Wed, Jan 28, 2026
[Archive]
Volume 18, Issue 7 (July 2020)
IJRM 2020, 18(7): 501-508 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Charostad J, Mokhtari-Azad T, Yavarian J, Ghavami N, Seyed Khorrami S M, Behboodi E, et al . Detection of human herpes viruses 1-5 in miscarriage: A case-control study. IJRM 2020; 18 (7) :501-508
URL: http://ijrm.ir/article-1-1339-en.html
URL: http://ijrm.ir/article-1-1339-en.html
Javad Charostad1 

 , Talat Mokhtari-Azad1
, Talat Mokhtari-Azad1 

 , Jila Yavarian1
, Jila Yavarian1 

 , Nastaran Ghavami1
, Nastaran Ghavami1 

 , Seyed Mahmood Seyed Khorrami2
, Seyed Mahmood Seyed Khorrami2 

 , Emad Behboodi1
, Emad Behboodi1 

 , Somayeh Jalilvand1
, Somayeh Jalilvand1 

 , Somayeh Shatizadeh Malekshahi2
, Somayeh Shatizadeh Malekshahi2 

 , Nazanin-Zahra Shafiei-Jandaghi *3
, Nazanin-Zahra Shafiei-Jandaghi *3 




 , Talat Mokhtari-Azad1
, Talat Mokhtari-Azad1 

 , Jila Yavarian1
, Jila Yavarian1 

 , Nastaran Ghavami1
, Nastaran Ghavami1 

 , Seyed Mahmood Seyed Khorrami2
, Seyed Mahmood Seyed Khorrami2 

 , Emad Behboodi1
, Emad Behboodi1 

 , Somayeh Jalilvand1
, Somayeh Jalilvand1 

 , Somayeh Shatizadeh Malekshahi2
, Somayeh Shatizadeh Malekshahi2 

 , Nazanin-Zahra Shafiei-Jandaghi *3
, Nazanin-Zahra Shafiei-Jandaghi *3 


1- Virology Department, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Virology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
3- Virology Department, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. ,nz-shafiei@tums.ac.ir
2- Department of Virology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
3- Virology Department, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. ,
Full-Text [PDF 269 kb]
(1311 Downloads)
| Abstract (HTML) (4060 Views)
Despite these evidence, the association of HCMV placental infection with miscarriage has not been fully clarified (14). In addition to HCMV, intrauterine infections by HSV1/2can lead to adverse consequences including miscarriage (15). Another member of HHVs, EBV, tropism to trophoblasts in the placenta (15, 16); however, the relationship between EBV infection and abortion remains controversial (17). Meanwhile, VZV can spread to the fetus via placental intrauterine infection, so the increased risk of miscarriage due to this infection has been investigated by some researchers (18). To date, several studies have been conducted to determine the effects of HSV1/2, VZV, EBV, and HCMV on pregnancy outcomes separately. But only a few studies have examined all of them simultaneously.
As mentioned before, viral infections are among the leading causes of spontaneous abortions, this case-control study concentrates on the detection of HHV1-5 DNAs in placental tissues and finding their association with miscarriage during the first 24 wk of pregnancy by evaluating spontaneous abortions as the case group and therapeutic abortions as the control group.
For the case group, specimens were obtained from the placentas of women with spontaneous pregnancy loss. The most consistent clinical symptoms of case group were abdominal pain and bleeding from the uterus. The DNA was extracted from 0.5gr of each placental sample by proteinase K digestion and the phenol/chloroform method. Briefly, placental samples were deparaffinized with xylene and resuspended in lysis buffer and treated overnight with proteinase K (20mg/ml). Then, genomic DNA was extracted by phenol/chloroform method followed by ethanol precipitation and dissolved in TE buffer. All extractions were aliquoted and preserved at -70ºC.
3. Discussion
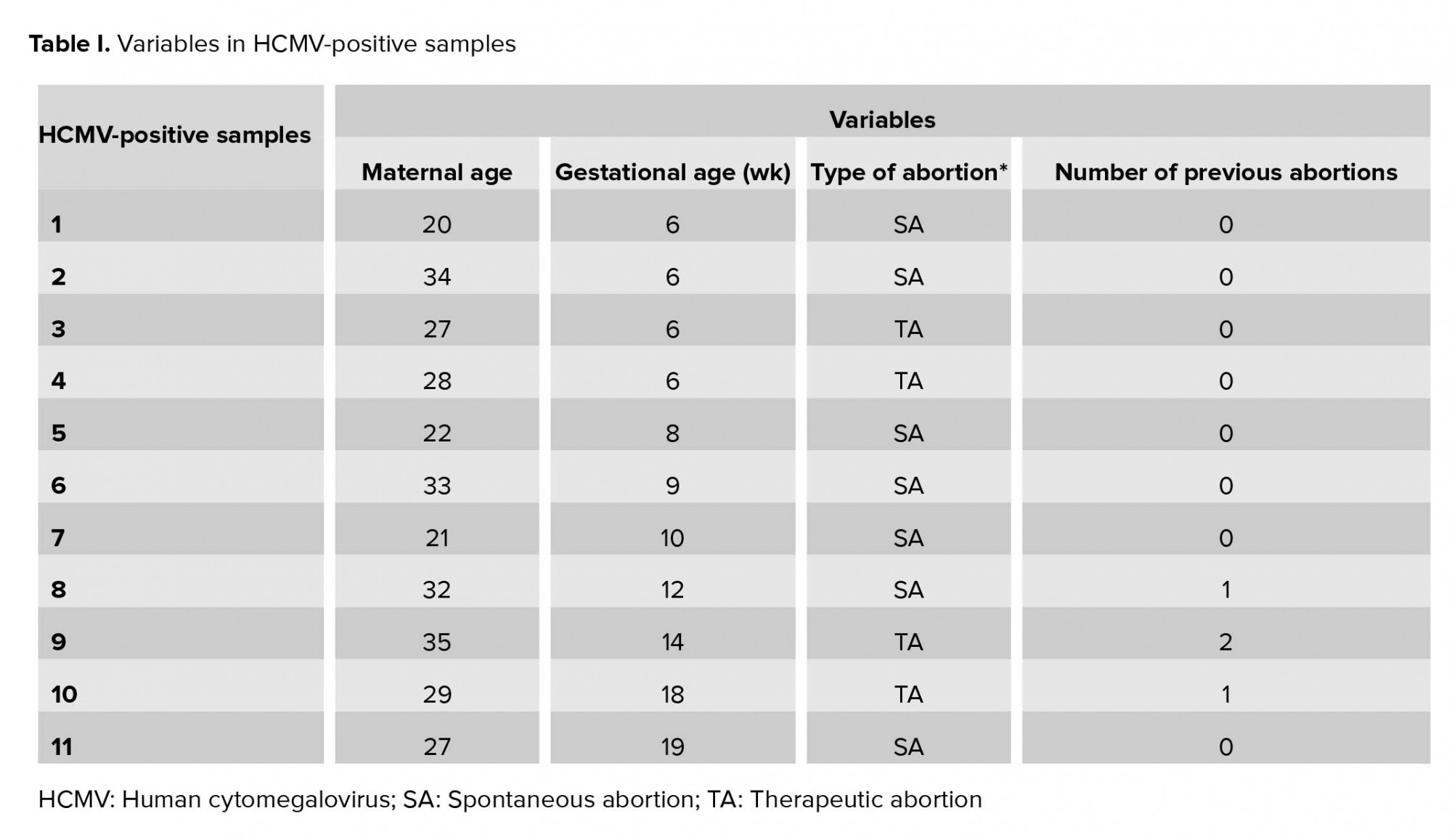
The present study evaluated and compared the prevalence of HHV1-5 DNAs in placental tissues of pregnant women with spontaneous vs. therapeutic abortions to assess their probable role in spontaneous abortion induction. Among these viruses, only the genome of HCMV was detected in the placenta of spontaneous (8.4%) and therapeutic (4.9%) abortions. Although the frequency of HCMV DNA detection was higher in the spontaneous abortions compared to the therapeutic ones, no significant association was found between the HCMV DNA detection in the placenta and the increased risk of miscarriage. Different studies have shown that certain intrauterine infections were recognized or assumed to play a role in miscarriage, the exact mechanism of which is still not fully clarified (4). Similar to the current study, a number of studies have attempted to reveal a possible correlation between miscarriage and HHVs placental infections in pregnant women. Some of these reports concluded similarly and some showed different results compared to the present study. Similar to this study, other studies have reported a higher rate of HCMV detection compared to the other HHVs in the placenta.
Al-Buhtori and colleagues evaluated 28 placental tissues from spontaneous abortion cases; they detected HCMV in 10 cases (35.7%) while 3 of them were co-infected with HSV1/2. No genome of VZV and EBV were found in their study (21). In a study conducted in China, HCMV DNA in cervicovaginal secretions was evaluated in 624 normal pregnancies and 440 miscarriages. HCMV DNA was detected in 10.9% and 8.5% of cases who had miscarriages and normal pregnancies, respectively. These findings suggested that HCMV infection of the genital tract in first trimester of pregnancy may affect pregnancy outcome and cause miscarriage (14).
Moreover, these findings were supported in a review article published in 2016 that named HCMV among the infectious agents which could increase the risk of miscarriage (4). However, similar to our findings, some other studies could not show any association between HCMV placental infection and increased risk of miscarriages. Gao and colleagues in China investigated HCMV spontaneous abortion cases in the first 12 wk of pregnancy. In their study, no HCMV DNA was detected in the placenta samples (22). In 2018, another Chinese research group investigated HCMV placental infection in miscarriages that occurred during the first trimester of pregnancy. They also could not find any significant correlation between infection and miscarriage as 9.1% of miscarriage cases (3/33) and 6.7% of normal cases (2/30) were positive for HCMV (14).
Formerly it was shown that the role of intrauterine infections in recurrent abortions was unclear, with a probable incidence of 0.5-5% (4). In a case-control study of women with recurrent pregnancy loss, anti-HCMV IgG and IgM and IgG avidity index were evaluated. They found that previous exposure to HCMV was significantly higher in patients with recurrent pregnancy loss than the control groups. However, no association was found between the IgG avidity index and recurrent pregnancy loss (23). In the present investigation, of the 12 recurrent miscarriage cases none were positive for HHV 1-5 DNAs. It should be noted that the rate of HCMV abortions was unclear (IgG positivity) in Iran and is estimated at around 90-98%in adults (24-26). So, the rate of HCMV primary infection during pregnancy would be low. However, the immunosuppression state during this period could result in HCMV reactivation, intrauterine fetal infection, and abnormalities in the fetus (8, 27).
As stated, in the present research, no HSV1/2, VZV, and EBV genomes were detected in 164 placental tissues. Previously, it has been shown that in spite of EBV reactivation during pregnancy, the rate of intrauterine infection remained low and miscarriage due to EBV infection was not reported. Haeri and colleagues examined EBV reactivation in serum samples of healthy pregnant women during second trimester. Their results demonstrated that 98% of women were EBV-seropositive with reactivation rate of 35% without adverse pregnancy outcomes (28). In a prospective cohort study on VZV over a 13-year period, the fetal infection rate was 0.8% but the prevalence of congenital varicella syndrome was 0.39% (29). Different rates of HSV1/2 infections have been reported in diverse studies. It was shown that intrauterine HSV1/2 infections caused growth restriction of the fetus, preterm delivery, and an increased risk of spontaneous abortions (15). Formerly, a study in Iran performing PCR on the placental tissue and curettage specimens of 35 full-term delivery cases and 35 spontaneous abortion casesshowed that HSV DNA was detected only in 2.8% of spontaneous abortions. They could not detect any significant differences between the case and control groups (30).
4. Conclusion
In conclusion, here, no correlation was detected between the HHVs placental infections and increased risk of spontaneous abortions. The divergent study results that have been reported in regards to HHVs placental infections and increased risk of spontaneous abortions could be due to the different types of specimens, populations, and differences in specificity and/or sensitivity of assays. In order to find the actual role of HHVs infection in this regard, further investigations should be performed with larger sample size and on populations in different countries.
Acknowledgments
The study has been funded by the School of Public Health, Tehran University of Medical Sciences (grant no. 95-03-27-31652).
Conflict of Interest
The authors declare no conflict of interests regarding the publication of this paper.
Full-Text: (636 Views)
- Introduction
Despite these evidence, the association of HCMV placental infection with miscarriage has not been fully clarified (14). In addition to HCMV, intrauterine infections by HSV1/2can lead to adverse consequences including miscarriage (15). Another member of HHVs, EBV, tropism to trophoblasts in the placenta (15, 16); however, the relationship between EBV infection and abortion remains controversial (17). Meanwhile, VZV can spread to the fetus via placental intrauterine infection, so the increased risk of miscarriage due to this infection has been investigated by some researchers (18). To date, several studies have been conducted to determine the effects of HSV1/2, VZV, EBV, and HCMV on pregnancy outcomes separately. But only a few studies have examined all of them simultaneously.
As mentioned before, viral infections are among the leading causes of spontaneous abortions, this case-control study concentrates on the detection of HHV1-5 DNAs in placental tissues and finding their association with miscarriage during the first 24 wk of pregnancy by evaluating spontaneous abortions as the case group and therapeutic abortions as the control group.
- Materials and Methods
- 1. Patients, specimens and DNA extraction
For the case group, specimens were obtained from the placentas of women with spontaneous pregnancy loss. The most consistent clinical symptoms of case group were abdominal pain and bleeding from the uterus. The DNA was extracted from 0.5gr of each placental sample by proteinase K digestion and the phenol/chloroform method. Briefly, placental samples were deparaffinized with xylene and resuspended in lysis buffer and treated overnight with proteinase K (20mg/ml). Then, genomic DNA was extracted by phenol/chloroform method followed by ethanol precipitation and dissolved in TE buffer. All extractions were aliquoted and preserved at -70ºC.
- 2. Molecular assays
- 3. Ethical consideration
- 4. Statistical analysis
- Results
- 1. Demographic characteristics
- 2. Viral DNA detection

3. Discussion
The present study evaluated and compared the prevalence of HHV1-5 DNAs in placental tissues of pregnant women with spontaneous vs. therapeutic abortions to assess their probable role in spontaneous abortion induction. Among these viruses, only the genome of HCMV was detected in the placenta of spontaneous (8.4%) and therapeutic (4.9%) abortions. Although the frequency of HCMV DNA detection was higher in the spontaneous abortions compared to the therapeutic ones, no significant association was found between the HCMV DNA detection in the placenta and the increased risk of miscarriage. Different studies have shown that certain intrauterine infections were recognized or assumed to play a role in miscarriage, the exact mechanism of which is still not fully clarified (4). Similar to the current study, a number of studies have attempted to reveal a possible correlation between miscarriage and HHVs placental infections in pregnant women. Some of these reports concluded similarly and some showed different results compared to the present study. Similar to this study, other studies have reported a higher rate of HCMV detection compared to the other HHVs in the placenta.
Al-Buhtori and colleagues evaluated 28 placental tissues from spontaneous abortion cases; they detected HCMV in 10 cases (35.7%) while 3 of them were co-infected with HSV1/2. No genome of VZV and EBV were found in their study (21). In a study conducted in China, HCMV DNA in cervicovaginal secretions was evaluated in 624 normal pregnancies and 440 miscarriages. HCMV DNA was detected in 10.9% and 8.5% of cases who had miscarriages and normal pregnancies, respectively. These findings suggested that HCMV infection of the genital tract in first trimester of pregnancy may affect pregnancy outcome and cause miscarriage (14).
Moreover, these findings were supported in a review article published in 2016 that named HCMV among the infectious agents which could increase the risk of miscarriage (4). However, similar to our findings, some other studies could not show any association between HCMV placental infection and increased risk of miscarriages. Gao and colleagues in China investigated HCMV spontaneous abortion cases in the first 12 wk of pregnancy. In their study, no HCMV DNA was detected in the placenta samples (22). In 2018, another Chinese research group investigated HCMV placental infection in miscarriages that occurred during the first trimester of pregnancy. They also could not find any significant correlation between infection and miscarriage as 9.1% of miscarriage cases (3/33) and 6.7% of normal cases (2/30) were positive for HCMV (14).
Formerly it was shown that the role of intrauterine infections in recurrent abortions was unclear, with a probable incidence of 0.5-5% (4). In a case-control study of women with recurrent pregnancy loss, anti-HCMV IgG and IgM and IgG avidity index were evaluated. They found that previous exposure to HCMV was significantly higher in patients with recurrent pregnancy loss than the control groups. However, no association was found between the IgG avidity index and recurrent pregnancy loss (23). In the present investigation, of the 12 recurrent miscarriage cases none were positive for HHV 1-5 DNAs. It should be noted that the rate of HCMV abortions was unclear (IgG positivity) in Iran and is estimated at around 90-98%in adults (24-26). So, the rate of HCMV primary infection during pregnancy would be low. However, the immunosuppression state during this period could result in HCMV reactivation, intrauterine fetal infection, and abnormalities in the fetus (8, 27).
As stated, in the present research, no HSV1/2, VZV, and EBV genomes were detected in 164 placental tissues. Previously, it has been shown that in spite of EBV reactivation during pregnancy, the rate of intrauterine infection remained low and miscarriage due to EBV infection was not reported. Haeri and colleagues examined EBV reactivation in serum samples of healthy pregnant women during second trimester. Their results demonstrated that 98% of women were EBV-seropositive with reactivation rate of 35% without adverse pregnancy outcomes (28). In a prospective cohort study on VZV over a 13-year period, the fetal infection rate was 0.8% but the prevalence of congenital varicella syndrome was 0.39% (29). Different rates of HSV1/2 infections have been reported in diverse studies. It was shown that intrauterine HSV1/2 infections caused growth restriction of the fetus, preterm delivery, and an increased risk of spontaneous abortions (15). Formerly, a study in Iran performing PCR on the placental tissue and curettage specimens of 35 full-term delivery cases and 35 spontaneous abortion casesshowed that HSV DNA was detected only in 2.8% of spontaneous abortions. They could not detect any significant differences between the case and control groups (30).
4. Conclusion
In conclusion, here, no correlation was detected between the HHVs placental infections and increased risk of spontaneous abortions. The divergent study results that have been reported in regards to HHVs placental infections and increased risk of spontaneous abortions could be due to the different types of specimens, populations, and differences in specificity and/or sensitivity of assays. In order to find the actual role of HHVs infection in this regard, further investigations should be performed with larger sample size and on populations in different countries.
Acknowledgments
The study has been funded by the School of Public Health, Tehran University of Medical Sciences (grant no. 95-03-27-31652).
Conflict of Interest
The authors declare no conflict of interests regarding the publication of this paper.
Type of Study: Original Article |
Subject:
Reproductive Epidemiology
References
1. Lang K. Nuevo-Chiquero A. Trends in self-reported spontaneous abortions: 1970-2000. Demography 2012; 49: 989-1009. [DOI:10.1007/s13524-012-0113-0] [PMID] [PMCID]
2. Nigro G, Mazzocco M, Mattia E, Di Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Neonatal Med 2011; 24: 983-989. [DOI:10.3109/14767058.2010.547963] [PMID]
3. El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE. Recurrent pregnancy loss: current perspectives. Int J Womens Health, 2017; 9: 331-345. [DOI:10.2147/IJWH.S100817] [PMID] [PMCID]
4. Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update 2016; 22: 116-133. [DOI:10.1093/humupd/dmv041] [PMID] [PMCID]
5. Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, et al. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PloS One 2012; 7: e52899. [DOI:10.1371/journal.pone.0052899] [PMID] [PMCID]
6. Finger-Jardim F, Teixeira LO, de Oliveira GR, Barral MF, da Hora VP, Gonçalves CV, et al. Herpes simplex virus: prevalence in placental tissue and incidence in neonatal cord blood samples. J Med Virol 2014; 86: 519-524. [DOI:10.1002/jmv.23817] [PMID]
7. Christian LM, Iams JD, Porter K, Glaser R. Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination. Brain Behav Immun 2012; 26: 1280-1287.
https://doi.org/10.1016/j.bbi.2012.08.006 [DOI:10.1016/j.bbi.2012.07.032] [PMID] [PMCID]
8. Britt WJ. Maternal immunity and the natural history of congenital human cytomegalovirus infection. Viruses 2018; 10: 405-422. [DOI:10.3390/v10080405] [PMID] [PMCID]
9. Tabata T, Petitt M, Fang-Hoover J, Rivera J, Nozawa N, Shiboski S, et al. Cytomegalovirus impairs cytotrophoblast-induced lymphangiogenesis and vascular remodeling in an in vivo human placentation model. Am J Pathol 2012; 181: 1540-1559. [DOI:10.1016/j.ajpath.2012.08.003] [PMID] [PMCID]
10. Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial vertical transmission during human pregnancy. Cell Host Microbe 2017; 21: 561-567. [DOI:10.1016/j.chom.2017.04.007] [PMID] [PMCID]
11. Zydek M, Petitt M, Fang-Hoover J, Adler B, Kauvar LM, Pereira L, et al. HCMV infection of human trophoblast progenitor cells of the placenta is neutralized by a human monoclonal antibody to glycoprotein B and not by antibodies to the pentamer complex. Viruses 2014; 6: 1346-1364. [DOI:10.3390/v6031346] [PMID] [PMCID]
12. Tabata T, Petitt M, Zydek M, Fang-Hoover J, Larocque N, Tsuge M, et al. Human cytomegalovirus infection interferes with the maintenance and differentiation of trophoblast progenitor cells of the human placenta. J Virol 2015; 89: 5134-5147. [DOI:10.1128/JVI.03674-14] [PMID] [PMCID]
13. Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction 2013; 146: R151-R162. [DOI:10.1530/REP-13-0232] [PMID] [PMCID]
14. Yan XC, Wang JH, Wang B, Huang LL, Zhou LQ, Zhu B, et al. Study of human cytomegalovirus replication in body fluids, placental infection, and miscarriage during the first trimester of pregnancy. J Med Virol 2015; 87: 1046-1053. [DOI:10.1002/jmv.24158] [PMID]
15. Straface G, Selmin A, Zanardo V, De Santis M, Ercoli A, Scambia G. Herpes simplex virus infection in pregnancy. Infectious Diseases in Obstetrics and Gynecology 2012; 2012: 1-6. [DOI:10.1155/2012/385697] [PMID] [PMCID]
16. Kim Y, Kim HS, Park JS, Kim CJ, Kim WH. Identification of Epstein-Barr Virus in the human placenta and its pathologic characteristics. J Korean Med Sci 2017; 32: 1959-1966. [DOI:10.3346/jkms.2017.32.12.1959] [PMID] [PMCID]
17. Tomai XH. Stillbirth following severe symmetric fetal growth restriction due to reactivation of Epstein-Barr virus infection in pregnancy. J Obstet Gynaecol Res 2011; 37: 1877-1882. [DOI:10.1111/j.1447-0756.2011.01662.x] [PMID]
18. Lamont RF, Sobel JD, Carrington D, Mazaki‐Tovi S, Kusanovic JP, Vaisbuch E, et al. Varicella‐zoster virus (chickenpox) infection in pregnancy. BJOG 2011; 118: 1155-1162. [DOI:10.1111/j.1471-0528.2011.02983.x] [PMID] [PMCID]
19. Shafiei-Jandaghi NZ, Yavarian J, Faghihloo E, Ghavami N, Ghalejoogh ZY, Kiani SJ, et al. Prevalence of adeno-associated virus and human papillomavirus DNA in Iranian women with and without cervical cancer. Pathol Res Pract 2017; 213: 457-460. [DOI:10.1016/j.prp.2017.02.010] [PMID]
20. Tafreshi NK, Sadeghizadeh M, Amini-Bavil-Olyaee S, Ahadi AM, Jahanzad I, Roostaee MH. Development of a multiplex nested consensus PCR for detection and identification of major human herpesviruses in CNS infections. J Clin Virol 2005; 32: 318-324. [DOI:10.1016/j.jcv.2004.05.018] [PMID]
21. Al-Buhtori M, Moore L, Benbow EW, Cooper RJ. Viral detection in hydrops fetalis, spontaneous abortion, and unexplained fetal death in utero. J Med Virol 2011; 83: 679-684. [DOI:10.1002/jmv.22007] [PMID]
22. Gao YL, Gao Z, He M, Liao P. Infection status of human parvovirus B19, cytomegalovirus and herpes simplex Virus-1/2 in women with first-trimester spontaneous abortions in Chongqing, China. Virol J 2018; 15: 74-81. [DOI:10.1186/s12985-018-0988-5] [PMID] [PMCID]
23. Sherkat R, Meidani M, Zarabian H, Rezaei A, Gholamrezaei A. Seropositivity of cytomegalovirus in patients with recurrent pregnancy loss. J Res Med Sci 2014; 19 (Suppl.): S22-S25.
24. Bagheri josheghani S, Moniri R, Baghbani Taheri F, Sadat S, Heidarrzadeh Z. Prevalence of serum antibodies to TORCH infection in the first trimester of the pregnancy in Kashan, Iran. Iranian Journal of Neonatology 2015; 6: 8-12.
25. Shaiegan M, Rasouli M, Zadsar M, Zolfaghari S. Meta-analysis of cytomegalovirus seroprevalence in volunteer blood donors and healthy subjects in Iran from 1992 to 2013. Iran J Basic Med Sci 2015; 18: 627-634.
26. Delfan-Beiranvand M, Sheikhian A, Birjandi M, Fazeli M. Seroprevalence of cytomegalovirus infection in pregnant women referred to Health Care Center of Khorramabad. Iranian Journal of Virology 2011; 5: 11-16. [DOI:10.21859/isv.5.4.11]
27. Nagamori T, Koyano S, Inoue N, Yamada H, Oshima M, Minematsu T, et al. Single cytomegalovirus strain associated with fetal loss and then congenital infection of a subsequent child born to the same mother. J Clin Virol 2010; 49: 134-136. [DOI:10.1016/j.jcv.2010.06.021] [PMID]
28. Haeri S, Baker AM, Boggess KA. Prevalence of epstein-barr virus reactivation in pregnancy. Am J Perinatol 2010; 27: 715-719. [DOI:10.1055/s-0030-1253098] [PMID]
29. Sanchez MA, Bello-Munoz JC, Cebrecos I, Sanz TH, Martinez JS, Moratonas EC, et al. The prevalence of congenital varicella syndrome after a maternal infection, but before 20 weeks of pregnancy: a prospective cohort study. J Matern Fetal Neonatal Med 2011; 24: 341-347. [DOI:10.3109/14767058.2010.497567] [PMID]
30. Borhani MS, Hoseini SM, Chamani M, Bagheri R, Kamali K, Aarabi M, et al. PCR Detection of Herpes Simplex Virus in Human Placenta and Aborted Materials in Patients with Spontaneous Abortion. Arch Clin Infect Dis 2012; 6: 17-20.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





