Wed, Feb 18, 2026
[Archive]
Volume 18, Issue 8 (August 2020)
IJRM 2020, 18(8): 571-578 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Tahtamouni L H, Hamdan M N, Al-Mazaydeh Z A, Bawadi R M, Rammaha M S, Zghoul A M, et al . Alu-repeat polymorphism in the tissue plasminogen activator (t-PA) gene, seminal t-PA concentration, and male fertility impairment: A case-control study. IJRM 2020; 18 (8) :571-578
URL: http://ijrm.ir/article-1-1510-en.html
URL: http://ijrm.ir/article-1-1510-en.html
Lubna Hamid Tahtamouni *1 

 , Mahmoud Nael Hamdan2
, Mahmoud Nael Hamdan2 

 , Zainab Ali Al-Mazaydeh2
, Zainab Ali Al-Mazaydeh2 

 , Randa Mahmoud Bawadi3
, Randa Mahmoud Bawadi3 

 , Majdoleen Sobhi Rammaha2
, Majdoleen Sobhi Rammaha2 

 , Ahmad Mohammad Zghoul2
, Ahmad Mohammad Zghoul2 

 , Mamoun Ahmad Ahram3
, Mamoun Ahmad Ahram3 

 , Salem Yasin2
, Salem Yasin2 




 , Mahmoud Nael Hamdan2
, Mahmoud Nael Hamdan2 

 , Zainab Ali Al-Mazaydeh2
, Zainab Ali Al-Mazaydeh2 

 , Randa Mahmoud Bawadi3
, Randa Mahmoud Bawadi3 

 , Majdoleen Sobhi Rammaha2
, Majdoleen Sobhi Rammaha2 

 , Ahmad Mohammad Zghoul2
, Ahmad Mohammad Zghoul2 

 , Mamoun Ahmad Ahram3
, Mamoun Ahmad Ahram3 

 , Salem Yasin2
, Salem Yasin2 


1- Department of Biology and Biotechnology, Faculty of Science, the Hashemite University, Zarqa, Jordan. Department of Biochemistry and Molecular Biology, College of Natural Sciences, Colorado State University, Fort Collins, Colorado, USA. , lubnatahtamuni@hu.edu.jo
2- Department of Biology and Biotechnology, Faculty of Science, the Hashemite University, Zarqa, Jordan.
3- Department of Physiology and Biochemistry, School of Medicine, the University of Jordan, Amman, Jordan.
2- Department of Biology and Biotechnology, Faculty of Science, the Hashemite University, Zarqa, Jordan.
3- Department of Physiology and Biochemistry, School of Medicine, the University of Jordan, Amman, Jordan.
Full-Text [PDF 377 kb]
(1403 Downloads)
| Abstract (HTML) (2683 Views)
The primary hormones involved in the male reproductive system are testosterone, luteinizing hormone, and follicle-stimulating hormone. Testosterone is responsible for the development of male characteristics and regulates the gene expression or activates signaling pathways in Sertoli cells that are required to maintain spermatogenesis. Luteinizing hormone stimulates the production of testosterone by Leydig cells, and follicle-stimulating hormone is necessary to induce Sertoli cells to secrete androgen binding-protein (4-8).
Infertility is defined as the inability of couples in reproductive age to achieve pregnancy after one year of unprotected intercourse. Worldwide, approximately 10-15% of couples are considered infertile. According to the World Health Organization (WHO), male factors are diagnosed in almost 50% of infertility cases, either solely (20%) or in combination with female factor (30-40%) (9-12).
Sertoli cells play critical roles during spermatogenesis by providing optimal environment for germ cell development as well phagocytosing residual bodies shed by developing germ cells (13).
In addition, Sertoli cells are responsible for secreting tissue plasminogen activator (t-PA) (14-15). t-PA is a protein involved in the fibrinolytic system that converts plasminogen, an inactive proenzyme, into the active protease plasmin that dissolves fibrin clot. The extracellular proteolysis mediated by plasminogen activators (PA) is associated with many important biological processes such as tissue remodeling, tissue destruction, and cell migration, in addition to some reproductive events such as ovulation, luteolysis, and embryo implantation. The activity of t-PA is controlled by plasminogen activator inhibitor-1 (PAI-1), which regulates t-PA activity by binding to t-PA’s active site, preventing the formation of plasmin (16-18).
In the human genome, t-PA is located at chromosome 8p12-p11.2 (19). Many studies on human population have indicated a 300-bp Alu repeat sequence insertion within intron 8 of the t-PA gene (20-23). One polymorphism was reported for this Alu repeat; either the presence of this repeat (Insertion/Alu+) or its absence (Deletion/Alu-) (21). The Alu polymorphism in the t-PA gene might play a role in the primary structure of the protein, and it may affect its secretion rate or plasma level (22). It was reported that there is an association between Alu polymorphism in the t-PA gene and t-PA plasma levels (23). However, the plasma level of t-PA is not only dependent on the secretion of t-PA but also on its rate and degree of complex-formation with PAI-1 (23). During spermatogenesis, t-PA has a role in the transport of preleptotene primary spermatocytes, through the blood-testis barrier, from the basal to the adluminal compartments of the seminiferous tubules. In addition, t-PA is involved during spermiation (14-15, 24-26). However, and as far as our knowledge is concerned, the association between t-PA Alu polymorphism and spermatogenesis has not been established. Thus, the current study aimed at studying the association between Alu polymorphism in the t-PA gene and male infertility.
DNA amplification by polymerase chain reaction (PCR) in a thermal cycler (MyCycler, Bio-Rad, USA) was performed following these conditions: initial denaturation for 2 min at 96ºC, followed by 35 cycles of denaturation for 30 sec at 96ºC, annealing for 30 sec at 65ºC and elongation for 30 sec at 96ºC. The total volume of each reaction was 30 µl containing 1 µl of each forward (GTAACCATTTAGTCCTCAGCTGTTCTCCT) and reverse (CCATGTAAGAGTAGAAGGAGACTCAGTCA) primers (28), 8 µl of nuclease-free water, 15 µl of the master mix (New England Biolabs, USA), and 5 µl of DNA sample.
PCR products were separated on 2% agarose gel electrophoresis containing 0.5 µg/ml ethidium bromide. Homozygote individuals carrying the t-PA Alu inserts are designated Alu +/+, heterozygotes as Alu +/-, and homozygotes for the absence of the insert as Alu -/-.
2.4. t-PA concentration and activity
Seminal total t-PA concentration was measured using the Human Total t-PA ELISA Kit (Assaypro LLC, USA). The absorbance on a microplate reader (Synergy HTX Multi-Mode Reader, USA) at a 450 nm was read immediately. On the other hand, seminal PAI-1/t-PA concentration was measured using the Human PAI-1/t-PA ELISA Kit (Assaypro LLC, USA). The activity of seminal t-PA was assayed using the Human t-PA Chromogenic Activity Kit (Assaypro LLC, USA). The absorbance was read at 405 nm for a zero-minute background reading and then was read every 1 hr on a microplate reader (Synergy HTX Multi-Mode Reader, USA) for 6 hr.
2.5. Ethical consideration
The study was approved by the Institutional Review Board (IRB) of the Hashemite University, which conforms to the World Medical Association Declaration of Helsinki (code: KTB/16/11/1800801).
2.6. Statistical analysis
All genotypes and frequencies for the insertion or deletion of the recruited individuals were calculated according to the counting method. The observed genotypes and alleles frequencies were compared with those expected in order to verify the Hardy-Weinberg equilibrium. To determine the differences between the two means, the Student’s t-test was performed, while the difference between the two proportions was calculated using the two-proportion test. Factorial ANOVA for higher orders (2-way or 3-way) was used to test for interactive effects for multiple categorical independent variables. Statistical analysis was performed using the Statistica software, StatSoft Inc., Tulsa, OK, USA (version 10). P-value < 0.05 was considered statistically significant.
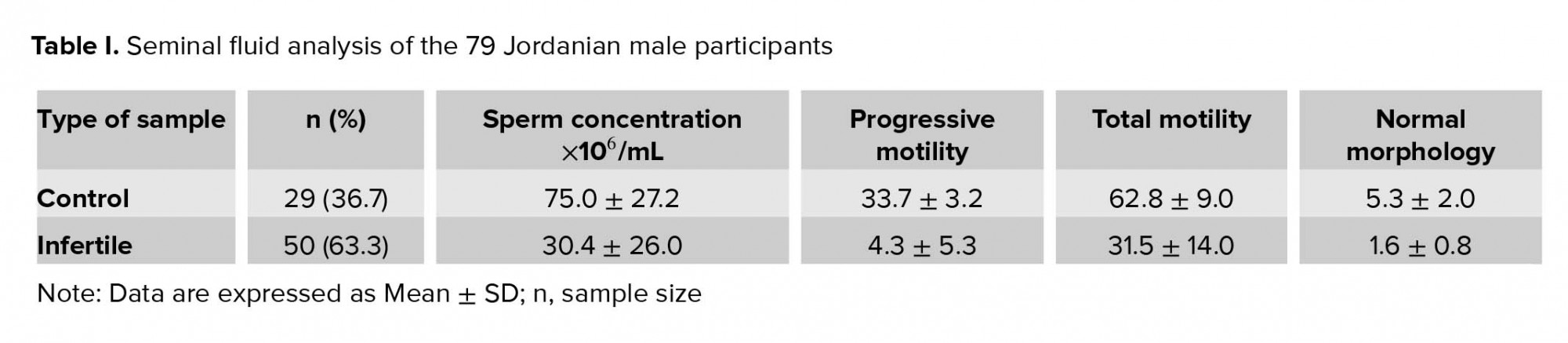
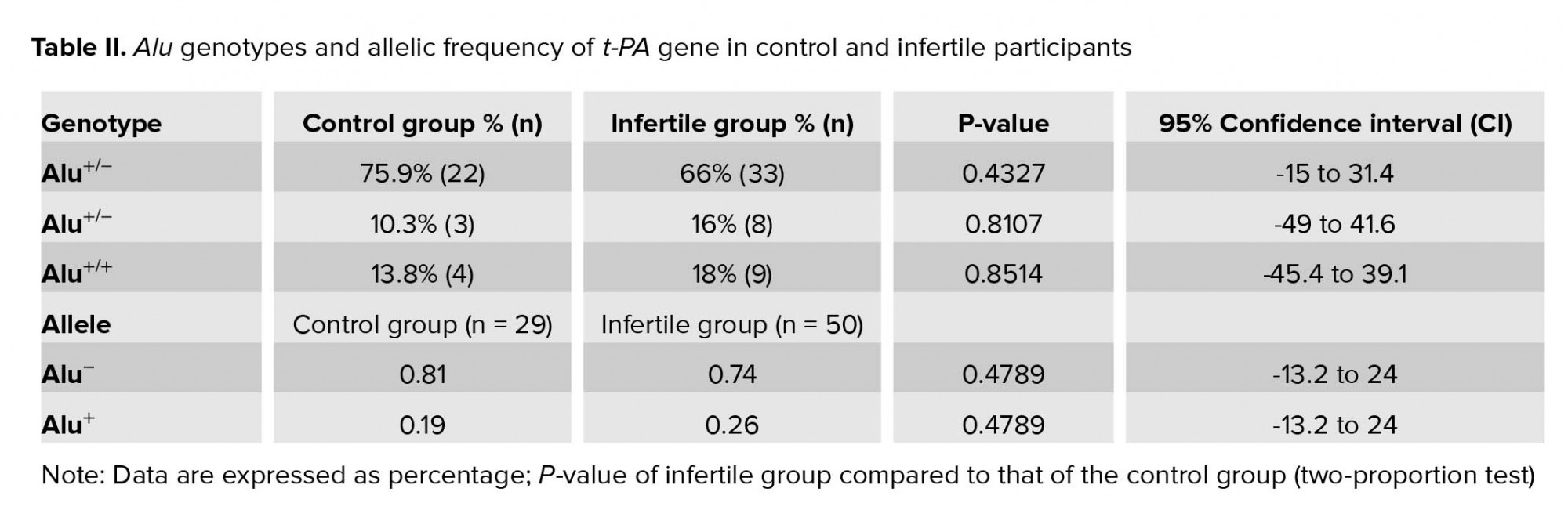
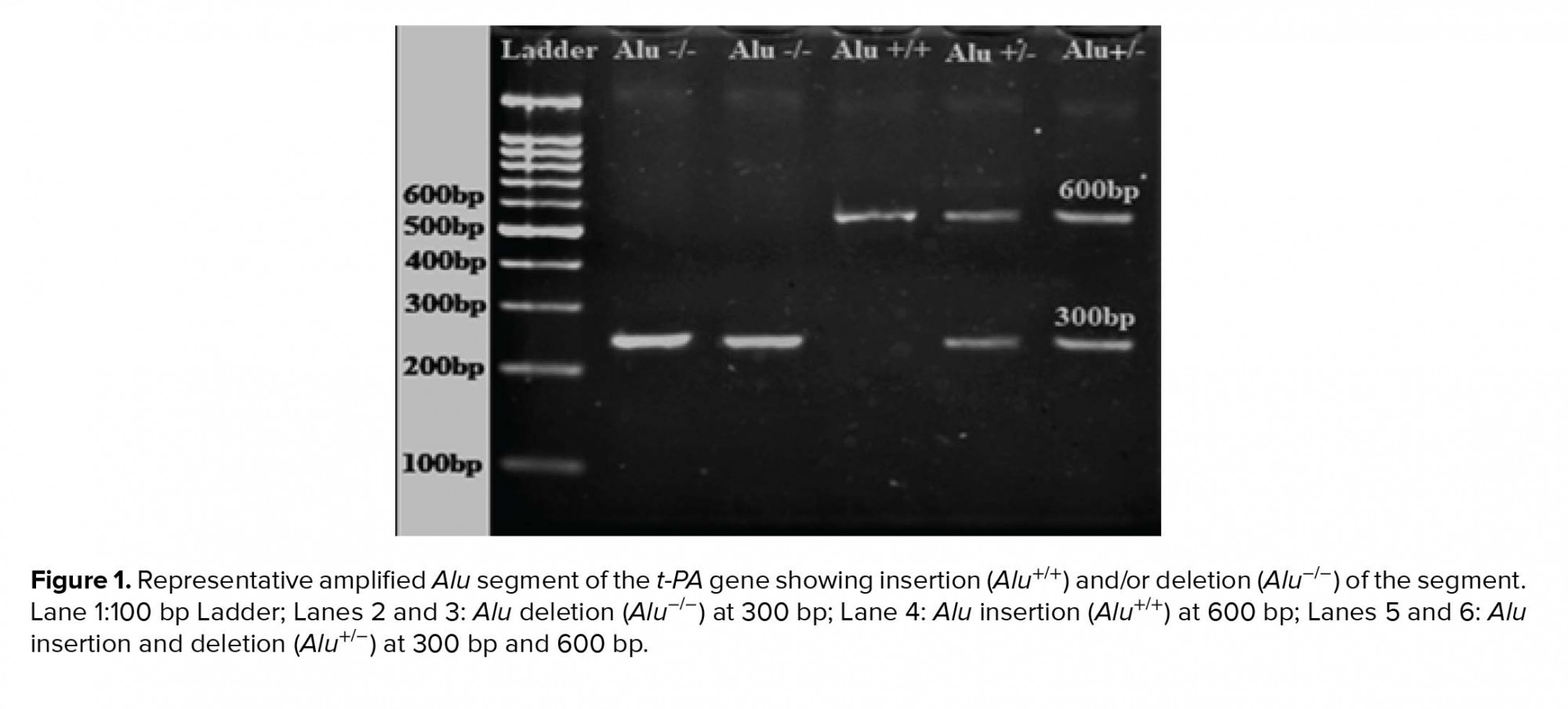
The Alu element of the t-PA gene was successfully amplified from all genomic DNA samples and PCR products were examined using 2% w/v agarose gel electrophoresis (Figure 1). Alu genotypes and allelic frequency of Alu insertion/deletion of the t-PA gene in control and infertile participation are shown in Table II. The results indicated that the percentage of infertile participants who were homozygotes for Alu+/+ was slightly decreased (66%) compared to the control group (75.9%); however, this decrease was insignificant (p = 0.43). On the other hand, the percentage of both Alu-/- homozygotes and heterozygotes Alu+/- were insignificantly increased in the infertile group (16%, and 18%, respectively) when compared to the control group (10.3% and 13.8%, respectively) (p = 0.81, and 0.85, respectively). The allelic frequency of the Alu‾ was decreased for the infertile group (0.74) when compared to the control group (0.81), though the decrease was insignificant (p = 0.48).
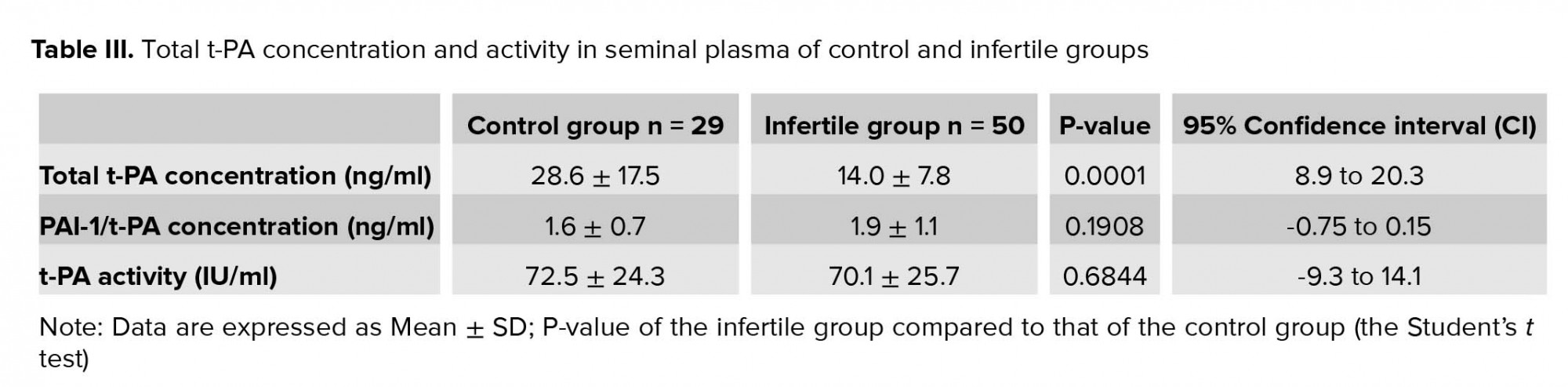
Total t-PA concentration, PAI-1/t-PA complex concentration, and t-PA activity in seminal plasma were assessed by ELISA assay for the control and infertile groups (Table III). The results show that the total seminal t-PA (free and complexed with PAI-1) was significantly lower in the infertile group in comparison to the control group (p = 0.0001, Table III). On the other hand, the concentration of seminal t-PA complexed with PAI-1 in the infertile group was higher than the control group; however, this increase was insignificant (p = 0.19). The decrease in concentration of total seminal t-PA and the increase in t-PA/PAI-1 reflected in reduced activity of t-PA in the infertile group, though this reduction was insignificant (p = 0.68, Table III).
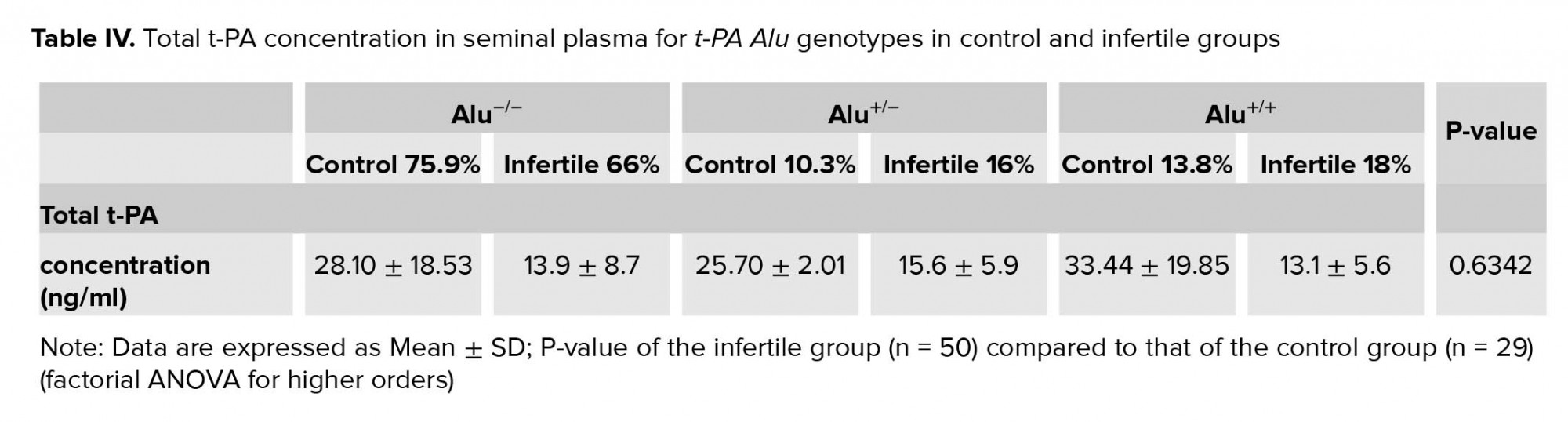
In order to assess if there was an association between Alu polymorphism in the t-PA gene and the total t-PA concentration in seminal plasma, multifactorial ANOVA test was performed. The results shown in Table IV indicate that the total t-PA concentration in the three t-PA Alu genotypes of the infertile group was lower than those of the control group. However, the statistical analysis of the results indicate that there was no association between the different t-PA Alu genotypes and the total t-PA seminal concentration in the infertile group when compared to the control group (p = 0.63, Table IV).
4. Discussion
In the current study, total t-PA concentration, PAI-1/t-PA complex concentration, and t-PA activity in seminal plasma for control and infertile groups were determined (Table III). The results show that the total seminal t-PA (free and complexed with PAI-1) was significantly lower in the infertile group in comparison with the control group (p= 0.0001, Table III). On the other hand, the concentration of seminal t-PA complexed with PAI-1 in the infertile group was higher than the control group; however, this increase was statistically insignificant (p = 0.19). The decrease in the concentration of the total seminal t-PA and the increase in the t-PA/PAI-1 were reflected in reduced activity of t-PA in the infertile group, although this reduction was insignificant (p = 0.68; Table III).
t-PA is a protein involved in the fibrinolytic system that converts the proenzyme plasminogen into the active protease plasmin that dissolves fibrin clot (17, 18). In addition, the extracellular proteolysis mediated by t-PA is associated with many important biological processes, such as tissue remodeling, tissue destruction, and cell migration. The activity of t-PA is controlled by PAI-1 (17, 18).
t-PA has been implicated in many diseases such as thrombotic disorders including strokes and myocardial infarction (29). In addition, it was found to be involved in the process of angiogenesis in cancer cells (30). Its concentration was reported to be increased in breast cancer and myocardial infarction patients. Furthermore, it has also been associated with polycystic ovary syndrome (PCOS) (29). Moreover, it was postulated that it has a role during spermatogenesis. Sertoli cells-secreted t-PA plays a role in the transport of preleptotene primary spermatocytes, through the blood-testis barrier, from the basal to the adluminal compartments of the seminiferous tubules. In addition, t-PA was also shown to play a role in the process of spermiation at stages IX-XII (14, 15, 24-26).
It was reported that the limiting factor of how much active t-PA is available in an organ is the capacity of the organ endothelium to increase its secretion of t-PA when required, that is, the major physiological regulator of local (organ level) fibrinolytic capacity is the secretion rate of t-PA (16, 18).
A single study reported the relation of t-PA concentration with male infertility. A higher concentration of t-PA was found in normal male compared with oligo/azoospermia patients. In addition, a higher number of immotile spermatozoa in the semen was associated with higher t-PA activities (31). The results presented in the current study is in accordance with their results in terms of concentration, but not activity (Table III). A possible explanation for the lower t-PA concentration could be due to the malfunction of the Sertoli cells, which it is the source of t-PA in the testis (14, 15, 24-26). However, testing this hypothesis is beyond the scope of this work.
Alu DNA fragment is a major driving force for evolution. The Alu element affects genes either negatively via inactivation of genes or positively by altering their function (32). The Alu (I/D) t-PA polymorphism occurs in intron 8 of the t-PA gene and consists of the presence (insertion; I or +) or absence (deletion; D or -) of Alu fragment. The three t-PA Alu genotypes are Alu⁺∕⁺, Alu-∕-, and the heterozygote genotype Alu⁺∕⁻ (21). A genetic polymorphism may affect the primary structure of a protein, but may also affect its synthetic rate or its plasma level if the protein involved is a secretory protein. However, several studies reported that Alu (I/D) polymorphism in t-PA locus gene does not affect the synthesis rate of t-PA (33, 34). Nevertheless, one study reported that homozygous carriers of Alu (I/I) t-PA polymorphism have an increased “in vivo” release rate of t-PA from vascular endothelial cells, even though this polymorphism is located in a non-coding area of the t-PA gene (22).
In the current study, the association between the total t-PA concentration and t-PA Alu genotypes in the normal and infertile participants was determined. The results indicate that there was no association between the different t-PA Alu genotypes and the total t-PA seminal concentration in the infertile group when compared to the control group (p = 0.63; Table IV). The small sample size of the current study could be the reason behind this insignificant association.
5. Conclusion
In conclusion, data obtained from the current study does not support the presence of an association between t-PA Alu polymorphism and t-PA seminal concentration or male infertility.
Acknowledgements
The authors are grateful to the Faculty of Graduate Studies, the Hashemite University for supporting the current work. They also want to acknowledge the effort of the attending physicians and nursing staff of the Assisted Reproductive Unit at the Jordan University Hospital, Farah Hospital, and Noor Fertility Center, Amman, Jordan.
Conflict of interest
The authors declare that there is no conflict of interest.
Full-Text: (770 Views)
- Introduction
The primary hormones involved in the male reproductive system are testosterone, luteinizing hormone, and follicle-stimulating hormone. Testosterone is responsible for the development of male characteristics and regulates the gene expression or activates signaling pathways in Sertoli cells that are required to maintain spermatogenesis. Luteinizing hormone stimulates the production of testosterone by Leydig cells, and follicle-stimulating hormone is necessary to induce Sertoli cells to secrete androgen binding-protein (4-8).
Infertility is defined as the inability of couples in reproductive age to achieve pregnancy after one year of unprotected intercourse. Worldwide, approximately 10-15% of couples are considered infertile. According to the World Health Organization (WHO), male factors are diagnosed in almost 50% of infertility cases, either solely (20%) or in combination with female factor (30-40%) (9-12).
Sertoli cells play critical roles during spermatogenesis by providing optimal environment for germ cell development as well phagocytosing residual bodies shed by developing germ cells (13).
In addition, Sertoli cells are responsible for secreting tissue plasminogen activator (t-PA) (14-15). t-PA is a protein involved in the fibrinolytic system that converts plasminogen, an inactive proenzyme, into the active protease plasmin that dissolves fibrin clot. The extracellular proteolysis mediated by plasminogen activators (PA) is associated with many important biological processes such as tissue remodeling, tissue destruction, and cell migration, in addition to some reproductive events such as ovulation, luteolysis, and embryo implantation. The activity of t-PA is controlled by plasminogen activator inhibitor-1 (PAI-1), which regulates t-PA activity by binding to t-PA’s active site, preventing the formation of plasmin (16-18).
In the human genome, t-PA is located at chromosome 8p12-p11.2 (19). Many studies on human population have indicated a 300-bp Alu repeat sequence insertion within intron 8 of the t-PA gene (20-23). One polymorphism was reported for this Alu repeat; either the presence of this repeat (Insertion/Alu+) or its absence (Deletion/Alu-) (21). The Alu polymorphism in the t-PA gene might play a role in the primary structure of the protein, and it may affect its secretion rate or plasma level (22). It was reported that there is an association between Alu polymorphism in the t-PA gene and t-PA plasma levels (23). However, the plasma level of t-PA is not only dependent on the secretion of t-PA but also on its rate and degree of complex-formation with PAI-1 (23). During spermatogenesis, t-PA has a role in the transport of preleptotene primary spermatocytes, through the blood-testis barrier, from the basal to the adluminal compartments of the seminiferous tubules. In addition, t-PA is involved during spermiation (14-15, 24-26). However, and as far as our knowledge is concerned, the association between t-PA Alu polymorphism and spermatogenesis has not been established. Thus, the current study aimed at studying the association between Alu polymorphism in the t-PA gene and male infertility.
- Materials and Methods
- 1. Study population
- 2. Sample collection
- 3. Alu polymorphism
DNA amplification by polymerase chain reaction (PCR) in a thermal cycler (MyCycler, Bio-Rad, USA) was performed following these conditions: initial denaturation for 2 min at 96ºC, followed by 35 cycles of denaturation for 30 sec at 96ºC, annealing for 30 sec at 65ºC and elongation for 30 sec at 96ºC. The total volume of each reaction was 30 µl containing 1 µl of each forward (GTAACCATTTAGTCCTCAGCTGTTCTCCT) and reverse (CCATGTAAGAGTAGAAGGAGACTCAGTCA) primers (28), 8 µl of nuclease-free water, 15 µl of the master mix (New England Biolabs, USA), and 5 µl of DNA sample.
PCR products were separated on 2% agarose gel electrophoresis containing 0.5 µg/ml ethidium bromide. Homozygote individuals carrying the t-PA Alu inserts are designated Alu +/+, heterozygotes as Alu +/-, and homozygotes for the absence of the insert as Alu -/-.
2.4. t-PA concentration and activity
Seminal total t-PA concentration was measured using the Human Total t-PA ELISA Kit (Assaypro LLC, USA). The absorbance on a microplate reader (Synergy HTX Multi-Mode Reader, USA) at a 450 nm was read immediately. On the other hand, seminal PAI-1/t-PA concentration was measured using the Human PAI-1/t-PA ELISA Kit (Assaypro LLC, USA). The activity of seminal t-PA was assayed using the Human t-PA Chromogenic Activity Kit (Assaypro LLC, USA). The absorbance was read at 405 nm for a zero-minute background reading and then was read every 1 hr on a microplate reader (Synergy HTX Multi-Mode Reader, USA) for 6 hr.
2.5. Ethical consideration
The study was approved by the Institutional Review Board (IRB) of the Hashemite University, which conforms to the World Medical Association Declaration of Helsinki (code: KTB/16/11/1800801).
2.6. Statistical analysis
All genotypes and frequencies for the insertion or deletion of the recruited individuals were calculated according to the counting method. The observed genotypes and alleles frequencies were compared with those expected in order to verify the Hardy-Weinberg equilibrium. To determine the differences between the two means, the Student’s t-test was performed, while the difference between the two proportions was calculated using the two-proportion test. Factorial ANOVA for higher orders (2-way or 3-way) was used to test for interactive effects for multiple categorical independent variables. Statistical analysis was performed using the Statistica software, StatSoft Inc., Tulsa, OK, USA (version 10). P-value < 0.05 was considered statistically significant.
- Results
The Alu element of the t-PA gene was successfully amplified from all genomic DNA samples and PCR products were examined using 2% w/v agarose gel electrophoresis (Figure 1). Alu genotypes and allelic frequency of Alu insertion/deletion of the t-PA gene in control and infertile participation are shown in Table II. The results indicated that the percentage of infertile participants who were homozygotes for Alu+/+ was slightly decreased (66%) compared to the control group (75.9%); however, this decrease was insignificant (p = 0.43). On the other hand, the percentage of both Alu-/- homozygotes and heterozygotes Alu+/- were insignificantly increased in the infertile group (16%, and 18%, respectively) when compared to the control group (10.3% and 13.8%, respectively) (p = 0.81, and 0.85, respectively). The allelic frequency of the Alu‾ was decreased for the infertile group (0.74) when compared to the control group (0.81), though the decrease was insignificant (p = 0.48).
Total t-PA concentration, PAI-1/t-PA complex concentration, and t-PA activity in seminal plasma were assessed by ELISA assay for the control and infertile groups (Table III). The results show that the total seminal t-PA (free and complexed with PAI-1) was significantly lower in the infertile group in comparison to the control group (p = 0.0001, Table III). On the other hand, the concentration of seminal t-PA complexed with PAI-1 in the infertile group was higher than the control group; however, this increase was insignificant (p = 0.19). The decrease in concentration of total seminal t-PA and the increase in t-PA/PAI-1 reflected in reduced activity of t-PA in the infertile group, though this reduction was insignificant (p = 0.68, Table III).
In order to assess if there was an association between Alu polymorphism in the t-PA gene and the total t-PA concentration in seminal plasma, multifactorial ANOVA test was performed. The results shown in Table IV indicate that the total t-PA concentration in the three t-PA Alu genotypes of the infertile group was lower than those of the control group. However, the statistical analysis of the results indicate that there was no association between the different t-PA Alu genotypes and the total t-PA seminal concentration in the infertile group when compared to the control group (p = 0.63, Table IV).





4. Discussion
In the current study, total t-PA concentration, PAI-1/t-PA complex concentration, and t-PA activity in seminal plasma for control and infertile groups were determined (Table III). The results show that the total seminal t-PA (free and complexed with PAI-1) was significantly lower in the infertile group in comparison with the control group (p= 0.0001, Table III). On the other hand, the concentration of seminal t-PA complexed with PAI-1 in the infertile group was higher than the control group; however, this increase was statistically insignificant (p = 0.19). The decrease in the concentration of the total seminal t-PA and the increase in the t-PA/PAI-1 were reflected in reduced activity of t-PA in the infertile group, although this reduction was insignificant (p = 0.68; Table III).
t-PA is a protein involved in the fibrinolytic system that converts the proenzyme plasminogen into the active protease plasmin that dissolves fibrin clot (17, 18). In addition, the extracellular proteolysis mediated by t-PA is associated with many important biological processes, such as tissue remodeling, tissue destruction, and cell migration. The activity of t-PA is controlled by PAI-1 (17, 18).
t-PA has been implicated in many diseases such as thrombotic disorders including strokes and myocardial infarction (29). In addition, it was found to be involved in the process of angiogenesis in cancer cells (30). Its concentration was reported to be increased in breast cancer and myocardial infarction patients. Furthermore, it has also been associated with polycystic ovary syndrome (PCOS) (29). Moreover, it was postulated that it has a role during spermatogenesis. Sertoli cells-secreted t-PA plays a role in the transport of preleptotene primary spermatocytes, through the blood-testis barrier, from the basal to the adluminal compartments of the seminiferous tubules. In addition, t-PA was also shown to play a role in the process of spermiation at stages IX-XII (14, 15, 24-26).
It was reported that the limiting factor of how much active t-PA is available in an organ is the capacity of the organ endothelium to increase its secretion of t-PA when required, that is, the major physiological regulator of local (organ level) fibrinolytic capacity is the secretion rate of t-PA (16, 18).
A single study reported the relation of t-PA concentration with male infertility. A higher concentration of t-PA was found in normal male compared with oligo/azoospermia patients. In addition, a higher number of immotile spermatozoa in the semen was associated with higher t-PA activities (31). The results presented in the current study is in accordance with their results in terms of concentration, but not activity (Table III). A possible explanation for the lower t-PA concentration could be due to the malfunction of the Sertoli cells, which it is the source of t-PA in the testis (14, 15, 24-26). However, testing this hypothesis is beyond the scope of this work.
Alu DNA fragment is a major driving force for evolution. The Alu element affects genes either negatively via inactivation of genes or positively by altering their function (32). The Alu (I/D) t-PA polymorphism occurs in intron 8 of the t-PA gene and consists of the presence (insertion; I or +) or absence (deletion; D or -) of Alu fragment. The three t-PA Alu genotypes are Alu⁺∕⁺, Alu-∕-, and the heterozygote genotype Alu⁺∕⁻ (21). A genetic polymorphism may affect the primary structure of a protein, but may also affect its synthetic rate or its plasma level if the protein involved is a secretory protein. However, several studies reported that Alu (I/D) polymorphism in t-PA locus gene does not affect the synthesis rate of t-PA (33, 34). Nevertheless, one study reported that homozygous carriers of Alu (I/I) t-PA polymorphism have an increased “in vivo” release rate of t-PA from vascular endothelial cells, even though this polymorphism is located in a non-coding area of the t-PA gene (22).
In the current study, the association between the total t-PA concentration and t-PA Alu genotypes in the normal and infertile participants was determined. The results indicate that there was no association between the different t-PA Alu genotypes and the total t-PA seminal concentration in the infertile group when compared to the control group (p = 0.63; Table IV). The small sample size of the current study could be the reason behind this insignificant association.
5. Conclusion
In conclusion, data obtained from the current study does not support the presence of an association between t-PA Alu polymorphism and t-PA seminal concentration or male infertility.
Acknowledgements
The authors are grateful to the Faculty of Graduate Studies, the Hashemite University for supporting the current work. They also want to acknowledge the effort of the attending physicians and nursing staff of the Assisted Reproductive Unit at the Jordan University Hospital, Farah Hospital, and Noor Fertility Center, Amman, Jordan.
Conflict of interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. Scanlon VC, Sanders T. Essentials of anatomy and physiology. 7th Ed. Philadelphia: FA Davis Company; 2015.
2. Neto FT, Bach PV, Najari BB, Li PS, Goldstein M. Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol 2016; 59: 10-26. [DOI:10.1016/j.semcdb.2016.04.009] [PMID]
3. Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011; 1: 116-120. [DOI:10.4161/spmg.1.2.16956] [PMID] [PMCID]
4. Sharpe R. Regulation of spermatogenesis. In: Knobil E, Neill JD (eds). The physiology of reproduction. New York: Raven Press; 1994: 1363-1434.
5. Ramaswamy S, Marshall GR, Pohl CR, Friedman RL, Plant TM. Inhibitory and stimulatory regulation of testicular inhibin B secretion by luteinizing hormone and follicle-stimulating hormone, respectively, in the rhesus monkey (Macaca mulatta). Endocrinology 2003; 144: 1175-1185. [DOI:10.1210/en.2002-221078] [PMID]
6. O'shaughnessy PJ, Monteiro A, Verhoeven G, De Gendt K, Abel MH. Effect of FSH on testicular morphology and spermatogenesis in gonadotrophin-deficient hypogonadal mice lacking androgen receptors. Reproduction 2010; 139: 177-184. [DOI:10.1530/REP-09-0377] [PMID] [PMCID]
7. Abel MH, Widen A, Wang X, Huhtaniemi I, Pakarinen P, Kumar TR, et al. Pituitary gonadotrophic hormone synthesis, secretion, subunit gene expression and cell structure in normal and follicle‐stimulating hormone β knockout, follicle‐stimulating hormone receptor knockout, luteinising hormone receptor knockout, hypogonadal and ovariectomised female mice. J Neuroendocrinol 2014; 26: 785-795. [DOI:10.1111/jne.12178] [PMID] [PMCID]
8. Ramaswamy S, Weinbauer GF. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 2015; 4: e996025. [DOI:10.1080/21565562.2014.996025] [PMID] [PMCID]
9. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International committee for monitoring assisted reproductive technology, World Health Organization. The international committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod 2009; 24: 2683-2687. [DOI:10.1093/humrep/dep343] [PMID]
10. Olayemi FO. A review on some causes of male infertility. Afr J Biotechnol 2010; 9: 2834-2842.
11. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci 2015; 8: 191-196. [DOI:10.4103/0974-1208.170370] [PMID] [PMCID]
12. Dimitriadis F, Adonakis G, Kaponis A, Mamoulakis C, Takenaka A, Sofikitis N. Pre-testicular, testicular, and post-testicular causes of male infertility. In: Simoni M, Huhtaniemi I (eds). Endocrinology of the testis and male reproduction. Cham: Springer; 2017: 1-47. [DOI:10.1007/978-3-319-29456-8_33-1]
13. Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol 1998; 9: 411-416. [DOI:10.1006/scdb.1998.0203] [PMID]
14. Liu YX, Liu K, Zhou HM, Du Q, Hu ZY, Zou RJ. Hormonal regulation of tissue-type plasminogen activator and plasminogen activator inhibitor type-1 in cultured monkey Sertoli cells. Hum Reprod 1995; 10: 719-727. [DOI:10.1093/oxfordjournals.humrep.a136022] [PMID]
15. Zhang T, Zhou HM, Liu YX. Expression of plasminogen activator and inhibitor, urokinase receptor and inhibin subunits in rhesus monkey testes. Mol Hum Reprod 1997; 3: 223-231.
https://doi.org/10.1093/molehr/3.11.945 [DOI:10.1093/molehr/3.3.223]
16. Hrafnkelsdottir T, Gudnason T, Wall U, Jern C, Jern S. Regulation of local availability of active tissue‐type plasminogen activator in vivo in man. J Thromb Haemost 2004; 2: 1960-1968. [DOI:10.1111/j.1538-7836.2004.00948.x] [PMID]
17. Collen D, Lijnen HR. The tissue-type plasminogen activator story. Arterioscler Thromb Vasc Boil 2009; 29: 1151-1155. [DOI:10.1161/ATVBAHA.108.179655] [PMID]
18. Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor‐1 (PAI‐1): a key factor linking fibrinolysis and age‐related subclinical and clinical conditions. Cardiovasc Ther 2010; 28: e72-e91. [DOI:10.1111/j.1755-5922.2010.00171.x] [PMID] [PMCID]
19. Ny T, Elgh F, Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci USA 1984; 81: 5355-5359. [DOI:10.1073/pnas.81.17.5355] [PMID] [PMCID]
20. Yang-Feng TL, Opdenakker G, Volckaert G, Franke U. Human tissue-type plasminogen activator gene located near chromosomal breakpoint in myeloproliferative disorder. Am J Hum Genet 1986; 39: 79-87.
21. Ludwig M, Wohn KD, Schleuning WD, Olek K. Allelic dimorphism in the human tissue-type plasminogen activator (TPA) gene as a result of an Alu insertion/deletion event. Hum Genet 1992; 88: 388-392. [DOI:10.1007/BF00215671] [PMID]
22. Jern C, Ladenvall P, Wall U, Jern S. Gene polymorphism of t-PA is associated with forearm vascular release rate of t-PA. Arterioscler Thromb Vasc Boil 1999; 19: 454-459. [DOI:10.1161/01.ATV.19.2.454] [PMID]
23. Chandler WL, Levy WC, Stratton JR. The circulatory regulation of TPA and UPA secretion, clearance, and inhibition during exercise and during the infusion of isoproterenol and phenylephrine. Circulation 1995; 92: 2984-2994. [DOI:10.1161/01.CIR.92.10.2984] [PMID]
24. Bourdon V, Defamie N, Fenichel P, Pointis G. Regulation of tissue-type plasminogen activator and its inhibitor (PAI-1) by lipopolysaccharide-induced phagocytosis in a Sertoli cell line. Exp Cell Res 1999; 247: 367-372. [DOI:10.1006/excr.1998.4369] [PMID]
25. Guo J, Shi YQ, Yang W, Li YC, Hu ZY, Liu YX. Testosterone upregulation of tissue type plasminogen activator expression in Sertoli cells. Endocrine 2007; 32: 83-89. [DOI:10.1007/s12020-007-9014-1] [PMID]
26. Liu YX. Involvement of plasminogen activator and plasminogen activator inhibitor type 1 in spermatogenesis, sperm capacitation, and fertilization. Semin Thromb Hemost 2007; 33: 29-40. [DOI:10.1055/s-2006-958459] [PMID]
27. World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th Ed. Switzerland: World Health Organization; 2010.
28. Hamdi HK, Reznik J, Castellon R, Atilano SR, Ong JM, Udar N, et al. Alu DNA polymorphism in ACE gene is protective for age-related macular degeneration. Biochem Biophys Res Comm 2002; 295: 668-672. [DOI:10.1016/S0006-291X(02)00728-3]
29. Valle-Garay E, Montes AH, Corte JR, Meana A, Fierer J, Asensi V. tPA Alu (I/D) polymorphism associates with bacterial osteomyelitis. J Infect Dis 2013; 208: 218-223. [DOI:10.1093/infdis/jit158] [PMID]
30. Nishida N, Yano H, Nishida T, Kamura T, Kojiro M. Angiogenesis in cancer. Vasc Health Risk Manag 2006; 2: 213-219. [DOI:10.2147/vhrm.2006.2.3.213] [PMID] [PMCID]
31. Liu K, Liu YX, Du Q, Zhou HM, Lin X, Hu ZY, et al. Preliminary studies on the role of plasminogen activator in seminal plasma of human and rhesus monkey. Mol Hum Reprod 1996; 2: 99-104. [DOI:10.1093/molehr/2.2.99] [PMID]
32. Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet 2002; 3: 370-379. [DOI:10.1038/nrg798] [PMID]
33. van den Eijnden-Schrauwen Y, Lakenberg N, Emeis JJ, de Knijff P. Alu-repeat polymorphism in the tissue-type plasminogen activator (tPA) gene does not affect basal endothelial tPA synthesis. Thromb Haemost 1995, 74: 1202. [DOI:10.1055/s-0038-1649907] [PMID]
34. Nikolopoulos GK, Bagos PG, Tsangaris I, Tsiara CG, Kopterides P, Vaiopoulos A, et al. The association between plasminogen activator inhibitor type 1 (PAI-1) levels, PAI-1 4G/5G polymorphism, and myocardial infarction: a Mendelian randomization meta-analysis. Clin Chem Lab Med 2014; 52: 937-950. [DOI:10.1515/cclm-2013-1124] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





