Sat, Feb 21, 2026
[Archive]
Volume 18, Issue 2 (February 2020)
IJRM 2020, 18(2): 105-112 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khadem Ghaebi N, Mahmoudiniya M, Najaf Najafi M, Zohdi E, Attaran M. Comparison of letrozole with gonadotropin-releasing hormone agonist in frozen embryo transfer after recurrent implantation failure: An RCT. IJRM 2020; 18 (2) :105-112
URL: http://ijrm.ir/article-1-1323-en.html
URL: http://ijrm.ir/article-1-1323-en.html
1- Department of Obstetrics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Imam Reza Clinical Research Units, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Obstetrics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. ,elnaz.zohdi61@gmail.com
2- Imam Reza Clinical Research Units, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Obstetrics, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. ,
Full-Text [PDF 402 kb]
(1262 Downloads)
| Abstract (HTML) (3714 Views)
Full-Text: (754 Views)
Introduction
Frozen embryo transfer (FET) has become a successful technique for in vitro fertilization (IVF). An important factor in the success in IVF is the proper synchronization between the fetus and endometrial growth (1-5). Previous studies have shown that endometrial preparation before FET was linked to better outcomes than was FET performed during the normal cycle (6, 7). The reason for the higher failure in FET in normal cycles might be the difficulty in identifying the optimal time for FET because of the irregularity in menstruation in some women and the difficulty in the assessment of the endometrial preparation (8). Therefore, different methods are used to prepare the endometrium for FET, including artificial-cycle FET (9-11).
A common method for artificial endometrium preparation is the use of estrogen and progesterone hormones with or without gonadotropin-releasing hormone (GnRH) agonist (9-11). This method (GnRH plus estrogen and progesterone) was shown to be effective especially in cases of previous IVF failures and may decrease the number of embryo transfer failures (9-11). An alternative to the use of GnRH agonists for endometrial induction is the use of letrozole (12). Letrozole is a third-generation aromatase inhibitor that does not have negative endometrial effects and preserves the normal response of the central feedback system for follicular growth and ovulation (12). In comparisons of the effect of letrozole and GnRH agonists on the FET outcome in infertile women, the findings have been controversial (7, 13, 14).
The aim of this study was to compare letrozole with combination therapy with GnRH agonist and estradiol valerate for endometrial induction in FET among infertile women with a history of FET failure.
Material and Methods
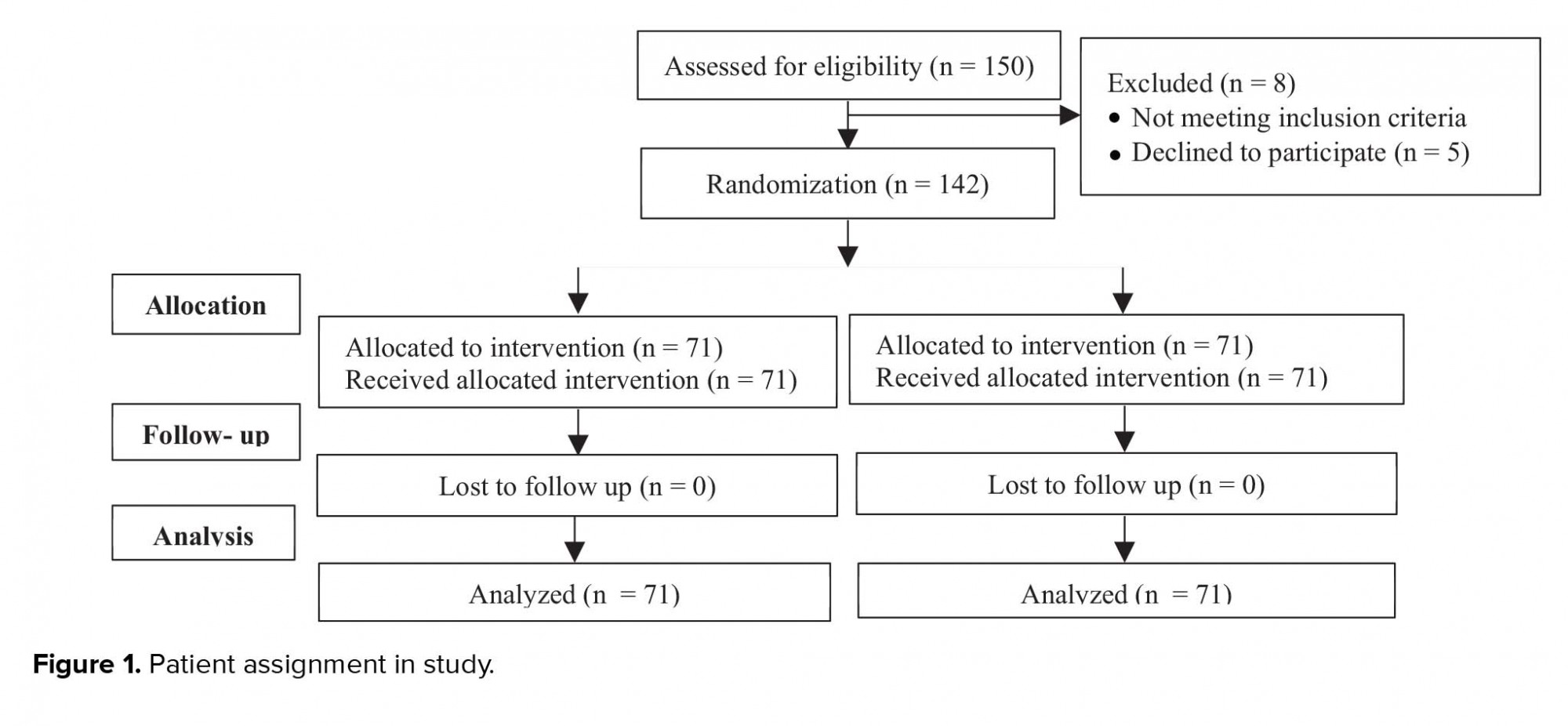
This randomized controlled trial was conducted in Milad Infertility Center, Mashhad, Iran, with 150 women who were referred for FET; 142 of them entered this study in January 2019 (Figure 1).
The inclusion criteria were willingness to participate in the study by giving written informed consent, age between 18 and 35 years, and a history of at least two cycles of FET failure. Women were excluded if severe infertility had been diagnosed in a spouse, if severe endometriosis (grade 3 or 4) were present, if a fibroma larger than 4 cm were present, or if the women were candidates for testicular sperm extraction for failure to otherwise produce a live embryo.
Demographic data were collected from patients’ medical records. Subjects were randomly divided into two groups based on simple random sampling method, where the first subject was randomly assigned to GnRH group and the consecutive subjects were assigned to the other group until the sample size was reached for each group. Subjects in the GnRH agonist group received 500 µg of buserelin daily subcutaneously from day 21 of the menstrual cycle. Estradiol was initiated at the time of cycle in the GnRH agonist group at a dose of 4 mg and increased to 8 mg if the endometrial thickness was less than 5 mm or the follicular diameter was less than 10 mm. Subjects underwent an ultrasound scan on day 3 of the next menstrual cycle. The first ovarian monitoring ultrasound examination was performed on day 12 of the menstruation cycle, at which point 600 mg of vaginal progesterone (Utrogestan; Besins Manufacturing Belgium, Brussels, Belgium) was administered. Subjects in the letrozole group received 2.5 mg of letrozole (Aburaihan Co., Tehran, Iran) daily, beginning on day 3 of the menstrual period, for 5 days. Subjects were then administered 75 IU of recombinant human follicle-stimulating hormone (CinnaGen Co., Tehran, Iran) subcutaneously starting on day 9 until the follicular size reached 18 mm. Then 10,000 IU of human chorionic gonadotropin (β-CG; Karma Pharmacology, Germany) was administered intramuscularly to induce ovulation; 2 days later, subjects began receiving progesterone, administered for 5 days at the dose of 100 mg/day to enhance formation of a blastocyst.
The quality of embryos was checked daily. Each 5-day old embryo was incubated in Blastocyst Medium (Origio, Måløv, Denmark). Embryos were defined as being of grade A quality if four blastomeres on day 2 or if seven or eight blastomeres were observed on day 3 with equal size and no more than 20% fragmentation, according to Gardner et al. criteria (15). Under ultrasound guidance, the Cook catheter (Cook Medical, Eight Mile Plains, Queensland, Australia) was used to transfer one or two embryos of the best quality. The most developed embryo was transferred in case no blastocyst existed on day 5. Serum β-hCG level was assessed 14 days after embryo transfer. Clinical pregnancy was defined as the observation of a gestational sac 6 weeks after embryo transfer. To calculate the implantation rate, the number of gestational sacs in all subjects was divided by the total number of transferred embryos. Continued pregnancy was defined as the presence of pregnancy until the 12th week of gestation.
Ethical consideration
The study protocol was approved by the Ethical Committee of the Mashhad University of Medical Sciences, Mashhad, Iran (Reg. no. IR.MUMS.MEDICAL.REC.1397.412). Subjects were approached by the researchers and received information regarding the aim and procedure of the study. Subjects who were willing to participate in the study were asked to sign an informed consent form.
Statistical analysis
Data were analyzed with SPSS software version 22 (IBM, Inc., Chicago, IL, USA). Continuous variables were checked for normality with the Shapiro-Wilk test. Normally distributed variables were calculated as means and standard deviations, whereas nonnormally distributed variables were calculated as medians with interquartile ranges. Categorical variables were calculated as frequencies and percentages. The distribution patterns of categorical variables of the groups were compared with the chi-square or Fisher’s exact test; while continuous variables were compared with the independent Student’s t test or the Mann-Whitney U test. The level of significance was defined as a p value smaller than 0.05.
Results
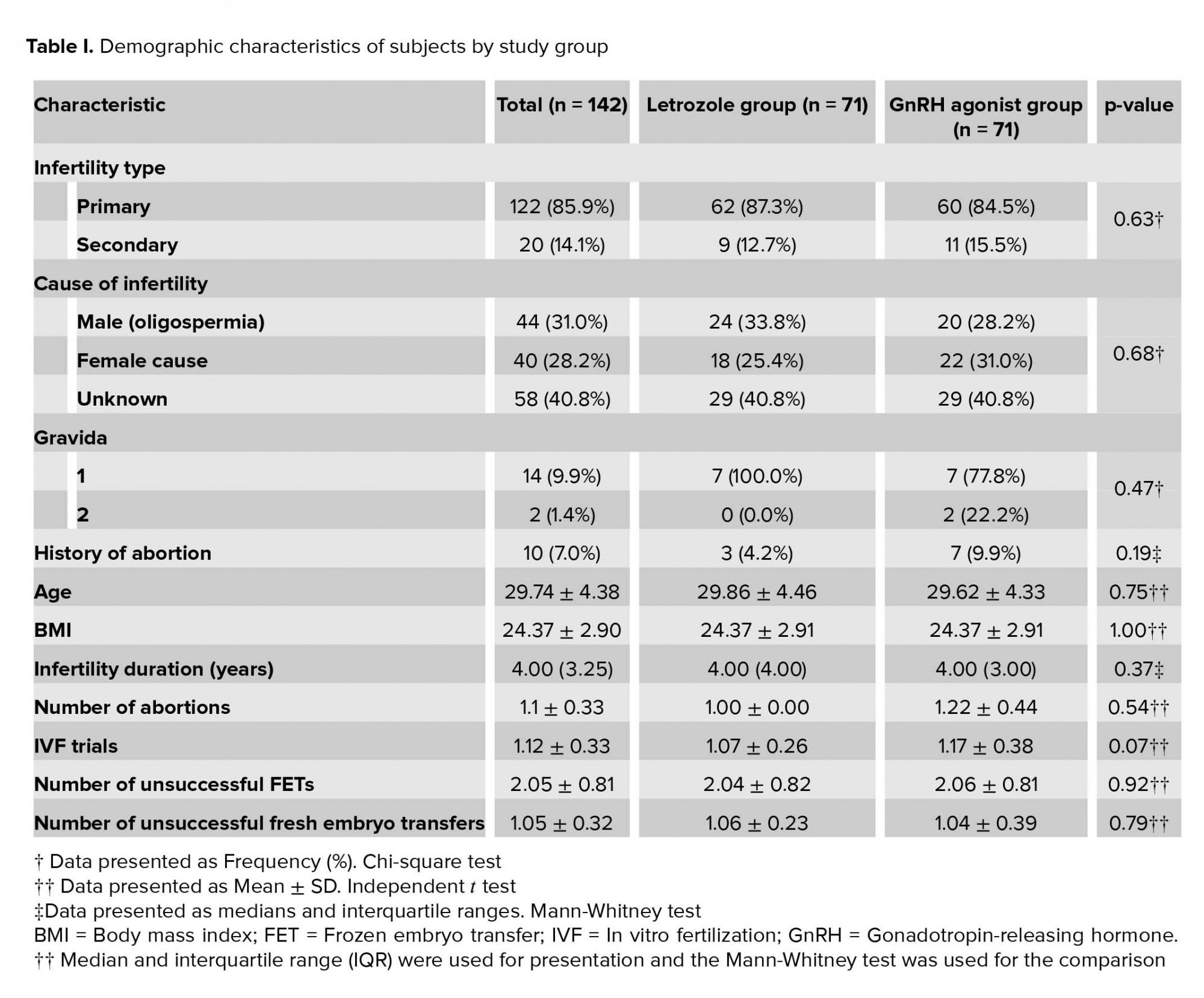
A total of 142 subjects participated in the study. The demographic characteristics of the subjects are listed in Table I. There was no significant difference in the distribution pattern of demographic characteristics between study groups. Of the subjects, 5 (3.5%) reported a history of using medications other than infertility medications, including levothyroxine in 4 (2.8%) and insulin in 1 (0.7%).
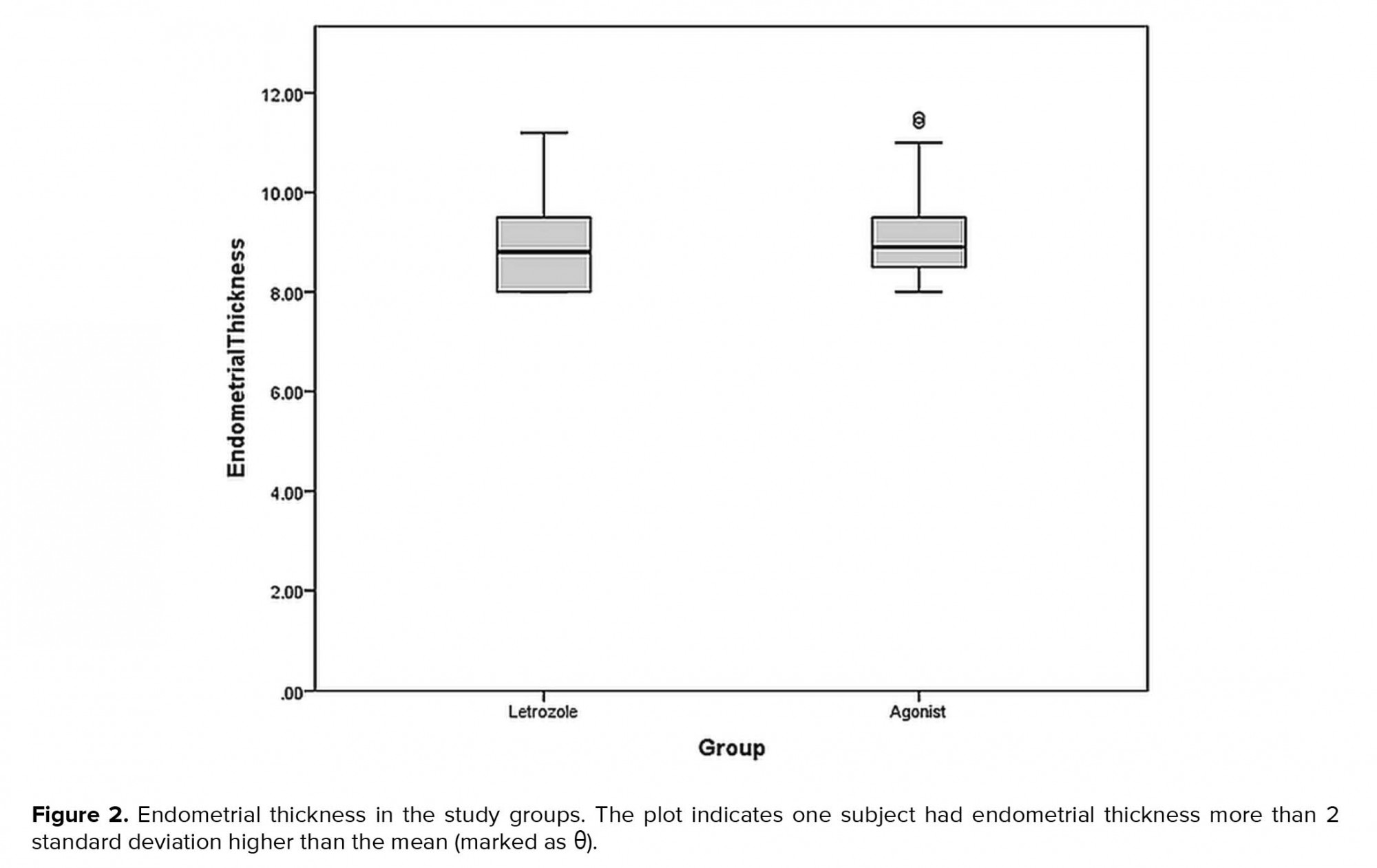
Mean endometrial thickness in the letrozole and GnRH agonist groups were 8.90±0.88 mm and 8.99±0.85 mm respectively. There was no significant difference in terms of endometrial thickens between study groups (p= 0.57) (Figure 2).
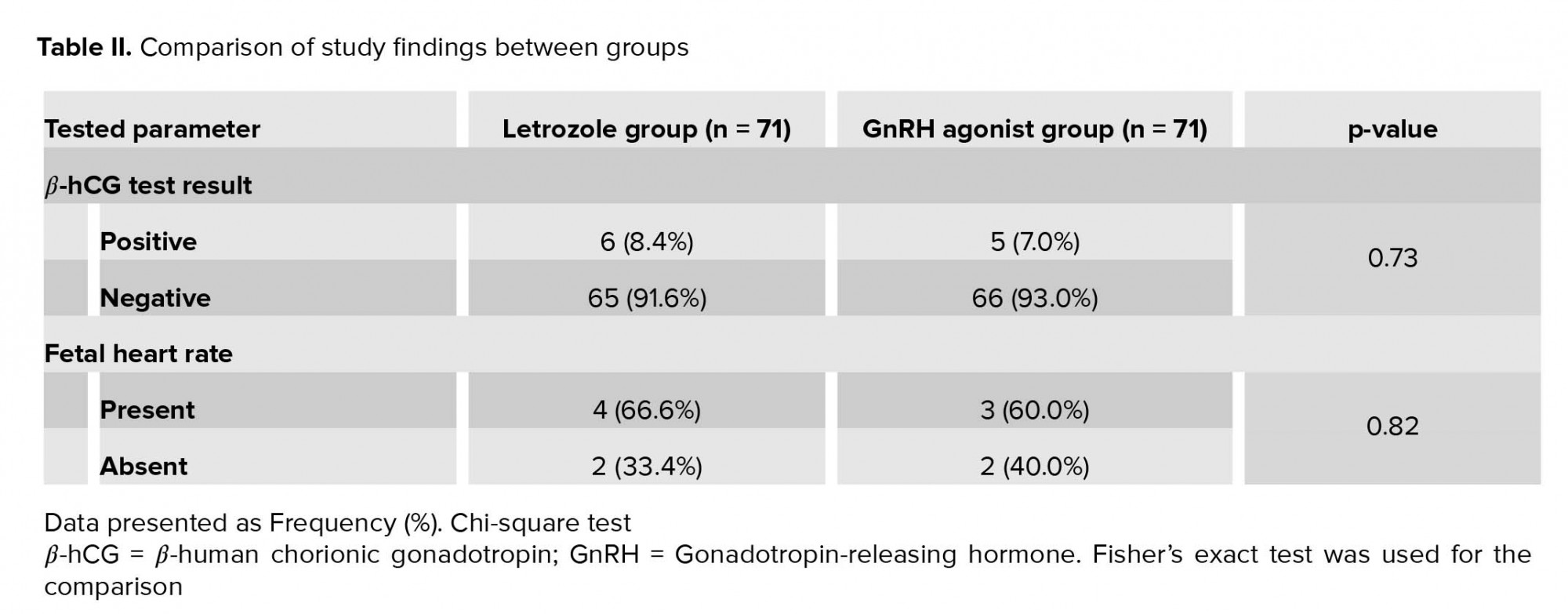
The prevalence of positive β-hCG test and detectable fetal heart rate were not significantly different between groups (Table II).
Discussion
The findings of this study revealed that the mean endometrial thicknesses did not significantly differ between the two groups; it was slightly higher in the GnRH agonist group (0.09 mm on average). Previous studies have yielded similar findings, which indicates that both preparation methods may increase endometrial thickness to a similar extent (7, 13). In a previous study, Huang and colleagues performed endometrial preparation by administering letrozole, followed by either GnRH agonist or β-hCG (13). They found no significant difference in endometrial thickness between patients receiving letrozole and those receiving GnRH agonist, although the endometrial thicknesses that they reported were higher than that observed in our study (10.77 mm in patients receiving letrozole and GnRH, 10.61 mm in those receiving letrozole and β-hCG) (13). The difference in the endometrial thickness between the two studies might have resulted from differences in the preparation method, inasmuch as both groups in Huang and colleagues’ study received letrozole, whereas in our study, letrozole was administrated to only one group. Aleyasin and coworkers observed no significant difference in endometrial thickness between patients receiving letrozole and those receiving GnRH group, and the mean endometrial thicknesses in their patients (8.08 mm in those receiving letrozole and in those receiving GnRH) were slightly lower than those observed in our study (7). It was previously shown that endometrial thickness and endometrial pattern were not associated with pregnancy outcome (16).
The rate of pregnancy achievement in this study was 8.4% in the letrozole group and 7.0% in the GnRH group. This pregnancy rate was lower than the 18.5% to 53.1% reported in previous studies (7, 10, 13, 17). The difference in the pregnancy rates between our study and the previous studies might have resulted from differences in the methodologies, study populations, duration of infertility, and comorbidities among study subjects. Furthermore, our study revealed no significant difference between the groups in rate of pregnancy achievement and FHR. This finding was in line with the findings of previous studies (7, 13, 18, 19). In a previous study, the pregnancy rate was significantly higher among patients receiving letrozole, but the rates of clinical pregnancy and pregnancy continuation did not differ significantly between the methods (20). Letrozole causes downregulation of estrogen production by inhibition of cytochrome P450, which in part results in increased secretion of follicle-stimulating hormone from the pituitary gland and in folliculogenesis (21). Letrozole also does not affect the central negative feedback of estrogen (21).
Our study revealed no significant difference in the rates of pregnancy and continuation of pregnancy between groups; however, because of the low pregnancy rate, the findings of this study may not apply to whole populations. Further studies with a larger sample size are needed in order to identify the pregnancy rate in FET cases in Iran.
Conclusion
Letrozole administration for endometrial preparation for FET might be as effective as GnRH administration.
Acknowledgments
The authors would like to thank the Milad infertility staffs for their kind support in conducting this research.
Conflicts of Interest
None
Frozen embryo transfer (FET) has become a successful technique for in vitro fertilization (IVF). An important factor in the success in IVF is the proper synchronization between the fetus and endometrial growth (1-5). Previous studies have shown that endometrial preparation before FET was linked to better outcomes than was FET performed during the normal cycle (6, 7). The reason for the higher failure in FET in normal cycles might be the difficulty in identifying the optimal time for FET because of the irregularity in menstruation in some women and the difficulty in the assessment of the endometrial preparation (8). Therefore, different methods are used to prepare the endometrium for FET, including artificial-cycle FET (9-11).
A common method for artificial endometrium preparation is the use of estrogen and progesterone hormones with or without gonadotropin-releasing hormone (GnRH) agonist (9-11). This method (GnRH plus estrogen and progesterone) was shown to be effective especially in cases of previous IVF failures and may decrease the number of embryo transfer failures (9-11). An alternative to the use of GnRH agonists for endometrial induction is the use of letrozole (12). Letrozole is a third-generation aromatase inhibitor that does not have negative endometrial effects and preserves the normal response of the central feedback system for follicular growth and ovulation (12). In comparisons of the effect of letrozole and GnRH agonists on the FET outcome in infertile women, the findings have been controversial (7, 13, 14).
The aim of this study was to compare letrozole with combination therapy with GnRH agonist and estradiol valerate for endometrial induction in FET among infertile women with a history of FET failure.
Material and Methods
This randomized controlled trial was conducted in Milad Infertility Center, Mashhad, Iran, with 150 women who were referred for FET; 142 of them entered this study in January 2019 (Figure 1).
The inclusion criteria were willingness to participate in the study by giving written informed consent, age between 18 and 35 years, and a history of at least two cycles of FET failure. Women were excluded if severe infertility had been diagnosed in a spouse, if severe endometriosis (grade 3 or 4) were present, if a fibroma larger than 4 cm were present, or if the women were candidates for testicular sperm extraction for failure to otherwise produce a live embryo.
Demographic data were collected from patients’ medical records. Subjects were randomly divided into two groups based on simple random sampling method, where the first subject was randomly assigned to GnRH group and the consecutive subjects were assigned to the other group until the sample size was reached for each group. Subjects in the GnRH agonist group received 500 µg of buserelin daily subcutaneously from day 21 of the menstrual cycle. Estradiol was initiated at the time of cycle in the GnRH agonist group at a dose of 4 mg and increased to 8 mg if the endometrial thickness was less than 5 mm or the follicular diameter was less than 10 mm. Subjects underwent an ultrasound scan on day 3 of the next menstrual cycle. The first ovarian monitoring ultrasound examination was performed on day 12 of the menstruation cycle, at which point 600 mg of vaginal progesterone (Utrogestan; Besins Manufacturing Belgium, Brussels, Belgium) was administered. Subjects in the letrozole group received 2.5 mg of letrozole (Aburaihan Co., Tehran, Iran) daily, beginning on day 3 of the menstrual period, for 5 days. Subjects were then administered 75 IU of recombinant human follicle-stimulating hormone (CinnaGen Co., Tehran, Iran) subcutaneously starting on day 9 until the follicular size reached 18 mm. Then 10,000 IU of human chorionic gonadotropin (β-CG; Karma Pharmacology, Germany) was administered intramuscularly to induce ovulation; 2 days later, subjects began receiving progesterone, administered for 5 days at the dose of 100 mg/day to enhance formation of a blastocyst.
The quality of embryos was checked daily. Each 5-day old embryo was incubated in Blastocyst Medium (Origio, Måløv, Denmark). Embryos were defined as being of grade A quality if four blastomeres on day 2 or if seven or eight blastomeres were observed on day 3 with equal size and no more than 20% fragmentation, according to Gardner et al. criteria (15). Under ultrasound guidance, the Cook catheter (Cook Medical, Eight Mile Plains, Queensland, Australia) was used to transfer one or two embryos of the best quality. The most developed embryo was transferred in case no blastocyst existed on day 5. Serum β-hCG level was assessed 14 days after embryo transfer. Clinical pregnancy was defined as the observation of a gestational sac 6 weeks after embryo transfer. To calculate the implantation rate, the number of gestational sacs in all subjects was divided by the total number of transferred embryos. Continued pregnancy was defined as the presence of pregnancy until the 12th week of gestation.

Ethical consideration
The study protocol was approved by the Ethical Committee of the Mashhad University of Medical Sciences, Mashhad, Iran (Reg. no. IR.MUMS.MEDICAL.REC.1397.412). Subjects were approached by the researchers and received information regarding the aim and procedure of the study. Subjects who were willing to participate in the study were asked to sign an informed consent form.
Statistical analysis
Data were analyzed with SPSS software version 22 (IBM, Inc., Chicago, IL, USA). Continuous variables were checked for normality with the Shapiro-Wilk test. Normally distributed variables were calculated as means and standard deviations, whereas nonnormally distributed variables were calculated as medians with interquartile ranges. Categorical variables were calculated as frequencies and percentages. The distribution patterns of categorical variables of the groups were compared with the chi-square or Fisher’s exact test; while continuous variables were compared with the independent Student’s t test or the Mann-Whitney U test. The level of significance was defined as a p value smaller than 0.05.
Results
A total of 142 subjects participated in the study. The demographic characteristics of the subjects are listed in Table I. There was no significant difference in the distribution pattern of demographic characteristics between study groups. Of the subjects, 5 (3.5%) reported a history of using medications other than infertility medications, including levothyroxine in 4 (2.8%) and insulin in 1 (0.7%).
Mean endometrial thickness in the letrozole and GnRH agonist groups were 8.90±0.88 mm and 8.99±0.85 mm respectively. There was no significant difference in terms of endometrial thickens between study groups (p= 0.57) (Figure 2).
The prevalence of positive β-hCG test and detectable fetal heart rate were not significantly different between groups (Table II).



Discussion
The findings of this study revealed that the mean endometrial thicknesses did not significantly differ between the two groups; it was slightly higher in the GnRH agonist group (0.09 mm on average). Previous studies have yielded similar findings, which indicates that both preparation methods may increase endometrial thickness to a similar extent (7, 13). In a previous study, Huang and colleagues performed endometrial preparation by administering letrozole, followed by either GnRH agonist or β-hCG (13). They found no significant difference in endometrial thickness between patients receiving letrozole and those receiving GnRH agonist, although the endometrial thicknesses that they reported were higher than that observed in our study (10.77 mm in patients receiving letrozole and GnRH, 10.61 mm in those receiving letrozole and β-hCG) (13). The difference in the endometrial thickness between the two studies might have resulted from differences in the preparation method, inasmuch as both groups in Huang and colleagues’ study received letrozole, whereas in our study, letrozole was administrated to only one group. Aleyasin and coworkers observed no significant difference in endometrial thickness between patients receiving letrozole and those receiving GnRH group, and the mean endometrial thicknesses in their patients (8.08 mm in those receiving letrozole and in those receiving GnRH) were slightly lower than those observed in our study (7). It was previously shown that endometrial thickness and endometrial pattern were not associated with pregnancy outcome (16).
The rate of pregnancy achievement in this study was 8.4% in the letrozole group and 7.0% in the GnRH group. This pregnancy rate was lower than the 18.5% to 53.1% reported in previous studies (7, 10, 13, 17). The difference in the pregnancy rates between our study and the previous studies might have resulted from differences in the methodologies, study populations, duration of infertility, and comorbidities among study subjects. Furthermore, our study revealed no significant difference between the groups in rate of pregnancy achievement and FHR. This finding was in line with the findings of previous studies (7, 13, 18, 19). In a previous study, the pregnancy rate was significantly higher among patients receiving letrozole, but the rates of clinical pregnancy and pregnancy continuation did not differ significantly between the methods (20). Letrozole causes downregulation of estrogen production by inhibition of cytochrome P450, which in part results in increased secretion of follicle-stimulating hormone from the pituitary gland and in folliculogenesis (21). Letrozole also does not affect the central negative feedback of estrogen (21).
Our study revealed no significant difference in the rates of pregnancy and continuation of pregnancy between groups; however, because of the low pregnancy rate, the findings of this study may not apply to whole populations. Further studies with a larger sample size are needed in order to identify the pregnancy rate in FET cases in Iran.
Conclusion
Letrozole administration for endometrial preparation for FET might be as effective as GnRH administration.
Acknowledgments
The authors would like to thank the Milad infertility staffs for their kind support in conducting this research.
Conflicts of Interest
None
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Aflatoonian A, Asgharnia M. Factors affecting the successful embryo transfer. Iran J Reprod Med 2006; 4: 45-50.
2. Nikzad H, Haddad Kashani H, Kabir-Salmani M, Akimoto Y, Iwashita M. Expression of galectin-8 on human endometrium: Molecular and cellular aspects. Iran J Reprod Med 2013; 11: 65-70.
3. Wirleitner B, Schuff M, Stecher A, Murtinger M, Vanderzwalmen P. Pregnancy and birth outcomes following fresh or vitrified embryo transfer according to blastocyst morphology and expansion stage, and culturing strategy for delayed development. Hum Reprod 2016; 31: 1685-1695. [DOI:10.1093/humrep/dew127] [PMID]
4. Gomaa H, Casper RF, Esfandiari N, Bentov Y. Non-synchronized endometrium and its correction in non-ovulatory cryopreserved embryo transfer cycles. Reprod Biomed Online 2015; 30: 378-384. [DOI:10.1016/j.rbmo.2014.12.005] [PMID]
5. Aflatoonian A, Mansoori Moghaddam F, Mashayekhy M, Mohamadian F. Comparison of early pregnancy and neonatal outcomes after frozen and fresh embryo transfer in ART cycles. J Assist Reprod Genet 2010; 27: 695-700. [DOI:10.1007/s10815-010-9470-z] [PMID] [PMCID]
6. Cerrillo M, Herrero L, Guillén A, Mayoral M, García-Velasco JA. Impact of endometrial preparation protocols for frozen embryo transfer on live birth rates. Rambam Maimonides Med J 2017; 8: doi: 10.5041/RMMJ.10297. [DOI:10.5041/RMMJ.10297] [PMID] [PMCID]
7. Aleyasin A, Aghahosseini M, Safdarian L, Noorzadeh M, Fallahi P, Rezaeian Z, et al. Can letrozole plus HMG protocol improve pregnancy outcomes in frozen-thawed embryo transfer? An RCT. Int J Reprod Biomed 2017; 15: 83-86. [DOI:10.29252/ijrm.15.2.83]
8. Yarali H, Polat M, Mumusoglu S, Yarali I, Bozdag G. Preparation of endometrium for frozen embryo replacement cycles: a systematic review and meta-analysis. J Assist Reprod Genet 2016; 33: 1287-1304. [DOI:10.1007/s10815-016-0787-0] [PMID] [PMCID]
9. Li H, Qiao J, Chi HB, Chen XN, Liu P, Ma CH. Comparison of the major malformation rate of children conceived from cryopreserved embryos and fresh embryos. Chin Med J 2010; 123: 1893-1897.
10. Basirat Z, Adib Rad H, Esmailzadeh S, Jorsaraei SG, Hajian-Tilaki K, Pasha H, et al. Comparison of pregnancy rate between fresh embryo transfers and frozen-thawed embryo transfers following ICSI treatment. Int J Reprod Biomed 2016; 14: 39-46. [DOI:10.29252/ijrm.14.1.39]
11. Aflatoonian A, Karimzadeh Maybodi MA, Aflatoonian N, Tabibnejad N, Amir-Arjmand MH, Soleimani M, et al. Perinatal outcome in fresh versus frozen embryo transfer in ART cycles. Int J Reprod Biomed 2016; 14: 167-172. [DOI:10.29252/ijrm.14.3.167]
12. Tatsumi T, Jwa SC, Kuwahara A, Irahara M, Kubota T, Saito H. Pregnancy and neonatal outcomes following letrozole use in frozen-thawed single embryo transfer cycles. Hum Reprod 2017; 32: 1244-1248. [DOI:10.1093/humrep/dex066] [PMID]
13. Huang PX, Wei JH, Wei LH. Gonadotropin-releasing hormone agonist for oocyte triggering in endometrial preparation of letrozole stimulation protocols does not affect clinical outcome of frozen-thawed embryo transfer. Int J Clin Exp Med 2015; 8: 19072-19078.
14. Dahan MH, Tannus S, Tan S. A comparison of pre-treatment with and without GnRH-agonist or letrozole in women with 2 failed embryo transfers undergoing a frozen cycle and no evidence of endometriosis. Fertil Steril 2018; 110 (Suppl.): e190-e191. [DOI:10.1016/j.fertnstert.2018.07.557]
15. Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 2000; 73: 1155-1158. [DOI:10.1016/S0015-0282(00)00518-5]
16. Gingold JA, Lee JA, Rodriguez-Purata J, Whitehouse MC, Sandler B, Grunfeld L, et al. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril 2015; 104: 620-628. e5. [DOI:10.1016/j.fertnstert.2015.05.036] [PMID] [PMCID]
17. Seikkula J, Ahinko K, Polo-Kantola P, Anttila L, Hurme S, Tinkanen H, et al. Mid-luteal phase gonadotropin-releasing hormone agonist support in frozen-thawed embryo transfers during artificial cycles: A prospective interventional pilot study. J Gynecol Obstet Hum Reprod 2018; 47: 391-395. [DOI:10.1016/j.jogoh.2018.04.009] [PMID]
18. Khayat S, Elliott B, Dahan MH. Management of recurrent implantation failure by gonadotropin-releasing hormone agonist and aromatase inhibitor suppression, in women without evidence of endometriosis. Gynecol Endocrinol 2019; 350: 267-270. [DOI:10.1080/09513590.2018.1519790] [PMID]
19. Konc J, Kanyo K, Varga E, Kriston R, Cseh S. The effect of cycle regimen used for endometrium preparation on the outcome of day 3 frozen embryo transfer cycle. Fertil Steril 2010; 94: 767-768. [DOI:10.1016/j.fertnstert.2009.09.053] [PMID]
20. Sibai H, El Husseiny A, Elgindy E. Letrozole versus artificial hormonal endometrial preparation for vitrified-warmed embryos transfer cycles. Middle East Fertility Society Journal; 21: 96-100. [DOI:10.1016/j.mefs.2015.09.002]
21. Franik S, Kremer JA, Nelen WL, Farquhar C. Aromatase inhibitors for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev 2014; 2: CD010287. [DOI:10.1002/14651858.CD010287.pub2]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








