BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijrm.ir/article-1-1495-en.html
2- Department of Anatomy and Cell Biology, School of Medicine, Mashhad University of Medical Science, Mashhad, Iran. ,
3- Immunology Research Center, School of Medicine, Mashhad University of Medical Science, Mashhad, Iran.
-
Introduction
The endocrine system is the second most important regulatory system in the body after the nervous system. Thyroid-stimulating hormone (THS) stimulates the thyroid gland to secrete thyroxin, and then triiodothyronine hormones. Although THS plays different roles in many cells and tissues of the body, the function of this hormone in sperm development requires further research (1). Thyroid hormone activates the function of a wide range of genes that guide nuclear receptors through complicated signaling pathways (2). The testicles are mainly composed of seminiferous tubules supported by the interstitial connective tissue. Each tubule consists of three layers, namely basement membrane, myoid cells, and elastic fibers. Sertoli cells in BM play multiple roles, including the establishment of a blood-testis barrier (3). Excessive thyroid hormones cause hyperthyroidism, which is associated with decreased testicular mass, testosterone level, and Sertoli cells (4, 5). Thyroid hormone receptors (TR) are located on Sertoli cells. Different patterns of TR gene expression include TRα and TRβ, which are the main factors in the regulation of the effect of the thyroid hormones on the testes (6-8).
Mammalian fertilization is one of the most amazing biological phenomena, involving the infusion of the sperm with the oocyte. Calcium channels play an important role in various mammalian sperm functions, such as capacitation and acrosome reaction (9, 10). The cation channel of sperm (CATSPER) is a type of voltage-sensitive ion channel that allows the entrance of calcium ions. Sperm motility is the most important factor in fertilization, which is mainly regulated by calcium signaling (11, 12). CATSPER1 is found in the tail of the sperm, and CATSPER2 is required for the entrance of calcium ions to the sperm (13-15). The CATSPER gene encodes a six-transmembrane-spanning repeat that its product are only expressed in testes. CATSPER gene produces the four repeat structure of voltage-dependent Ca+2-chanel and cyclic-nucleotide-gated before duplication. Computer-assisted sperm analysis approved that every mutant of CATSPER gene reduced sperm motility and male fertility function for sperm penetration of the zona pellucida in mice. In addition, investigation showed that hyperactivated motility needs a sperm-specific pH-gated calcium channel in mice. So that increasing intracellular pH induced a rise in intracellular calcium. The amount of calcium regulate sperm motility through the motor proteins of the tail sperm (16, 17).
According to the importance of cation channel (CATSPER1 and CATSPER2) in motility and the function of sperm, the aim of the present study was to investigate the effect of hyperthyroidism on the expressions of CATSPER1 and CATSPER2 genes in the seminiferous tubules of mice.
-
Materials and Methods
-
1. Treatments of animals
-
This experimental study was conducted on 20 adult BALB/C male mice weighing 25-30 gr. The animals were obtained from the animal house of the Faculty of Medicine of Mashhad University of Medical Sciences, Mashhad, Iran, and kept under standard animal housing conditions (12 hr light-12 hr dark cycle at a temperature range of 22-24ºC and humidity of 50-55%). During the experiment, the mice had enough access to food and water. All protocol of immunohistochemistry and real-time PCR were carried out at a specialized laboratory of histochemistry and Buali (Avicenna) at the Research Institute of Mashhad University of Medical Sciences.
After the adaptation to the environment, the mice were randomly divided into two groups of hyperthyroidism and control. The control did not receive any interventions, while the hyperthyroidism group was administered with 500 mg/l levothyroxine (L-thyroxine) liquid solution for two 2 months (18). After the treatment period, the hyperthyroidism was confirmed by means of radioimmunoassay after three days in laboratory. Then the mice were kept in the normal conditions of the animal house. After four days, all mice were sacrificed, and their testes were extracted and fixed in formalin. Based on the common histological methods, the samples were molded in paraffin.
-
2. Epididymal smear preparation
For supplying smear, epididymis were removed and divided into small pieces and incubated in 1 ml normal saline, 5% CO2 at 37oC for 30 min. A drop of sperm suspension was placed on a poly-L-lysine lam and fixed for CATSPER1 and CATSPER2 immunohistochemical investigation.
-
3. Immunohistochemistry
The animals were sacrificed through cervical dislocation under anesthesia induced with chloroform; subsequently, the epididymis was removed, followed by the incubation of the epididymis fragments mixed with physiological serum at 370ºC for 30 min. Next, 10 μl of the suspension containing sperm was spread on a poly-L-lysine slide and then fixed with methanol. For immunohistochemical study, the smears were washed with phosphate-buffered saline (PBS) thrice for 15 min. Subsequently, a standard method was applied for performing heat-induced antigen retrieval. After placing the samples in EDTA/PBS solution at 100°C for 30 min, they were washed in PBS for 15 min. In order to control the peroxidase activity, the samples were immersed in 3% oxygen water, in addition to methanol in darkness for 20 min. Then, they were incubated with PBS for 15 min and 1.5% bovine serum albumin buffer for 30 min at room temperature. Next, the primary CATSPER1 or CATSPER2 antibodies were deposited on the samples at a concentration of 1:50 and then incubated (overnight) at 40° for 24 hr. After washing the samples with PBS for 15 min, they were incubated with the donkey anti-goat polyclonal secondary antibody at a concentration of 1: 200 at 37°C for 2 hr. The samples were washed again with PBS, and then placed in a solution containing 0.3 g of 3,3'-diaminobenzidine, 200 μl water, oxygenated water, and PBS for 15 min. After washing the specimens with running water for 1.5 min, they were rinsed with water for 12 min. The samples were then digested using ascending alcohol series (700-1000). Finally, they were sealed for clarification in xylene and between the slide and coverslip. To study the brown color intensity of the samples, an optical microscope (BX51, Olympus, Japan) was connected to a camera with a 100´ magnification. The images were reviewed by two researchers in a double-blind manner. The color intensity of the samples in different groups was categorized semiquantitatively (Table I). As indicated in Table I, the samples were analyzed for anti-CATSPER1 or anti-CATSPER2 considering light to dark brown staining based on their immunohistochemically staining intensity.
-
4. Messenger RNA expression of CATSPER1 and CATSPER2 genes
Total RNA was isolated from the testicular samples by means of the Total RNA Purification kit (Parstous Biotechnology, Iran) following the manufacturer’s instructions. The integrity and purity of the obtained RNA were evaluated using electrophoresis on 1.5% agarose gel (Green Viewer, Betagen) and visualized by an ultraviolet transilluminator. The synthesis of the first-strand complementary DNA (cDNA) of the extracted messenger RNA (mRNA) was performed using the Easy cDNA Synthesis kit in the presence of Oligo d (T)16 primer (Parstous Biotechnology, Iran). The mRNA transcripts of CATSPER1 and CATSPER2 were examined by the SYBR Green quantitative real-time polymerase chain reaction kit (Parstous Biotechnology, Iran) using the mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as the housekeeping gene.
Table II lists the primers applied in the present research. Each reaction (10 µl) contained 5 µl SYBR 2X Mater Mix and 0.5 µM of each specific primer. Amplification was performed with an initial denaturation at 95°C for 10 min, followed by 45 cycles of amplification at 94°C for 20 sec and 60°C for 30 sec using the Rotor-Gene Q (Qiagen, Germany). The reaction mixtures were warmed up at 72°C for 3 min; subsequently, the temperature was ramped to 99°C to analyze the melting curves. To evaluate the gene expression, the cycle thresholds (Ct) for CATSPER1 and CATSPER2 genes were normalized to the Ct of the housekeeping gene (i.e., GAPDH). The relative quantification value of the target is expressed as follows:
2−ΔΔCt, where ΔCt = Ct (specific gene) - Ct (GAPDH) and ΔΔCt = ΔCt (sample) - ΔCt (calibrator)
ΔCt = CT (target sample) - CT (Referenses)
ΔΔCt = ΔCT (target sample) - ΔCT (control sample)
Ratio= 2−ΔΔCt


3. Results
After the confirmation of hyperthyroidism in the experimental mice by the relevant tests, the intensity of immunohistochemical staining was determined in the three parts of the sperm (i.e., head, middle piece, and tail) for both hyperthyroidism and control groups. With regard to CATSPER1, there was no reaction in all the three segments of the spermatozoa in the hyperthyroidism group. However, this intensity of coloration was clearly observed in all the three parts of spermatozoa in the control group. In this regard, the head and middle pieces showed high reactions, and the tail demonstrated a moderate reaction (Figure 1). Considering CATSPER2 in the hyperthyroidism group, the severity of the immunohistochemical staining of the head, middle piece of the sperms was moderate, while that of the tail of the spermatozoa was obtained as low. In the control group, the head and middle piece showed high reactions, while the tail demonstrated a moderate reaction. This comparison showed variation in color from bright to dark brown (Figure 2). The results of the Mann-Whitney U-test revealed no significant differences between the hyperthyroidism and control groups in terms of the severity of immunohistochemical reactions in both CATSPER1 (p = 0.17) and CATSPER2 (p = 0.22). Similarly, the results of the t-test also showed no significant difference between the hyperthyroidism and control groups regarding the real-time PCR results of the mRNA gene expression in both CATSPER1 (p = 0.45; Figure 3) and CATSPER2 (p = 0.34; Figure 4).
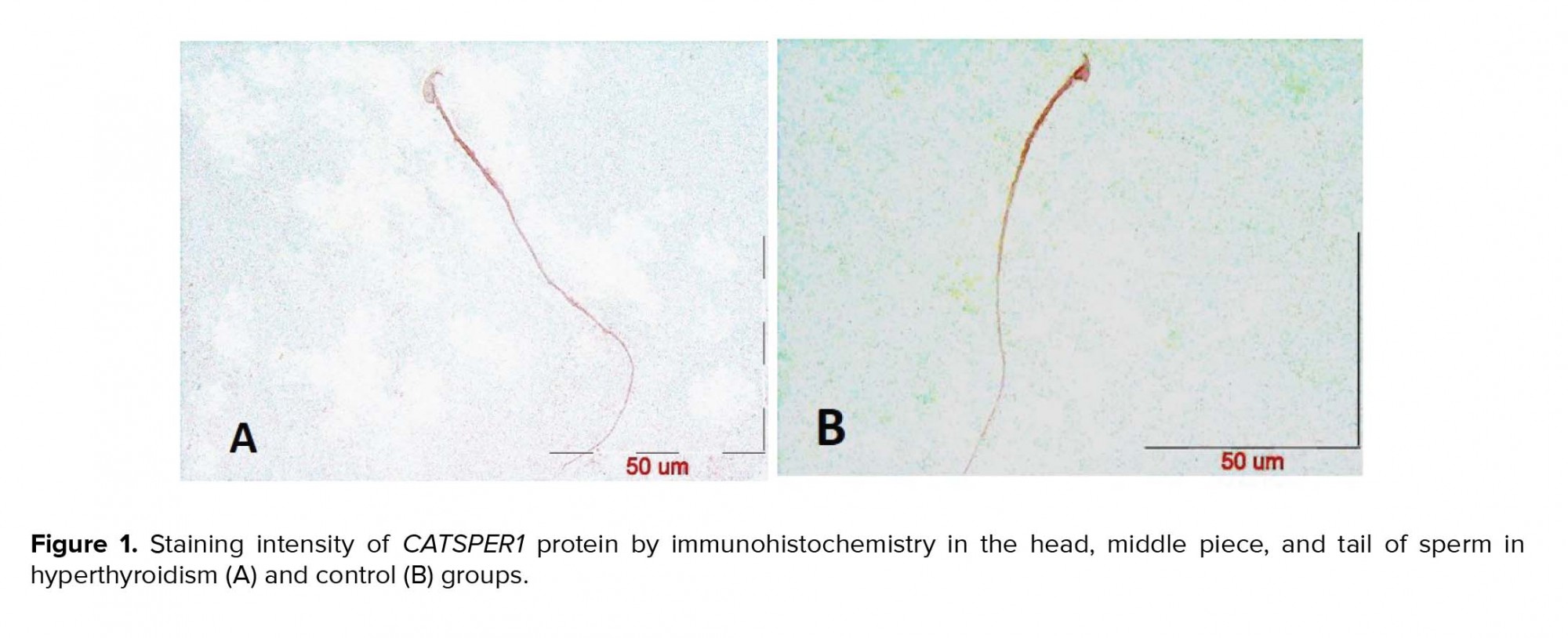
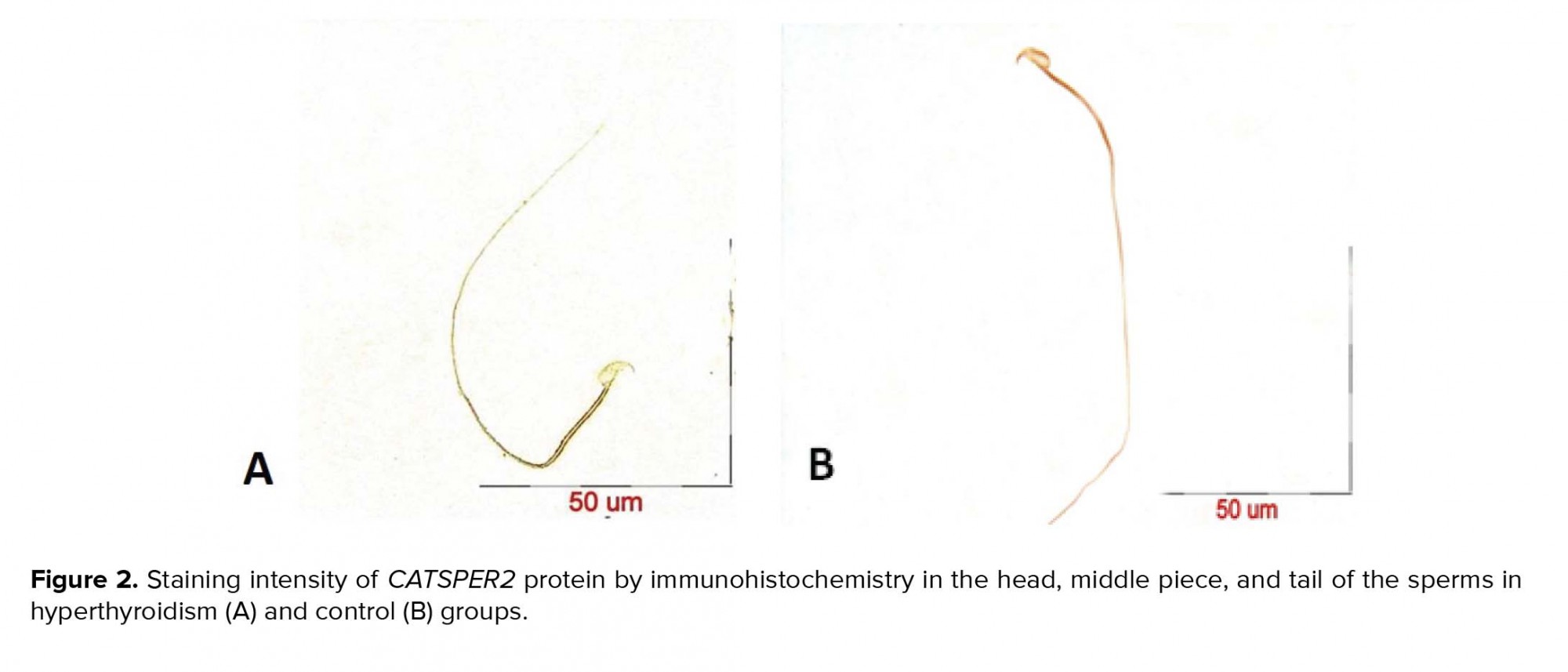
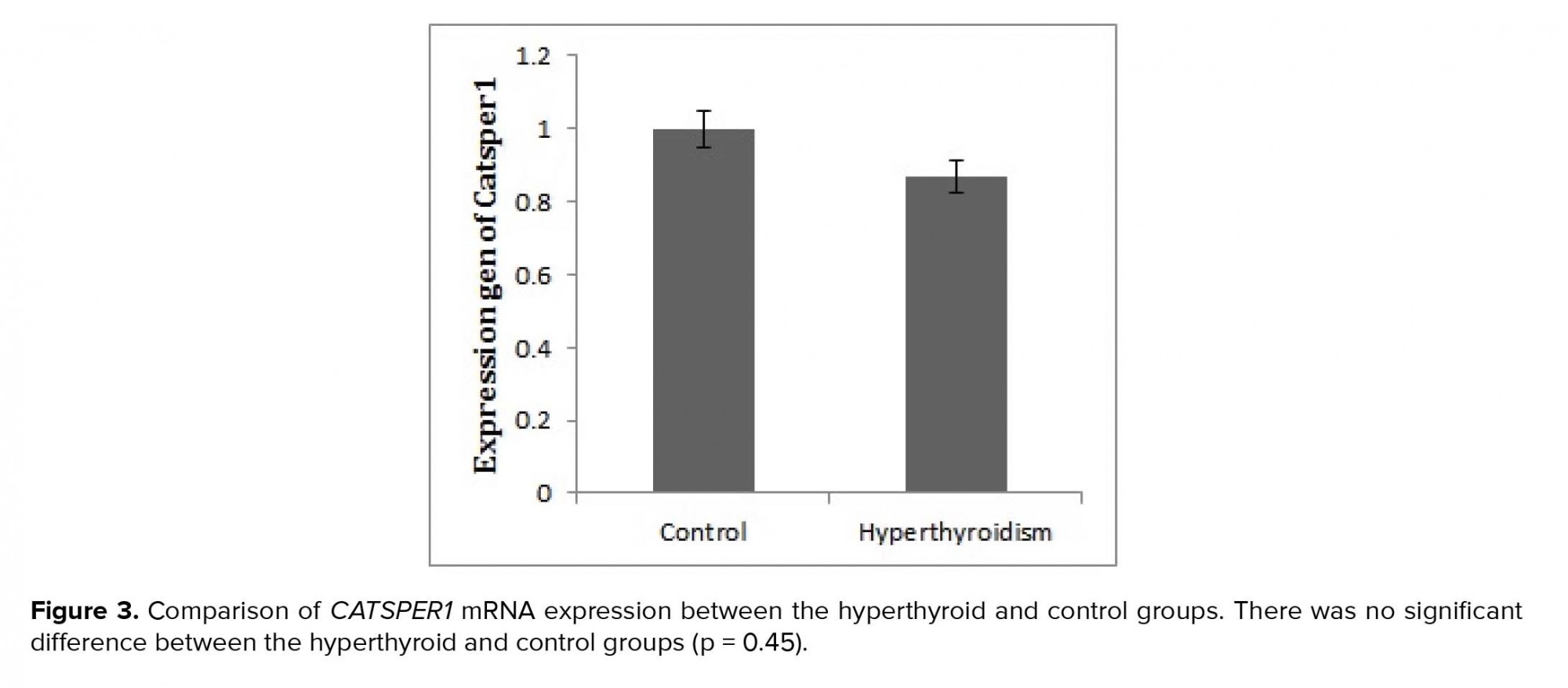
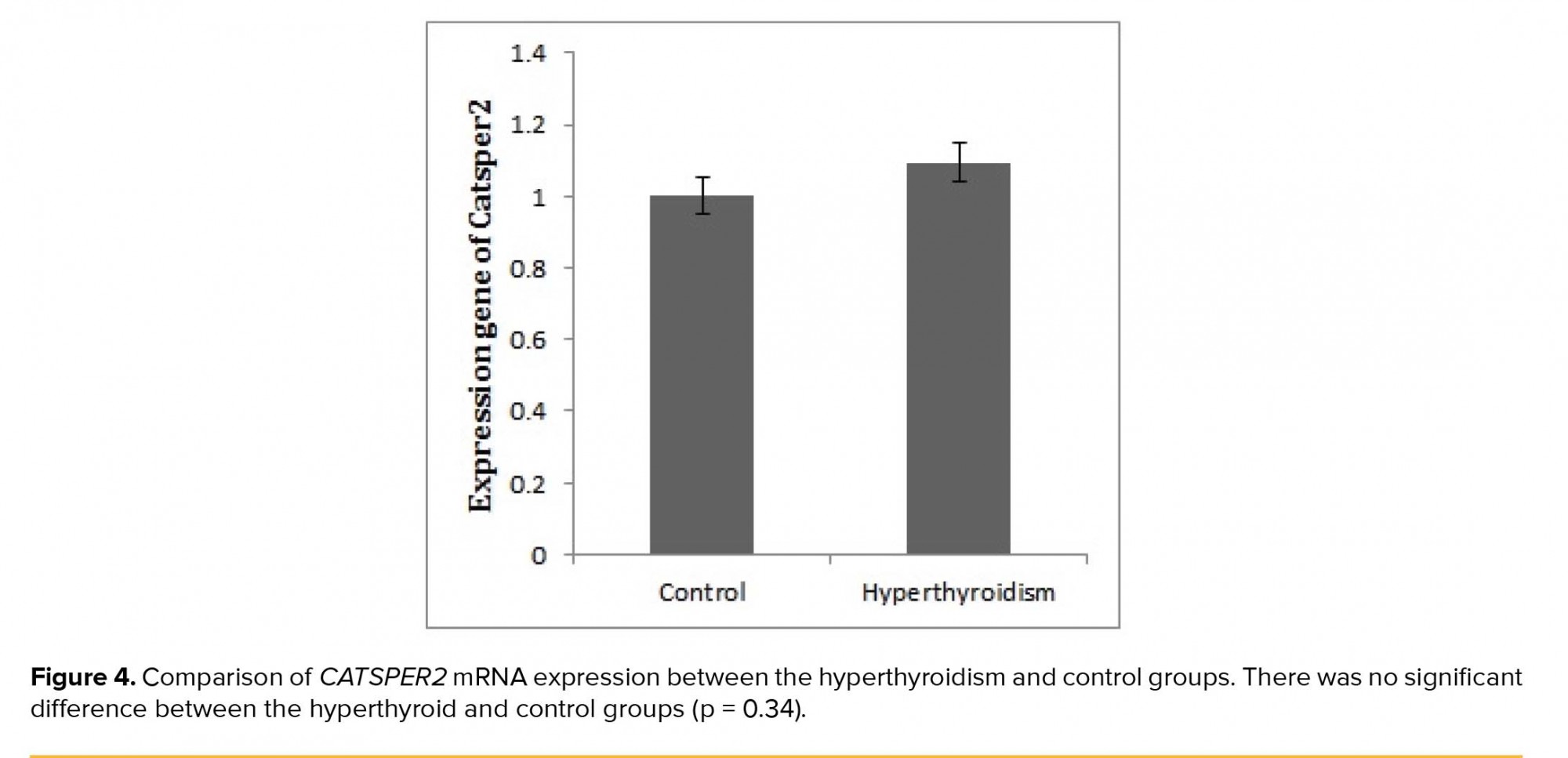
4. Discussion
According to the previous study, hyperthyroidism leads to the reduction of some parameters in the Sertoli and Leydig cells in the testes and cellular function in the spermatogenesis process (19). Considering the significant role of CATSPER in the molecular and functional structure of the sperm and its motility (11), the present study was targeted toward the investigation of changes in CATSPER gene and protein in hyperthyroidism cases. In our study, not only the expression rates of both CATSPER1 and CATSPER2 genes were not significantly different between the hyperthyroidism and control groups, but also the immunohistochemically reactions of anti- CATSPER1 and CATSPER2 antibodies between the two groups. Western blot analysis indicates that the expression level of the CATSPER1 gene is directly related to sperm with progressive motility. In addition, the expression of this gene in sperm with normal seminal fluid environmental conditions increased as compared to sperm that do not have these Optima conditions (20).
Meanwhile, the expression of CATSPER1 has a direct relationship with the sperm speed and quality and an inverse relationship with aging. Accordingly, the dead sperms have a low percentage of CATSPER1 expression (21). In a couple of studies performed by Mohammedi and colleagues on male mice treated with cadmium and nickel, there was a significant difference between the experimental and control groups regarding the expressions of CATSPER1 and CATSPER2 genes, which is not similar to our results. Aside from the toxic effects of selenium on most cells and tissues, it seems that it also has its undesirable effects on CATSPER1,2 gene and protein expression (22, 23). In another study on adult male mice treated with L-carnitine, the expression rate of CATSPER1 protein and immunohistochemical reaction of the sperm (head, middle piece, and tail) increased in the experimental group compared to those in the control group. They also obtained similar results regarding CATSPER2 protein. On the contrary, our results showed a reduction in staining intensity in the hyperthyroidism mice compared to that in the control group (24). Previous findings showed that in hypothyroid mice, hypothyroidism has an effect on the expression CATSPER1,2 gene and protein that results in significant difference between the experimental group and the control group (25). However, the results of this study on the effects of hyperthyroidism on the aforementioned factors did not show any significant differences in the experimental group compared to the control group. Therefore, it can be concluded that the decrease in thyroid hormone levels may affect the CATSPER1,2 gene and protein expression (25). In varicocele disease, sgnificant increase was observed in the CATSPER1,2 gene and protein expression in the experimental group compared to the control group, which did not match with the results of our research in terms of significance. In this regard, study on varicocele was associated with increased testicular temperature. So, increasing temperature may also, in addition to destructive effect on testicular tissue, effect on increasing significant difference on CATSPER1,2 gene and protein expression (26).
Limitations
Blood collection from mice was challenging due to their low blood volume; thus, we used gel tubes provided by the laboratory and performed the required evaluations.
5. Conclusion
As the findings of the present study indicated, hyperthyroidism has no significant changes in the expressions of both CATSPER1 and CATSPER2 genes. In addition, no reaction was observed in the histochemical staining intensity of CATSPER1. Regarding the CATSPER2, the staining intensity in hyperthyroid specimens was weak.Acknowledgments
The authors would like to thank all their colleagues and assistances who helped them in conducting the present study. This study was funded by a grant from the Research Council of Mashhad University of Medical Sciences. It was conducted under contract no. 940347.
Conflict of Interest
There is no conflict of interests.
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








