Sat, Feb 21, 2026
[Archive]
Volume 22, Issue 12 (December 2024)
IJRM 2024, 22(12): 975-984 |
Back to browse issues page
Ethics code: IR.TUMS.DENTISTRY.REC.1402.041
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Gholizadeh N, Koopaie M, Aleyasin A, Mortazavi Milani A, Aghahosseini M, Kharrazifard M J et al . The role of salivary antioxidant level in polycystic ovary syndrome women under assisted reproductive technology treatment: A case-control study. IJRM 2024; 22 (12) :975-984
URL: http://ijrm.ir/article-1-3461-en.html
URL: http://ijrm.ir/article-1-3461-en.html
Narges Gholizadeh1 

 , Maryam Koopaie1
, Maryam Koopaie1 

 , Ashraf Aleyasin2
, Ashraf Aleyasin2 

 , Atousa Mortazavi Milani3
, Atousa Mortazavi Milani3 

 , Marziyeh Aghahosseini4
, Marziyeh Aghahosseini4 

 , Mohammad Javad Kharrazifard5
, Mohammad Javad Kharrazifard5 

 , Mohadeseh Bahmaee *6
, Mohadeseh Bahmaee *6 




 , Maryam Koopaie1
, Maryam Koopaie1 

 , Ashraf Aleyasin2
, Ashraf Aleyasin2 

 , Atousa Mortazavi Milani3
, Atousa Mortazavi Milani3 

 , Marziyeh Aghahosseini4
, Marziyeh Aghahosseini4 

 , Mohammad Javad Kharrazifard5
, Mohammad Javad Kharrazifard5 

 , Mohadeseh Bahmaee *6
, Mohadeseh Bahmaee *6 


1- Department of Oral and Maxillofacial Medicine, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Infertility, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Clinical Research Development Unit of Imam Khomeini Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Obstetrics and Gynecology and Female Infertility Unit, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Epidemiology and Biostatistics, Faculty of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
6- School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran. ,md.bahmaee@yahoo.com
2- Department of Infertility, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran.
3- Clinical Research Development Unit of Imam Khomeini Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Obstetrics and Gynecology and Female Infertility Unit, Tehran University of Medical Sciences, Tehran, Iran.
5- Department of Epidemiology and Biostatistics, Faculty of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
6- School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran. ,
Abstract: (910 Views)
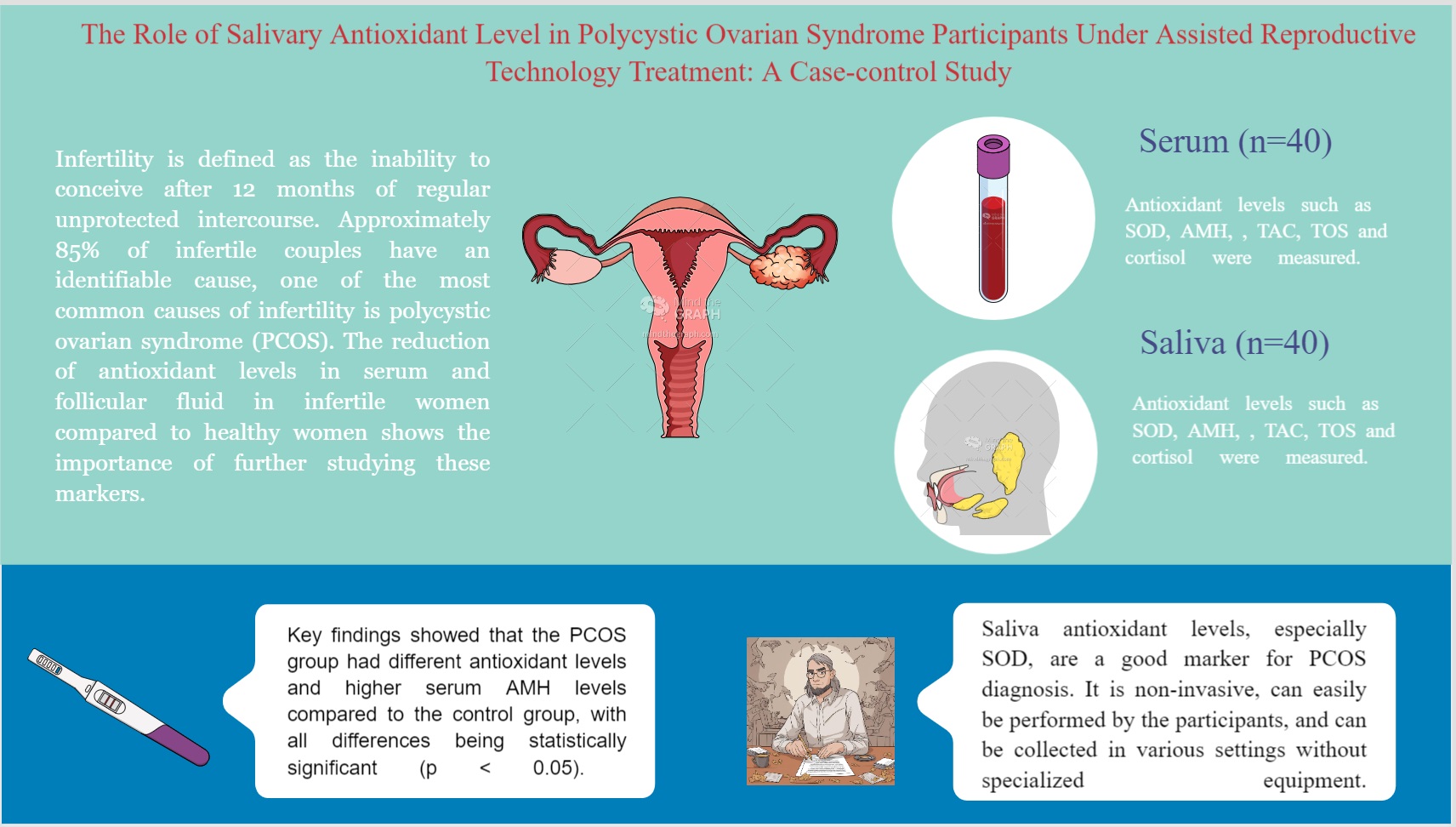
Background: Infertility is defined as the inability to conceive after 12 months of regular unprotected intercourse. Approximately 85% of infertile couples have an identifiable cause, one of the most common causes of infertility is polycystic ovary syndrome (PCOS). The reduction of antioxidant levels in serum and follicular fluid in infertile women compared to healthy women shows the importance of further studying these markers.
Objective: To study salivary and serum antioxidant levels in PCOS participants under assisted reproductive technology.
Materials and Methods: This case-control study was conducted on 80 women in 2 groups including normal participants as control and PCOS groups (n = 40/each). Serum and salivary antioxidant levels such as saliva superoxide dismutase (SOD), saliva anti-Müllerian hormone (AMH), serum SOD, serum total oxidant status, and serum AMH were measured.
Results: The average age of participants was 31.6 ± 5.4 yr. In both the saliva and serum, antioxidant levels differed significantly between the PCOS and control groups. Key findings showed that the PCOS group had different antioxidant levels and higher serum AMH levels compared to the control group, with all differences being statistically significant (p < 0.05).
Conclusion: Our finding underscored that saliva antioxidant levels, especially SOD, are a good marker for PCOS diagnosis. It is noninvasive, can easily be performed by the participants, and can be collected in various settings without specialized equipment.
Objective: To study salivary and serum antioxidant levels in PCOS participants under assisted reproductive technology.
Materials and Methods: This case-control study was conducted on 80 women in 2 groups including normal participants as control and PCOS groups (n = 40/each). Serum and salivary antioxidant levels such as saliva superoxide dismutase (SOD), saliva anti-Müllerian hormone (AMH), serum SOD, serum total oxidant status, and serum AMH were measured.
Results: The average age of participants was 31.6 ± 5.4 yr. In both the saliva and serum, antioxidant levels differed significantly between the PCOS and control groups. Key findings showed that the PCOS group had different antioxidant levels and higher serum AMH levels compared to the control group, with all differences being statistically significant (p < 0.05).
Conclusion: Our finding underscored that saliva antioxidant levels, especially SOD, are a good marker for PCOS diagnosis. It is noninvasive, can easily be performed by the participants, and can be collected in various settings without specialized equipment.
This article has been extracted from the D.D.S. Thesis. (Mohadeseh Bahmaee)
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
References
1. Carson SA, Kallen AN. Diagnosis and management of infertility: A review. JAMA 2021; 326: 65-76. [DOI:10.1001/jama.2021.4788] [PMID] [PMCID]
2. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010; 8: 41. [DOI:10.1186/1741-7015-8-41] [PMID] [PMCID]
3. Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016; 2: 16057. [DOI:10.1038/nrdp.2016.57] [PMID]
4. Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017; 102: 604-612.
5. Dokras A, Saini S, Gibson-Helm M, Schulkin J, Cooney L, Teede H. Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome. Fertil Steril 2017; 107: 1380-1386. [DOI:10.1016/j.fertnstert.2017.04.011] [PMID]
6. Teede H, Gibson-Helm M, Norman RJ, Boyle J. Polycystic ovary syndrome: Perceptions and attitudes of women and primary health care physicians on features of PCOS and renaming the syndrome. J Clin Endocrinol Metab 2014; 99: E107-E111. [DOI:10.1210/jc.2013-2978] [PMID]
7. Chang S, Dunaif A. Diagnosis of polycystic ovary syndrome: Which criteria to use and when? Endocrinol Metab Clin North Am 2021; 50: 11-23. [DOI:10.1016/j.ecl.2020.10.002] [PMID] [PMCID]
8. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev 2017; 2017: 8416763. [DOI:10.1155/2017/8416763] [PMID] [PMCID]
9. Christodoulakos G, Augoulea A, Lambrinoudaki I, Sioulas V, Creatsas G. Pathogenesis of endometriosis: The role of defective 'immunosurveillance'. Eur J Contracept Reprod Health Care 2007; 12: 194-202. [DOI:10.1080/13625180701387266] [PMID]
10. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol 2012; 10: 49. [DOI:10.1186/1477-7827-10-49] [PMID] [PMCID]
11. Mohammadi M. Oxidative stress and polycystic ovary syndrome: A brief review. Int J Prevent Med 2019; 10: 86. [DOI:10.4103/ijpvm.IJPVM_576_17] [PMID] [PMCID]
12. Rudnicka E, Duszewska AM, Kucharski M, Tyczyński P, Smolarczyk R. Oxidative stress and reproductive function: Oxidative stress in polycystic ovary syndrome. Reproduction 2022; 164: F145-154. [DOI:10.1530/REP-22-0152] [PMID]
13. Li W, Liu C, Yang Q, Zhou Y, Liu M, Shan H. Oxidative stress and antioxidant imbalance in ovulation disorder in patients with polycystic ovary syndrome. Front Nutr 2022; 9: 1018674. [DOI:10.3389/fnut.2022.1018674] [PMID] [PMCID]
14. Jamil M, Debbarh H, Aboulmaouahib S, Filali OA, Mounaji K, Zarqaoui M, et al. Reactive oxygen species in reproduction: Harmful, essential or both? Zygote 2020; 28: 255-269. [DOI:10.1017/S0967199420000179] [PMID]
15. Lin J, Wang L. Oxidative stress in oocytes and embryo development: Implications for in vitro systems. Antioxid Redox Signal 2021; 34: 1394-1406. [DOI:10.1089/ars.2020.8209] [PMID]
16. Venetis CA, Storr A, Chua SJ, Mol BW, Longobardi S, Yin X, et al. What is the optimal GnRH antagonist protocol for ovarian stimulation during ART treatment? A systematic review and network meta-analysis. Hum Reprod Update 2023; 29: 307-326. [DOI:10.1093/humupd/dmac040] [PMID] [PMCID]
17. van der Ham K, Laven JS, Tay CT, Mousa A, Teede H, Louwers YV. Anti-Müllerian hormone as a diagnostic biomarker for polycystic ovary syndrome (PCOS) and polycystic ovarian morphology (PCOM): A systematic review and meta-analysis. Fertil Steril 2024; 122: 727-739. [DOI:10.1016/j.fertnstert.2024.05.163] [PMID]
18. Vagios S, Sacha CR, Hammer KC, Dimitriadis I, James KE, Bormann CL, et al. Response to ovulation induction treatments in women with polycystic ovary syndrome as a function of serum anti-Müllerian hormone levels. J Assist Reprod Genet 2021; 38: 1827-1833. [DOI:10.1007/s10815-021-02217-x] [PMID] [PMCID]
19. Bizoń A, Tchórz A, Madej P, Leśniewski M, Wójtowicz M, Piwowar A, et al. The activity of superoxide dismutase, its relationship with the concentration of zinc and copper and the prevalence of rs2070424 superoxide dismutase gene in women with polycystic ovary syndrome- preliminary study. J Clin Med 2022; 11: 2548. [DOI:10.3390/jcm11092548] [PMID] [PMCID]
20. Talat A, Satyanarayana P, Anand P. Association of superoxide dismutase level in women with polycystic ovary syndrome. J Obstet Gynecol India 2022; 72: 6-12. [DOI:10.1007/s13224-021-01430-z] [PMID] [PMCID]
21. Pekel A, Gönenç A, Turhan NÖ, Kafalı H. Changes of sFas and sFasL, oxidative stress markers in serum and follicular fluid of patients undergoing IVF. J Assist Reprod Genet 2015; 32: 233-241. [DOI:10.1007/s10815-014-0396-8] [PMID] [PMCID]
22. Becatti M, Fucci R, Mannucci A, Barygina V, Mugnaini M, Criscuoli L, et al. A new biochemical approach to detect oxidative stress in infertile women undergoing assisted reproductive technology procedures. Int J Mol Sci 2018; 19: 592. [DOI:10.3390/ijms19020592] [PMID] [PMCID]
23. Miller N, Herzberger EH, Pasternak Y, Klement AH, Shavit T, Yaniv RT, et al. Does stress affect IVF outcomes? A prospective study of physiological and psychological stress in women undergoing IVF. Reprod Biomed Online 2019; 39: 93-101. [DOI:10.1016/j.rbmo.2019.01.012] [PMID]
24. Debbarh H, Louanjli N, Aboulmaouahib S, Jamil M, Ahbbas L, Kaarouch I, et al. Antioxidant activities and lipid peroxidation status in human follicular fluid: Age-dependent change. Zygote 2021; 29: 490-494. [DOI:10.1017/S0967199421000241] [PMID]
25. Fiers T, Dielen C, Somers S, Kaufman JM, Gerris J. Salivary estradiol as a surrogate marker for serum estradiol in assisted reproduction treatment. Clin Biochem 2017; 50: 145-149. [DOI:10.1016/j.clinbiochem.2016.09.016] [PMID]
26. Velthut A, Zilmer M, Zilmer K, Kaart T, Karro H, Salumets A. Elevated blood plasma antioxidant status is favourable for achieving IVF/ICSI pregnancy. Reprod Biomed Online 2013; 26: 345-352. [DOI:10.1016/j.rbmo.2012.12.012] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |