Sun, Jul 13, 2025
[Archive]
Volume 19, Issue 3 (March 2021)
IJRM 2021, 19(3): 271-282 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khani S, Abdollahi M, Khalaj A, Heidari H, Zohali S. The effect of hydroalcoholic extract of Nigella Sativa seed on dehydroepiandrosterone-induced polycystic ovarian syndrome in rats: An experimental study. IJRM 2021; 19 (3) :271-282
URL: http://ijrm.ir/article-1-1777-en.html
URL: http://ijrm.ir/article-1-1777-en.html
1- Neuroscience Research Center, Qom University of Medical Sciences, Qom, Iran.
2- Department of Anatomical Sciences, Medical Sciences Faculty, Tarbiat Modares University, Tehran, Iran.
3- Department of Physiology, School of Medicine, Qom University of Medical Sciences, Qom, Iran.
4- Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran. Department of Physiology and Pharmacology, Faculty of Medicine, Qom University of Medical Sciences, Qom, Iran. ,physiology_86@yahoo.com
5- Student Research Committee, Qom University of Medical Sciences, Qom, Iran.
2- Department of Anatomical Sciences, Medical Sciences Faculty, Tarbiat Modares University, Tehran, Iran.
3- Department of Physiology, School of Medicine, Qom University of Medical Sciences, Qom, Iran.
4- Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran. Department of Physiology and Pharmacology, Faculty of Medicine, Qom University of Medical Sciences, Qom, Iran. ,
5- Student Research Committee, Qom University of Medical Sciences, Qom, Iran.
Keywords: Nigella sativa seed, Oxidative stress, Insulin resistance, Polycystic ovary syndrome, Rat.
Full-Text [PDF 12704 kb]
(1380 Downloads)
| Abstract (HTML) (3049 Views)
Full-Text: (563 Views)
- Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders among women (1). The causes of PCOS can be attributed to defects in the hypothalamic-pituitary function and insulin action (2).
Increased androgens and obesity, insulin resistance, and type 2 diabetes, and oxidative stress are the long-term consequences of this syndrome (3, 4). Disturbance in antioxidant systems may lead to pathological outcomes such as PCOS and disruption of the synthesis of ovarian steroids in women (5). An impaired release of gonadotropin associated with enhanced secretion of luteinizing hormone (LH) compared to the follicle-stimulating hormone (FSH) are observed in these patients (6). Several medical treatments are available for PCOS, however, most of them are temporary and not definitive treatment. Given the side effects of these drugs, identifying and providing alternative drugs are very important (7).
In this study, the effects of Nigella sativa seeds (N. sativa also known as black cumin) used in traditional medicine have been investigated. The seeds of this plant have antimicrobial and antifungal properties, in addition to being menstrual regulators and milk boosters (8), and are used in Iranian traditional medicine for treating several inflammatory and painful disorders (9).
The compounds isolated from N. sativa, including thymoquinone, t-anethole, carvacrol, and 4-terpinol, have detectable free radical scavenging, antioxidant, and anti-inflammatory properties (10). Regarding the effect of this plant on the hypothalamic-pituitary axis and to determine its effect on PCOS for the first time, we decided to evaluate the effect of the extract of this seed on the histological and hormonal levels and the appropriate dosage of the extract to reduce ovarian cysts, insulin resistance, and oxidative stress in rats model of the PCOS.
Increased androgens and obesity, insulin resistance, and type 2 diabetes, and oxidative stress are the long-term consequences of this syndrome (3, 4). Disturbance in antioxidant systems may lead to pathological outcomes such as PCOS and disruption of the synthesis of ovarian steroids in women (5). An impaired release of gonadotropin associated with enhanced secretion of luteinizing hormone (LH) compared to the follicle-stimulating hormone (FSH) are observed in these patients (6). Several medical treatments are available for PCOS, however, most of them are temporary and not definitive treatment. Given the side effects of these drugs, identifying and providing alternative drugs are very important (7).
In this study, the effects of Nigella sativa seeds (N. sativa also known as black cumin) used in traditional medicine have been investigated. The seeds of this plant have antimicrobial and antifungal properties, in addition to being menstrual regulators and milk boosters (8), and are used in Iranian traditional medicine for treating several inflammatory and painful disorders (9).
The compounds isolated from N. sativa, including thymoquinone, t-anethole, carvacrol, and 4-terpinol, have detectable free radical scavenging, antioxidant, and anti-inflammatory properties (10). Regarding the effect of this plant on the hypothalamic-pituitary axis and to determine its effect on PCOS for the first time, we decided to evaluate the effect of the extract of this seed on the histological and hormonal levels and the appropriate dosage of the extract to reduce ovarian cysts, insulin resistance, and oxidative stress in rats model of the PCOS.
- Materials and Methods
- 1. Animals
In this experimental study ,which was carried out in 2017 at the Qom University of Medical Sciences, 36 female Wistar rats (60 ± 10 gr, aged 21 days) (11) obtained from the animal house of the Qom Medical University were used. The rats were housed in a room with controlled temperature (23 ± 2ºC) and luminosity (12-hr light/dark cycles) with free access to food and water.
- 2. Experimental groups
Rats were randomly divided into six groups (n = 6/each):
Group 1 (control): rats received no medication;
Group 2 (PCOS): dehydroepiandrosterone (DHEA)-induced PCOS-control or PCOS;
Group 3 (PCOS+ Met) as a positive control: rats with PCOS receiving 30 mg/100 g of Metformin (Chemidarou Co. Tehran, Iran) through gavage for one month;
Groups 4 (PCOS + N.S 50), Groups 5 (PCOS + N.S 100) and Groups 6 (PCOS + N.S 200): PCOS rats which received a hydroalcoholic extract of N. Sativa seeds in doses of 50, 100, and 200 mg/kg respectively, through gavage for one month.
Group 1 (control): rats received no medication;
Group 2 (PCOS): dehydroepiandrosterone (DHEA)-induced PCOS-control or PCOS;
Group 3 (PCOS+ Met) as a positive control: rats with PCOS receiving 30 mg/100 g of Metformin (Chemidarou Co. Tehran, Iran) through gavage for one month;
Groups 4 (PCOS + N.S 50), Groups 5 (PCOS + N.S 100) and Groups 6 (PCOS + N.S 200): PCOS rats which received a hydroalcoholic extract of N. Sativa seeds in doses of 50, 100, and 200 mg/kg respectively, through gavage for one month.
- 3. Plant preparation and extract
The seeds of N. sativa were purchased from a local market in Qom (Iran) and authenticated scientifically by the Department of Medicinal Plants, Qom University of Medical Science (voucher specimen: PMP-735). The maceration method was used for the preparation of the extract. To prepare the hydroalcoholic extract, 50 gr of seed powder was soaked in 200 ml of hydroalcoholic solvent (distilled water-ethanol 96% mixture; 60-40) and kept for 72 hr in a closed container at room temperature. Then, the contents of the container were filtered using a Buchner funnel (Whatman's filter paper, No. 1), after removing the solvent and drying at room temperature, to obtain the semisolid mass which was kept at 4ºC prior to use (12, 13).
- 4. Induction of PCOS model
PCOS was induced by a subcutaneous injection of DHEA (60 mg/kg, Cayman, USA) dissolved in sesame oil for 3 wk. According to other similar studies on PCOS induction, after 21 days of DHEA injection, the rats were subjected to vaginal smear tests to determine the irregularity of the estrous cycle and the occurrence of persistent vaginal carnification (PVC), which is one of the symptoms of follicular cysts in the ovary (14). Moreover, the induction of PCOS was confirmed by serological tests (such as increase in LH, testosterone, and HOMA-IR levels and decrease in FSH and progesterone levels) and histomorphometric studies (wherein one or both ovaries can contain multiple small, immature ovarian follicles as cysts).
- 5. Serum analysis
At the end of the treatment period and 24 hr after the last gavage, the animals were subjected to deep anesthesia with diethyl ether. Blood samples were directly collected by cardiac puncture and centrifuged (Eppendorf centrifuge 5702, Germany) at 3,000 rpm for 15 min to separate serum samples (Eppendorf centrifuge 5702, Germany) (15, 16). Serum levels of FSH, LH, progesterone, estradiol, and testosterone were measured by the ELISA kit (Pars Azmoon, Iran) according to the manufacturer's kit.
Moreover, while the fasting blood glucose (FBG) was determined through a lateral tail vein using an Elegance glucometer (CT-X10, Convergent Technologies, Germany) and serum insulin levels were also measured using commercial ELISA kit (Monobimd, California, USA).
Furthermore, the indices of insulin resistance at the beginning and end of the treatment using HOMA-IR (Homeostasis Model Assessment of Insulin Resistance) were calculated according to the following equation: fasting glucose (mg/dl) × fasting insulin )µIU/ml)/405 (17).
Moreover, while the fasting blood glucose (FBG) was determined through a lateral tail vein using an Elegance glucometer (CT-X10, Convergent Technologies, Germany) and serum insulin levels were also measured using commercial ELISA kit (Monobimd, California, USA).
Furthermore, the indices of insulin resistance at the beginning and end of the treatment using HOMA-IR (Homeostasis Model Assessment of Insulin Resistance) were calculated according to the following equation: fasting glucose (mg/dl) × fasting insulin )µIU/ml)/405 (17).
- 6. Antioxidant assessment
Serum antioxidant enzyme superoxide dismutase (SOD) (ZB-SOD-96A), glutathione peroxidase (GPX) (ZB-GPX-96A) (18), and catalase (CAT) (ZB-CAT-96A) activities were measured using ELISA assays kits (ZellBio GmbH, Germany), according to the provider’s instructions.
Also, malondialdehyde (MDA) level as a biomarker of lipid peroxidation was assayed according of the kit protocol (ZB-MDA-96A).
Also, malondialdehyde (MDA) level as a biomarker of lipid peroxidation was assayed according of the kit protocol (ZB-MDA-96A).
- 7. Ovarian morphology
After the blood collection, uterus and ovaries were removed from the body of the rats for histopathological study.
After removing the adherent connective adipose tissue, then the ovaries tissues were embedded in a solution containing 10% formaldehyde for at least 48 hr for fixation. Afterwards, the tissue preparation phase were carried out according to the standard protocols. The samples were dehydrated, embedded in paraffin, serially sectioned, and stained with hematoxylin and eosin. Follicles were counted in all section and classified according to the study of Luo and collaborators (19).
After removing the adherent connective adipose tissue, then the ovaries tissues were embedded in a solution containing 10% formaldehyde for at least 48 hr for fixation. Afterwards, the tissue preparation phase were carried out according to the standard protocols. The samples were dehydrated, embedded in paraffin, serially sectioned, and stained with hematoxylin and eosin. Follicles were counted in all section and classified according to the study of Luo and collaborators (19).
- 8. Ethical considerations
Procedures related to the animals were approved by the Ethical Committee of Qom University of Medical Sciences (code: IR.MUQ.REC.1394.119) and the Ethical Principles of work on animals were taken in accordance with the agenda of the Ethics Committee of the Medical Sciences University.
- 9. Statistical analysis
Data were indicated as mean ± SEM. Statistical evaluation was done by analysis of variance (one-way ANOVA), followed by Tukey᾽s test. The test was performed using the SPSS Statistics, v. 17.01 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, USA). P < 0.05 was considered statistically significant.
- Results
- 1. Effect of hydroalcoholic extract of N. sativa seed on hormonal levels
3.1.1. Serum LH and FSH levels
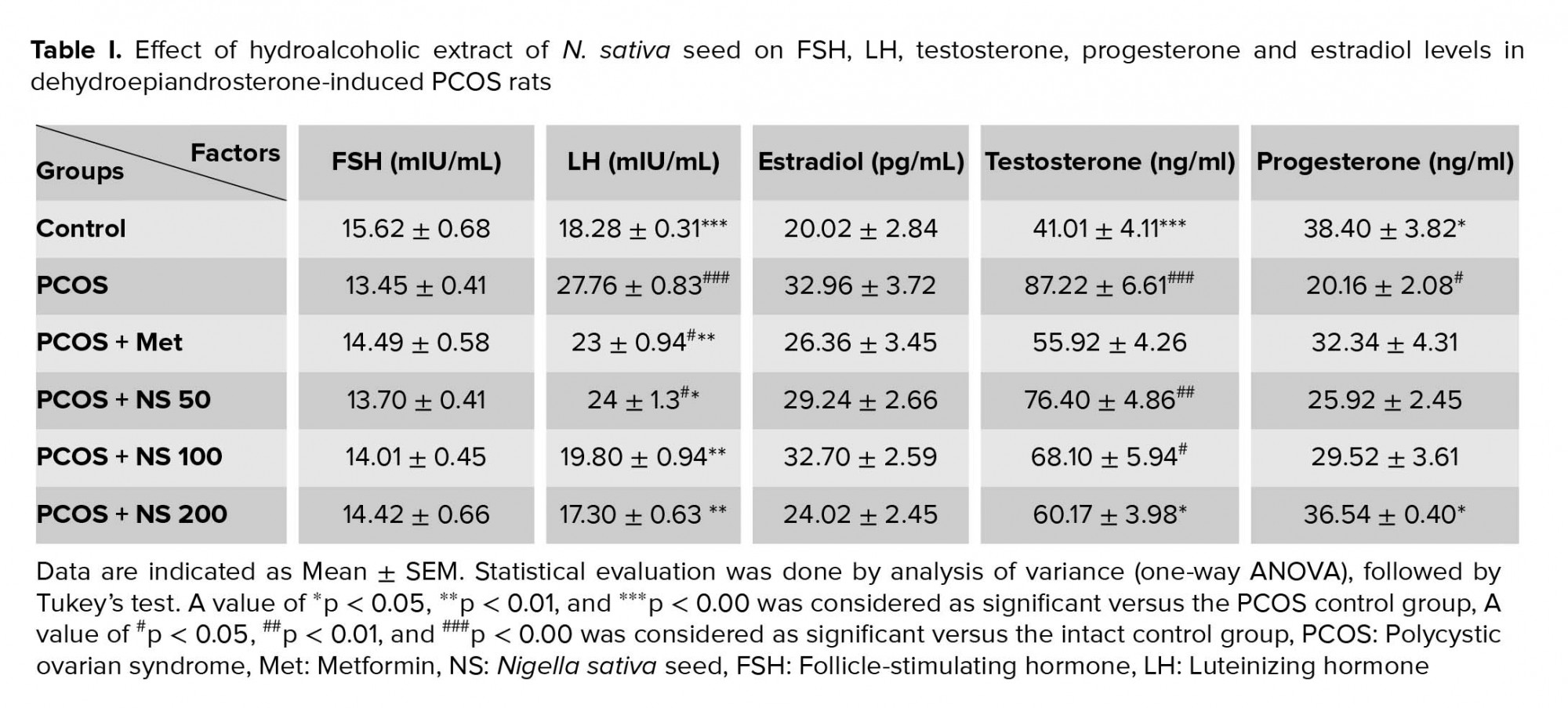
The results of this study showed that LH level increased in PCOS group (p < 0.001). The treatment of PCO animals with metformin and doses of 50, 100, and 200 mg/kg of the N. sativa extract decreased LH levels (p < 0.001and p = 0.05 respectively). The level of FSH decreased in PCOS rats, while it and FSH increased in other groups (Table I).
3.1.2. Serum testosterone level
The testosterone levels elevated in PCOS rats (p < 0.001) and treatment with metformin and a dose of 200 mg/kg N. sativa reduced it (p = 0.003 and p = 0.01, respectively) (Table I).
3.1.3. Serum progesterone and estrogen levels
The mean serum level of progesterone downregulated in the PCOS group (p = 0.01). Receiving a dose of 200 mg/kg of N. sativa extract increased the progesterone level (p = 0.03). Data of the present study showed that the level of estrogen upregulated in the PCOS group while it decreased in all treatment groups (Table I).
3.1.4. Glucose, insulin levels, and HOMA-IR
Following the induction of PCOS, the insulin and FBG levels increased (p = 0.001). The treatment of PCO rats with metformin and a dose of 200 mg/kg of N. sativa extract decreased the FBG levels (p = 0.03), while the doses of 100 and 200 mg/kg (p = 0.03 and p = 0.002, respectively) decreased the insulin level. In addition, the treatment of PCO rats with metformin and doses of 100 and 200 mg /kg (p = 0.01, p = 0.02 and, p < 0.001, respectively) of the extract reduced the insulin resistance (Table II).
3.2. Effect of hydroalcoholic extract of N. sativa seed on oxidative stress markers
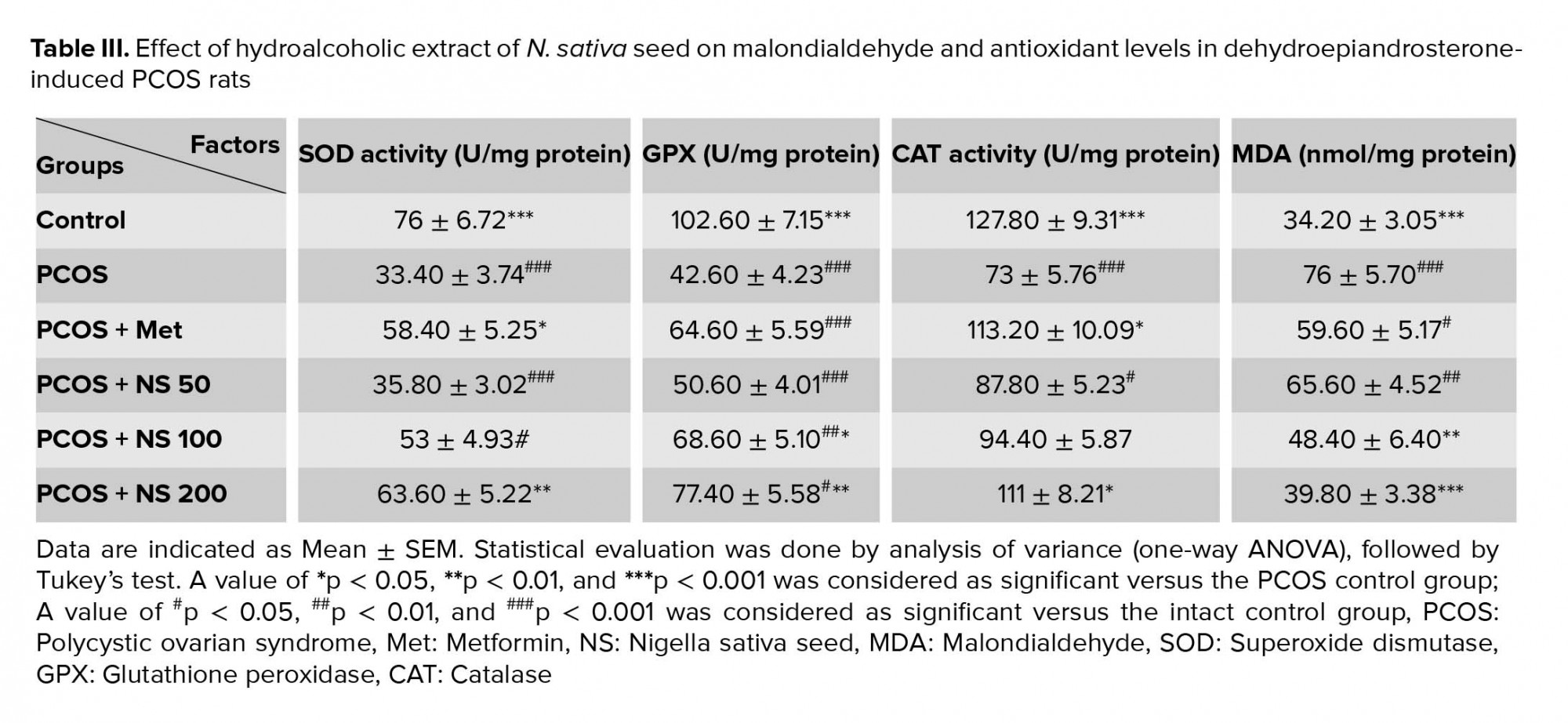
The induction of PCOS decreased the levels of SOD, GPX and CAT (p < 0.001) and increased the level of MDA (p < 0.001) in rat's serum. Treating the PCOS rats with a dose of 200 mg/kg of the N. sativa extract increased the SOD (p = 0.003), GPX (p = 0.002), and CAT (p = 0.02) levels. Also, a dose of 100 mg/kg of the extract increased the GPX level (p = 0.02). In addition, receiving the extract in doses 100 (p = 0.006) and 200 mg/kg (p < 0.001) in the PCOS groups decreased MDA levels. Moreover, the administration of metformin in the PCOS group increased the level of SOD (p = 0.01) and CAT (p = 0.12) (Table III).
3.3. Effect of hydroalcoholic extract of N. sativa seed on body weight
After 4 wk, the final body weight of PCOS rats increased in the PCOS group compared to the intact control group. Despite the decreased body weight in other groups, there were no significant differences in this item (Figure 1).
3.4. Results of histopathological study
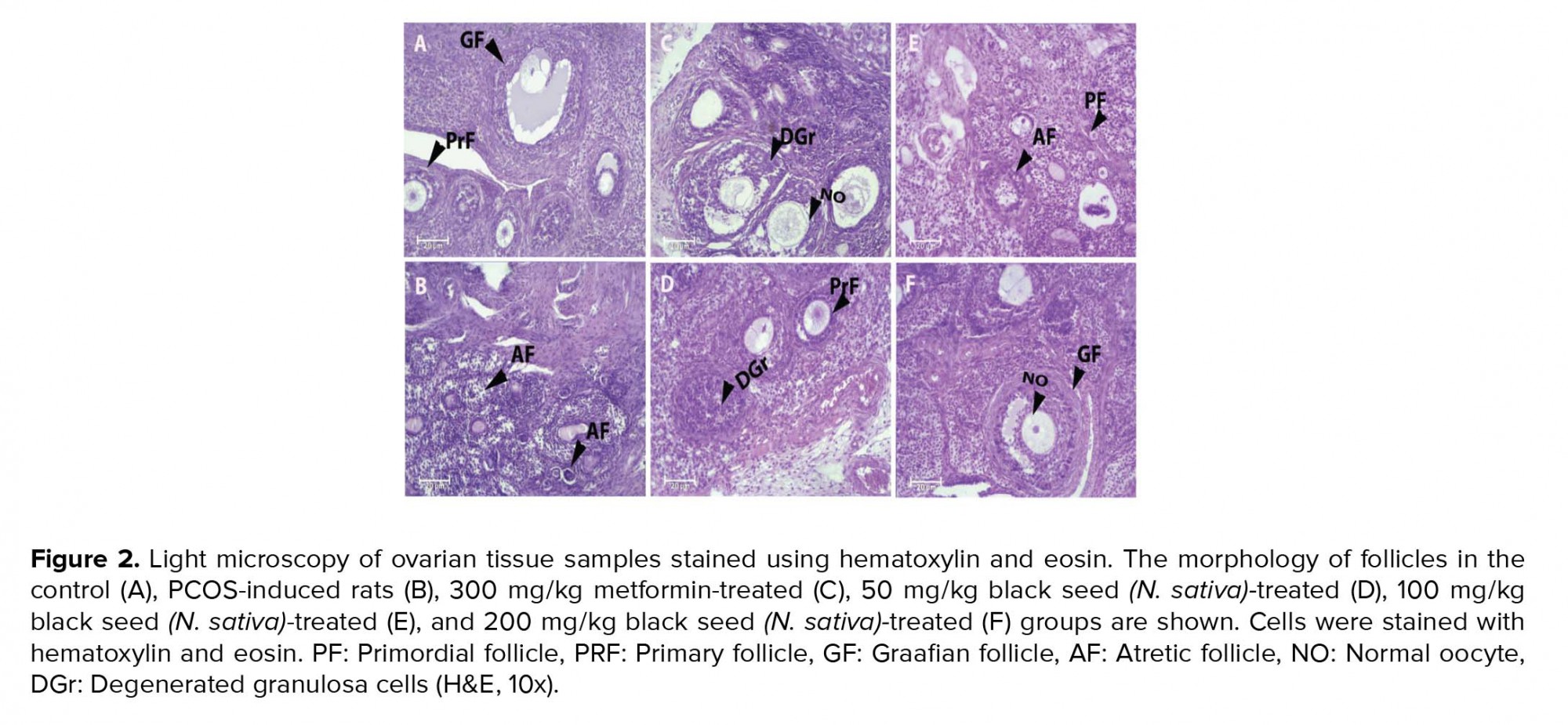
The normal histological view of the ovary was observed in the control group (Figure 2). The histopathological evaluation of ovarian tissues showed damaging changes in PCOS rats. A slight decrease was observed in the mean number of primordial and primary follicles of the PCOS group in comparison to the control group. The mean number of secondary and graffian follicles were decreased in the PCOS rats (p ≤ 0.001). However, a clear increase in cystic (p ≤ 0.001) and atretic follicles and a decrease in corpus luteum (p ≤ 0.001) was perceived in the PCOS-induced rats. Treatment with metformin and N. sativa seed extract, to some extent, improved the pathological changes. A dose of 200 mg/kg of the extract reversed these changes related to primary follicles (p ≤ 0.001). While administration 200 mg/kg of the extract increased the number of graafian follicles (p ≤ 0.001) it decreased the number of cystic follicles (p = 0.01). Receiving dose of 100 mg/kg of extract decreased the atretic follicles (p ≤ 0.001). Moreover administration of doses of 50, 200 mg/kg of extract and also receiving metformin reduced the atretic follicles in comparison to PCO group (p = 0.03, p = 0.04 and p = 0.02, respectively). Also, the doses of 50 and 100 mg/kg of the extract increased the corpus luteum (p ≤ 0.001 and p ≤ 0.001, respectively). Table IV presents the number of different ovarian follicles.



4. Discussion
In women with PCOS, the serum levels of testosterone, estradiol, and LH are increased and FSH and progesterone levels are decreased (17) .In this study, the treatment of rats with DHEA elevated the LH, estrogen, and testosterone levels, and decreased the FSH and progesterone levels, which is consistent with the results of Walters study Walters study following the same model of inducing PCOS (20). Therefore, it seems that the factors which can lower LH and estrogen levels and increase the level of FSH and progesterone can be used to treat this disease. The N. sativa seed, which is a plant about which the Prophet Muhammad (peace and blessings be upon him) said, "There is healing in the black seed for all diseases except death," was used in this study (21). In this study, the administration of N. sativa extract decreased the estrogen levels in the PCOS rats. Phytoestrogens are the active compounds of this extract which are able to, directly and indirectly, attach to estrogen receptors and cause significant estrogen-like effects (22). This effect, by negative feedback, reduces the plasma levels of estrogen. Also, the administration of the extract decreased the LH levels in PCOS rats. According to research, this may be related to linoleic acid (CLA) in N. sativa, which has a decreasing effect on LH levels. This effect of CLA is through the inhibition of nitric oxide and leptin, which are important controllers for the release of LH (23). In DHEA-induced PCOS model, the co-occurrence of hyperandrogenism and decrease in FSH level, observed in our study, is very common. We believe that it can be claimed that the hydroalcoholic extract of N. sativa seed showed remarkable antiandrogenic effects by reducing elevated testosterone levels. This property of the extract is possibly due to the presence of phytoestrogens in the extract. More studies are required to elucidate the exact functional mechanism of the extract. Furthermore, increasing the level of androgens can increase the level of FSH receptors in PCOS patients )24(, thus lowering the FSH serum level by negative feedback. Previous studies in PCOS indicate the relationship between the increased levels of LH or decreased FSH levels and creating insulin resistance. Disruption in the usual hypothalamic-pituitary-gonadal axis increases both the levels of testosterone and LH leading to the disease state )24(. LH stimulates testosterone secretion from ovarian theca cells through the PI3K/Akt pathway further elevating the activity of 17-a hydroxylase enzyme which catalyzes the conversion of progesterone to androgens, culminates in diminishing the progesterone level, and elevates the androgens level )25(. Thus, the anti-androgenic effects of N. sativa seed may be due to its potentials obstructing the PK13 pathway. The results of the tests showed that the level of glucose, insulin, and insulin resistance significantly increased in the PCOS rats compared to the healthy control group. Reduction in serum glucose observed in the PCOS rats treated with N. sativa can occur for various reasons. Thymoquinone in N. sativa seed can reduce the expression of gluconeogenic enzymes and the production of hepatic glucose (26). In addition, it has been shown that the liquid intake of N. sativa seed extract reduces glucose absorption and inhibits glucose carriers in diabetic rats (27). Our results verify the positive and dose-dependent effect of N. sativa seed in improving insulin resistance and serum level changes of insulin in PCOS rats. The findings suggests that hyperinsulinemia may enhance the chemerin gene expression in polycystic ovaries in which chemerin may play a role in pathophysiology of PCOS by direct action on ovary (28). In addition, the mechanism responsible for insulin resistance is obscure. Insulin resistance is worsened by extreme fat mass or unusual secretion or function of adipocytokines such as chemerin (29). Furthermore, according to our results, in a human study it was shown that receiving N. sativa caused a decrease in the insulin resistance (26). In our study, the induction of PCOS led to a decrease in progesterone levels. This may be due to the expression of the chemerin gene which inhibited FSH-induced progesterone secretion in granulosa cells by prevention of aromatase and p450scc expression (30). The results of this study showed that as expected, metformin as standard drug for PCOS treatment could significantly decrease testosterone, LH, insulin resistance, and glucose serum level compared to the PCOS-induced groups. However, it seems that one of the mechanisms involved in the function of metformin in the treatment of PCOS is its effect on reducing the gene expression of chemerin, which has been reported in various studies (31). In our study, the weight of PCOS rats increased, and treatment with N. sativa caused a drop in weight. In a human study, similar to our research, two months of N. sativa administration to menopausal women led to weight loss compared to the PCOS group (32). study has indicated the anti-obesity results of Nigella, due to its affirmative effects against insulin sensitivity and its immune-modular effects (33). In addition, N. sativa might possess anorectic effects and can cause a decrease in food intake. Despite these dates, more controlled intervention researches are necessary for higher comprehension the effects of N. sativa on weight loss. The increased androgens and insulin resistance can lead to oxidative stress (34), and the role of oxidative stress in PCOS pathogenesis can never be neglected (35). Therefore, reducing oxidative stress by increasing the activity of antioxidant enzymes can be one of the effective therapies in these patients. The results of this study showed that antioxidant enzymes levels increased and the malondialdehyde level decreased in rat’s model of PCOS after treatment with the extract of N. sativa seed. This result is similar to the results of Leong and colleagues (36).
Recent studies have shown that thymoquinone in black seeds have inhibitory effects on free radicals (37, 38) and the potential antihyperglycemic properties of N. Sativa is based on its antioxidant content. Moreover, N. sativa is reported to stimulate paraoxonase enzyme, which functions as an antioxidant (27). Furthermore, flavonoids, active ingredients in N. sativa seed (21), have antioxidant properties (39). The results of hormonal analysis were also supported by histopathological findings of the uterus as histopathological changes reached up in the PCOS group relative to the control group. In PCOS rats follicular atresia increased. Corpus luteum is a necessary factor for the synthesis of progesterone which affects the reproductive cycles and supports the uterus for implantation if conception happen, as in this study, in the PCOS group, the Corpus luteum was decreased accompanied by decline in progesterone level (40). Decrement in secondary follicles number because of the overproduction of androgens prevents normal follicular maturation process in PCOS group. However, the extract and metformin groups indicated a remarkable improvement of ovarian tissue with the appearance of reduction in cysts and outstanding regular luteinization (35).
5. Conclusion
The results of this study suggest that N. sativa seed extract has hypoglycemic, antioxidant effect and can modulate hormones related to fertility in PCOS rats. However, its use in the long-term treatment of PCOS, requires more investigation.
Acknowledgements
This study was financially supported by the Vice Chancellor of Research Affairs of the Qom University of Medical Sciences. The authors are also deeply thankful from collaboration of the Qom Danesh Laboratory.
Conflict of interest
The authors have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
The results of this study showed that LH level increased in PCOS group (p < 0.001). The treatment of PCO animals with metformin and doses of 50, 100, and 200 mg/kg of the N. sativa extract decreased LH levels (p < 0.001and p = 0.05 respectively). The level of FSH decreased in PCOS rats, while it and FSH increased in other groups (Table I).
3.1.2. Serum testosterone level
The testosterone levels elevated in PCOS rats (p < 0.001) and treatment with metformin and a dose of 200 mg/kg N. sativa reduced it (p = 0.003 and p = 0.01, respectively) (Table I).
3.1.3. Serum progesterone and estrogen levels
The mean serum level of progesterone downregulated in the PCOS group (p = 0.01). Receiving a dose of 200 mg/kg of N. sativa extract increased the progesterone level (p = 0.03). Data of the present study showed that the level of estrogen upregulated in the PCOS group while it decreased in all treatment groups (Table I).
3.1.4. Glucose, insulin levels, and HOMA-IR
Following the induction of PCOS, the insulin and FBG levels increased (p = 0.001). The treatment of PCO rats with metformin and a dose of 200 mg/kg of N. sativa extract decreased the FBG levels (p = 0.03), while the doses of 100 and 200 mg/kg (p = 0.03 and p = 0.002, respectively) decreased the insulin level. In addition, the treatment of PCO rats with metformin and doses of 100 and 200 mg /kg (p = 0.01, p = 0.02 and, p < 0.001, respectively) of the extract reduced the insulin resistance (Table II).
3.2. Effect of hydroalcoholic extract of N. sativa seed on oxidative stress markers
The induction of PCOS decreased the levels of SOD, GPX and CAT (p < 0.001) and increased the level of MDA (p < 0.001) in rat's serum. Treating the PCOS rats with a dose of 200 mg/kg of the N. sativa extract increased the SOD (p = 0.003), GPX (p = 0.002), and CAT (p = 0.02) levels. Also, a dose of 100 mg/kg of the extract increased the GPX level (p = 0.02). In addition, receiving the extract in doses 100 (p = 0.006) and 200 mg/kg (p < 0.001) in the PCOS groups decreased MDA levels. Moreover, the administration of metformin in the PCOS group increased the level of SOD (p = 0.01) and CAT (p = 0.12) (Table III).
3.3. Effect of hydroalcoholic extract of N. sativa seed on body weight
After 4 wk, the final body weight of PCOS rats increased in the PCOS group compared to the intact control group. Despite the decreased body weight in other groups, there were no significant differences in this item (Figure 1).
3.4. Results of histopathological study
The normal histological view of the ovary was observed in the control group (Figure 2). The histopathological evaluation of ovarian tissues showed damaging changes in PCOS rats. A slight decrease was observed in the mean number of primordial and primary follicles of the PCOS group in comparison to the control group. The mean number of secondary and graffian follicles were decreased in the PCOS rats (p ≤ 0.001). However, a clear increase in cystic (p ≤ 0.001) and atretic follicles and a decrease in corpus luteum (p ≤ 0.001) was perceived in the PCOS-induced rats. Treatment with metformin and N. sativa seed extract, to some extent, improved the pathological changes. A dose of 200 mg/kg of the extract reversed these changes related to primary follicles (p ≤ 0.001). While administration 200 mg/kg of the extract increased the number of graafian follicles (p ≤ 0.001) it decreased the number of cystic follicles (p = 0.01). Receiving dose of 100 mg/kg of extract decreased the atretic follicles (p ≤ 0.001). Moreover administration of doses of 50, 200 mg/kg of extract and also receiving metformin reduced the atretic follicles in comparison to PCO group (p = 0.03, p = 0.04 and p = 0.02, respectively). Also, the doses of 50 and 100 mg/kg of the extract increased the corpus luteum (p ≤ 0.001 and p ≤ 0.001, respectively). Table IV presents the number of different ovarian follicles.






4. Discussion
In women with PCOS, the serum levels of testosterone, estradiol, and LH are increased and FSH and progesterone levels are decreased (17) .In this study, the treatment of rats with DHEA elevated the LH, estrogen, and testosterone levels, and decreased the FSH and progesterone levels, which is consistent with the results of Walters study Walters study following the same model of inducing PCOS (20). Therefore, it seems that the factors which can lower LH and estrogen levels and increase the level of FSH and progesterone can be used to treat this disease. The N. sativa seed, which is a plant about which the Prophet Muhammad (peace and blessings be upon him) said, "There is healing in the black seed for all diseases except death," was used in this study (21). In this study, the administration of N. sativa extract decreased the estrogen levels in the PCOS rats. Phytoestrogens are the active compounds of this extract which are able to, directly and indirectly, attach to estrogen receptors and cause significant estrogen-like effects (22). This effect, by negative feedback, reduces the plasma levels of estrogen. Also, the administration of the extract decreased the LH levels in PCOS rats. According to research, this may be related to linoleic acid (CLA) in N. sativa, which has a decreasing effect on LH levels. This effect of CLA is through the inhibition of nitric oxide and leptin, which are important controllers for the release of LH (23). In DHEA-induced PCOS model, the co-occurrence of hyperandrogenism and decrease in FSH level, observed in our study, is very common. We believe that it can be claimed that the hydroalcoholic extract of N. sativa seed showed remarkable antiandrogenic effects by reducing elevated testosterone levels. This property of the extract is possibly due to the presence of phytoestrogens in the extract. More studies are required to elucidate the exact functional mechanism of the extract. Furthermore, increasing the level of androgens can increase the level of FSH receptors in PCOS patients )24(, thus lowering the FSH serum level by negative feedback. Previous studies in PCOS indicate the relationship between the increased levels of LH or decreased FSH levels and creating insulin resistance. Disruption in the usual hypothalamic-pituitary-gonadal axis increases both the levels of testosterone and LH leading to the disease state )24(. LH stimulates testosterone secretion from ovarian theca cells through the PI3K/Akt pathway further elevating the activity of 17-a hydroxylase enzyme which catalyzes the conversion of progesterone to androgens, culminates in diminishing the progesterone level, and elevates the androgens level )25(. Thus, the anti-androgenic effects of N. sativa seed may be due to its potentials obstructing the PK13 pathway. The results of the tests showed that the level of glucose, insulin, and insulin resistance significantly increased in the PCOS rats compared to the healthy control group. Reduction in serum glucose observed in the PCOS rats treated with N. sativa can occur for various reasons. Thymoquinone in N. sativa seed can reduce the expression of gluconeogenic enzymes and the production of hepatic glucose (26). In addition, it has been shown that the liquid intake of N. sativa seed extract reduces glucose absorption and inhibits glucose carriers in diabetic rats (27). Our results verify the positive and dose-dependent effect of N. sativa seed in improving insulin resistance and serum level changes of insulin in PCOS rats. The findings suggests that hyperinsulinemia may enhance the chemerin gene expression in polycystic ovaries in which chemerin may play a role in pathophysiology of PCOS by direct action on ovary (28). In addition, the mechanism responsible for insulin resistance is obscure. Insulin resistance is worsened by extreme fat mass or unusual secretion or function of adipocytokines such as chemerin (29). Furthermore, according to our results, in a human study it was shown that receiving N. sativa caused a decrease in the insulin resistance (26). In our study, the induction of PCOS led to a decrease in progesterone levels. This may be due to the expression of the chemerin gene which inhibited FSH-induced progesterone secretion in granulosa cells by prevention of aromatase and p450scc expression (30). The results of this study showed that as expected, metformin as standard drug for PCOS treatment could significantly decrease testosterone, LH, insulin resistance, and glucose serum level compared to the PCOS-induced groups. However, it seems that one of the mechanisms involved in the function of metformin in the treatment of PCOS is its effect on reducing the gene expression of chemerin, which has been reported in various studies (31). In our study, the weight of PCOS rats increased, and treatment with N. sativa caused a drop in weight. In a human study, similar to our research, two months of N. sativa administration to menopausal women led to weight loss compared to the PCOS group (32). study has indicated the anti-obesity results of Nigella, due to its affirmative effects against insulin sensitivity and its immune-modular effects (33). In addition, N. sativa might possess anorectic effects and can cause a decrease in food intake. Despite these dates, more controlled intervention researches are necessary for higher comprehension the effects of N. sativa on weight loss. The increased androgens and insulin resistance can lead to oxidative stress (34), and the role of oxidative stress in PCOS pathogenesis can never be neglected (35). Therefore, reducing oxidative stress by increasing the activity of antioxidant enzymes can be one of the effective therapies in these patients. The results of this study showed that antioxidant enzymes levels increased and the malondialdehyde level decreased in rat’s model of PCOS after treatment with the extract of N. sativa seed. This result is similar to the results of Leong and colleagues (36).
Recent studies have shown that thymoquinone in black seeds have inhibitory effects on free radicals (37, 38) and the potential antihyperglycemic properties of N. Sativa is based on its antioxidant content. Moreover, N. sativa is reported to stimulate paraoxonase enzyme, which functions as an antioxidant (27). Furthermore, flavonoids, active ingredients in N. sativa seed (21), have antioxidant properties (39). The results of hormonal analysis were also supported by histopathological findings of the uterus as histopathological changes reached up in the PCOS group relative to the control group. In PCOS rats follicular atresia increased. Corpus luteum is a necessary factor for the synthesis of progesterone which affects the reproductive cycles and supports the uterus for implantation if conception happen, as in this study, in the PCOS group, the Corpus luteum was decreased accompanied by decline in progesterone level (40). Decrement in secondary follicles number because of the overproduction of androgens prevents normal follicular maturation process in PCOS group. However, the extract and metformin groups indicated a remarkable improvement of ovarian tissue with the appearance of reduction in cysts and outstanding regular luteinization (35).
5. Conclusion
The results of this study suggest that N. sativa seed extract has hypoglycemic, antioxidant effect and can modulate hormones related to fertility in PCOS rats. However, its use in the long-term treatment of PCOS, requires more investigation.
Acknowledgements
This study was financially supported by the Vice Chancellor of Research Affairs of the Qom University of Medical Sciences. The authors are also deeply thankful from collaboration of the Qom Danesh Laboratory.
Conflict of interest
The authors have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Type of Study: Original Article |
Subject:
Reproductive Physiology
References
1. Amooee S, Akbarzadeh-Jahromi M, Motavas M, Zarei F. Comparing endometrial hysteroscopic and histological findings of infertile women with polycystic ovary syndrome and unexplained infertility: A cross-sectional study. Int J Reprod BioMed 2020; 18: 33-40. [DOI:10.18502/ijrm.v18i1.6195] [PMID] [PMCID]
2. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2014; 6: 1-13. [DOI:10.2147/CLEP.S37559] [PMID] [PMCID]
3. Banaszewska B, Duleba AJ, Spaczynski RZ, Pawelczyk L. Lipids in polycystic ovary syndrome: role of hyperinsulinemia and effects of metformin. Am J Obstet Gynecol 2006; 194: 1266-1272. [DOI:10.1016/j.ajog.2005.11.009] [PMID]
4. Duleba AJ. Medical management of metabolic dysfunction in PCOS. Steroids 2012; 77: 306-311. [DOI:10.1016/j.steroids.2011.11.014] [PMID] [PMCID]
5. Omidi M, Ahangarpour A, Mard SA, Khorsandi L. The effects of myricitrin and vitamin E against reproductive changes induced by D-galactose as an aging model in female mice: An experimental study. Int J Reprod BioMed 2019; 17: 789-798. [DOI:10.18502/ijrm.v17i10.5486] [PMID] [PMCID]
6. Atiomo WU, El-Mahdi E, Hardiman P. Familial associations in women with polycystic ovary syndrome. Fertil Steril 2003; 80: 143-145. [DOI:10.1016/S0015-0282(03)00502-8]
7. Amirghofran Z. Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran J Immunol 2010; 7: 65-73.
8. Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res 2003; 17: 299-305. [DOI:10.1002/ptr.1309] [PMID]
9. Ghannadi A, Hajhashemi V, Jafarabadi H. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food 2005; 8: 488-493. [DOI:10.1089/jmf.2005.8.488] [PMID]
10. Panahi M, Namjoyan F, Shakerin Z. Evaluation of antioxidant effects of nigella sativa extract on the ultra structure of neural tube defects in diabetic rats's offspring. Jundishapur J Nat Pharm Products 2011; 6: 16-23.
11. Kim EJ, Jang M, Choi JH, Park KS, Cho IH. An improved dehydroepiandrosterone-induced rat model of polycystic ovary syndrome (PCOS): Post-pubertal improve PCOS's features. Front Endocrinol 2018; 9: 1-28 [DOI:10.3389/fendo.2018.00735] [PMID] [PMCID]
12. Ahangarpour A, Heidari H, Oroojan AA, Mirzavandi F, Nasr Esfehani K, Dehghan Mohammadi Z. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root's hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J Phytomed 2017; 7: 169-179.
13. Javadi I, Rashidi Nooshabadi M, Goudarzi M, Roudbari R. Protective effects of celery (Apium graveloens) seed extract on bleomycin-induced pulmonary fibrosis in rats. J Babol Univ Med Sci 2015; 17: 70-76.
14. Jelodar Gh, Masoomi S, Rahmanifar F. Hydroalcoholic extract of flaxseed improves polycystic ovary syndrome in a rat model. Iran J Basic Med Sci 2018; 21: 645-650.
15. Goudarzi M, Mombeini MA, Fatemi I, Aminzadeh A, Kalantari H, Nesari A, et al. Neuroprotective effects of Ellagic acid against acrylamide-induced neurotoxicity in rats. Neurol Res 2019; 41: 419-428. [DOI:10.1080/01616412.2019.1576319] [PMID]
16. Zamami Y, Takatori S, Goda M, Koyama T, Iwatani Y, Jin X, et al. Royal jelly ameliorates insulin resistance in fructose-drinking rats. Biol Pharm Bull 2008; 31: 2103-2107. [DOI:10.1248/bpb.31.2103] [PMID]
17. Ahangarpour A, Heidari H, Salehizade Junghani M, Absari R, Khoogar M, Ghaedi E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin-induced type II diabetic male mice. Res Pharm Sci 2017; 12: 416-424. [DOI:10.4103/1735-5362.213987] [PMID] [PMCID]
18. Mehrzadi S, Bahrami N, Mehrabani M, Motevalian M, Mansouri E, Goudarzi M. Ellagic acid: A promising protective remedy against testicular toxicity induced by arsenic. Biomed Pharmacother 2018; 103: 1464-1472. [DOI:10.1016/j.biopha.2018.04.194] [PMID]
19. Luo LL, Huang J, Fu YC, Xu JJ, Qian YS. Effects of tea polyphenols on ovarian development in rats. J Endocrinol Invest 2008; 31: 1110-1118. [DOI:10.1007/BF03345661] [PMID]
20. Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod 2012; 86: 149. 1-12. [DOI:10.1095/biolreprod.111.097808] [PMID]
21. Sudhir SP, Deshmukh VO, Verma HN. Nigella sativa seed, a novel beauty care ingredient: A review. Int J Pharm Sci Res 2016; 7: 3185-3196.
22. Parhizkar S, Latiff LA, Rahman SA, Dollah MA, Parichehr H. Assessing estrogenic activity of Nigella sativa in ovariectomized rats using vaginal cornification assay. Afr J Pharm Pharmacol 2011; 5: 137-142. [DOI:10.5897/AJPP10.276]
23. Khodaii H, Chamani M, Sadeghi A, Hejazi H. Effects of conjugated linoleic acid on mouse factors and hormones in the process of ovulation in miceintint. J Fertil 2009; 2: 101-109.
24. Atashpour S, Jahromi HK, Jahromi ZK, Maleknasab M. Comparison of the effects of Ginger extract with clomiphene citrate on sex hormones in rats with polycystic ovarian syndrome. Int J Reprod BioMed 2017; 15: 561-568. [DOI:10.29252/ijrm.15.9.561] [PMID] [PMCID]
25. Jahan S, Abid A, Khalid S, Afsar T, Ul-Ain Q, Shaheen G, et al. Therapeutic potentials of Quercetin in management of polycystic ovarian syndrome using Letrozole induced rat model: A histological and a biochemical study. J Ovarian Res 2018; 11: 26-35. [DOI:10.1186/s13048-018-0400-5] [PMID] [PMCID]
26. Heshmati J, Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: A systematic review. Complement Ther Med 2015; 23: 275-282. [DOI:10.1016/j.ctim.2015.01.013] [PMID]
27. Ermumcu MŞ, Şanlıer N. black cumin (Nigella sativa) and its active component of thymoquinone: effects on health. Food and Health 2017; 3: 170-183. [DOI:10.3153/JFHS17020]
28. Rezvanfar MA, Shojaei Saadi HA, Gooshe M, Abdolghaffari AH, Baeeri M, Abdollahi M. Ovarian aging-like phenotype in the hyperandrogenism-induced murine model of polycystic ovary. Oxid Med Cell Longev 2014; 2014: 1-10. [DOI:10.1155/2014/948951] [PMID] [PMCID]
29. Bozaoglu K, Segal D, Shields KA, Cummings N, Curran JE, Comuzzie AG, et al. Chemerin is associated with metabolic syndromephenotypes in a Mexican-American population. J Clin Endocrinol Metab 2009; 94: 3085-3088. [DOI:10.1210/jc.2008-1833] [PMID] [PMCID]
30. Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007; 148: 4687-4694. [DOI:10.1210/en.2007-0175] [PMID]
31. Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, et al. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes 2009; 58: 1971-1977. [DOI:10.2337/db08-1528] [PMID] [PMCID]
32. Ibrahim RM, Hamdan NS, Ismail M, Saini SM, Rashid SNA, Latiff LA, et al. Protective effects of Nigella sativa on metabolic syndrome in menopausal women. Adv Pharm Bull 2014; 4: 29-33.
33. Vanamala J, Kester AC, Heuberger AL, Reddivari L. Mitigation of obesity-promoted diseases by Nigella sativa and thymoquinone. Plant Foods Hum Nutr 2012; 67: 111-119. [DOI:10.1007/s11130-012-0279-z] [PMID]
34. Zuo T, Zhu M, Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid Med Cell Longev 2016; 2016: 8589318: 1-15. [DOI:10.1155/2016/8589318] [PMID] [PMCID]
35. Rezvanfar MA, Rezvanfar MA, Ahmadi A, Shojaei Saadi HA, Baeeri M, Abdollahi M. Mechanistic links between oxidative/nitrosative stress and tumor necrosis factor alpha in letrozole-induced murine polycystic ovary: biochemical and pathological evidences for beneficial effect of pioglitazone. Hum Exp Toxicol 2012; 31: 887-897. [DOI:10.1177/0960327111426589] [PMID]
36. Leong XF, Rais Mustafa M, Jaarin K. Nigella sativa and its protective role in oxidative stress and hypertension. Evidence-Based Complementary and Alternative Medicine 2013; 2013: 120732: 1-10. [DOI:10.1155/2013/120732] [PMID] [PMCID]
37. Nader MA, El-Agamy DS, Suddek GhM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res 2010; 33: 637-643. [DOI:10.1007/s12272-010-0420-1] [PMID]
38. Inci M, Davarci M, Inci M, Motor S, Yalcinkaya FR, Nacar E, et al. Anti-inflammatory and antioxidant activity of thymoquinone in a rat model of acute bacterial prostatitis. Hum Exp Toxicol 2013; 32: 354-361. [DOI:10.1177/0960327112455068] [PMID]
39. Banjarnahor SDS, Artanti N. Antioxidant properties of flavonoids. Med J Indones 2014; 23: 239-244. [DOI:10.13181/mji.v23i4.1015]
40. Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev 2007; 28: 117-149. [DOI:10.1210/er.2006-0022] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








