Thu, Feb 19, 2026
[Archive]
Volume 18, Issue 8 (August 2020)
IJRM 2020, 18(8): 605-610 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mercy Sylus A, Nandeesha H, Thiagaraju C. Matrix metalloproteinase-9 increases and Interleukin-10 reduces with increase in body mass index in polycystic ovary syndrome: A cross-sectional study. IJRM 2020; 18 (8) :605-610
URL: http://ijrm.ir/article-1-1533-en.html
URL: http://ijrm.ir/article-1-1533-en.html
1- Department of Biochemistry, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
2- Department of Biochemistry, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India. ,nandijipmer@gmail.com
3- Obstetrics and Gynecology, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
2- Department of Biochemistry, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India. ,
3- Obstetrics and Gynecology, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
Full-Text [PDF 265 kb]
(1119 Downloads)
| Abstract (HTML) (2608 Views)
Several investigators have emphasized the role of obesity as a contributing factor in the pathogenesis of PCOS. Body mass index (BMI) was found to be increased in 50-60% of women with PCOS (3). Obesity is known to be associated with infertility and the influence of obesity on the exacerbation of clinical features and hormonal disturbances of PCOS has been revealed the past by many earlier studies (4). Healthy dietary habits and weight reduction have shown a beneficial impact on the metabolic state, hyperandrogenemia, ovulatory function and pregnancy rates in PCOS suggesting the importance of maintaining a healthy body weight for women with PCOS (5).
Matrix metalloproteinase-9 (MMP-9) is a zinc-dependent enzyme involved in the tissue remodeling of the ovary and uterus, which is responsible for growth of the follicle, formation of corpus luteum and blastocyst implantation (6). Elevated MMP-9 expression has been reported to be involved in trophoblast invasion during pregnancy (7). Previous studies have hypothesized that increased MMP-9 levels might be related to menstrual irregularities and increased risk of cardiovascular disease in PCOS patients (6, 8).
Nitric oxide (NO) plays a crucial role in pregnancy and it promotes embryonic development, maintains the fetoplacental unit and sustains pregnancy (9). Reduced NO levels in women with PCOS were found to be related with spontaneous pregnancy (10).
Inflammation is usually associated with PCOS, which influences ovarian folliculogenesis, altered steroidogenesis in ovary and hyperinsulinemia in these women (11). Interleukin-10 (IL-10), an anti-inflammatory cytokine regulates the action of pro-inflammatory cytokines during inflammation. IL-10 gene polymorphism has been reported in PCOS and reduced IL-10 levels have been documented in women with PCOS (11, 12)
Since obesity and alteration in inflammation and matrix metalloproteinases are important underlying factors associated with the metabolic, hormonal and reproductive abnormalities in PCOS (13-15), this study was intended to determine the influence of increase in body mass index on MMP-9, nitric oxide and interleukin-10 in PCOS.
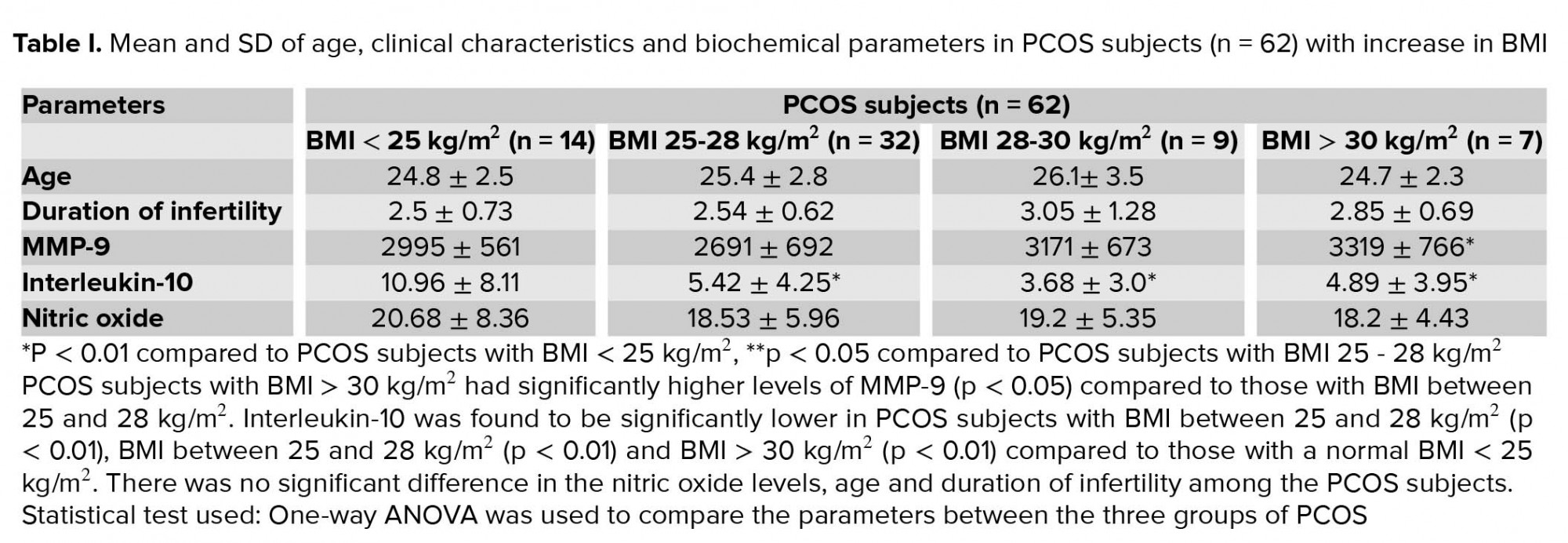
Table II shows the correlation of MMP-9 with age, BMI and biochemical parameters like IL-10 and NO in the PCOS subjects. MMP-9 was positively correlated with the duration of infertility (r = 0.253, p = 0.047) and negatively correlated with NO (r = -0.259, p = 0.042). IL-10 was negatively associated with BMI (r = -0.272, p = 0.033) and the duration of infertility was positively associated with age (r = 0.374, p = 0.003) in PCOS subjects.

4. Discussion
In the present study, MMP-9 was increased and IL-10 was reduced in PCOS women with increase in BMI. MMP-9 was associated with NO and duration of infertility in PCOS.
Several factors such as inflammation, endothelial dysfunction and altered ovarian remodelling are known to play a role in the pathogenesis of PCOS and its complications including infertility (17-19). Obesity has got a major influence on the clinical outcome of PCOS as evident by the presence of hyperandrogenism, insulin resistance, decreased ovulation as well as lower pregnancy rates in PCOS women with obesity in comparison with non-obese PCOS women (20).
MMP-9, a matrix metalloproteinase produced by the ovary is involved in various phases of female reproduction. High MMP-9 levels in PCOS adversely affects ovulation and fertility, as it alters the extracellular matrix remodelling thus causing aberrant follicular atresia and increased ovarian stromal tissue (21). MMP-9 is reported to be strongly associated with obesity and furthermore the presence of a high BMI has also been hypothesized to promote poor ovulation and thereby lesser chances of pregnancy (22). In our study, we observed that MMP-9 levels were higher in infertile women with PCOS having a BMI >30 kg/m2 compared to those with BMI between 25 and 28 kg/m2. These findings were supported by previous studies that have reported high MMP-9 in obese subjects (22, 23). As body weight increases, fat cells are enlarged and adipose tissue is expanded and differentiated. During differentiation of adipose tissue, the activity of MMP-9 is induced resulting in remodeling of stromal matrix (24).
NO has a pivotal role in endothelial function by which it regulates the uterine blood flow, relaxes the uterine myometrium and reduces the feto-placental vascular resistance during pregnancy (25) NO levels are found to be reduced both in obesity and PCOS subjects which might be associated with adverse pregnancy outcome (26, 27). In our study, we observed a reduction in the nitric oxide levels as BMI increased however, it was not statistically significant. Decreased serum NO levels in PCOS have been attributed to elevated asymmetrical dimethyl arginine (ADMA) in PCOS subjects associated with hyperandrogenemia and insulin resistance (28, 29).
Extensive studies have documented the role of inflammation in PCOS and reported an imbalance in the levels of pro and anti-inflammatory cytokines in these subjects (11). Obesity and inflammation are known to occur concurrently (30). The presence of hyperandrogenemia in PCOS stimulates adipocyte hypertrophy and hypoperfusion, release of inflammatory mediators and development of chronic inflammation, consequently resulting in insulin resistance and cardiovascular complications (28). Low levels of IL-10 were observed in PCOS and it was found to affect the pregnancy rates and outcomes (31). Our data demonstrated a significant reduction in the serum IL-10 levels of infertile women with PCOS who had a BMI in the range of 25-28 kg/m2, 28-30 kg/m2, and > 28 kg/m2 compared to those who had a normal BMI. Also IL-10 was negatively associated with BMI suggesting that as BMI increases, IL-10 reduces in PCOS subjects. These findings were in agreement with previous reports that demonstrate reduction in IL-10 levels in obesity and PCOS (31, 32). Since weight gain is associated with inflammation (33), there is reduction of anti-inflammatory cytokines like IL-10.
In the present study, MMP-9 levels negatively correlated with NO levels and positively correlated with the duration of infertility in PCOS subjects. These findings suggest that as the duration of infertility increases, the MMP-9 level increases which in turn reduces NO levels leading to the complications of PCOS.
In the present study we have investigated whether these markers are altered in PCOS women with increase in body weight / BMI. Alteration in these markers can lead to reduction in ovulation. Based on the findings of our study we suggest that weight reduction in PCOS can reduce MMP-9 and increase IL-10, which may improve ovulation in these subjects. The main limitations of the study are the small sample size and non-inclusion of healthy control groups. Moreover we did not estimate other inflammatory cytokines due to financial constraints.
5. Conclusion
The present study concludes that as BMI increases MMP-9 is increased and IL-10 is reduced in PCOS subjects. The association of MMP-9 with NO and duration of infertility indicates that high MMP-9 levels may cause endothelial dysfunction which might be responsible for complications in PCOS subjects with obesity. Further studies are required to investigate whether weight reduction reduces MMP-9 levels and the complications of PCOS in obese subjects.
Acknowledgements
The authors would like to thank JIPMER intramural fund for supporting this study.
Conflict of interest
The authors report no conflict of interest.
Full-Text: (555 Views)
- Introduction
Several investigators have emphasized the role of obesity as a contributing factor in the pathogenesis of PCOS. Body mass index (BMI) was found to be increased in 50-60% of women with PCOS (3). Obesity is known to be associated with infertility and the influence of obesity on the exacerbation of clinical features and hormonal disturbances of PCOS has been revealed the past by many earlier studies (4). Healthy dietary habits and weight reduction have shown a beneficial impact on the metabolic state, hyperandrogenemia, ovulatory function and pregnancy rates in PCOS suggesting the importance of maintaining a healthy body weight for women with PCOS (5).
Matrix metalloproteinase-9 (MMP-9) is a zinc-dependent enzyme involved in the tissue remodeling of the ovary and uterus, which is responsible for growth of the follicle, formation of corpus luteum and blastocyst implantation (6). Elevated MMP-9 expression has been reported to be involved in trophoblast invasion during pregnancy (7). Previous studies have hypothesized that increased MMP-9 levels might be related to menstrual irregularities and increased risk of cardiovascular disease in PCOS patients (6, 8).
Nitric oxide (NO) plays a crucial role in pregnancy and it promotes embryonic development, maintains the fetoplacental unit and sustains pregnancy (9). Reduced NO levels in women with PCOS were found to be related with spontaneous pregnancy (10).
Inflammation is usually associated with PCOS, which influences ovarian folliculogenesis, altered steroidogenesis in ovary and hyperinsulinemia in these women (11). Interleukin-10 (IL-10), an anti-inflammatory cytokine regulates the action of pro-inflammatory cytokines during inflammation. IL-10 gene polymorphism has been reported in PCOS and reduced IL-10 levels have been documented in women with PCOS (11, 12)
Since obesity and alteration in inflammation and matrix metalloproteinases are important underlying factors associated with the metabolic, hormonal and reproductive abnormalities in PCOS (13-15), this study was intended to determine the influence of increase in body mass index on MMP-9, nitric oxide and interleukin-10 in PCOS.
- Materials and Methods
- 1. Subjects
- 2. Clinical evaluation
- 3. Sample collection
- 4. Biochemical analysis
- 5. Ethical consideration
- 6. Statistical analysis
- Results
Table II shows the correlation of MMP-9 with age, BMI and biochemical parameters like IL-10 and NO in the PCOS subjects. MMP-9 was positively correlated with the duration of infertility (r = 0.253, p = 0.047) and negatively correlated with NO (r = -0.259, p = 0.042). IL-10 was negatively associated with BMI (r = -0.272, p = 0.033) and the duration of infertility was positively associated with age (r = 0.374, p = 0.003) in PCOS subjects.


4. Discussion
In the present study, MMP-9 was increased and IL-10 was reduced in PCOS women with increase in BMI. MMP-9 was associated with NO and duration of infertility in PCOS.
Several factors such as inflammation, endothelial dysfunction and altered ovarian remodelling are known to play a role in the pathogenesis of PCOS and its complications including infertility (17-19). Obesity has got a major influence on the clinical outcome of PCOS as evident by the presence of hyperandrogenism, insulin resistance, decreased ovulation as well as lower pregnancy rates in PCOS women with obesity in comparison with non-obese PCOS women (20).
MMP-9, a matrix metalloproteinase produced by the ovary is involved in various phases of female reproduction. High MMP-9 levels in PCOS adversely affects ovulation and fertility, as it alters the extracellular matrix remodelling thus causing aberrant follicular atresia and increased ovarian stromal tissue (21). MMP-9 is reported to be strongly associated with obesity and furthermore the presence of a high BMI has also been hypothesized to promote poor ovulation and thereby lesser chances of pregnancy (22). In our study, we observed that MMP-9 levels were higher in infertile women with PCOS having a BMI >30 kg/m2 compared to those with BMI between 25 and 28 kg/m2. These findings were supported by previous studies that have reported high MMP-9 in obese subjects (22, 23). As body weight increases, fat cells are enlarged and adipose tissue is expanded and differentiated. During differentiation of adipose tissue, the activity of MMP-9 is induced resulting in remodeling of stromal matrix (24).
NO has a pivotal role in endothelial function by which it regulates the uterine blood flow, relaxes the uterine myometrium and reduces the feto-placental vascular resistance during pregnancy (25) NO levels are found to be reduced both in obesity and PCOS subjects which might be associated with adverse pregnancy outcome (26, 27). In our study, we observed a reduction in the nitric oxide levels as BMI increased however, it was not statistically significant. Decreased serum NO levels in PCOS have been attributed to elevated asymmetrical dimethyl arginine (ADMA) in PCOS subjects associated with hyperandrogenemia and insulin resistance (28, 29).
Extensive studies have documented the role of inflammation in PCOS and reported an imbalance in the levels of pro and anti-inflammatory cytokines in these subjects (11). Obesity and inflammation are known to occur concurrently (30). The presence of hyperandrogenemia in PCOS stimulates adipocyte hypertrophy and hypoperfusion, release of inflammatory mediators and development of chronic inflammation, consequently resulting in insulin resistance and cardiovascular complications (28). Low levels of IL-10 were observed in PCOS and it was found to affect the pregnancy rates and outcomes (31). Our data demonstrated a significant reduction in the serum IL-10 levels of infertile women with PCOS who had a BMI in the range of 25-28 kg/m2, 28-30 kg/m2, and > 28 kg/m2 compared to those who had a normal BMI. Also IL-10 was negatively associated with BMI suggesting that as BMI increases, IL-10 reduces in PCOS subjects. These findings were in agreement with previous reports that demonstrate reduction in IL-10 levels in obesity and PCOS (31, 32). Since weight gain is associated with inflammation (33), there is reduction of anti-inflammatory cytokines like IL-10.
In the present study, MMP-9 levels negatively correlated with NO levels and positively correlated with the duration of infertility in PCOS subjects. These findings suggest that as the duration of infertility increases, the MMP-9 level increases which in turn reduces NO levels leading to the complications of PCOS.
In the present study we have investigated whether these markers are altered in PCOS women with increase in body weight / BMI. Alteration in these markers can lead to reduction in ovulation. Based on the findings of our study we suggest that weight reduction in PCOS can reduce MMP-9 and increase IL-10, which may improve ovulation in these subjects. The main limitations of the study are the small sample size and non-inclusion of healthy control groups. Moreover we did not estimate other inflammatory cytokines due to financial constraints.
5. Conclusion
The present study concludes that as BMI increases MMP-9 is increased and IL-10 is reduced in PCOS subjects. The association of MMP-9 with NO and duration of infertility indicates that high MMP-9 levels may cause endothelial dysfunction which might be responsible for complications in PCOS subjects with obesity. Further studies are required to investigate whether weight reduction reduces MMP-9 levels and the complications of PCOS in obese subjects.
Acknowledgements
The authors would like to thank JIPMER intramural fund for supporting this study.
Conflict of interest
The authors report no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004; 89: 2745-2749.
https://doi.org/10.1210/jcem.89.9.9990 [DOI:10.1210/jc.2003-032046] [PMID]
3. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. The Lancet 2007; 370: 685-697. [DOI:10.1016/S0140-6736(07)61345-2]
4. Franks S. Polycystic ovary syndrome. Trends Endocrinol Metab 1989; 1: 60-63. [DOI:10.1016/1043-2760(89)90003-9]
5. Diamanti-Kandarakis E. Role of obesity and adiposity in polycystic ovary syndrome. Int J Obes 2007; 31 (Suppl.): S8-S13. [DOI:10.1038/sj.ijo.0803730] [PMID]
6. Naderpoor N, Shorakae S, Joham A, Boyle J, De Courten B, Teede HJ. Obesity and polycystic ovary syndrome. Minerva Endocrinol 2015; 40: 37-51.
7. Curry TE Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428-465. [DOI:10.1210/er.2002-0005] [PMID]
8. Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol 2004; 2: 59-71. [DOI:10.1186/1477-7827-2-59] [PMID] [PMCID]
9. Lewandowski KC, Komorowski J, O'Callaghan CJ, Tan BK, Chen J, Prelevic GM, et al. Increased circulating lLevels of matrix metalloproteinase-2 and -9 in women with the polycystic ovary syndrome. J Clin Endocrinol Metab 2006; 91: 1173-1177. [DOI:10.1210/jc.2005-0648] [PMID]
10. Zullino S, Buzzella F, Simoncini T. Nitric oxide and the biology of pregnancy. Vascul Pharmacol 2018; 110: 71-74. [DOI:10.1016/j.vph.2018.07.004] [PMID]
11. Mahran A, Abdelmeged A, Shawki H, Moheyelden A, Ahmed AM. Nitric oxide donors improve the ovulation and pregnancy rates in anovulatory women with polycystic ovary syndrome treated with clomiphene citrate: A RCT. Int J Reprod Biomed 2016; 14: 9-14. [DOI:10.29252/ijrm.14.1.9]
12. Elkholi DGEY, Hammoudah SF. Subclinical inflammation in obese women with polycystic ovary syndrome. Middle East Fertility Society Journal 2012; 17: 195-202. [DOI:10.1016/j.mefs.2012.02.004]
13. Talaat RM, Mohamed YA, Mohamad EH, Elsharkawy M, Guirgis AA. Interleukin 10 (-1082 G/A) and (-819 C/T) gene polymorphisms in Egyptian women with polycystic ovary syndrome (PCOS). Meta Gene 2016; 9: 254-258. [DOI:10.1016/j.mgene.2016.08.001] [PMID] [PMCID]
14. Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 2015; 149: R219-R227. [DOI:10.1530/REP-14-0435] [PMID]
15. Li L, Feng Q, Ye M, He Y, Yao A, Shi K. Metabolic effect of obesity on polycystic ovary syndrome in adolescents: a meta-analysis. J Obstet Gynaecol 2017; 37: 1036-1047. [DOI:10.1080/01443615.2017.1318840] [PMID]
16. Baka S, Zourla K, Kouskouni E, Makrakis E, Demeridou S, Tzanakaki D, et al. Matrix metalloproteinases 2 and 9 and their tissue inhibitors in the follicular fluid of patients with polycystic ovaries undergoing in vitro fertilisation. In Vivo 2010; 24: 293-296.
17. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41-47. [DOI:10.1093/humrep/deh098] [PMID]
18. Ojeda-Ojeda M, Murri M, Insenser M, Escobar-Morreale HF. Mediators of low-grade chronic inflammation in polycystic ovary syndrome (PCOS). Curr Pharm Des 2013; 19: 5775-5791. [DOI:10.2174/1381612811319320012] [PMID]
19. Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation 2001; 103: 1410-1415. [DOI:10.1161/01.CIR.103.10.1410] [PMID]
20. Goldman S, Shalev E. MMPS and TIMPS in ovarian physiology and pathophysiology. Front Biosci 2004; 9: 2474-2483. [DOI:10.2741/1409] [PMID]
21. Messinis IE, Messini CI, Anifandis G, Dafopoulos K. Polycystic ovaries and obesity. Best Pract Res Clin Obstet Gynaecol 2015; 29: 479-488. [DOI:10.1016/j.bpobgyn.2014.11.001] [PMID]
22. Nissi R, Talvensaari-Mattila A, Kotila V, Niinimäki M, Järvelä I, Turpeenniemi-Hujanen T. Circulating matrix metalloproteinase MMP-9 and MMP-2/TIMP-2 complex are associated with spontaneous early pregnancy failure. Reprod Biol Endocrinol 2013; 11: 2-7. [DOI:10.1186/1477-7827-11-2] [PMID] [PMCID]
23. Derosa G, Ferrari I, D'Angelo A, Tinelli C, Salvadeo SA, Ciccarelli L, et al. Matrix metalloproteinase-2 and -9 levels in obese patients. Endothelium 2008; 15: 219-224. [DOI:10.1080/10623320802228815] [PMID]
24. Andrade VL, Petruceli E, Belo VA, Andrade-Fernandes CM, Caetano Russi CV, Bosco AA, et al. Evaluation of plasmatic MMP-8, MMP-9, TIMP-1 and MPO levels in obese and lean women. Clin Biochem 2012; 45: 412-415. [DOI:10.1016/j.clinbiochem.2012.01.008] [PMID]
25. Gomes VA, Vieira CS, Jacob-Ferreira AL, Belo VA, Soares GM, Fernandes JBF, et al. Imbalanced circulating matrix metalloproteinases in polycystic ovary syndrome. Mol Cell Biochem 2011; 353: 251-257. [DOI:10.1007/s11010-011-0793-6] [PMID]
26. Gouge RC, Marshburn P, Gordon BE, Nunley W, Huet-Hudson YM. Nitric oxide as a regulator of embryonic development. Biol Reprod 1998; 58: 875-879. [DOI:10.1095/biolreprod58.4.875] [PMID]
27. Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med 2014; 73: 383-399. [DOI:10.1016/j.freeradbiomed.2014.05.016] [PMID] [PMCID]
28. Paradisi R, Fabbri R, Battaglia C, Facchinetti F, Venturoli S. Nitric oxide levels in women with missed and threatened abortion: results of a pilot study. Fertil Steril 2007; 88: 744-748. [DOI:10.1016/j.fertnstert.2006.12.026] [PMID]
29. Deligeoroglou E, Vrachnis N, Athanasopoulos N, Iliodromiti Z, Sifakis S, Iliodromiti S, et al. Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol Endocrinol 2012; 28: 974-978. [DOI:10.3109/09513590.2012.683082] [PMID]
30. Heutling D, Schulz H, Nickel I, Kleinstein J, Kaltwasser P, Westphal S, et al. Asymmetrical dimethylarginine, inflammatory and metabolic parameters in women with polycystic ovary syndrome before and after metformin treatment. J Clin Endocrinol Metab 2008; 93: 82-90. [DOI:10.1210/jc.2007-0842] [PMID]
31. Nehir Aytan A, Bastu E, Demiral I, Bulut H, Dogan M, Buyru F. Relationship between hyperandrogenism, obesity, inflammation and polycystic ovary syndrome. Gynecol Endocrinol 2016; 32: 709-713. [DOI:10.3109/09513590.2016.1155208] [PMID]
32. Benson S, Janssen OE, Hahn S, Tan S, Dietz T, Mann K, et al. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav Immun 2008; 22: 177-184. [DOI:10.1016/j.bbi.2007.07.003] [PMID]
33. Leon-Cabrera S, Arana-Lechuga Y, Esqueda-León E, Terán-Pérez G, Gonzalez-Chavez A, Escobedo G, et al. Reduced systemic levels of IL-10 are associated with the severity of obstructive sleep apnea and insulin resistance in morbidly obese humans. Mediators Inflamm 2015; 2015: 493409-493418. [DOI:10.1155/2015/493409] [PMID] [PMCID]
34. Attie AD, Scherer PE. Adipocyte metabolism and obesity. J Lipid Res 2009; 50 (Suppl.): S395-S399. [DOI:10.1194/jlr.R800057-JLR200] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








