Sat, Apr 27, 2024
[Archive]
Volume 6, Issue 5 (7-2008)
IJRM 2008, 6(5): 199-204 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Javed A, Rezaei-Zarchi S, Anvari M, Javeed Ghani M, Barzegari Firouzabadi F, Jamil A, et al . Determination of effective dosage of FSH and hCG in the maturation of preantral follicles and enclosed oocytes in mice. IJRM 2008; 6 (5) :199-204
URL: http://ijrm.ir/article-1-124-en.html
URL: http://ijrm.ir/article-1-124-en.html
Aisha Javed1 
 , Saeed Rezaei-Zarchi2
, Saeed Rezaei-Zarchi2 
 , Morteza Anvari1
, Morteza Anvari1 
 , Madiha Javeed Ghani3
, Madiha Javeed Ghani3 
 , Fatemeh Barzegari Firouzabadi2
, Fatemeh Barzegari Firouzabadi2 
 , Amer Jamil4
, Amer Jamil4 
 , Seyed Mehdi Kalantar *
, Seyed Mehdi Kalantar * 
 5, Habibollah Nazem2
5, Habibollah Nazem2 


 , Saeed Rezaei-Zarchi2
, Saeed Rezaei-Zarchi2 
 , Morteza Anvari1
, Morteza Anvari1 
 , Madiha Javeed Ghani3
, Madiha Javeed Ghani3 
 , Fatemeh Barzegari Firouzabadi2
, Fatemeh Barzegari Firouzabadi2 
 , Amer Jamil4
, Amer Jamil4 
 , Seyed Mehdi Kalantar *
, Seyed Mehdi Kalantar * 
 5, Habibollah Nazem2
5, Habibollah Nazem2 

1- Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2- Department of Biology, Payam-e-Noor University, Yazd, Iran
3- Department of Bioinformatics, Government College University, Faisalabad, Pakistan
4- Department of Biochemistry, University of Agriculture, Faisalabad, Pakistan
5- Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran , smkalantar@yahoo.com
2- Department of Biology, Payam-e-Noor University, Yazd, Iran
3- Department of Bioinformatics, Government College University, Faisalabad, Pakistan
4- Department of Biochemistry, University of Agriculture, Faisalabad, Pakistan
5- Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran , smkalantar@yahoo.com
Full-Text [PDF 152 kb]
(839 Downloads)
| Abstract (HTML) (2686 Views)
Full-Text: (438 Views)
Introduction
There was an increasing interest in in vitro maturation (IVM) as well as natural-cycle in in vitro fertilization (IVF) and minimal stimulation regimes in the past few years (1).
Although numerous studies on IVM of immature oocytes have been performed, the efficiency of current IVM techniques is still suboptimal in terms of the number of mature oocytes obtained, embryo developmental competence and implantation rates. Therefore, several attempts have been made to improve the viability of IVM oocytes by gonadotrophin stimulation (2). Chian et al (2000) reported good pregnancy rates by hCG priming (10,000 IU) before immature oocyte retrieval from women with polycystic ovary syndrome (PCOS) and this has been confirmed by some other groups (3,4). There are several studies evaluating the advantages of using hCG priming in IVM practices (1, 4). Research in this area of human biology is difficult to carry out but in vitro maturation of immature oocytes has been achieved in small mammals, using a number of methods. Reliable results have only been obtained using oocytes, derived from mouse, in their final stages of growth (5, 6). Follicle stimulating hormone (FSH) exists as a family of isohormones, which is composed of and ß-subunit with two possible N-linked glycosylation sites located on each of the two subunits. Pituitary FSH supports in vitro follicular growth (5). FSHß deficient female mice are infertile (7).
and ß-subunit with two possible N-linked glycosylation sites located on each of the two subunits. Pituitary FSH supports in vitro follicular growth (5). FSHß deficient female mice are infertile (7).
Furthermore, the presence of FSH induces the inhibition of apoptosis by granulosa cells in vivo and in vitro (8). Oocytes of several animal species undergo spontaneous maturation when they are removed from their follicles (9). hCG is given before oocyte retrieval in IVM, which appears to be useful (10).
In these cases, FSH may help in the timing of oocyte retrieval (11).The signal for maturation is mediated through the receptors for FSH and human chorionic gonadotrophin (hCG), which are situated on the granulosa cells surrounding the oocyte. The binding of different gonadotrophins to the receptor induces an intracellular rise in cyclic AMP levels (12). The present study was designed to evaluate the effect of hCG, in the presence or absence of FSH, on follicle maturation, ovulation capacity and its proper timing in Syrian mice oocytes.
Materials and methods
Chemicals and hormones
Tissue Culture Medium 199 (TCM199) was used as a culture medium for the in vitro growth and maturation of Syrian mice preantral follicles. FSH (HP Metrodin; Serono, Welwyn Garden City, UK) was prepared in un-supplemented culture medium and stored in 100ml aliquots at -20oC until used to produce final concentrations of 10-200 mIU/ml. Hundred IU hCG (Pregnyl; Organon, Oss, The Netherlands) was dissolved in 4 ml culture medium and the stock was stored at 4oC for up to one week until used for adding to the cultured follicles to a final concentration of 1.5 IU/ml. All other chemicals were of analytical grade or the highest quality commercially available.
Animal model and ovary collection
Female Syrian mice were housed and bred in Central Animal House of the Animal Biotechnology Laboratory, Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Animals were kept under controlled conditions, fed with water and food pellets ad libitum. In order to maintain stable biological rhythms, 12 hours of artificial light and 12 hours of darkness were provided. Six-weeks-old mice were used for the isolation of follicle-enclosed oocytes.
The mice were killed by cervical dislocation as described by Conti (2002) and Mahmoudi et al. (2005) (13, 14). The ovaries were removed aseptically and placed in Falcon plastic petri dishes filled at room temperature with the basal medium, which was TCM199 (HEPES buffered, GIBCO BRL, Tokyo, Japan), supplemented with sodium pyruvate (2 mM), glutamine (2 mM), penicillin G (75 μg/ml) and streptomycin (50 μg/ml) and overlaid with 75 µl light mineral oil (Sigma) at 25-30 oC (9). Follicles were cultured in an incubator at 37 °C, 92 % humidity and 5% CO2 in air.
Pilot study of FSH and hCG effect
Firstly, the preantral follicles were grown in the presence of 10, 25, 50, 75, 100, 150 and 200 mIU/ml FSH, in TCM199 under the above described conditions, for 6 days. Then, to check the effect of hCG on the ovulation and normal recovery of oocytes, 1.5 IU/ml of the gonadotrophin was added to the preantral follicles on day 8, which was counted as Day zero. Ovulation rate was also seen with and without the addition of 100 mIU/ml FSH, hCG and the combination of both in the separate experiments. The control group was cultured with the same conditions as for the experimental groups except for the addition of FSH, hCG or the combination of both of them. Follicles were cultured in the presence of 100 mIU/ml FSH and 1.5 IU/ml hCG along with other parameters (15, 16). Oocytes were examined after 8, 16, 24, 32, 40, 48 and 56 hours and percentage of normally ovulating oocytes was evaluated without disrupting the follicles. Measurements were the average of 6 identical experiments. The measurements were made using an inverted microscope after stripping off any cumulus cells but including the zona pellucida.
Statistical analysis
Every experiment contained 30 follicles with the initial diameter of 95 ± 5 µm. Follicle survival was determined and considered positive as long as the oocyte remained surrounded by the granulosa cells, attached to the culture dish during in vitro culture.
Premature release of oocytes, follicle degeneration and loss of growth was also determined during the experiment. All of the experiments were done via an inverted microscope with the Hoffmann contrast modulation system (IMT-2, Olympus Corp., Tokyo, Japan) (17, 18). Maximum and minimum lengths (diameter) of each follicle were also measured with inverted microscope equipped with a micrometer. The mean diameter of the follicle was calculated by averaging these two measurements. The influence of FSH, on the extent of oocyte maturation, germinal vesicle breakdown, increase in follicle diameter and survival rates, was compared with one way ANOVA. p<0.05 was considered to be statistically significant.
Results
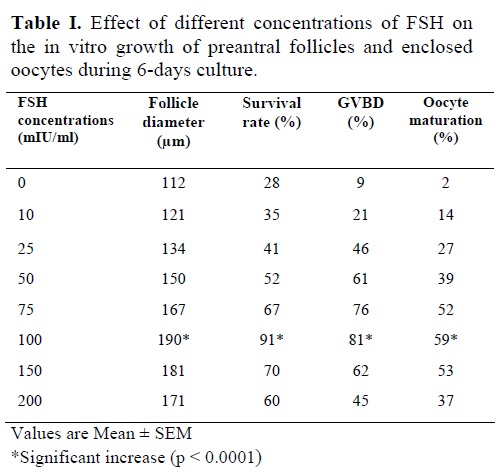
Morphological changes in preantral follicles of immature mice were studied during a culture period of 6 days in the presence of 10, 25, 50, 75, 100, 150 and 200 mIU/ml FSH (Table I). FSH concentrations of 10, 25, 50, 75, 150 and 200 mIU/ml did not show significant changes in follicle diameter, survival, GVBD and oocyte maturation rates as compared to control. On the contrary, 100 mIU/ml FSH showed a significant increase in follicular diameter (190 µm), survival rate (91%), GVBD (81%) and oocyte maturation (59%) rates as compared to control (p<0.0001).
A small number of follicles, ovulated later than 24 hours post hCG, had cumulus cells attached to their oocytes. However, 97% of the follicles that ovulated within 16 hours post hCG stimulus had attached mucified cumulus cells. A significantly higher number of follicles had mucified the cumulus cells, attached to the oocytes when ovulation started within 16-24 hours post hCG (97% and 80% respectively; p<0.0001), as shown in figure 1.
Successful ovulation failed to occur when the follicles were allowed to ovulate without hCG administration or more than 24 hours post hCG administration. Ovulation of 32 hours post hCG yielded approximately a half number (42%) of the oocytes attached to cumulus cells as compared to that carried out after 16-24 hours post hCG addition (p<0.0001).

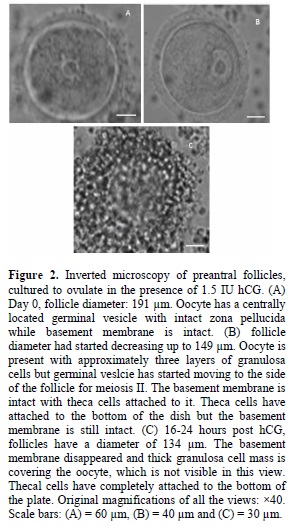
In this culture system, when appropriate conditions were applied, ovulation occurred with a high level of success. The cultured follicles initially became attached to the culture dish via theca cells, with granulosa cell proliferation causing rupture of the basement membrane and subsequent migration over the theca cells and a phenomenon analogous to ovulation occurred in response to hCG. Increased number of granulosa cells could be seen when ovulation was carried out for 16-24 hours. Large sized follicle and a healthy oocyte with a central visible germinal vesicle can be seen in figure 2. After this period, theca cells started attaching to the dish and the follicle size had started diminishing (~ 149 µm).
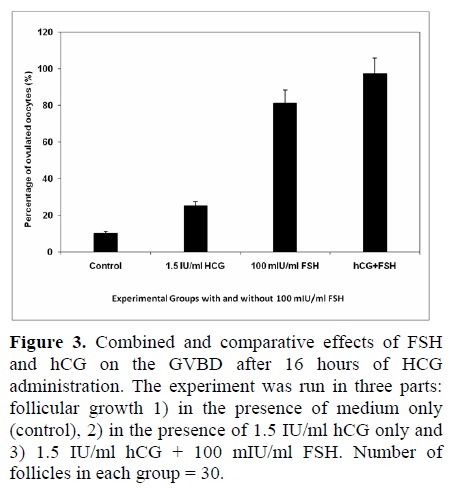
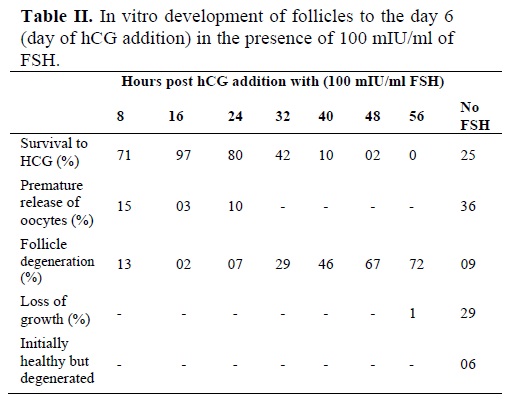
The effect of FSH-addition was seen on the ovulation of follicle-enclosed oocytes. As seen in figure 3, 100 mIU/ml FSH had a significant impact on the ovulation process when hCG was also administered. Table II shows the in vitro development of the follicles up to the optimum time of hCG addition. Only 25% of the cultured follicles could release their oocyte normally with a thick layer of granulosa cells while, a majority of the follicles (36%) prematurely released their oocyte between day 4 and day 9 in the cultures without FSH. Other follicles appeared degenerate (9%), did not grow (29%) or follicular cells were sparsely attached to the bottom of the dish (4%).
Some follicles initially appeared healthy but then degenerated (6%). While in the medium containing FSH and 1.5 IU hCG, the ovulation percentage reached a maximum of 97% as compared to that seen for FSH-containing medium only (81%) or in control experiment (10%). Therefore, the effect of FSH + hCG was highly significant over the control medium (p<0.0001).
Discussion
FSH is essential for the steroidogenesis by stimulating aromatase enzyme activity (P450 aromatase). FSH receptors appear on granulosa cells of preantral follicles and the follicles become gonadotropin dependent (19). Therefore, FSH is usually added to the in vitro culture medium of preantral follicules in mice and large mammals (20). Several studies have shown that FSH has potential of affecting the ovarian functions especially follicular development (21). In this study, we have shown that mouse isolated preantral follicles could undergo in vitro maturation; followed by successful ovulation. Gonadotropins are necessary for follicular cell proliferation and ovulation (22). Demeestre et al. demonstrated that isolated mouse preantral follicles cultured in a medium with FSH were able to support follicular growth and maturation (23). Our results showed that during in vitro maturation of isolated follicles, the diameter and the percentages of follicle survival, oocyte maturation and GVBD rates showed remarkable increase when 100 mIU/ml FSH was added. This may be due to some mechanisms including direct and indirect effects of FSH on the granulosa cells and oocyte (17, 21).
During the present experiments, we have characterized the response of mouse follicles during in vitro culture to different conditions in terms of their survival, ovulation efficiency and exact ovulation timing. Growth of Syrian mice pre-ovulatory follicles, from a preantral population with the diameter averaging ~95 ± 5 µm, took ~ 7 days to normally ovulate, as compared with ~12-14 days at 37 °C, 92% humidity and 5% CO2 in air. It seems that in vitro development may be accelerated as compared with the in vivo one, where small preantral follicles take ~16 days to become pre-ovulatory. Our optimal dose of FSH (100 mIU/ml) conforms to the results of Marilyn et al, which measured growth in cultured follicles over a 6 day period (24). While it is already known that the absence of FSH is incompatible with normal in vitro follicular growth (24), however, the excessive exposure to gonadotrophins (FSH and hCG) would result in receptor down-regulation, potentially leading to a suboptimal follicular response (25).
The significance of late ovulation is not known. Since in vivo ovulation occurs promptly (1), we thought that ovulation within 16 hours indicated good hCG responsiveness, and anything later to be potentially indicative of suboptimal follicular development. The proportion of follicles showing this delayed response varies with the culture conditions, as is evident from the above experiments, in which the timings and proper requirements of the cultured follicles, for ovulation, are indicated. Many late-ovulated oocytes had reduced or no cumulus surrounding, which indicates the loss of follicle/oocyte communication. Late ovulation is potentially associated with suboptimal growth or post-maturity of the oocyte or the follicle. Our data has shown that the oocyte cultures with ovulation period beyond or more than 16 hours post hCG, or devoid of sufficient concentration of FSH, showed a significantly lower survival rate, suggesting that ovulation timing and supplementation of the medium is an effective indicator of follicle viability and survival during in vitro culture. The response to hCG requires the presence of receptors on granulosa cells, which are known to be stimulated by the action of FSH, indicating the importance of appropriate priming. Weon-Young et al, (2008) has demonstrated that in programmed IVM cycles an extension of the interval between hCG administration and oocyte collection from 35 to 38 h results in a higher number of in vivo and in vitro matured oocytes (1). Furthermore, the presence of gonadotropins (FSH and hCG) induces the expression of inhibitor of apoptosis proteins (IAP) by GCs in vivo and in vitro (9). These intraovarian regulators mediate the effect of gonadotropins in regulating cellular interactions by autocrine and paracrine mechanisms (26).
Conclusion
We have demonstrated the ability of preantral follicles to grow, form antra and ovulate in vitro. The present study has revealed that the proper dose and administration timing of hCG has a considerable impact on the ovulation capacity of mice oocytes during the in vitro conditions. On the other hand, a combined administration of hCG and FSH increases the follicle survival and oocyte maturation as well as accelerating the follicles to grow and ovulate in vitro. The efficiency of these key markers for follicle function may be affected by gonadotrophic support and the culture conditions to which it is exposed.
Acknowledgements
Financial supports from the University of Agriculture, Faisalabad, Pakistan and, Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran are gratefully acknowledged.
There was an increasing interest in in vitro maturation (IVM) as well as natural-cycle in in vitro fertilization (IVF) and minimal stimulation regimes in the past few years (1).
Although numerous studies on IVM of immature oocytes have been performed, the efficiency of current IVM techniques is still suboptimal in terms of the number of mature oocytes obtained, embryo developmental competence and implantation rates. Therefore, several attempts have been made to improve the viability of IVM oocytes by gonadotrophin stimulation (2). Chian et al (2000) reported good pregnancy rates by hCG priming (10,000 IU) before immature oocyte retrieval from women with polycystic ovary syndrome (PCOS) and this has been confirmed by some other groups (3,4). There are several studies evaluating the advantages of using hCG priming in IVM practices (1, 4). Research in this area of human biology is difficult to carry out but in vitro maturation of immature oocytes has been achieved in small mammals, using a number of methods. Reliable results have only been obtained using oocytes, derived from mouse, in their final stages of growth (5, 6). Follicle stimulating hormone (FSH) exists as a family of isohormones, which is composed of
Furthermore, the presence of FSH induces the inhibition of apoptosis by granulosa cells in vivo and in vitro (8). Oocytes of several animal species undergo spontaneous maturation when they are removed from their follicles (9). hCG is given before oocyte retrieval in IVM, which appears to be useful (10).
In these cases, FSH may help in the timing of oocyte retrieval (11).The signal for maturation is mediated through the receptors for FSH and human chorionic gonadotrophin (hCG), which are situated on the granulosa cells surrounding the oocyte. The binding of different gonadotrophins to the receptor induces an intracellular rise in cyclic AMP levels (12). The present study was designed to evaluate the effect of hCG, in the presence or absence of FSH, on follicle maturation, ovulation capacity and its proper timing in Syrian mice oocytes.
Materials and methods
Chemicals and hormones
Tissue Culture Medium 199 (TCM199) was used as a culture medium for the in vitro growth and maturation of Syrian mice preantral follicles. FSH (HP Metrodin; Serono, Welwyn Garden City, UK) was prepared in un-supplemented culture medium and stored in 100ml aliquots at -20oC until used to produce final concentrations of 10-200 mIU/ml. Hundred IU hCG (Pregnyl; Organon, Oss, The Netherlands) was dissolved in 4 ml culture medium and the stock was stored at 4oC for up to one week until used for adding to the cultured follicles to a final concentration of 1.5 IU/ml. All other chemicals were of analytical grade or the highest quality commercially available.
Animal model and ovary collection
Female Syrian mice were housed and bred in Central Animal House of the Animal Biotechnology Laboratory, Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Animals were kept under controlled conditions, fed with water and food pellets ad libitum. In order to maintain stable biological rhythms, 12 hours of artificial light and 12 hours of darkness were provided. Six-weeks-old mice were used for the isolation of follicle-enclosed oocytes.
The mice were killed by cervical dislocation as described by Conti (2002) and Mahmoudi et al. (2005) (13, 14). The ovaries were removed aseptically and placed in Falcon plastic petri dishes filled at room temperature with the basal medium, which was TCM199 (HEPES buffered, GIBCO BRL, Tokyo, Japan), supplemented with sodium pyruvate (2 mM), glutamine (2 mM), penicillin G (75 μg/ml) and streptomycin (50 μg/ml) and overlaid with 75 µl light mineral oil (Sigma) at 25-30 oC (9). Follicles were cultured in an incubator at 37 °C, 92 % humidity and 5% CO2 in air.
Pilot study of FSH and hCG effect
Firstly, the preantral follicles were grown in the presence of 10, 25, 50, 75, 100, 150 and 200 mIU/ml FSH, in TCM199 under the above described conditions, for 6 days. Then, to check the effect of hCG on the ovulation and normal recovery of oocytes, 1.5 IU/ml of the gonadotrophin was added to the preantral follicles on day 8, which was counted as Day zero. Ovulation rate was also seen with and without the addition of 100 mIU/ml FSH, hCG and the combination of both in the separate experiments. The control group was cultured with the same conditions as for the experimental groups except for the addition of FSH, hCG or the combination of both of them. Follicles were cultured in the presence of 100 mIU/ml FSH and 1.5 IU/ml hCG along with other parameters (15, 16). Oocytes were examined after 8, 16, 24, 32, 40, 48 and 56 hours and percentage of normally ovulating oocytes was evaluated without disrupting the follicles. Measurements were the average of 6 identical experiments. The measurements were made using an inverted microscope after stripping off any cumulus cells but including the zona pellucida.
Statistical analysis
Every experiment contained 30 follicles with the initial diameter of 95 ± 5 µm. Follicle survival was determined and considered positive as long as the oocyte remained surrounded by the granulosa cells, attached to the culture dish during in vitro culture.
Premature release of oocytes, follicle degeneration and loss of growth was also determined during the experiment. All of the experiments were done via an inverted microscope with the Hoffmann contrast modulation system (IMT-2, Olympus Corp., Tokyo, Japan) (17, 18). Maximum and minimum lengths (diameter) of each follicle were also measured with inverted microscope equipped with a micrometer. The mean diameter of the follicle was calculated by averaging these two measurements. The influence of FSH, on the extent of oocyte maturation, germinal vesicle breakdown, increase in follicle diameter and survival rates, was compared with one way ANOVA. p<0.05 was considered to be statistically significant.
Results
Morphological changes in preantral follicles of immature mice were studied during a culture period of 6 days in the presence of 10, 25, 50, 75, 100, 150 and 200 mIU/ml FSH (Table I). FSH concentrations of 10, 25, 50, 75, 150 and 200 mIU/ml did not show significant changes in follicle diameter, survival, GVBD and oocyte maturation rates as compared to control. On the contrary, 100 mIU/ml FSH showed a significant increase in follicular diameter (190 µm), survival rate (91%), GVBD (81%) and oocyte maturation (59%) rates as compared to control (p<0.0001).

A small number of follicles, ovulated later than 24 hours post hCG, had cumulus cells attached to their oocytes. However, 97% of the follicles that ovulated within 16 hours post hCG stimulus had attached mucified cumulus cells. A significantly higher number of follicles had mucified the cumulus cells, attached to the oocytes when ovulation started within 16-24 hours post hCG (97% and 80% respectively; p<0.0001), as shown in figure 1.
Successful ovulation failed to occur when the follicles were allowed to ovulate without hCG administration or more than 24 hours post hCG administration. Ovulation of 32 hours post hCG yielded approximately a half number (42%) of the oocytes attached to cumulus cells as compared to that carried out after 16-24 hours post hCG addition (p<0.0001).

In this culture system, when appropriate conditions were applied, ovulation occurred with a high level of success. The cultured follicles initially became attached to the culture dish via theca cells, with granulosa cell proliferation causing rupture of the basement membrane and subsequent migration over the theca cells and a phenomenon analogous to ovulation occurred in response to hCG. Increased number of granulosa cells could be seen when ovulation was carried out for 16-24 hours. Large sized follicle and a healthy oocyte with a central visible germinal vesicle can be seen in figure 2. After this period, theca cells started attaching to the dish and the follicle size had started diminishing (~ 149 µm).


The effect of FSH-addition was seen on the ovulation of follicle-enclosed oocytes. As seen in figure 3, 100 mIU/ml FSH had a significant impact on the ovulation process when hCG was also administered. Table II shows the in vitro development of the follicles up to the optimum time of hCG addition. Only 25% of the cultured follicles could release their oocyte normally with a thick layer of granulosa cells while, a majority of the follicles (36%) prematurely released their oocyte between day 4 and day 9 in the cultures without FSH. Other follicles appeared degenerate (9%), did not grow (29%) or follicular cells were sparsely attached to the bottom of the dish (4%).
Some follicles initially appeared healthy but then degenerated (6%). While in the medium containing FSH and 1.5 IU hCG, the ovulation percentage reached a maximum of 97% as compared to that seen for FSH-containing medium only (81%) or in control experiment (10%). Therefore, the effect of FSH + hCG was highly significant over the control medium (p<0.0001).

Discussion
FSH is essential for the steroidogenesis by stimulating aromatase enzyme activity (P450 aromatase). FSH receptors appear on granulosa cells of preantral follicles and the follicles become gonadotropin dependent (19). Therefore, FSH is usually added to the in vitro culture medium of preantral follicules in mice and large mammals (20). Several studies have shown that FSH has potential of affecting the ovarian functions especially follicular development (21). In this study, we have shown that mouse isolated preantral follicles could undergo in vitro maturation; followed by successful ovulation. Gonadotropins are necessary for follicular cell proliferation and ovulation (22). Demeestre et al. demonstrated that isolated mouse preantral follicles cultured in a medium with FSH were able to support follicular growth and maturation (23). Our results showed that during in vitro maturation of isolated follicles, the diameter and the percentages of follicle survival, oocyte maturation and GVBD rates showed remarkable increase when 100 mIU/ml FSH was added. This may be due to some mechanisms including direct and indirect effects of FSH on the granulosa cells and oocyte (17, 21).
During the present experiments, we have characterized the response of mouse follicles during in vitro culture to different conditions in terms of their survival, ovulation efficiency and exact ovulation timing. Growth of Syrian mice pre-ovulatory follicles, from a preantral population with the diameter averaging ~95 ± 5 µm, took ~ 7 days to normally ovulate, as compared with ~12-14 days at 37 °C, 92% humidity and 5% CO2 in air. It seems that in vitro development may be accelerated as compared with the in vivo one, where small preantral follicles take ~16 days to become pre-ovulatory. Our optimal dose of FSH (100 mIU/ml) conforms to the results of Marilyn et al, which measured growth in cultured follicles over a 6 day period (24). While it is already known that the absence of FSH is incompatible with normal in vitro follicular growth (24), however, the excessive exposure to gonadotrophins (FSH and hCG) would result in receptor down-regulation, potentially leading to a suboptimal follicular response (25).
The significance of late ovulation is not known. Since in vivo ovulation occurs promptly (1), we thought that ovulation within 16 hours indicated good hCG responsiveness, and anything later to be potentially indicative of suboptimal follicular development. The proportion of follicles showing this delayed response varies with the culture conditions, as is evident from the above experiments, in which the timings and proper requirements of the cultured follicles, for ovulation, are indicated. Many late-ovulated oocytes had reduced or no cumulus surrounding, which indicates the loss of follicle/oocyte communication. Late ovulation is potentially associated with suboptimal growth or post-maturity of the oocyte or the follicle. Our data has shown that the oocyte cultures with ovulation period beyond or more than 16 hours post hCG, or devoid of sufficient concentration of FSH, showed a significantly lower survival rate, suggesting that ovulation timing and supplementation of the medium is an effective indicator of follicle viability and survival during in vitro culture. The response to hCG requires the presence of receptors on granulosa cells, which are known to be stimulated by the action of FSH, indicating the importance of appropriate priming. Weon-Young et al, (2008) has demonstrated that in programmed IVM cycles an extension of the interval between hCG administration and oocyte collection from 35 to 38 h results in a higher number of in vivo and in vitro matured oocytes (1). Furthermore, the presence of gonadotropins (FSH and hCG) induces the expression of inhibitor of apoptosis proteins (IAP) by GCs in vivo and in vitro (9). These intraovarian regulators mediate the effect of gonadotropins in regulating cellular interactions by autocrine and paracrine mechanisms (26).
Conclusion
We have demonstrated the ability of preantral follicles to grow, form antra and ovulate in vitro. The present study has revealed that the proper dose and administration timing of hCG has a considerable impact on the ovulation capacity of mice oocytes during the in vitro conditions. On the other hand, a combined administration of hCG and FSH increases the follicle survival and oocyte maturation as well as accelerating the follicles to grow and ovulate in vitro. The efficiency of these key markers for follicle function may be affected by gonadotrophic support and the culture conditions to which it is exposed.
Acknowledgements
Financial supports from the University of Agriculture, Faisalabad, Pakistan and, Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran are gratefully acknowledged.
Type of Study: Original Article |
References
1. Weon-Young S, Jin-Tae C, Ri-Cheng C, Belen H, Ezgi D, Shai E, et al. 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Human Reprod 2008; 23: 2010-2016. [DOI:10.1093/humrep/den210]
2. Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod 2000; 15: 165-170. [DOI:10.1093/humrep/15.1.165]
3. Lin YH, Hwang JL, Huang LW, Mu SC, Seow KM, Chung J, et al. Combination of FSH priming and hCG priming for in-vitro maturation of human oocytes. Hum Reprod 2003; 18: 1632-1636. [DOI:10.1093/humrep/deg335]
4. Son WY, Lee SY, Yoon SH, Lim JH. Pregnancies and deliveries after transfer of human blastocysts derived from in vitro matured oocytes in in vitro maturation cycles. Fertil Steril 2007; 87: 1491-1493. [DOI:10.1016/j.fertnstert.2006.11.027]
5. Smitz JEJ, Cortvrindt GR. The earliest stages of folliculogenesis in vitro. Reprod 2002; 123: 185-202. [DOI:10.1530/rep.0.1230185]
6. O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68: 1682-1686. [DOI:10.1095/biolreprod.102.013029]
7. Liu X, Andoh K, Mizunuma H, Kamijo T, Kikuchi N, Yamada K, et al. Effects of recombinant human FSH (rhFSH), urinary purified FSH (uFSH) and hMG on small preantral follicles and tertiary follicles from normal adult and androgen-sterilized female mice. Fertil Steril 2000; 73: 372-380. [DOI:10.1016/S0015-0282(99)00494-X]
8. Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinol 2000; 141: 1795-1803. [DOI:10.1210/endo.141.5.7456]
9. Wang Y, Rippstein PU, Tsang BK. Role and gonadotrophic regulation of X-linked inhibitor of apoptosis protein expression during rat ovarian follicular development in vitro. Biol Reprod 2003; 68: 610-619. [DOI:10.1095/biolreprod.102.007807]
10. Tan SL, Child TJ, Gulekli B. In vitro maturation and fertilization of oocytes from unstimulated ovaries: predicting the number of immature oocytes retrieved by early follicular phase ultrasonography. Am J Obstet Gynecol 2002; 186: 684-689. [DOI:10.1067/mob.2002.122146]
11. Son WY, Yoon SH, Lee SW, Ko Y, Yoon HG, Lim JH. Blastocyst development and pregnancies after IVF of mature oocytes retrieved from un-stimulated patients with PCOS after in-vivo HCG priming: case report. Hum Reprod 2002; 17: 134-136. [DOI:10.1093/humrep/17.1.134]
12. Mikkelsen AL, Lindenberg S. Influence of the dominant follicle on in-vitro maturation of human oocytes: a prospective non-randomized study. RBM Online 2001; 3: 199-204. [DOI:10.1016/S1472-6483(10)62036-6]
13. Conti M. Specificity of the cyclic adenosine 3', 5'-monophosphate signal in granulosa cell function. Biol Reprod 2002; 67: 1653-1661. [DOI:10.1095/biolreprod.102.004952]
14. Mahmoudi R, Subhani A, Pasbakhsh, Abolhasani F, Amiri I, Salehnia M, et al. The Effects of cumulus cells on in vitro maturation of mouse germinal vesicle stage oocytes. Ir J Reprod Med 2005; 3: 74-78.
15. Javed A, Jamil A, Rezaei-Zarchi S, Anvari M, Nazem H, Kalantar SM. An in vitro comparative study of follicle stimulating hormone (FSH) and activin A effects on the maturation of preantral follicle-enclosed oocytes from immature Syrian mice. Ir Biomed J 2008; 12: 85-92.
16. Javed A, Rezaei-Zarchi S, Jamil A, Kalantar SM. An in vitro comparative study of the effects of ascorbic acid and FSH on the maturation of Syrian mice follicles. Mid East Fretil Soc J 2008; 13: 135-142.
17. Mitchell LM, Kennedy CR, Hartshorne GM. Effects of varying gonadotrophin dose and timing on antrum formation and ovulation efficiency of mouse follicles in vitro. Hum Reprod 2002; 17: 1181-1188. [DOI:10.1093/humrep/17.5.1181]
18. Bishonga C, Takashi Y, Katagiri S, Nagano M, Ishikawa A. In vitro growth of ovarian peantral follicles and the capacity of their oocytes to develop to the blastocyst stage. J Vet Med Sci 2001; 63: 619-624. [DOI:10.1292/jvms.63.619]
19. Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reprod 2001; 121: 647-653. [DOI:10.1530/rep.0.1210647]
20. Mao J, Wu G, Smith MF, McCauley MC, Cantley TC, Prather RS, et al. Effects of culture medium, serum type, and various concentrations of follicle-stimulating hormone on porcine preantral follicular development and antrum formation in vitro. Biol Reprod 2002; 67: 1197-1203. [DOI:10.1095/biolreprod67.4.1197]
21. Demeestere I, Centner J, Gervy C, Englert Y, Delbaere A. Impact of various endocrine and paracrine factors on in vitro culture of preantral follicles in rodents. Reprod 2005; 130: 147-156. [DOI:10.1530/rep.1.00648]
22. Cecconi S, Rossi G, Coticchio G, Macchiarelli G, Borini A, Canipari R. Influence of thyroid hormone on mouse preantral follicle development in vitro. Fertil Steril 2004; 81: 919-924. [DOI:10.1016/j.fertnstert.2003.11.014]
23. Demeestere I, Gervy C, Centner J, Devreker F, Englert Y, Delbaere A. Effect of insulin-like growth factor-I during preantral follicular culture on steroidogenesis, in vitro oocyte maturation and embryo development in mice. Biol Reprod 2004; 70: 1664-1669. [DOI:10.1095/biolreprod.103.023317]
24. Marilyn JO, Janice KP, John J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68: 1682-1686. [DOI:10.1095/biolreprod.102.013029]
25. Hreinsson J, Bjoern R, Barbro F, Lev L, Ingvar E, Anne-Maria S, et al. Recombinant LH is equally effective as recombinant hCG in promoting oocyte maturation in a clinical in-vitro maturation programme: a randomized study. Human Reprod 2003; 18: 2131-2136. [DOI:10.1093/humrep/deg422]
26. Erickson GF, Shimasaki S. The physiology of folliculogenesis: the role of novel growth factors. Fertil Steril 2001; 76: 943-949. [DOI:10.1016/S0015-0282(01)02859-X]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


