Sun, Jul 6, 2025
[Archive]
Volume 6, Issue 4 (7-2008)
IJRM 2008, 6(4): 133-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Niknafs B, Afshari F, Dezfulian A. Morphological and morphometrical changes of endometrim after application of estrogen and progesterone during luteal phase in the superovulated mice. IJRM 2008; 6 (4) :133-0
URL: http://ijrm.ir/article-1-121-en.html
URL: http://ijrm.ir/article-1-121-en.html
1- Department of Anatomy, Medical Faculty, Tabriz University of Medical Sciences, Tabriz, Iran , niknafsbeh@yahoo.com
2- Department of Histology, Medical Faculty, Islamic Azad University, Tabriz Branch, Iran
3- Department of Histology, Medical Faculty, Ahvaz University of Medical Sciences, Ahvaz, Iran
2- Department of Histology, Medical Faculty, Islamic Azad University, Tabriz Branch, Iran
3- Department of Histology, Medical Faculty, Ahvaz University of Medical Sciences, Ahvaz, Iran
Full-Text [PDF 233 kb]
(526 Downloads)
| Abstract (HTML) (2791 Views)
Full-Text: (434 Views)
Introduction
Ovarian hormones are responsible for induction of endometrial receptivity. In all mammals, the uterus differentiates into receptivity state, and then blastocyst is capable to initiate the implantation.
After this period the uterus is incapable of blastocyst reception (1). The maximal endometrial receptivity in the mouse is after day 5 of pregnancy and duration of window implantation is 24 hour (2).
The endometrium in response to ovarian hormones undergoes the morphological and biochemical modifications (3). In the pseudopregnant mice, the hormonal milieu in the uterus is similar to normal pregnancy, thus in the pseudopregnant mice, implantation on the day 4 is quite similar to normal pregnancy (1).
Estrogen activates epithelial cell proliferation and stromal inflammation while progesterone has antiproliferative role and antiinflamatory function (4). Lack of progesterone receptors in the mice led to infertility and sexual behavior alterations (5-7). Ablaition of progesterone by RU 486 caused the changes of endometrial cells and reduction of decidual reaction (8).
Many ovarian hyperstimulation cycles are associated with failure in implantation. Hormone therapy affects the endometrial receptivity (9). Inadequate uterine receptivity is responsible for two- third of implantation failures whereas one – third of these failures are related to embryo (10). Fossum demonstrates that ovarian hyperstimulation with PMSG and HCG in the mouse decreases the implantation rate (11).
Although ovarian hormones (E2, P4) are necessary for implantation in rat and mouse but absolute requirement for estrogen in some species is on debate.
The recent data show that uterine receptivity remains open for an extended period at lower estrogen concentration and rapidly closes at higher levels (1). Endrwes et al have shown that estrogen injection in rat increased high of luminal and glandular epithelium and also increased the granulocytic infiltration in the stroma (12). The results suggest that careful regulation of estrogen concentration could improve implantation
rate in IVF protocols (1).
The roles of estrogen and estrogen + progesterone on the endometrial epithelium and different stages of endometrial cycle are known. The roles of these hormones to support endometrium after ovarian hyperstimulation at the luteal phase are unknown. There are reports that progesterone and HCG injection at luteal phase can not increase the implantation rate after ovarian hyperstimulation (13). In addition increasing of the E /P ratio in serum has high prognosis in ovarian hyperstimulation patients for prognosis of pregnancy rate (9)
Although the progesterone is routinely used at luteal phase as support hormone for in IVF cycle, addition of estrogen to progesterone during luteal phase is controversial in implantation and in rising of pregnancy rate (14). There are some reports that the progesterone cannot supply suitable uterine for implantation (15,16). In this study the morphological and morphometrical alterations in the luminal and glandular epithelium as well as stromal changes after application of different hormones such as estrogen and progesterone were investigated.
The animals were obtained from animal house of Tabirz University of Medical Sciences. Adult male and female mice (8-10 weeks) were housed under temperature and light controlled condition with free access to food and water.
The female mice in the experimental groups were superovulated by injection of a single dose of 10 I.U. PMSG (pregnant mare serum gonadotropin) and after 48 hours, 10 I .U. HCG (human chorionic gonadotropin). The mice were mated with the vasectomised mice to produce psudopregnancy.
In the control group, psudopregnancy was induced in natural cycle without any superovulation. Female mice of control and experimental groups were housed over night with vasectomised males and the presence of vaginal plaque was checked in the following morning; a successful mating was considered to be the first day of psudopregnancy.
Experimental group based on hormone therapy at luteal phase was divided into five groups:
1) Sham group: The superovulated mice that were induced for pseudopregnancy were not received any hormones for luteal phase .This group was received only vehicle (olive oil)
2) E group: The psudopregnant mice that were superovulated were received consecutive daily estrogen (10ng in vehicle /mouse) injection until day 4 (17).
3) P group: The psudopregnant mice that were superovulated were received consecutive daily progesterone (1 mg in vehicle /mouse) injection until day 4 (15).
4) E +P group: The psudopregnant mice that were superovulated were received consecutive daily estrogen+progesterone (10 ng+1mg) injection until day 4.
5) RU 486+E group: The psudopregnant mice that were superovulated were received consecutive daily antiprogesterone + estrogen (1mg+10 ng) injection until day 4.
Tissue preparation
Animals in all groups were scarificed by cervical dislocation after 4.5 day of psudopregnancy. The samples were obtained from the 1/3 middle part of uterine horns and immediately were fixed in formaldehyde then were embedded in paraffin wax .After preparation of 5 micrometer sections , the sections were stained with H & E method and were studied by light microscopy.
Morphometrical studying
For the assessment of morphometrical parameters the extracted uterine was divided into 4 pieces. The pieces were embedded in paraffin wax separeatly in defined direction. Five sections were provided from each pieces. In order to assess the high of luminal and glandular epithelium and stromal thickness each section were stained with H & E and measured by graded eye piece in four directions (18). Then the data were changed to micron by slide measurement and were analyzed by statistical method.
Statistical analysis
The collected data from each group were analyzed by SPSS software with One Way ANOVA method.
The results of this study are presented into two parts; morphology and morphometry.
I) morphology
1) Control group: After 4.5 day of psudopregnancy the form of epithelium was columnar and nucleus was located in the base of cells.
The height of glandular epithelium was decreased in comparison with luminal epithelium. The stromal cellular compaction was low and decidual reaction was seen. Luminal surface of endometrium was seen in normal folding (figure 1.a).
2) Experimental group:
A) Sham group: In this group the luminal epithelium was columnar and luminal surface of the epithelium was irregular with folding. The height of glandular epithelium was decreased in comparison to luminal epithelium. The number of gland and secretion was similar to control group. Decidual reaction was higher than control group (figure 1.b), (Table I).
B) Progesterone group: The shape of epithelium was cuboidal and the height of luminal epithelium was decreased in comparison to control group. The nucleus was located in the base of cells and occupied the main portion of the cytoplasems. The apical border of cells was regular and stroma was seen compacted. Stromal desidulization was lower than control group and their intercellular spaces were narrow. The endometrial folding was not seen in this group (figure 1.c), (figure 2 .a).
C) Estrogen group: The form of epithelium was pseudostratified and the epithelial height was higher than control group. The glands mainly contained secretions .The cellularity of stroma was low and the intercellular spaces were increased and the decidual reaction was higher than control group. The endometrial folding was severe (figure 1.d).
D) E+P group: The shape of epithelium was columnar and the nucleus was located in the middle of cells. The height of epithelium was increased in comparison to control group.
Development of glands high and the majority of glands contained secretion. Cellularity of stroma was low and decidual reaction was higher than control group .The endometrial folding was severe (figure 1.e and 2.b).
E) RU 486+E group: The shape of epithelium was pseudostratified and the epithelial height was higher than control group. In comparison to control group, stroma in this group contained inflammatory and pyknotic cells that mainly were located in the subepithelium. The stromal thickness and decidual reaction were decreased in comparison to control group. The endometrial folding was moderate (figure 1.f), (Table I).
II) morphometry
The morphometrical obtained data were prepared in the three parts: height of luminal epithelium, height of glandular epithelium and stromal thickness.
Height of luminal epithelium
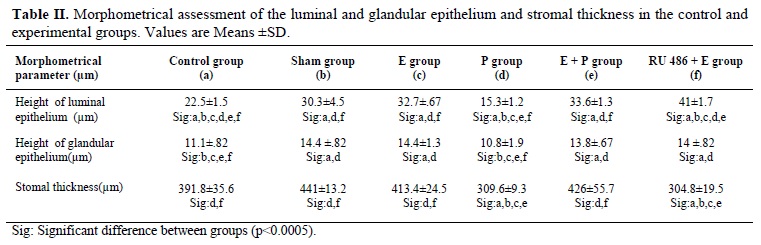
The height of luminal epithelium in the progesterone group was the lowest in comparison to other groups. The comparison of luminal epithelium in the control and sham groups showed that there was significant increase in the height of luminal epithelium in sham group. Comparison of luminal epithelium in the E, E+P and sham groups demonstrated that there were no significant differences among these groups, while they have shown significant differences in comparison to control group (Table II).The height of luminal epithelium in the RU 486 + E group was higher than other groups. The estrogen had a critical role in the increasing of height of the luminal epithelium.
Height of glandular epithelium
The lowest height of glandular epithelium was seen in the progesterone group. The comparison of the height of glandular epithelium in progesterone group with control group has not shown any differences, while the comparison of progesterone group has shown
significant differences with other groups.
Stromal thickness
Ovarian hormones are responsible for induction of endometrial receptivity. In all mammals, the uterus differentiates into receptivity state, and then blastocyst is capable to initiate the implantation.
After this period the uterus is incapable of blastocyst reception (1). The maximal endometrial receptivity in the mouse is after day 5 of pregnancy and duration of window implantation is 24 hour (2).
The endometrium in response to ovarian hormones undergoes the morphological and biochemical modifications (3). In the pseudopregnant mice, the hormonal milieu in the uterus is similar to normal pregnancy, thus in the pseudopregnant mice, implantation on the day 4 is quite similar to normal pregnancy (1).
Estrogen activates epithelial cell proliferation and stromal inflammation while progesterone has antiproliferative role and antiinflamatory function (4). Lack of progesterone receptors in the mice led to infertility and sexual behavior alterations (5-7). Ablaition of progesterone by RU 486 caused the changes of endometrial cells and reduction of decidual reaction (8).
Many ovarian hyperstimulation cycles are associated with failure in implantation. Hormone therapy affects the endometrial receptivity (9). Inadequate uterine receptivity is responsible for two- third of implantation failures whereas one – third of these failures are related to embryo (10). Fossum demonstrates that ovarian hyperstimulation with PMSG and HCG in the mouse decreases the implantation rate (11).
Although ovarian hormones (E2, P4) are necessary for implantation in rat and mouse but absolute requirement for estrogen in some species is on debate.
The recent data show that uterine receptivity remains open for an extended period at lower estrogen concentration and rapidly closes at higher levels (1). Endrwes et al have shown that estrogen injection in rat increased high of luminal and glandular epithelium and also increased the granulocytic infiltration in the stroma (12). The results suggest that careful regulation of estrogen concentration could improve implantation
rate in IVF protocols (1).
The roles of estrogen and estrogen + progesterone on the endometrial epithelium and different stages of endometrial cycle are known. The roles of these hormones to support endometrium after ovarian hyperstimulation at the luteal phase are unknown. There are reports that progesterone and HCG injection at luteal phase can not increase the implantation rate after ovarian hyperstimulation (13). In addition increasing of the E /P ratio in serum has high prognosis in ovarian hyperstimulation patients for prognosis of pregnancy rate (9)
Although the progesterone is routinely used at luteal phase as support hormone for in IVF cycle, addition of estrogen to progesterone during luteal phase is controversial in implantation and in rising of pregnancy rate (14). There are some reports that the progesterone cannot supply suitable uterine for implantation (15,16). In this study the morphological and morphometrical alterations in the luminal and glandular epithelium as well as stromal changes after application of different hormones such as estrogen and progesterone were investigated.
Materials and methods
AnimalsThe animals were obtained from animal house of Tabirz University of Medical Sciences. Adult male and female mice (8-10 weeks) were housed under temperature and light controlled condition with free access to food and water.
Preparation of animals
Male mice were vasectomized and after recovery were used for induction of psudopregnancy. Female mice were kept separately until the estrous cycles of mice become similar. The female mice based on superovulation were divided into two groups: control and experimental groups. Five mice were studied in each group.The female mice in the experimental groups were superovulated by injection of a single dose of 10 I.U. PMSG (pregnant mare serum gonadotropin) and after 48 hours, 10 I .U. HCG (human chorionic gonadotropin). The mice were mated with the vasectomised mice to produce psudopregnancy.
In the control group, psudopregnancy was induced in natural cycle without any superovulation. Female mice of control and experimental groups were housed over night with vasectomised males and the presence of vaginal plaque was checked in the following morning; a successful mating was considered to be the first day of psudopregnancy.
Experimental group based on hormone therapy at luteal phase was divided into five groups:
1) Sham group: The superovulated mice that were induced for pseudopregnancy were not received any hormones for luteal phase .This group was received only vehicle (olive oil)
2) E group: The psudopregnant mice that were superovulated were received consecutive daily estrogen (10ng in vehicle /mouse) injection until day 4 (17).
3) P group: The psudopregnant mice that were superovulated were received consecutive daily progesterone (1 mg in vehicle /mouse) injection until day 4 (15).
4) E +P group: The psudopregnant mice that were superovulated were received consecutive daily estrogen+progesterone (10 ng+1mg) injection until day 4.
5) RU 486+E group: The psudopregnant mice that were superovulated were received consecutive daily antiprogesterone + estrogen (1mg+10 ng) injection until day 4.
Tissue preparation
Animals in all groups were scarificed by cervical dislocation after 4.5 day of psudopregnancy. The samples were obtained from the 1/3 middle part of uterine horns and immediately were fixed in formaldehyde then were embedded in paraffin wax .After preparation of 5 micrometer sections , the sections were stained with H & E method and were studied by light microscopy.
Morphometrical studying
Statistical analysis
The collected data from each group were analyzed by SPSS software with One Way ANOVA method.
Results
The results of this study are presented into two parts; morphology and morphometry.
I) morphology
The height of glandular epithelium was decreased in comparison with luminal epithelium. The stromal cellular compaction was low and decidual reaction was seen. Luminal surface of endometrium was seen in normal folding (figure 1.a).
2) Experimental group:
A) Sham group: In this group the luminal epithelium was columnar and luminal surface of the epithelium was irregular with folding. The height of glandular epithelium was decreased in comparison to luminal epithelium. The number of gland and secretion was similar to control group. Decidual reaction was higher than control group (figure 1.b), (Table I).
B) Progesterone group: The shape of epithelium was cuboidal and the height of luminal epithelium was decreased in comparison to control group. The nucleus was located in the base of cells and occupied the main portion of the cytoplasems. The apical border of cells was regular and stroma was seen compacted. Stromal desidulization was lower than control group and their intercellular spaces were narrow. The endometrial folding was not seen in this group (figure 1.c), (figure 2 .a).
C) Estrogen group: The form of epithelium was pseudostratified and the epithelial height was higher than control group. The glands mainly contained secretions .The cellularity of stroma was low and the intercellular spaces were increased and the decidual reaction was higher than control group. The endometrial folding was severe (figure 1.d).
D) E+P group: The shape of epithelium was columnar and the nucleus was located in the middle of cells. The height of epithelium was increased in comparison to control group.
Development of glands high and the majority of glands contained secretion. Cellularity of stroma was low and decidual reaction was higher than control group .The endometrial folding was severe (figure 1.e and 2.b).
E) RU 486+E group: The shape of epithelium was pseudostratified and the epithelial height was higher than control group. In comparison to control group, stroma in this group contained inflammatory and pyknotic cells that mainly were located in the subepithelium. The stromal thickness and decidual reaction were decreased in comparison to control group. The endometrial folding was moderate (figure 1.f), (Table I).
II) morphometry
Height of luminal epithelium
The height of luminal epithelium in the progesterone group was the lowest in comparison to other groups. The comparison of luminal epithelium in the control and sham groups showed that there was significant increase in the height of luminal epithelium in sham group. Comparison of luminal epithelium in the E, E+P and sham groups demonstrated that there were no significant differences among these groups, while they have shown significant differences in comparison to control group (Table II).The height of luminal epithelium in the RU 486 + E group was higher than other groups. The estrogen had a critical role in the increasing of height of the luminal epithelium.
Height of glandular epithelium
The lowest height of glandular epithelium was seen in the progesterone group. The comparison of the height of glandular epithelium in progesterone group with control group has not shown any differences, while the comparison of progesterone group has shown
significant differences with other groups.
Stromal thickness
The assessment of stromal thickness showed that the height of stroma in the sham group was the highest and the thickness in the RU 486+ E group was the lowest. This has suggested that the growth of stroma was dependent on both estrogen and progesterone. Assessment of stromal thickness in the progesterone group showed that there was significant reduction in comparison to control group. There were not significant differences between stromal thickness in E and in E + P groups but in comparison to control group this was significant (Table II).



The results of present study showed that ovarian hyperstimulation without hormone therapy induced the severe modifications in the luminal and glandular epithelium as well as stroma. They included increasing in the luminal epithelial height and stroma thickness. The modifications could be related to alterations in the concentration of hormones in serum. The data show that gonadotrophins administration increases the concentration of estrogen. The estrogen decreases the implantation rate in comparison to normal pregnancy (11).
Also the gonadotropins injections in the mouse increase the stromal thickness and vessel growth as well as development of glands at day 4.5 of pregnancy (20). These results confirm our results. In contrast to our findings, Beverley shows that ovarian hyperstimulation causes the reduction in the decidual reaction which is related to reduction
of vessel permeability (21). The shape of epithelial layer in the sham group was irregular and this is suggested that ovarian hyperstimulation induced the morphological alterations in the luminal part of uterine. Therefore, ovarian hyperstimulation without any luteal support hormones solely incapable to provide endometrial receptivity. The studies of Kramer and Foosum confirm our results that ovarian hyperstimulation causes the modification in the endometrium and decreases the implantation rate (11, 22).
The morphometrical data showed that using progesterone after ovarian hyperstimulation decreased the height of luminal and glandular epithelium and also decreased the stromal thickness. Also the morphological results indicated that epithelium in this group was cuboidal form. This form of epithelium was seen only in this group. Furthermore the cellularity of stroma was increased and stromal thickness was decreased. The progesterone reduced height of the luminal and glandular epithelium as well as decidual reaction. The morphological and morphometrical data showed that endometrium in the progesterone group was unsuitable for implantation in comparison to control group. Pervious investigations have confirmed our results (15, 16).
The assessment of morphological analysis in the glandular and luminal epithelium in the estrogen (E group) and estrogen + progesterone group (E+P group) revealed that in the E+P group the nucleus located in the middle part of cells but in the E group epithelium was pseudostratified. In addition the decidual reaction in the E+P group was higher than E group while the cellularity of stroma was reduced. In agreement whit our results Andrwas et al show that E has increased the height of epithelial cells and also has caused the proliferation of epithelial cells as well as increasing the number of glands. They show that estrogen has increased the glandular secretion and granulocyte infiltration in the stroma in the ovariectomized mouse (12).
The estrogen induces epithelial cell proliferation by a paracrine mechanism through estrogen receptor alpha (ERα) in the stromal cells. Estrogen connects to ER α in the stroma and stimulates the releasing of paracrine factors including Epidermal Growth Factor (EGF), and Insulin Like Growth Factor (IGF). The released factors increase the proliferation of epithelial cells (23). On the other hand, estrogen has induced the proliferation in the epithelium. E+P administration have increased the stromal cell proliferation and also has accelerated the decidual reaction in comparison to estrogen injection (24).
The obtained results from RU 486+E administration showed that the inflammatory and pyknotic cells were seen in the stroma. Moreover, the ablaition of progesterone associated with estrogen using showed that decidual reaction is dependent on both estrogen and progesterone hormones while the epithelial cells were affected by estrogen. Therefore, progesterone application requires for luteal phase.
Rotello et al showed that progesterone is necessary for the differentiation and growth of stroma (25). They concern the RU 486 in the ovariectomized rabbit with psudopregnancy cause the apoptosis in the stroma, whereas progesterone inhibits the apoptosis. It is confirmed that the decidual reaction in the presence of E +P is higher than P group (26).
The comparison of RU 486+E, P, and E+P groups indicated that the progesterone injection caused the antiproliferation in the luminal epithelium.The increase of E / P ratio in serum at the embryo transfer time indicates higher pregnancy rate and decreasing of the E / P ratio associates with lower pregnancy rate (9). Also E+P administration in the luteal phase in the IVF protocols increased the pregnancy rate (27).
The additional of E2 to P4 during luteal phase results in an increase in implantation and pregnancy rate .The pregnancy rate was dependent on dose of E2. Our morphometrical and morphological results confirm the clinical results of Krzysztof (28). However, Lewin does not find any advantages in the live birth and pregnancy rate when adding E2+P4 at luteal phase (29). Some researches show no difference in clinical pregnancy rate when P4 administration are compared with combination of P4 and E2 (30, 31). Morphological and morphometrical results of this study showed that P could not provide suitable condition of endometrium for implantation in comparison to other groups. Although in most studies, there are not any significant differences in success rates in administration of P, E+P, P+HCG and HCG alone (14). The data obtained from this study indicated that E+P supplied morphologically an appropriate endometrial condition for embryo implantation compare to P supplementation alone.
Conclusion
This study showed that progesterone application at luteal phase did not solely supply an appropriate endometrial condition for implantation. Addition of estrogen to progesterone provided an improved endometrial state to implantation. It was suggested that estrogen plus progesterone may be used instead of progesterone alone at luteal phase.




Discussion
The endometrium in the menstrual cycles undergoes modifications for blactocyste reception. Ovarian hormones (E2, P4) are the first hormones that are responsible for induction of endometrial receptivity (1). At endometrial receptivity state, endometrium undergoes some modifications that are not seen in the pre implantation state .These changes include: columnar shape in luminal epithelium and nucleus in the basal portion of cells. The shape of glandular epithelium and position of nucleus is similar to luminal epithelium. Stroma is affected by decidulal reaction and inflammation .Our results confirmed these modifications in normal group (19).The results of present study showed that ovarian hyperstimulation without hormone therapy induced the severe modifications in the luminal and glandular epithelium as well as stroma. They included increasing in the luminal epithelial height and stroma thickness. The modifications could be related to alterations in the concentration of hormones in serum. The data show that gonadotrophins administration increases the concentration of estrogen. The estrogen decreases the implantation rate in comparison to normal pregnancy (11).
Also the gonadotropins injections in the mouse increase the stromal thickness and vessel growth as well as development of glands at day 4.5 of pregnancy (20). These results confirm our results. In contrast to our findings, Beverley shows that ovarian hyperstimulation causes the reduction in the decidual reaction which is related to reduction
of vessel permeability (21). The shape of epithelial layer in the sham group was irregular and this is suggested that ovarian hyperstimulation induced the morphological alterations in the luminal part of uterine. Therefore, ovarian hyperstimulation without any luteal support hormones solely incapable to provide endometrial receptivity. The studies of Kramer and Foosum confirm our results that ovarian hyperstimulation causes the modification in the endometrium and decreases the implantation rate (11, 22).
The morphometrical data showed that using progesterone after ovarian hyperstimulation decreased the height of luminal and glandular epithelium and also decreased the stromal thickness. Also the morphological results indicated that epithelium in this group was cuboidal form. This form of epithelium was seen only in this group. Furthermore the cellularity of stroma was increased and stromal thickness was decreased. The progesterone reduced height of the luminal and glandular epithelium as well as decidual reaction. The morphological and morphometrical data showed that endometrium in the progesterone group was unsuitable for implantation in comparison to control group. Pervious investigations have confirmed our results (15, 16).
The assessment of morphological analysis in the glandular and luminal epithelium in the estrogen (E group) and estrogen + progesterone group (E+P group) revealed that in the E+P group the nucleus located in the middle part of cells but in the E group epithelium was pseudostratified. In addition the decidual reaction in the E+P group was higher than E group while the cellularity of stroma was reduced. In agreement whit our results Andrwas et al show that E has increased the height of epithelial cells and also has caused the proliferation of epithelial cells as well as increasing the number of glands. They show that estrogen has increased the glandular secretion and granulocyte infiltration in the stroma in the ovariectomized mouse (12).
The estrogen induces epithelial cell proliferation by a paracrine mechanism through estrogen receptor alpha (ERα) in the stromal cells. Estrogen connects to ER α in the stroma and stimulates the releasing of paracrine factors including Epidermal Growth Factor (EGF), and Insulin Like Growth Factor (IGF). The released factors increase the proliferation of epithelial cells (23). On the other hand, estrogen has induced the proliferation in the epithelium. E+P administration have increased the stromal cell proliferation and also has accelerated the decidual reaction in comparison to estrogen injection (24).
The obtained results from RU 486+E administration showed that the inflammatory and pyknotic cells were seen in the stroma. Moreover, the ablaition of progesterone associated with estrogen using showed that decidual reaction is dependent on both estrogen and progesterone hormones while the epithelial cells were affected by estrogen. Therefore, progesterone application requires for luteal phase.
Rotello et al showed that progesterone is necessary for the differentiation and growth of stroma (25). They concern the RU 486 in the ovariectomized rabbit with psudopregnancy cause the apoptosis in the stroma, whereas progesterone inhibits the apoptosis. It is confirmed that the decidual reaction in the presence of E +P is higher than P group (26).
The comparison of RU 486+E, P, and E+P groups indicated that the progesterone injection caused the antiproliferation in the luminal epithelium.The increase of E / P ratio in serum at the embryo transfer time indicates higher pregnancy rate and decreasing of the E / P ratio associates with lower pregnancy rate (9). Also E+P administration in the luteal phase in the IVF protocols increased the pregnancy rate (27).
The additional of E2 to P4 during luteal phase results in an increase in implantation and pregnancy rate .The pregnancy rate was dependent on dose of E2. Our morphometrical and morphological results confirm the clinical results of Krzysztof (28). However, Lewin does not find any advantages in the live birth and pregnancy rate when adding E2+P4 at luteal phase (29). Some researches show no difference in clinical pregnancy rate when P4 administration are compared with combination of P4 and E2 (30, 31). Morphological and morphometrical results of this study showed that P could not provide suitable condition of endometrium for implantation in comparison to other groups. Although in most studies, there are not any significant differences in success rates in administration of P, E+P, P+HCG and HCG alone (14). The data obtained from this study indicated that E+P supplied morphologically an appropriate endometrial condition for embryo implantation compare to P supplementation alone.
Conclusion
This study showed that progesterone application at luteal phase did not solely supply an appropriate endometrial condition for implantation. Addition of estrogen to progesterone provided an improved endometrial state to implantation. It was suggested that estrogen plus progesterone may be used instead of progesterone alone at luteal phase.
Type of Study: Original Article |
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




