Sat, Jul 12, 2025
[Archive]
Volume 18, Issue 5 (May 2020)
IJRM 2020, 18(5): 367-374 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Novia D, Putri Lubis H, Halim B, Pustimbara A, Lestari R, Abinawanto A et al . The impact of late follicular progesterone level on in vitro fertilization-intracytoplasmic sperm injection outcome: Case-control study. IJRM 2020; 18 (5) :367-374
URL: http://ijrm.ir/article-1-1419-en.html
URL: http://ijrm.ir/article-1-1419-en.html
Diana Novia1 

 , Hilma Putri Lubis2
, Hilma Putri Lubis2 

 , Binarwan Halim2
, Binarwan Halim2 

 , Anantya Pustimbara3
, Anantya Pustimbara3 

 , Retno Lestari3
, Retno Lestari3 

 , Abinawanto Abinawanto3
, Abinawanto Abinawanto3 

 , Anom Bowolaksono *4
, Anom Bowolaksono *4 




 , Hilma Putri Lubis2
, Hilma Putri Lubis2 

 , Binarwan Halim2
, Binarwan Halim2 

 , Anantya Pustimbara3
, Anantya Pustimbara3 

 , Retno Lestari3
, Retno Lestari3 

 , Abinawanto Abinawanto3
, Abinawanto Abinawanto3 

 , Anom Bowolaksono *4
, Anom Bowolaksono *4 


1- Department of Biology, Faculty of Mathematics and Science, University of Indonesia, Depok, Indonesia. Halim Fertility Center, Stella Maris Women and Children Hospital, Medan, Indonesia.
2- Halim Fertility Center, Stella Maris Women and Children Hospital, Medan, Indonesia. Faculty of Medicine University of Sumatera Utara, Haji Adam Malik General Hospital, Medan, Indonesia.
3- Department of Biology, Faculty of Mathematics and Science, University of Indonesia, Depok, Indonesia.
4- Department of Biology, Faculty of Mathematics and Science, University of Indonesia, Depok, Indonesia. ,alaksono@sci.ui.ac.id
2- Halim Fertility Center, Stella Maris Women and Children Hospital, Medan, Indonesia. Faculty of Medicine University of Sumatera Utara, Haji Adam Malik General Hospital, Medan, Indonesia.
3- Department of Biology, Faculty of Mathematics and Science, University of Indonesia, Depok, Indonesia.
4- Department of Biology, Faculty of Mathematics and Science, University of Indonesia, Depok, Indonesia. ,
Full-Text [PDF 1227 kb]
(1098 Downloads)
| Abstract (HTML) (2698 Views)
According to the practice committee of the American Society for Reproductive Medicine (5), infertility in female can be caused by a few factors, such as ovulation disorders where there are abnormalities in the female’s menstrual cycles, tubal and pelvic disorders that are caused by infection and endometriosis, and uterine disorders that include submucosal myomas, endometrial polyps, leiomyomas, and Asherman’s syndrome. Meanwhile, the infertility in male often occurs due to the poor quality of sperm.
Since the birth of Louise Brown in 1978, many researches have been conducted to improve the pregnancy rate through IVF program. In recent years, researchers have been focusing on finding the relationship between serum progesterone level on the day of human chorionic gonadotropin (hCG) administration and endometrial receptivity or live birth rate (6-9). Elevated serum progesterone level on the day of hCG injection is known as premature luteinization (PL) and is frequently found in GnRH antagonist cycle. PL on the day of hCG injection has a negative effect on clinical pregnancy rate probably due to embryo-endometrial asynchrony (10).
The assessment of oocyte and embryo quality based on the progesterone serum levels on the day of hCG injection has been rarely studied with a very limited number of cases (7, 11). Some studies state that there is no association between progesterone elevation level and fertilization rates (FRs) as well as the oocyte and embryo quality (12, 13). However, Huang et al. stated that serum progesterone concentration on the day of hCG injection is positively and significantly correlated with oocyte fertilization failure (9).
Since there are still very few studies on the effect of progesterone level on oocyte and embryo quality, hence, this study aims to assess the effect of late follicular progesterone level on the outcomes of IVF-ICSI. The outcomes assessed will be the number of oocytes retrieved (OR), maturation rate (MR), fertilization rate (FR), number of good embryos (GE), number of fair embryos (FE), and number of poor embryos (PE).
On the day of oocyte(s) retrieval, spouse collected the sperm samples using masturbation method. The semen samples were analyzed for concentration, motility, as well as morphology. The semen was processed and selected by using a density gradient method, layer concentration used was 45:90% SpermGrad (Vitrolife; Sweden). The solution was centrifuged at 300-600 g for 10-20 min and re-suspended with 1 ml of MOPS-buffered medium (GMOPS Plus, Vitrolife; Sweden). For ICSI purposes, a single spermatozoon was immobilized using a polyvinylpyrrolidone (PVP) solution (Medicult; Denmark).
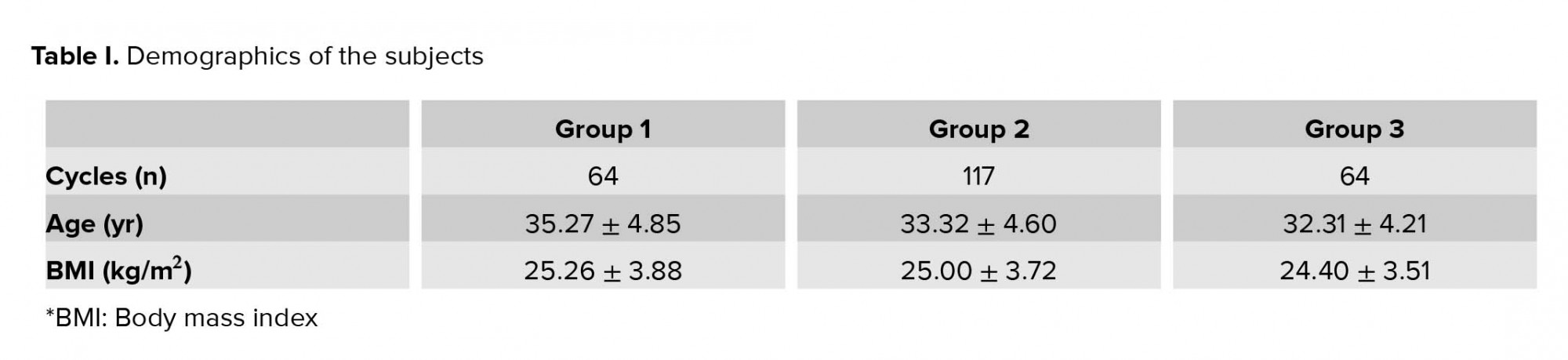
As shown in table I, the subjects that mainly belonged to group 2 had a higher cycles numbers compared to other groups (n = 117). The age range of subjects were 22-47 yr and the mean age is as shown in table I. The mean body mass index of the three groups was 25.26 ± 3.88 kg/m2; 25.00 ± 3.72 kg/m2; and 24.40 ± 3.51 kg/m2, respectively.
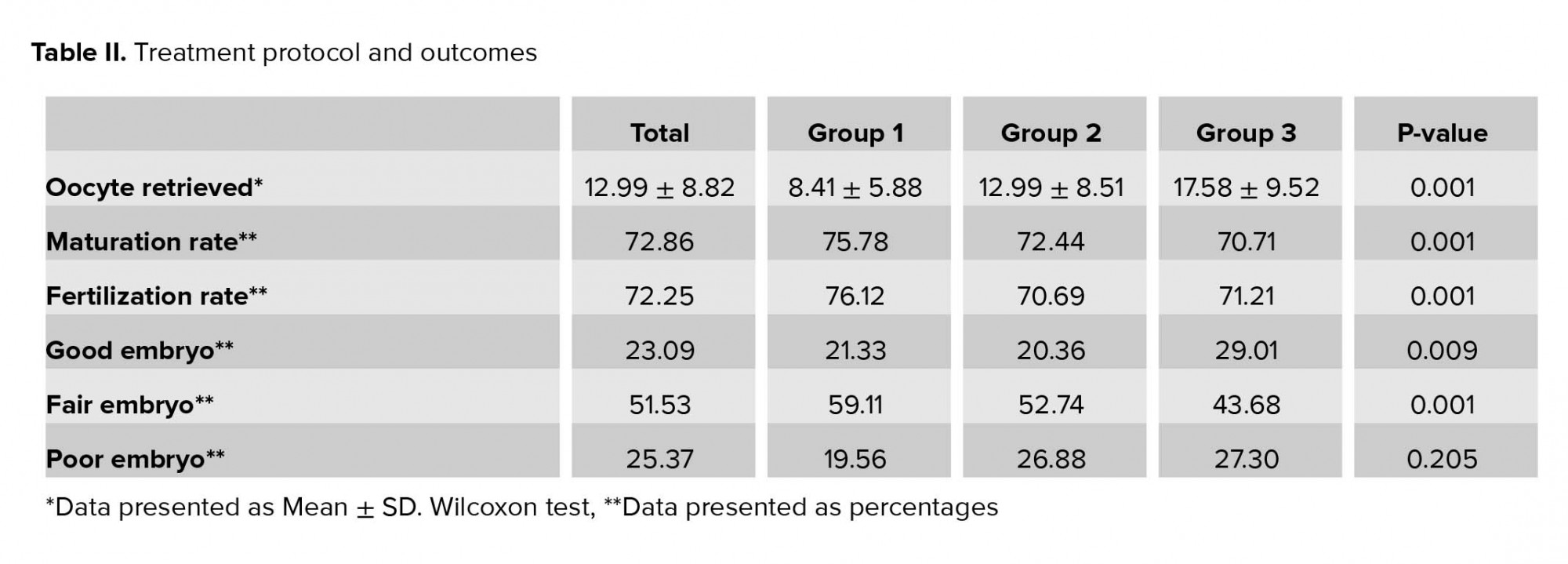
As shown in Table II, the number of OR was significantly higher in group 3 compared to the other groups. It also shows that oocytes in group 1 had a significantly higher maturation and FRs (75.78%; 76.12%, respectively; p = 0.001) but had the lowest percent of GE (p = 0.009). Group 2 had the highest percent of FE and also the lowest FR (p = 0.001). Although group 3 had the lowest maturation rate compared to the other groups, it had a significantly higher percent of GE. Apart from the low maturation rate and the high percent of GE, group 3 also had the highest percent of PE compared to the other groups although it was not significant (p = 0.205). The estradiol serum level on the day of hCG injection was 1247.09 ± 730.56, 2092.39 ± 1276.42, and 3266.43 ± 319.06 in group 1, 2, and 3, respectively where the highest level is in group 3 (p = 0.001).
4. Discussion
Our result suggests that progesterone level on the day of human chorionic gonadotropin injection may have an impact on the outcome of in vitro fertilization IVF-ICSI. Oocytes are not able to express gonadotropin to induce their own maturation. Hence, LH is responsible in activating theca cells and granulosa cells so that oocytes can return to the meiotic stage and begin the maturation process (15). Human chorionic gonadotropin (hCG) is a homologue of LH which can activate LH receptors, a primary, and also the most commonly used, trigger for oocyte maturation (16). In IVF, hCG is a hormone used to create an expression for LH-like exposure. The influence of serum progesterone level on the day of hCG injection on the outcome of the IVF program has long been a matter of debate.
In this study, we found a significant difference in the serum progesterone level with regards to oocyte maturation and FRs among the groups. This result is in line with a recent study which stated that serum progesterone concentration on the day of hCG injection was positively and significantly correlated with the rescue ICSI rate (9). A high progesterone level (> 1.50 ng/ml) on the day of hCG injection may have negatively affected oocyte fertilization, which indicates that greater attention will be needed to avoid fertilization failure. Some studies have reported that an elevation of serum progesterone level as a result of premature oocyte maturation or aging may reduce FR. This oocyte aging may lead to disorganization of the oocyte spindle and cytoplasm as well as the retention of the second polar body which may result in chromosomal anomalies (9).
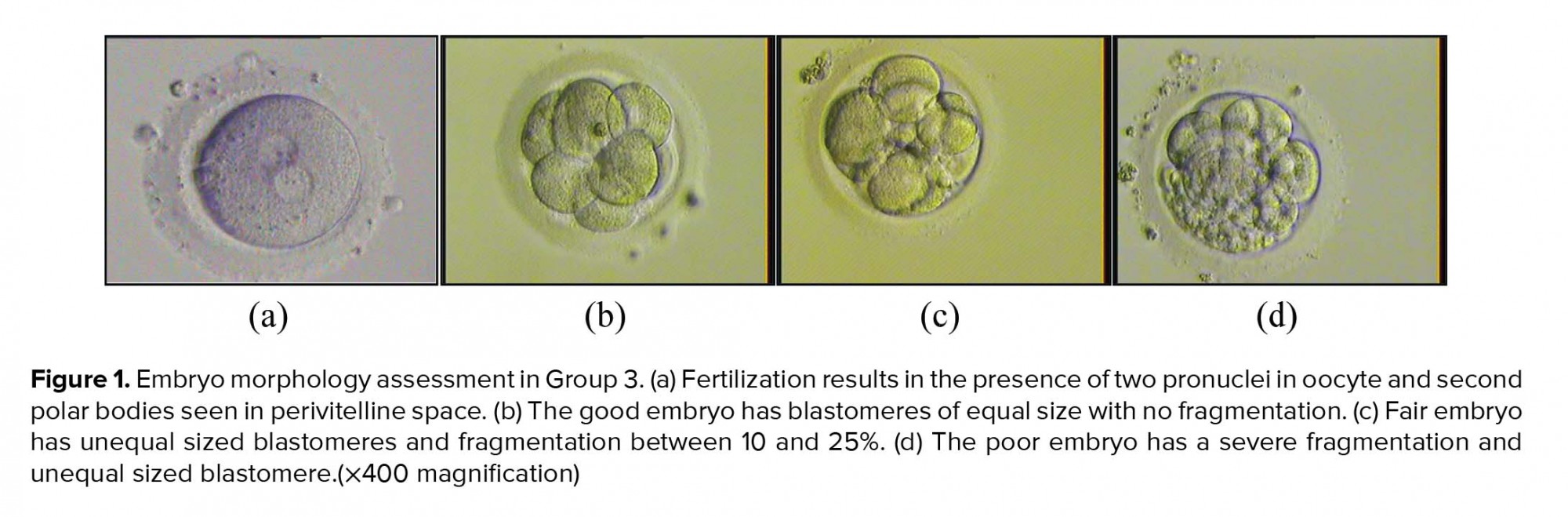
Our study also showed that high level of progesterone on the day of hCG injection did not have an adverse effect on embryo quality. As shown in Figure 1, embryo in group 3 can also develop into a good-quality embryo. In contrast, however, some study showed that high progesterone level may have detrimental effects on oocyte and embryo quality (17, 18). Sonigo et al. (13) stated that embryo cleavage and blastocyst rate are not affected by the elevated progesterone level. Slight impact of the elevated progesterone level on oocyte or embryo quality is found consistent with the evidence that female gametes do not express progesterone receptor. Previous study showed that the quality of embryo is mostly determined by the quality of oocytes, which include the first polar body, meiotic spindle, cumulus cells, and mitochondria (19).
Women who are high responders of gonadotropin, with > 10 follicles in the size of > 14 mm and estradiol level of > 2500 pg/ml on the day of hCG injection may also have a significantly high rise of progesterone. Serum progesterone level on the day of hCG injection reported closely related to the dose of gonadotropin injected irrespectively to the type given. Some study also explained that this event is able to occur due to the initial intense recombinant FSH dose administered during the first six days of stimulation, which in return, may increase the steroidogenetic activity of granulosa cell. According to the study conducted by MERiT, the rise of serum progesterone appears to be more often in the FSH recombinant treatment group compared to the HMG treatment group (10, 20).
PL may negatively impact the success rate of IVF/ICSI fresh transfer, not through an ovarian event but its influence on the endometrium, which may possibly lead to impaired endometrial receptivity. Elevated progesterone level may cause embryo/endometrial asynchrony, which in return may reduce the probability of implantation (21). Hence, clinicians will need to make use of some strategies in controlling PL with the aim of avoiding its possible deleterious effects in fresh IVF cycles. One of the strategies to improve the pregnancy rate in PL cases is to cancel the fresh ET and replacing the fresh embryo with frozen-thawed embryo (22).
The limitation in this study is related to the number of samples. Due to the short duration of the study, we only involved 245 women. Since there are some points in the study that are still being debated, hence, further research with a longer duration is needed to improve the result of this study, which allows the researcher to recruit more samples. For further research, we would like to suggest a study to assess the association between progesterone receptor polymorphism and the outcome of IVF.
5. Conclusion
Increased progesterone level on the day of follicle maturation may not affect embryo quality but may have an adverse effect on oocyte maturation and FRs. Elevated estradiol and progesterone level on the day of hCG injection were the effects of the increasing number and size of follicles.
Acknowledgments
This research was funded by the Direktorat Riset dan Pengabdian Masyarakat Universitas Indonesia and supported by the International Publication Grant: PIT-9 (NKB-0018/UN2.R3.1/HKP.05.00/2019) of year 2019.
Conflict of Interest
There is no conflict of interest that could affect the impartiality of the study conducted.
Full-Text: (663 Views)
- Introduction
According to the practice committee of the American Society for Reproductive Medicine (5), infertility in female can be caused by a few factors, such as ovulation disorders where there are abnormalities in the female’s menstrual cycles, tubal and pelvic disorders that are caused by infection and endometriosis, and uterine disorders that include submucosal myomas, endometrial polyps, leiomyomas, and Asherman’s syndrome. Meanwhile, the infertility in male often occurs due to the poor quality of sperm.
Since the birth of Louise Brown in 1978, many researches have been conducted to improve the pregnancy rate through IVF program. In recent years, researchers have been focusing on finding the relationship between serum progesterone level on the day of human chorionic gonadotropin (hCG) administration and endometrial receptivity or live birth rate (6-9). Elevated serum progesterone level on the day of hCG injection is known as premature luteinization (PL) and is frequently found in GnRH antagonist cycle. PL on the day of hCG injection has a negative effect on clinical pregnancy rate probably due to embryo-endometrial asynchrony (10).
The assessment of oocyte and embryo quality based on the progesterone serum levels on the day of hCG injection has been rarely studied with a very limited number of cases (7, 11). Some studies state that there is no association between progesterone elevation level and fertilization rates (FRs) as well as the oocyte and embryo quality (12, 13). However, Huang et al. stated that serum progesterone concentration on the day of hCG injection is positively and significantly correlated with oocyte fertilization failure (9).
Since there are still very few studies on the effect of progesterone level on oocyte and embryo quality, hence, this study aims to assess the effect of late follicular progesterone level on the outcomes of IVF-ICSI. The outcomes assessed will be the number of oocytes retrieved (OR), maturation rate (MR), fertilization rate (FR), number of good embryos (GE), number of fair embryos (FE), and number of poor embryos (PE).
- Materials and Methods
- 1. Study design
- 2. Ovarian hyperstimulation controlled
- 3. Oocyte morphology assessment and ICSI procedure
On the day of oocyte(s) retrieval, spouse collected the sperm samples using masturbation method. The semen samples were analyzed for concentration, motility, as well as morphology. The semen was processed and selected by using a density gradient method, layer concentration used was 45:90% SpermGrad (Vitrolife; Sweden). The solution was centrifuged at 300-600 g for 10-20 min and re-suspended with 1 ml of MOPS-buffered medium (GMOPS Plus, Vitrolife; Sweden). For ICSI purposes, a single spermatozoon was immobilized using a polyvinylpyrrolidone (PVP) solution (Medicult; Denmark).
- 4. Embryo morphology assessment
- 5. Hormones measurements
- 6. Ethical consideration
- 7. Data analysis
- Results
As shown in table I, the subjects that mainly belonged to group 2 had a higher cycles numbers compared to other groups (n = 117). The age range of subjects were 22-47 yr and the mean age is as shown in table I. The mean body mass index of the three groups was 25.26 ± 3.88 kg/m2; 25.00 ± 3.72 kg/m2; and 24.40 ± 3.51 kg/m2, respectively.
As shown in Table II, the number of OR was significantly higher in group 3 compared to the other groups. It also shows that oocytes in group 1 had a significantly higher maturation and FRs (75.78%; 76.12%, respectively; p = 0.001) but had the lowest percent of GE (p = 0.009). Group 2 had the highest percent of FE and also the lowest FR (p = 0.001). Although group 3 had the lowest maturation rate compared to the other groups, it had a significantly higher percent of GE. Apart from the low maturation rate and the high percent of GE, group 3 also had the highest percent of PE compared to the other groups although it was not significant (p = 0.205). The estradiol serum level on the day of hCG injection was 1247.09 ± 730.56, 2092.39 ± 1276.42, and 3266.43 ± 319.06 in group 1, 2, and 3, respectively where the highest level is in group 3 (p = 0.001).


4. Discussion
Our result suggests that progesterone level on the day of human chorionic gonadotropin injection may have an impact on the outcome of in vitro fertilization IVF-ICSI. Oocytes are not able to express gonadotropin to induce their own maturation. Hence, LH is responsible in activating theca cells and granulosa cells so that oocytes can return to the meiotic stage and begin the maturation process (15). Human chorionic gonadotropin (hCG) is a homologue of LH which can activate LH receptors, a primary, and also the most commonly used, trigger for oocyte maturation (16). In IVF, hCG is a hormone used to create an expression for LH-like exposure. The influence of serum progesterone level on the day of hCG injection on the outcome of the IVF program has long been a matter of debate.
In this study, we found a significant difference in the serum progesterone level with regards to oocyte maturation and FRs among the groups. This result is in line with a recent study which stated that serum progesterone concentration on the day of hCG injection was positively and significantly correlated with the rescue ICSI rate (9). A high progesterone level (> 1.50 ng/ml) on the day of hCG injection may have negatively affected oocyte fertilization, which indicates that greater attention will be needed to avoid fertilization failure. Some studies have reported that an elevation of serum progesterone level as a result of premature oocyte maturation or aging may reduce FR. This oocyte aging may lead to disorganization of the oocyte spindle and cytoplasm as well as the retention of the second polar body which may result in chromosomal anomalies (9).
Our study also showed that high level of progesterone on the day of hCG injection did not have an adverse effect on embryo quality. As shown in Figure 1, embryo in group 3 can also develop into a good-quality embryo. In contrast, however, some study showed that high progesterone level may have detrimental effects on oocyte and embryo quality (17, 18). Sonigo et al. (13) stated that embryo cleavage and blastocyst rate are not affected by the elevated progesterone level. Slight impact of the elevated progesterone level on oocyte or embryo quality is found consistent with the evidence that female gametes do not express progesterone receptor. Previous study showed that the quality of embryo is mostly determined by the quality of oocytes, which include the first polar body, meiotic spindle, cumulus cells, and mitochondria (19).
Women who are high responders of gonadotropin, with > 10 follicles in the size of > 14 mm and estradiol level of > 2500 pg/ml on the day of hCG injection may also have a significantly high rise of progesterone. Serum progesterone level on the day of hCG injection reported closely related to the dose of gonadotropin injected irrespectively to the type given. Some study also explained that this event is able to occur due to the initial intense recombinant FSH dose administered during the first six days of stimulation, which in return, may increase the steroidogenetic activity of granulosa cell. According to the study conducted by MERiT, the rise of serum progesterone appears to be more often in the FSH recombinant treatment group compared to the HMG treatment group (10, 20).
PL may negatively impact the success rate of IVF/ICSI fresh transfer, not through an ovarian event but its influence on the endometrium, which may possibly lead to impaired endometrial receptivity. Elevated progesterone level may cause embryo/endometrial asynchrony, which in return may reduce the probability of implantation (21). Hence, clinicians will need to make use of some strategies in controlling PL with the aim of avoiding its possible deleterious effects in fresh IVF cycles. One of the strategies to improve the pregnancy rate in PL cases is to cancel the fresh ET and replacing the fresh embryo with frozen-thawed embryo (22).
The limitation in this study is related to the number of samples. Due to the short duration of the study, we only involved 245 women. Since there are some points in the study that are still being debated, hence, further research with a longer duration is needed to improve the result of this study, which allows the researcher to recruit more samples. For further research, we would like to suggest a study to assess the association between progesterone receptor polymorphism and the outcome of IVF.

5. Conclusion
Increased progesterone level on the day of follicle maturation may not affect embryo quality but may have an adverse effect on oocyte maturation and FRs. Elevated estradiol and progesterone level on the day of hCG injection were the effects of the increasing number and size of follicles.
Acknowledgments
This research was funded by the Direktorat Riset dan Pengabdian Masyarakat Universitas Indonesia and supported by the International Publication Grant: PIT-9 (NKB-0018/UN2.R3.1/HKP.05.00/2019) of year 2019.
Conflict of Interest
There is no conflict of interest that could affect the impartiality of the study conducted.
Type of Study: Original Article |
Subject:
Assisted Reproductive Technologies
References
1. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glosarry on infertility and fertility care, 2017. Fertil Steril 2017; 108: 393-406. [DOI:10.1016/j.fertnstert.2017.06.005] [PMID]
2. Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem 2018; 62: 2-10. [DOI:10.1016/j.clinbiochem.2018.03.012] [PMID]
3. Persson J. The ART of assisted reproductive technology. Aust Fam Physician 2005; 34: 119-122.
4. Lestari L, Pratama G, Maidarti M, Harzif AK, B. Wiweko. Characteristic and pregnancy rate of IVF patient: A retrospective analysis from two centres. ASPIRE conference proceedings, the 6th congress of the asia pacific initiative on reproduction, KnE Medicine 2016: 43-48.
5. Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103: 44-50. [DOI:10.1016/j.fertnstert.2015.03.019]
6. Santos-Ribeiro S, Polyzos NP, Haentjens P, Smitz J, Camus M, Tournaye H, et al. Live birth rates after IVF are reduced by both low and high progesterone levels on the day of human chorionic gonadotrophin administration. Hum Reprod 2014; 29: 1698-1705. [DOI:10.1093/humrep/deu151] [PMID]
7. Lawrenz B, Fatemi HM. Effect of progesterone elevation in follicular phase of IVF-cycles on the endometrial receptivity. Reprod Biomed Online 2017; 34: 422-428. [DOI:10.1016/j.rbmo.2017.01.011] [PMID]
8. Segal S, Glatstein I, McShane P, Hotamisligil S, Ezcurra D, Carson R. Premature luteinization and in vitro fertilization outcome in gonadotropin/gonadotropin-releasing hormone antagonist cycles in women with polycystic ovary syndrome. Fertil Steril 2009; 91: 1755-1759. [DOI:10.1016/j.fertnstert.2008.02.009] [PMID]
9. Huang B, Li Z, Zhu L, Hu D, Liu Q, Zhu G, et al. Progesterone elevation on the day of HCG administration may affect rescue ICSI. Reprod Biomed Online 2014; 29: 88-93. [DOI:10.1016/j.rbmo.2014.03.015] [PMID]
10. M. Choudhary, Sharma S, Swarankar M L, Bhardwaj S L. Risk of premature luteinization in IVF cycles and its impact on clinical pregnancy rate. Int J Res Med Sci 2016; 4: 139-143. [DOI:10.18203/2320-6012.ijrms20160020]
11. Lawrenz B, Labarta E, Fatemi H, Bosch E. Premature progesterone elevation: targets and rescue strategies. Fertil Steril 2018; 109: 577-582. [DOI:10.1016/j.fertnstert.2018.02.128] [PMID]
12. Liu Y, Copeland C, Chapple V, Roberts P, Feenan K, Matson P. The relationship between embryo quality assessed using routine embryology or time-lapse videography and serum progesterone concentration on the day of ovulatory trigger in in vitro fertilization cycles. Asian Pacific Journal of Reproduction 2015; 4: 140-146. [DOI:10.1016/S2305-0500(15)30011-7]
13. Sonigo C, Dray G, Roche C, Cédrin-Durnerin I, Hugues JN. Impact of high serum progesterone during the late follicular phase on IVF outcome. Reprod Biomed Online 2014; 29: 177-186. [DOI:10.1016/j.rbmo.2014.03.027] [PMID]
14. Alpha scientists in reproductive medicine and ESHRE special interest group of embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 2011; 26: 1270-1283. [DOI:10.1093/humrep/der037] [PMID]
15. Trounson A, Gosden R, Eichenlaub-Ritter U. Second edition biology and pathology of the oocyte role in fertility, medicine, and nuclear reprogramming. Cambridge University Press, New York; 2013. [DOI:10.1017/CBO9781139135030]
16. Abbara A, Clarke SA, Dhillo WS. Novel concepts for inducing final oocyte maturation in in vitro fertilization treatment. Endocr Rev 2018; 39: 593-628. [DOI:10.1210/er.2017-00236] [PMID] [PMCID]
17. Rehman R, Khan R, Baig M, Hussain M, Fatima SS. Estradiol progesterone ratio on ovulation induction day: a determinant of successful pregnancy outcome after intra cytoplasmic sperm injection. Iran J Reprod Med 2014; 12: 633-640.
18. Bu Z, Zhao F, Wang K, Guo Y, Su Y, Zhai J, et al. Serum progesterone elevation adversely affects cumulative live birth rate in different ovarian responders during in vitro fertilization and embryo transfer: a large retrospective study. PloS One 2014; 9: e100011. [DOI:10.1371/journal.pone.0100011] [PMID] [PMCID]
19. Hoshino Y. Updating the markers for oocyte quality evaluation: intracellular temperature as a new index. Reprod Med Biol 2018; 17: 434-441. [DOI:10.1002/rmb2.12245] [PMID] [PMCID]
20. Huang B, Ren X, Wu L, Zhu L, Xu B, Li Y, et al. Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. PLoS One 2016; 11: e0145895. [DOI:10.1371/journal.pone.0145895] [PMID] [PMCID]
21. Huang PC, Chen MJ, Guu HF, Yi YC, Ho JY, Chen YF, et al. Effect of premature serum progesterone rise on embryo transfer outcomes and the role of blastocyst culture and transfer in assisted reproductive technology cycles with premature progesterone rise. Taiwan J Obstet Gynecol 2015; 54: 641-646. [DOI:10.1016/j.tjog.2014.03.014] [PMID]
22. Huang Y, Wang EY, Du QY, Xiong YJ, Guo XY, Yu YP, et al. Progesterone elevation on the day of human chorionic gonadotropin administration adversely affects the outcome of IVF with transferred embryos at different developmental stages. Reprod Biol Endocrinol 2015; 13: 82-91. [DOI:10.1186/s12958-015-0075-3] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





