Fri, Feb 20, 2026
[Archive]
Volume 18, Issue 9 (September 2020)
IJRM 2020, 18(9): 693-700 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Davar R, Pourmasumi S, Mohammadi B, Mortazavi Lahijani M. The effect of low-dose aspirin on the pregnancy rate in frozen-thawed embryo transfer cycles: A randomized clinical trial. IJRM 2020; 18 (9) :693-700
URL: http://ijrm.ir/article-1-1747-en.html
URL: http://ijrm.ir/article-1-1747-en.html
1- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2- Non-Communicable Diseases Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran. Clinical Research Development Unit (CRDU), Moradi Hospital, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
3- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Department of Obstetrics and Gynecology, Rafsanjan University of Medical Sciences, Rafsanjan, Iran. ,m_mortazavi58@yahoo.com
2- Non-Communicable Diseases Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran. Clinical Research Development Unit (CRDU), Moradi Hospital, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
3- Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Department of Obstetrics and Gynecology, Rafsanjan University of Medical Sciences, Rafsanjan, Iran. ,
Full-Text [PDF 296 kb]
(1573 Downloads)
| Abstract (HTML) (10053 Views)
Full-Text: (1483 Views)
1. Introduction
Human reproduction depends on a successful implantation and the development of an embryo on the endometrial surface. A receptive endometrium is a main factor for embryo implantation. Adjuvant therapy has been utilized to improve the thickness and vascularity of the endometrium with the purpose of improving implantation rates. One of this adjuvant therapies is low-dose aspirin administration (1).
There have been several studies on the effect of low-dose aspirin in assisted reproductive technology (ART) cycles, but contradictory and limited results have been reported on the effect of aspirin on the endometrial thickness as well as on the frozen-thaw embryo transfer (FET) cycles (2, 3).
Several studies showed that there was a direct correlation between an increased uterine vascular resistance and decreased endometrial and sub-endometrial blood flow with poor implantation and pregnancy rate (4-6). Aspirin can inhibit platelet aggregation and increases blood flow by changing the balance between thromboxane (which is a vasoconstrictor mediator) and prostaglandin (which is vasodilator mediator) (1). As per the recent studies, low-dose aspirin appears to be safe and well-tolerated by pregnant women (7, 8). Other aspirin mechanism is increasing it in the level of integrin B3 and Leukemia inhibitory factor (LIF) expression in the endometrium (9).
In a meta-analysis Wang and colleagues suggested that "low-dose aspirin may increase the pregnancy rate in In vitro fertilization/intra cytoplasmic sperm injection (IVF/ICSI) and recommended 100 mg/day ASA clinical use. Because of the limitation of the included studies, more well-designed large-scaled RCTs (randomized clinical trial) are necessary to confirm the effect of aspirin on the endometrial thickness in the FET cycles" (10).
Madani and colleagues performed a pilot clinical trial, where 60 patients who were candidates for FET cycle were divided into two groups. Group A received 100 mg aspirin orally and the other group received placebo. Finally, they reported that the administration of aspirin in FET cycles improved implantation rate, clinical pregnancy rate, and live birth rate. They also recommended that a large sample size is also necessary to confirm the effect of aspirin on the FET cycles (11). Fatemi and colleagues , on the other hand in a review study reported contradictory results about the effect of aspirin on the IVF cycles (12). Haapsamo and colleagues, reported that the administration of aspirin in ovarian stimulation cycles improved uterine hemodynamic status compared to the control group (13). In another study, the authors showed that the administration of aspirin in patients undergoing IVF cycle reduced uterine artery pulsatility index (PI) in the early and mid-pregnancy and reduced the risk of preeclampsia and intrauterine growth restriction (IUGR) (14).
In a systematic review, researchers concluded that there is insufficient evidence for routine administration of aspirin in IVF cycles. On the other hand, they reported that the administration of aspirin has no positive significant effects on IVF outcome and needs further studies (15). Dirckx and co-worker in Belgium found no positive effect of aspirin in improving clinical pregnancy in IVF cycles and recommended that it should not be used routinely (3). Also, in a recent Cochrane review, the authors concluded that the use of empirical aspirin for general IVF population cannot be recommended for routine use (16).
Based on the contradictory results and the lack of sufficient studies on the effect of aspirin on the pregnancy rate in FET, in our study we aimed to investigate the effect of low-dose aspirin adjuvant therapy in women undergoing FET cycles.
2. Materials and Methods
This study was a randomized clinical trial performed in the Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute from January 2019 to February 2020.
The inclusion criteria were age<40 yr, at least two frozen-thawed embryos available for another transfer, no contraindications for aspirin administration, no uterine disorders, no endometriosis, no history of uterine surgery, and no history of recurrent abortion (two or more than two abortions).
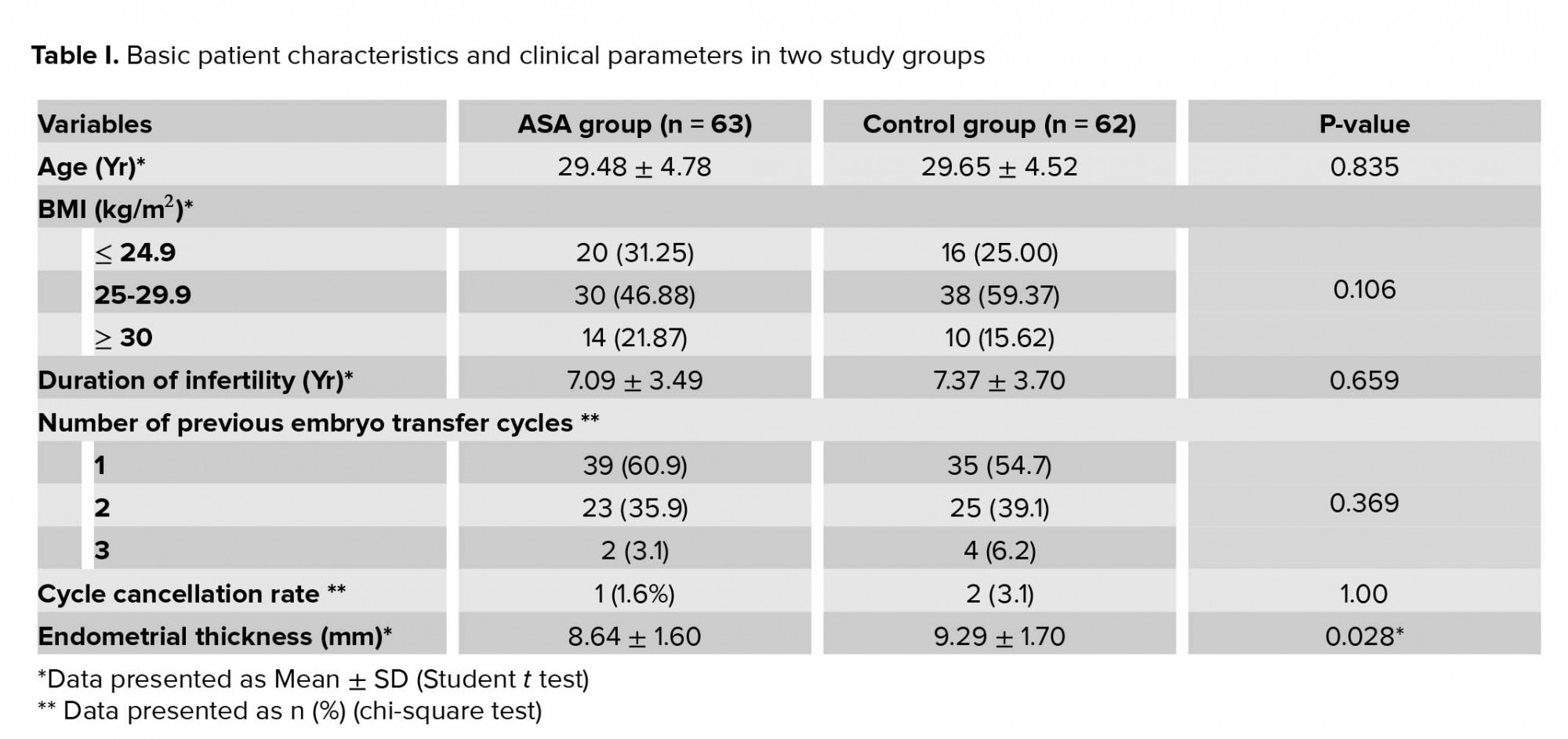
128 eligible women who were assigned for FET participated in the study. They were allocated into two groups randomly and equally. Group A (n = 64) was prescribed 80 mg aspirin, and for group B (n = 64), no treatment was prescribed to the routine FET protocols (Table I).
First, all patients underwent transvaginal ultrasonography on the second day of their cycles for excluding functional ovarian cysts. For endometrial preparation, patients received 6 mg estradiol valerate (aburahan co.) per day from second day of their cycles, and if the endometrial thickness was 7 mm or less on the thirteenth day of their cycles, the dose was increased to 10 mg.
When the optimal endometrial thickness (> 8 mm) was obtained, vaginal progesterone 400 mg BID was started and continued until the 12 week of pregnancy. For endometrial thickness measurement, transvaginal ultrasonography (fillips affinity 70) was performed from the 13th day of their cycles and every 4 days if the endometrial thickness of 8 mm was not obtained on the 13th day.
The two selected embryos were transferred at cleavage stage by Cook catheter. 5000 IU HCG (human chorionic gonadotropin) was administered on the first, third, and sixth days of embryo transfer.
In our study 20 days after the embryo transfer beta human chorionic gonadotropin (βHCG) was checked. The primary outcome was clinical pregnancy rate that determined as presence of an intrauterine gestational sac with fetal heart beat (28-42 days after the embryo transfer). We requested women to continue taking aspirin for until 12 weeks of gestation. Secondary outcomes were implantation and miscarriage rates. The implantation rate was determined as the total number of gestational sacs per total number of transferred embryos, and the miscarriage rate was determined as the number of miscarriage that took place until the 20th week of pregnancy.
2.1. Ethical consideration
The study has been approved by the ethical committee of the Yazd Reproductive Sciences Institute (IR.SSU.RSI.REC.1398.003). All participants gave informed consent being included in the study.
2.2. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences 15.0 software. The baseline characteristics of the two groups of patients were compared using the student t test. Differences in the pregnancy outcomes of the two groups were analyzed using the Chi-square test. P ≤ 0.05 was considered statistically significant.
3. Results
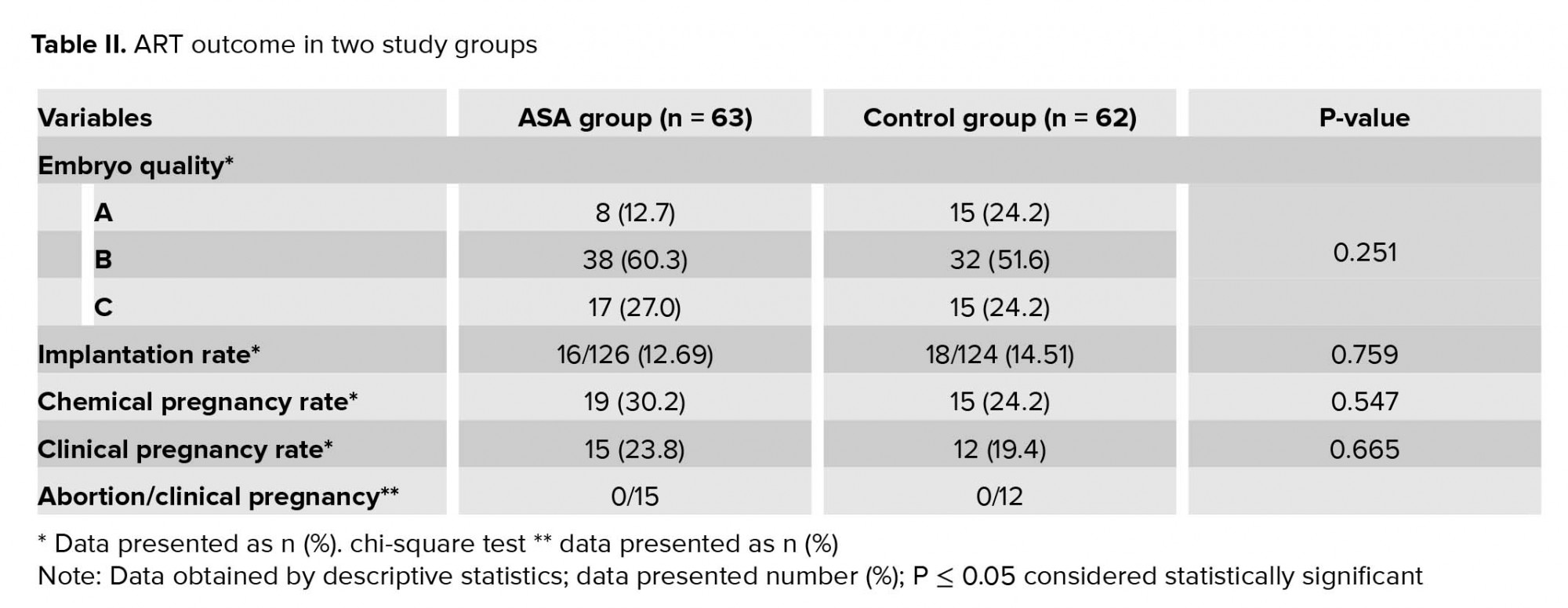
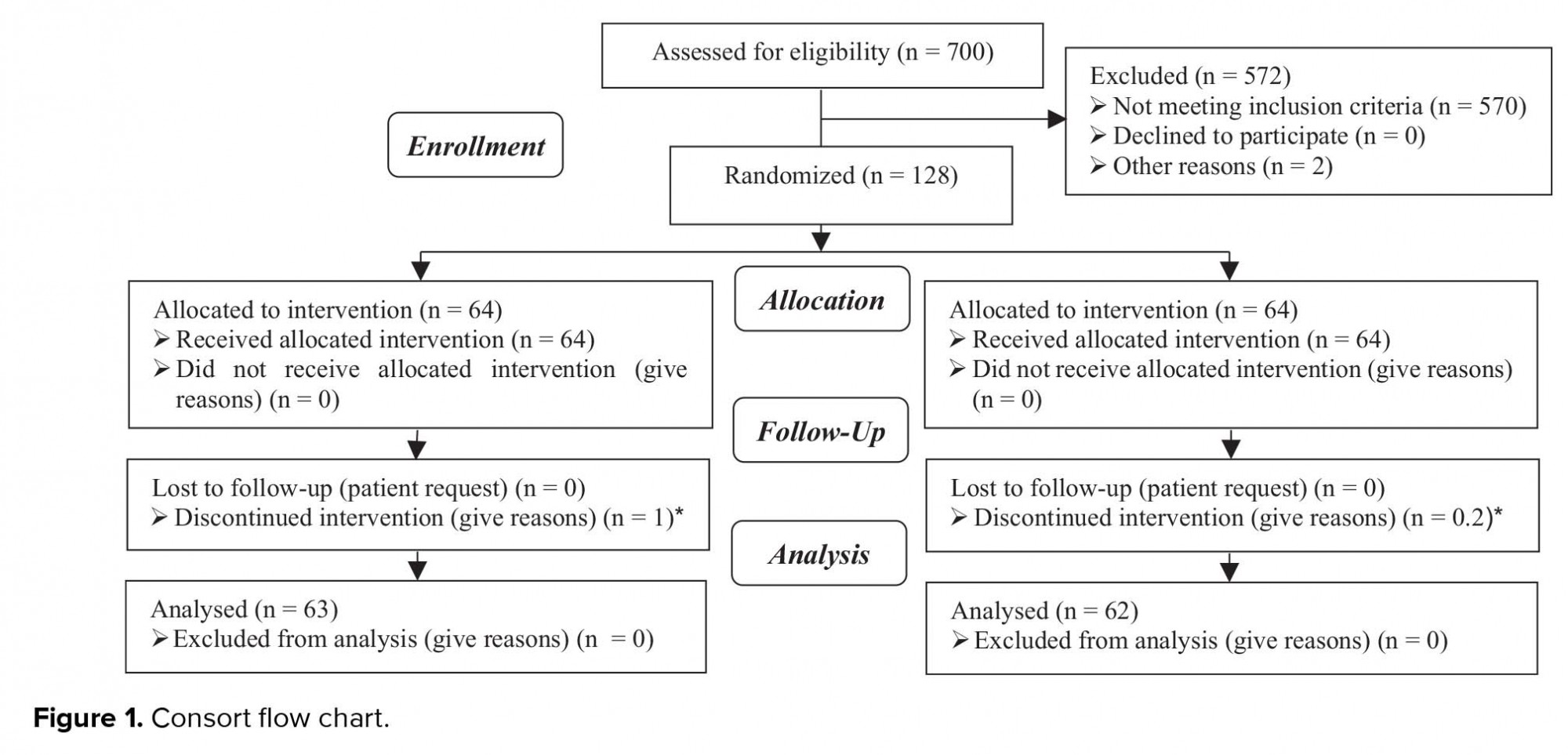
In the present study, from 700 women who candidate for FET 572 were excluded from our study and we evaluated a total of 128 patients in two study groups: 64 cases in ASA (amino salicylic acid) group and 64 in control group. There was one cancelled cycle in case group (1/64) and two in control group (2/64) due to thin endometrium (< 7 mm) (Figure 1). As shown in table I, the age, BMI, infertility duration, and the number of previous embryo transfer cycles had similarity in the two study groups, and there was no statistically significant difference between the two groups. The endometrial thickness was lower in the group receiving ASA in comparison to the control group. The difference was statistically significant between the two groups (p = 0.028; Table I). ART outcomes in the two study groups are summarized in table II. The quality of transferred embryo was similar in the two groups and the highest number of embryos were in good quality (A and B). In all cycles, we transferred two embryo. From 126 transferred embryo in case group, 16 pregnancy sacs were observed in ultrasonography, and from 124 transferred embryo in control group, 18 pregnancy sacs were observed in ultrasonography. The Implantation rate was higher in the control group (14.5% vs. 12.6%), but this difference was not statistically significant. Chemical and clinical pregnancy rates were similar in the two groups, and there was no statistically significant difference.
4. Discussion
The results of our study showed that there was no difference in the pregnancy outcome and the clinical pregnancy rate in patients who received aspirin in comparison with the control group in patients undergoing FET cycles. The endometrial thickness was significantly lower in the aspirin group than the control group. Some studies have investigated the role of aspirin in ART cycles and reported contradictory results (17, 18).
The majority of previous studies investigated the role of aspirin as an adjuvant therapy on ART outcome in fresh cycles (stimulated cycles) (17, 19). In fresh cycles, the estradiol levels are higher than in the freeze-thaw cycles. Also, in a fresh cycle, embryo is exposed to supra-physiological levels of estrogen during implantation but in the frozen-thaw cycles endometrial preparation is done artificially by estrogen and progesterone administration and the embryo is not exposed to supra-physiological level of estrogen. Supra-physiologic levels of estrogen may have a negative impact on endometrial receptivity and maybe responsible for implantation failure in ART (20, 21). Therefore, the results of aspirin adjuvant therapy in the fresh and frozen cycles are noncomparable. The results of the past studies on the effect of low-dose aspirin in FET cycles are limited.
The result of ART outcome in the present study was in contrast with Madani’s study. They reported that the administration of aspirin in FET cycles improved the implantation, clinical pregnancy, and live birthrates, however, we found no statistically significant improvement in the biochemical, clinical pregnancy, and abortion rates in the aspirin group in comparison with the control. However, their study was a pilot study and the difference between the results of our study and the Madani’s study may be the low sample size of their study (11).
Hsieh and colleagues showed higher clinical pregnancy rate and better endometrial pattern in patients with thin endometrium after aspirin administration, and because in this group they selected patients who had thin endometrium their results is in contrast to our findings (22).
Conforming to our study, a meta-analysis (15) and reports by Cochrane reviews (16, 23) also establish that there was no improvement in pregnancy rates with the use of low-dose aspirin. Jeromeh demonstrated that the clinical pregnancy rate was lower in the aspirin group compared with control group (11.1% vs 33.3% respectively) for, and the implantation rates were 2.9 and 10.9%, respectively. In this study, low-dose aspirin administration did not cause positive effect on pregnancy rates in FET cycles (24). Gelbaya and co-workers suggested that in FET cycles, there was no significant difference in pregnancy rate between untreated women with normal uterine perfusion and those that uterine perfusion was improved after aspirin administration. This result is similar with our study (25). In another study, Doppler ultrasound was used for uterine perfusion assessment and patients were classified as two groups: normal uterine perfusion and impaired uterine perfusion. administration of low-dose aspirin to hormone replacement therapy in women with impaired uterine perfusion was associated with improved uterine perfusion and acceptable pregnancy rates but without any benefits in patients with normal perfusion (26). In our study, the assessment of uterine perfusion and patients classification was not done.
Conclusion
Our study concludes that the administration of aspirin in frozen-thaw cycles had no positive effect on the implantation, chemical, and clinical pregnancy rates, and is in accordance with the current Cochrane review that does not recommend aspirin administration as a routine in ART cycles.
Acknowledgments
The authors would like to thank the staff from the Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran, for their skillful technical assistance during the course of this study. The study was financially supported by the Research Deputy of the Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflict of interest
The authors have no financial or nonfinancial conflicts of interest.
Human reproduction depends on a successful implantation and the development of an embryo on the endometrial surface. A receptive endometrium is a main factor for embryo implantation. Adjuvant therapy has been utilized to improve the thickness and vascularity of the endometrium with the purpose of improving implantation rates. One of this adjuvant therapies is low-dose aspirin administration (1).
There have been several studies on the effect of low-dose aspirin in assisted reproductive technology (ART) cycles, but contradictory and limited results have been reported on the effect of aspirin on the endometrial thickness as well as on the frozen-thaw embryo transfer (FET) cycles (2, 3).
Several studies showed that there was a direct correlation between an increased uterine vascular resistance and decreased endometrial and sub-endometrial blood flow with poor implantation and pregnancy rate (4-6). Aspirin can inhibit platelet aggregation and increases blood flow by changing the balance between thromboxane (which is a vasoconstrictor mediator) and prostaglandin (which is vasodilator mediator) (1). As per the recent studies, low-dose aspirin appears to be safe and well-tolerated by pregnant women (7, 8). Other aspirin mechanism is increasing it in the level of integrin B3 and Leukemia inhibitory factor (LIF) expression in the endometrium (9).
In a meta-analysis Wang and colleagues suggested that "low-dose aspirin may increase the pregnancy rate in In vitro fertilization/intra cytoplasmic sperm injection (IVF/ICSI) and recommended 100 mg/day ASA clinical use. Because of the limitation of the included studies, more well-designed large-scaled RCTs (randomized clinical trial) are necessary to confirm the effect of aspirin on the endometrial thickness in the FET cycles" (10).
Madani and colleagues performed a pilot clinical trial, where 60 patients who were candidates for FET cycle were divided into two groups. Group A received 100 mg aspirin orally and the other group received placebo. Finally, they reported that the administration of aspirin in FET cycles improved implantation rate, clinical pregnancy rate, and live birth rate. They also recommended that a large sample size is also necessary to confirm the effect of aspirin on the FET cycles (11). Fatemi and colleagues , on the other hand in a review study reported contradictory results about the effect of aspirin on the IVF cycles (12). Haapsamo and colleagues, reported that the administration of aspirin in ovarian stimulation cycles improved uterine hemodynamic status compared to the control group (13). In another study, the authors showed that the administration of aspirin in patients undergoing IVF cycle reduced uterine artery pulsatility index (PI) in the early and mid-pregnancy and reduced the risk of preeclampsia and intrauterine growth restriction (IUGR) (14).
In a systematic review, researchers concluded that there is insufficient evidence for routine administration of aspirin in IVF cycles. On the other hand, they reported that the administration of aspirin has no positive significant effects on IVF outcome and needs further studies (15). Dirckx and co-worker in Belgium found no positive effect of aspirin in improving clinical pregnancy in IVF cycles and recommended that it should not be used routinely (3). Also, in a recent Cochrane review, the authors concluded that the use of empirical aspirin for general IVF population cannot be recommended for routine use (16).
Based on the contradictory results and the lack of sufficient studies on the effect of aspirin on the pregnancy rate in FET, in our study we aimed to investigate the effect of low-dose aspirin adjuvant therapy in women undergoing FET cycles.
2. Materials and Methods
This study was a randomized clinical trial performed in the Research and Clinical Center for Infertility, Yazd Reproductive Sciences Institute from January 2019 to February 2020.
The inclusion criteria were age<40 yr, at least two frozen-thawed embryos available for another transfer, no contraindications for aspirin administration, no uterine disorders, no endometriosis, no history of uterine surgery, and no history of recurrent abortion (two or more than two abortions).
128 eligible women who were assigned for FET participated in the study. They were allocated into two groups randomly and equally. Group A (n = 64) was prescribed 80 mg aspirin, and for group B (n = 64), no treatment was prescribed to the routine FET protocols (Table I).
First, all patients underwent transvaginal ultrasonography on the second day of their cycles for excluding functional ovarian cysts. For endometrial preparation, patients received 6 mg estradiol valerate (aburahan co.) per day from second day of their cycles, and if the endometrial thickness was 7 mm or less on the thirteenth day of their cycles, the dose was increased to 10 mg.
When the optimal endometrial thickness (> 8 mm) was obtained, vaginal progesterone 400 mg BID was started and continued until the 12 week of pregnancy. For endometrial thickness measurement, transvaginal ultrasonography (fillips affinity 70) was performed from the 13th day of their cycles and every 4 days if the endometrial thickness of 8 mm was not obtained on the 13th day.
The two selected embryos were transferred at cleavage stage by Cook catheter. 5000 IU HCG (human chorionic gonadotropin) was administered on the first, third, and sixth days of embryo transfer.
In our study 20 days after the embryo transfer beta human chorionic gonadotropin (βHCG) was checked. The primary outcome was clinical pregnancy rate that determined as presence of an intrauterine gestational sac with fetal heart beat (28-42 days after the embryo transfer). We requested women to continue taking aspirin for until 12 weeks of gestation. Secondary outcomes were implantation and miscarriage rates. The implantation rate was determined as the total number of gestational sacs per total number of transferred embryos, and the miscarriage rate was determined as the number of miscarriage that took place until the 20th week of pregnancy.

2.1. Ethical consideration
The study has been approved by the ethical committee of the Yazd Reproductive Sciences Institute (IR.SSU.RSI.REC.1398.003). All participants gave informed consent being included in the study.
2.2. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences 15.0 software. The baseline characteristics of the two groups of patients were compared using the student t test. Differences in the pregnancy outcomes of the two groups were analyzed using the Chi-square test. P ≤ 0.05 was considered statistically significant.
3. Results
In the present study, from 700 women who candidate for FET 572 were excluded from our study and we evaluated a total of 128 patients in two study groups: 64 cases in ASA (amino salicylic acid) group and 64 in control group. There was one cancelled cycle in case group (1/64) and two in control group (2/64) due to thin endometrium (< 7 mm) (Figure 1). As shown in table I, the age, BMI, infertility duration, and the number of previous embryo transfer cycles had similarity in the two study groups, and there was no statistically significant difference between the two groups. The endometrial thickness was lower in the group receiving ASA in comparison to the control group. The difference was statistically significant between the two groups (p = 0.028; Table I). ART outcomes in the two study groups are summarized in table II. The quality of transferred embryo was similar in the two groups and the highest number of embryos were in good quality (A and B). In all cycles, we transferred two embryo. From 126 transferred embryo in case group, 16 pregnancy sacs were observed in ultrasonography, and from 124 transferred embryo in control group, 18 pregnancy sacs were observed in ultrasonography. The Implantation rate was higher in the control group (14.5% vs. 12.6%), but this difference was not statistically significant. Chemical and clinical pregnancy rates were similar in the two groups, and there was no statistically significant difference.


4. Discussion
The results of our study showed that there was no difference in the pregnancy outcome and the clinical pregnancy rate in patients who received aspirin in comparison with the control group in patients undergoing FET cycles. The endometrial thickness was significantly lower in the aspirin group than the control group. Some studies have investigated the role of aspirin in ART cycles and reported contradictory results (17, 18).
The majority of previous studies investigated the role of aspirin as an adjuvant therapy on ART outcome in fresh cycles (stimulated cycles) (17, 19). In fresh cycles, the estradiol levels are higher than in the freeze-thaw cycles. Also, in a fresh cycle, embryo is exposed to supra-physiological levels of estrogen during implantation but in the frozen-thaw cycles endometrial preparation is done artificially by estrogen and progesterone administration and the embryo is not exposed to supra-physiological level of estrogen. Supra-physiologic levels of estrogen may have a negative impact on endometrial receptivity and maybe responsible for implantation failure in ART (20, 21). Therefore, the results of aspirin adjuvant therapy in the fresh and frozen cycles are noncomparable. The results of the past studies on the effect of low-dose aspirin in FET cycles are limited.
The result of ART outcome in the present study was in contrast with Madani’s study. They reported that the administration of aspirin in FET cycles improved the implantation, clinical pregnancy, and live birthrates, however, we found no statistically significant improvement in the biochemical, clinical pregnancy, and abortion rates in the aspirin group in comparison with the control. However, their study was a pilot study and the difference between the results of our study and the Madani’s study may be the low sample size of their study (11).
Hsieh and colleagues showed higher clinical pregnancy rate and better endometrial pattern in patients with thin endometrium after aspirin administration, and because in this group they selected patients who had thin endometrium their results is in contrast to our findings (22).
Conforming to our study, a meta-analysis (15) and reports by Cochrane reviews (16, 23) also establish that there was no improvement in pregnancy rates with the use of low-dose aspirin. Jeromeh demonstrated that the clinical pregnancy rate was lower in the aspirin group compared with control group (11.1% vs 33.3% respectively) for, and the implantation rates were 2.9 and 10.9%, respectively. In this study, low-dose aspirin administration did not cause positive effect on pregnancy rates in FET cycles (24). Gelbaya and co-workers suggested that in FET cycles, there was no significant difference in pregnancy rate between untreated women with normal uterine perfusion and those that uterine perfusion was improved after aspirin administration. This result is similar with our study (25). In another study, Doppler ultrasound was used for uterine perfusion assessment and patients were classified as two groups: normal uterine perfusion and impaired uterine perfusion. administration of low-dose aspirin to hormone replacement therapy in women with impaired uterine perfusion was associated with improved uterine perfusion and acceptable pregnancy rates but without any benefits in patients with normal perfusion (26). In our study, the assessment of uterine perfusion and patients classification was not done.
Conclusion
Our study concludes that the administration of aspirin in frozen-thaw cycles had no positive effect on the implantation, chemical, and clinical pregnancy rates, and is in accordance with the current Cochrane review that does not recommend aspirin administration as a routine in ART cycles.
Acknowledgments
The authors would like to thank the staff from the Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran, for their skillful technical assistance during the course of this study. The study was financially supported by the Research Deputy of the Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflict of interest
The authors have no financial or nonfinancial conflicts of interest.
Type of Study: Original Article |
Subject:
Fertility & Infertility
References
1. de Mola JRL. Principles and practice of assisted reproductive technology. Fertility and Sterility 2014; 102: 610. [DOI:10.1016/j.fertnstert.2014.06.018]
2. Pakkila M, Rasanen J, Heinonen S, Tinkanen H, Tuomivaara L, Makikallio K, et al. Low-dose aspirin does not improve ovarian responsiveness or pregnancy rate in IVF and ICSI patients: a randomized, placebo-controlled double-blind study. Hum Reprod 2005; 20: 2211-2214. [DOI:10.1093/humrep/dei020] [PMID]
3. Dirckx K, Cabri P, Merien A, Galajdova L, Gerris J, Dhont M, et al. Does low-dose aspirin improve pregnancy rate in IVF/ICSI? A randomized double-blind placebo controlled trial. Hum Reprod 2009; 24: 856-860. [DOI:10.1093/humrep/den476] [PMID]
4. Zaidi J, Pittrof R, Shaker A, Kyei-Mensah A, Campbell S, Tan SL. Assessment of uterine artery blood flow on the day of human chorionic gonadotropin administration by transvaginal color Doppler ultrasound in an in vitro fertilization program. Fertil Steril 1996; 65: 377-381. [DOI:10.1016/S0015-0282(16)58103-5]
5. Cacciatore B, Simberg N, Fusaro P, Tiitinen A. Transvaginal Doppler study of uterine artery blood flow in in vitro fertilization-embryo transfer cycles. Fertil Steril 1996; 66: 130-134. [DOI:10.1016/S0015-0282(16)58400-3]
6. Chien LW, Au HK, Chen PL, Xiao J, Tzeng CR. Assessment of uterine receptivity by the endometrial-subendometrial blood flow distribution pattern in women undergoing in vitro fertilization-embryo transfer. Fertil Steril 2002; 78: 245-251. [DOI:10.1016/S0015-0282(02)03223-5]
7. Ahrens KA, Silver RM, Mumford SL, Sjaarda LA, Perkins NJ, Wactawski-Wende J, et al. Complications and safety of preconception low-dose aspirin among women with prior pregnancy losses. Obstet Gynecol 2016; 127: 689-698. [DOI:10.1097/AOG.0000000000001301] [PMID] [PMCID]
8. Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, Townsend JM, et al. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet 2014; 384: 29-36. [DOI:10.1016/S0140-6736(14)60157-4]
9. Zhao M, Chang C, Liu Z, Chen LM, Chen Q. Treatment with low-dose aspirin increased the level LIF and integrin beta3 expression in mice during the implantation window. Placenta 2010; 31: 1101-1105. [DOI:10.1016/j.placenta.2010.10.002] [PMID]
10. Wang L, Huang X, Li X, Lv F, He X, Pan Y, et al. Efficacy evaluation of low-dose aspirin in IVF/ICSI patients evidence from 13 RCTs: A systematic review and meta-analysis. Medicine 2017; 96: e7720-e7726. [DOI:10.1097/MD.0000000000007720] [PMID] [PMCID]
11. Madani T, Ahmadi F, Jahangiri N, Bahmanabadi A, Bagheri Lankarani N. Does low-dose aspirin improve pregnancy rate in women undergoing frozen-thawed embryo transfer cycle? A pilot double-blind, randomized placebo-controlled trial. J Obstet Gynaecol Res 2019; 45: 156-163. [DOI:10.1111/jog.13802] [PMID]
12. Fatemi HM, Popovic-Todorovic B. Implantation in assisted reproduction: a look at endometrial receptivity. Reprod Biomed Online 2013; 27: 530-538. [DOI:10.1016/j.rbmo.2013.05.018] [PMID]
13. Haapsamo M, Martikainen H, Rasanen J. Low-dose aspirin and uterine haemodynamics on the day of embryo transfer in women undergoing IVF/ICSI: a randomized, placebo-controlled, double-blind study. Hum Reprod 2009; 24: 861-866. [DOI:10.1093/humrep/den489] [PMID]
14. Haapsamo M, Martikainen H, Rasanen J. Low-dose aspirin reduces uteroplacental vascular impedance in early and mid gestation in IVF and ICSI patients: a randomized, placebo-controlled double-blind study. Ultrasound Obstet Gynecol 2008; 32: 687-693. [DOI:10.1002/uog.6215] [PMID]
15. Khairy M, Banerjee K, El-Toukhy T, Coomarasamy A, Khalaf Y. Aspirin in women undergoing in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril 2007; 88: 822-831. [DOI:10.1016/j.fertnstert.2006.12.080] [PMID]
16. Siristatidis CS, Dodd SR, Drakeley AJ. Aspirin is not recommended for women undergoing IVF. Hum Reprod Update 2012; 18: 233. [DOI:10.1093/humupd/dmr049] [PMID]
17. Ruopp MD, Collins TC, Whitcomb BW, Schisterman EF. Evidence of absence or absence of evidence? A reanalysis of the effects of low-dose aspirin in in vitro fertilization. Fertil Steril 2008; 90: 71-76. [DOI:10.1016/j.fertnstert.2007.06.033] [PMID] [PMCID]
18. Duvan CI, Ozmen B, Satiroglu H, Atabekoglu CS, Berker B. Does addition of low-dose aspirin and/or steroid as a standard treatment in nonselected intracytoplasmic sperm injection cycles improve in vitro fertilization success? A randomized, prospective, placebo-controlled study. J Assist Reprod Genet 2006; 23: 15-21. [DOI:10.1007/s10815-005-9003-3] [PMID] [PMCID]
19. Frattarelli JL, McWilliams GD, Hill MJ, Miller KA, Scott RT Jr. Low-dose aspirin use does not improve in vitro fertilization outcomes in poor responders. Fertil Steril 2008; 89: 1113-1117. [DOI:10.1016/j.fertnstert.2007.05.007] [PMID]
20. Imudia AN, Goldman RH, Awonuga AO, Wright DL, Styer AK, Toth TL. The impact of supraphysiologic serum estradiol levels on peri-implantation embryo development and early pregnancy outcome following in vitro fertilization cycles. J Assist Reprod Genet 2014; 31: 65-71. [DOI:10.1007/s10815-013-0117-8] [PMID] [PMCID]
21. Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure? J Assist Reprod Genet 2016; 33: 1419-1430. [DOI:10.1007/s10815-016-0773-6] [PMID] [PMCID]
22. Hsieh YY, Tsai HD, Chang CC, Lo HY, Chen CL. Low-dose aspirin for infertile women with thin endometrium receiving intrauterine insemination: a prospective, randomized study. J Assist Reprod Genet 2000; 17: 174-177.
https://doi.org/10.1023/A:1009474307376
https://doi.org/10.1023/A:1009458215962 [DOI:10.1023/A:1009426303742]
23. Siristatidis CS, Basios G, Pergialiotis V, Vogiatzi P. Aspirin for in vitro fertilisation. Cochrane Database Syst Rev 2016; 11: CD004832. [DOI:10.1002/14651858.CD004832.pub4] [PMID] [PMCID]
24. Check JH, Dietterich C, Lurie D, Nazari A, Chuong J. A matched study to determine whether low-dose aspirin without heparin improves pregnancy rates following frozen embryo transfer and/or affects endometrial sonographic parameters. J Assist Reprod Genet 1998; 15: 579-582. [DOI:10.1023/A:1020373009043] [PMID] [PMCID]
25. Gelbaya TA, Kyrgiou M, Li TC, Stern C, Nardo LG. Low-dose aspirin for in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update 2007; 13: 357-364. [DOI:10.1093/humupd/dmm005] [PMID]
26. Wada I, Hsu CC, Williams G, Macnamee MC, Brinsden PR. The benefits of low-dose aspirin therapy in women with impaired uterine perfusion during assisted conception. Hum Reprod 1994; 9: 1954-1957. [DOI:10.1093/oxfordjournals.humrep.a138366] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








