for 3 hr (15-17).
Melatonin (10mg/kg) that is a white lipophil powder was dissolved in 1% ethanol (total volume =10 cc) just before use and injected intra peritoneally 30 min before reperfusion in the Melatonin group while saline was administered (10cc) in the saline group. Reperfusion was achieved by releasing the occlusion and restoring the circulation for 3 hr. After 3 hr, cats in both groups were reanesthesized, laparatomy was performed and right ovaries were surgically removed. The ovaries were preserved in formalin for histopathological examination.
Histopathological examination
At the end of each experiment, the ovaries were removed and fixed in 10% neutral buffered formalin solution and then were embedded in paraffin as usual. Serial sections were cut using the microtome at a thickness of 4
The changes seen were graded as follows (16):
Grade I: Mild edema / Mild vascular congestion/No hemorrhage /No PMN.
Grade II: Moderate edema / Moderate vasudar congestion / No hemorrhage / No PMN.
Grade III: Severe edema/Severe vascular Congestion /Minimal hemorrhage / Minimal PMN.
Grade IV: Severe edema / Severe vascular congestion / Severe hemorrhage / Severe PMN.
Statistical analysis
The Statistical package for social sciences (SPSS) version 10 was used for statistical analysis. Individual group parameters were assessed with Manwhitney test. The spearman's test determined the correlation between ischemic indices.
Results
Macroscopically, torsioned ovaries had a cherry–red color. Microscopic examination of ovaries in both groups showed: interstitial edema, vascular congestion, hemorrhage and acute infiltration by PMNs.

The Manwhitney test demonstrated edema and vascular congestion in saline group were more severe than Melatonin group (p–value=0.009), also hermorrhage and leukocyte infiltration were more obvious in saline group (p–value=0.018). The histological changes, graded as described in the methods, are summarized in Table I. Thehistological grades of the ovaries in melatonin. Group were lower than those of the saline group (p-value=0.015). Further more, there was a correlation between edema and vascular congestion (r =1, p-value =0.001), hemorrhage and PMN infiltration (r=1, p-value=0.001) and the other ischemia indices, for example vascular congestion and hemorrhage (r =0.857, p-value< 0.001).
Discussion
Ovarian injury resulting from torsion and detorsion resembles the phenomenon of Ischemia – Reperfusion injuries in other organs (1-3). It has been demonstrated that oxygen free radical generation is a critical mechanism causing injury in post ischemic cells and tissues , therefore oxygen , despite its vital properties, can be the most toxic elements known to human (6,7,18,19). Detorsion of the ovary, a conservative management that save it for future fertility ,is one of the most important factors in future injury (1,3) Lipid peroxidation by active oxygen radicals can alter both membrane structure and function thus reperfusion injury may increase vascular permeability. Neutrophil infiltration might be regarded as another source of free radicals in the ischemic tissue, because they stimulate inflammatory mediators such as Tumor Necrozing Factor (TNF) and Interluekin-1(IL1) which are involved in the pathogenesis of I/R injury. Edema and increased permeability occurred in vivo after ischemia reperfusion, due to disruption of endothelial cell junction integrity (20, 21). In the present study, Melatonin significantly prevented hemorrhage and greatly reduced the number of PMN and degree of vascular congestion and edema. These effects of Melatonin may occur not only because of its anti oxidant and Ros scavenger properties, but also its stimulatory effect on endogenous anti oxidant enzyme such as: catalase and SOD (super oxide dismutase) which bind to cell membrane receptors and decrease Ca and CAMP concentrations. Thus it is superior to other scavengers (22-26).
The results of the histopathological parameters in our study indicate that administration of Melatonin has beneficial effects in the prevention of reperfusion injury of ovary. Hara et al (1996) demonstrated that melatonin given in advance of severe exercise prevented the reduction in muscle GSH (an antioxidant enzyme in normal tissues) (27).
Another study reported that melatonin preventes the paraquat induced reduction in GSH in both lung and liver (28).Although melatonin scavenging actions have been demonstrated in a number of tissues (29-33), only Turkoz et al demonstrated



the effects of melatonin on reducing ischemic indices such as xanthine oxidase (p-value <0.001), MDA (Malondialdehyde) (p-value <0.001) , acute PMN infiltration, edema and Vascular dilatation in ovaries . In this study, Melatonin administration reduced the morphological changes by induced I/R; in particular, PMN infiltration, edema and vascular dilatation were much lower but had not been totally prevented by i.p Melatonin administration (15). In addition melatonin has an ameliorating effect on oxidative stress-induced renal tubular damage in diabetic nephropathy via its antioxidant properties (34). High dose of melatonin (50mg/kg), physiologically, biochemically and morphologically can be useful to normalize contractility injured by oxidative stress in intestinal ischemia/reperfusion (35).
Conclusion
We demonstrated that Melatonin reduced tissue damage induced by I/R in cat ovaries. This means that administration of Melatonin could be helpful in protection of ovaries from torsion – detorsion induced damage in humans.
Type of Study: Original Article |
References
1. Chen M, Chen CD, Yang YS. Torsion of the previously normal uterine adnexa. Evaluation of the correlation between the pathological changes and the clinical characteristics. Acta obstet Gynecol scand 2001; 85:58-61. [DOI:10.1034/j.1600-0412.2001.800111.x]
2. Haskins T, Shull B. Adnexal torsion: a mind - twisting diagnosis. South Med J 1986; 79: 576- 577. [DOI:10.1097/00007611-198605000-00013]
3. Meyer JS, Harman CM, Harty MP , Markowitz RI, Hubba AM, Bellah RD. Ovarian torsion: Clinical and imaging presentation in children. J Pediatr Surg 1995; 30:1433 -1436. [DOI:10.1016/0022-3468(95)90399-2]
4. Taskin O, Birincioglu M, Aydin A, Buhur A, Burak F, Yilmaz I, et al. The effects of twisted ischemic adnexa managed by detorsion on Ovarion viability and histology: an ischemia-reperfusion rodent model. Hum Reprod 1998; 13: 2823-2827. [DOI:10.1093/humrep/13.10.2823]
5. Kaakaji Y, Nghiem HV, Nodell C, Winter TC. Sonography of obstetric and Gynecologic emergencies: part II, gynecologic emergencies . AM J Roentgend 2000; 174:651-656. [DOI:10.2214/ajr.174.3.1740651]
6. Bird JE, Milhoan K, Wilson CB, Young SG, Mundy CA. Ischemic acute renal failure and antioxidant therapy in the rat: the relation between glomerular and tubular dysfurction. J Din Invest 1988; 81:1630-1638. [DOI:10.1172/JCI113498]
7. Baker GL, Corry RJ, Autor AP. Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion: protection effect of superoxide dismutase. Ann Surg 1985; 202:628-641. [DOI:10.1097/00000658-198511000-00016]
8. Gilham B, Papa Chri Stodoulou Dk, Thomas Jh. Will's Biochemical Basis of Medicine 3rd Ed. Butterworth - Heinemann, London 1997: 343-354.
9. Breen AP, Murphy JA. Reactions of oxyl radicals with DNA. Free Radic Biol Med 1995 ; 18: 1033-1077. [DOI:10.1016/0891-5849(94)00209-3]
10. Ames BN. Endogenous dna damage as related to cancer and aging. Mutat Res 1989 ; 214: 41-46 [DOI:10.1016/0027-5107(89)90196-6]
11. Tan Dx, Chen Ld. Poeggeller B. Melatonin: a potent endogenous hydroxyl radical scavenger. Endocr J 1993; 1:57-60.
12. Allegra M, Reiter RJ, Tan DX. The chemistry of melatonin's interaction with reactive species, J Pineal Res 2003; 34:1. [DOI:10.1034/j.1600-079X.2003.02112.x]
13. Reiter RJ. Pineal Melatonin: Cell biology of its synthesis and its physiolosical interactions . Endocr Rev 1991; 12: 151-156. [DOI:10.1210/edrv-12-2-151]
14. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B. Evaluation of the antioxidant activity of melatonin in vitro. Free Radic Biol Med 1996; 21:307-315. [DOI:10.1016/0891-5849(96)00046-9]
15. Turkoz Y, Celik O , Hascalik S, Cigremis Y, Hascalik M, Mizrak B, et al. Melatonin reduces torsion - detorsion injury in rat ovary: Biochemical And Histopathologic Evaluation. J .Pineal Res 2004; 37: 137-141. [DOI:10.1111/j.1600-079X.2004.00146.x]
16. Celik O, Turkoz Y, Hascalik S, Hascalik M, Cigremis Y, Mizrak B, et al. The protective effect of caffeic acid phenethyl ester on ischemia-reperfusion injury in rat ovary: European Journal of Obstetrics & Gynecology and Reproductive Biology 2004; 117: 183-188. [DOI:10.1016/j.ejogrb.2004.05.007]
17. Hascalik S, Celik O, Turkoz Y, Hascalik M, Cigremis Y, Mizrak B, et al. Resveratrol, a red wine constituent polyphenol, protects from ischemia-reperfusion damage of the ovaries. Gynecol Obstet Invest 2004; 57: 218-223. [DOI:10.1159/000076760]
18. Melchiorri D, Reiter RJ, Sewerynek E, Hara M, Chen I, Nistico G. Paraquat toxicity and oxidative damage reduction by melatonin. Biochem Biochem Pharmacol 1996; 51: 1095-1099. [DOI:10.1016/0006-2952(96)00055-X]
19. MC Cord JM. Oxygen - derived free radicals in post ischemic tissue injury. N Engl J Med 1985; 312:159 - 163. [DOI:10.1056/NEJM198501173120305]
20. Koltuksuz U , Ozen S , Vz E, Aydinc M , Karaman A , Gultek A, et al. Caffeic acid phenethyl ester prevents intestinal reperfusion injury in rats. J Pediatr Surg 1999; 34:1458-1462. [DOI:10.1016/S0022-3468(99)90103-3]
21. Suzuki S, Toledo - Pereyra LH, Rodriguez F, Lopez F. Role of kupffer cells in neutrophil activation and infiltration following total hepatic ischemia and reperfusion. Circ Shock 1994; 42:204-209.
22. Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species. Cell Biochem Biophys 2001; 34:237-256. [DOI:10.1385/CBB:34:2:237]
23. Tan Dx, Reiter RJ, Manchester LC. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum anti oxidant and free radical scavenser. Curr Top Med Chem 2002; 2:181-197. [DOI:10.2174/1568026023394443]
24. Reiter RJ, Guerrero JM, Garcia JJ, Acuna-Castroviejo D. Reactive oxygen intermediates, molecular damage, and aging relation to melatonin. Ann NY Acad Sci 1998; 854:410-424. [DOI:10.1111/j.1749-6632.1998.tb09920.x]
25. Okatani Y. Melatonin increases activities of glutathione peroxidase and super oxide dismutase in fetal rat brain. J Pineal Res 2000; 28:89-96. [DOI:10.1034/j.1600-079X.2001.280204.x]
26. Rodriguez C, Mayo JC , Sains RM, Antolin I, Herrera F, Martin V, et al. Regulation of anti oxidant enzymes: a signficant role for melatonin . J Pineal Res 2004; 36: 1-9 [DOI:10.1046/j.1600-079X.2003.00092.x]
27. Hara M, Abe M, Suzuki T, Rieter RJ. Tissue changes in glutathione metabolism and lipid peroxidation induced by swimming are partially prevented by melatonin. Pharmacol Toxicol 1996; 78:308-312. [DOI:10.1111/j.1600-0773.1996.tb01380.x]
28. Durmus O, Aricioglu A, Guven T, Oguz M, Yel M, Turkozkan N. The effects of allopurinol on the liver ultrastructure, reduced glutathione and lipid percxide levels during liver ischemia in guinea pigs. Gen Pharmacol 1994; 25:781-786. [DOI:10.1016/0306-3623(94)90260-7]
29. Bertuglia S, Marchiafava Pl, Colantuoni A. Melatonin prevents ischemia reperfusion injury in hamster cheek. Pouch Micro Circulation Cardiovasc Res 1996; 31:947-952. [DOI:10.1016/S0008-6363(96)00030-2]
30. Sewerynek E, Reiter RJ, Melchiorri D, Ortiz GG, Lewinski A. Oxidative damage in the liver induced by ischemia-reperfusion: Protective by melatonin .Hepato - Gasteroenterology 1996; 43:898-905.
31. Celik H, Ayar A,Tug N, Simsek M, Ozercan I, Cikim G, et al . Effects of melatonin on noncardiogenic pulmonary edema secondary to adnexial ischemia- reperfusion in guinea pig. Neuroendocrinol Lett 2002; 23:115-118.
32. Torri K, Uneyama H, Nishino H, Kondoh T. Melatonin suppresses cerebral edema caused by middle cerebral artery occlusion/reperfusion in rats assesed by magnetic resonance imaging. J Pineal Res 2004; 36: 18-24. [DOI:10.1046/j.1600-079X.2003.00097.x]
33. Lahiri OK, Ghosh C. Interactions between melatonin, reactive oxygen species, and nitric oxide. Ann Ny Acad Sci 1999; 893: 325-330. [DOI:10.1111/j.1749-6632.1999.tb07847.x]
34. Oktem F, Ozguner F, Yilmaz Hr, Uz E. Melatonin reduces urinary excretion of n-acetyl-beta-d-glucosaminidase, albumin and renal oxidative markers in diabetic rats. Clin Exp Pharmacol Physiol 2006; 33:95-101. [DOI:10.1111/j.1440-1681.2006.04330.x]
35. Ozacmak VH, Sayan H, Arslan SO, Altaner S, Aktas RG. Protective effect of melatonin on contractile activity and oxidative injury induced by ischemia and reperfusion of rat ileum. Life Sci 2005; 76:1575-1588. [DOI:10.1016/j.lfs.2004.08.031]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




