Mon, May 13, 2024
[Archive]
Volume 19, Issue 10 (October 2021)
IJRM 2021, 19(10): 921-928 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Banafshi O, Nasseri S, Farhadi L, Alasvand M, Khadem-Erfan M B, Hosseini J, et al . The effects of supplemented sericin on in vitro maturation and preimplantation development of mouse embryos: An experimental study. IJRM 2021; 19 (10) :921-928
URL: http://ijrm.ir/article-1-1795-en.html
URL: http://ijrm.ir/article-1-1795-en.html
Omid Banafshi1 

 , Sherko Nasseri1
, Sherko Nasseri1 

 , Leila Farhadi1
, Leila Farhadi1 

 , Masoud Alasvand2
, Masoud Alasvand2 

 , Mohammad Bagher Khadem-Erfan1
, Mohammad Bagher Khadem-Erfan1 

 , Javad Hosseini1
, Javad Hosseini1 

 , Saber Miraki1
, Saber Miraki1 

 , Fardin Fathi *
, Fardin Fathi * 

 3
3


 , Sherko Nasseri1
, Sherko Nasseri1 

 , Leila Farhadi1
, Leila Farhadi1 

 , Masoud Alasvand2
, Masoud Alasvand2 

 , Mohammad Bagher Khadem-Erfan1
, Mohammad Bagher Khadem-Erfan1 

 , Javad Hosseini1
, Javad Hosseini1 

 , Saber Miraki1
, Saber Miraki1 

 , Fardin Fathi *
, Fardin Fathi * 

 3
3
1- Cellular and Molecular Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
2- Cancer and Immunology Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
3- Cellular and Molecular Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran. , farfath@gmail.com
2- Cancer and Immunology Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran.
3- Cellular and Molecular Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran. , farfath@gmail.com
Keywords: Sericin, In vitro maturation, In vitro fertilization, Preimplantation embryo, Culture medium, Mice.
Full-Text [PDF 270 kb]
(1059 Downloads)
| Abstract (HTML) (1641 Views)
Full-Text: (398 Views)
- Introduction
In vitro culture of murine embryos has traditionally been a two-step process of retrieval and cultivation, requiring appropriate unique media for culture conditions. Embryos are routinely harvested from oviducts and placed into an embryo culture medium. In the next step, the embryos are transferred into a bicarbonate-based buffered medium placed in a culture incubator (1). Different types of primary culture media used for in vitro embryo culture are often provided with sera such as fetal bovine serum or bovine serum albumin for the preparation of growth factors, amino acids, hormones, and proteins which are useful for embryonic development (2). The high risk of sera contamination with pathogens, prions, viruses, and bovine spongiform encephalopathy, etc. has prompted researchers to try to find alternative supplements that can be used instead of serum during in vitro embryo culture (3, 4). Dash and colleagues Sericin has been reported to have an antioxidant effect on skin fibroblast cells exposed to hydrogen peroxide. They showed the potential to inhibit the production of intracellular hydrogen peroxide in ultraviolet-treated keratinocytes (5).
Sericin is a water-soluble silk protein extracted from cocoons and it is the second major protein component (besides fibroin) of silk used in biological materials due to their anti-UV and antibacterial properties. Sericin is composed of 18 types of amino acids as well as polar side groups such as carboxyl, hydroxyl, and amino groups and it is rich in serine and aspartic acid (6). Izobe et al. Found that culturing two-cell embryos separately for seven days in an environment with 0.5% sericin resulted in the highest rate of blastocyst formation and development into expanded blastocysts. It has been shown that preimplantation development and the quality of cultured bovine embryos increased following the addition of sericin to an in vitro medium, which prevented oxidative stress (7). It was reported that supplementation of a medium with 0.1% sericin during in vitro maturation (IVM) improved the nuclear maturation and fertilizability of sheep oocytes. Also, because of its antibacterial and UV-resistant properties, as a biomaterial. It may replace bovine serum albumin with Sericin in chemical environments without risk of disease transmission. Several studies have suggested the benefits of using sericin as a protein replacement (8). Also, some studies have suggested that sericin can have a role in cryoprotection in the freezing of mice, human, and buffalo sperm (9-11).
The present study is the first to investigate the effect of 0.1%, 0.5%, and 1% sericin as a medium supplement on IVM, the rate of in vitro fertilization (IVF), and in vitro development of mouse embryos.
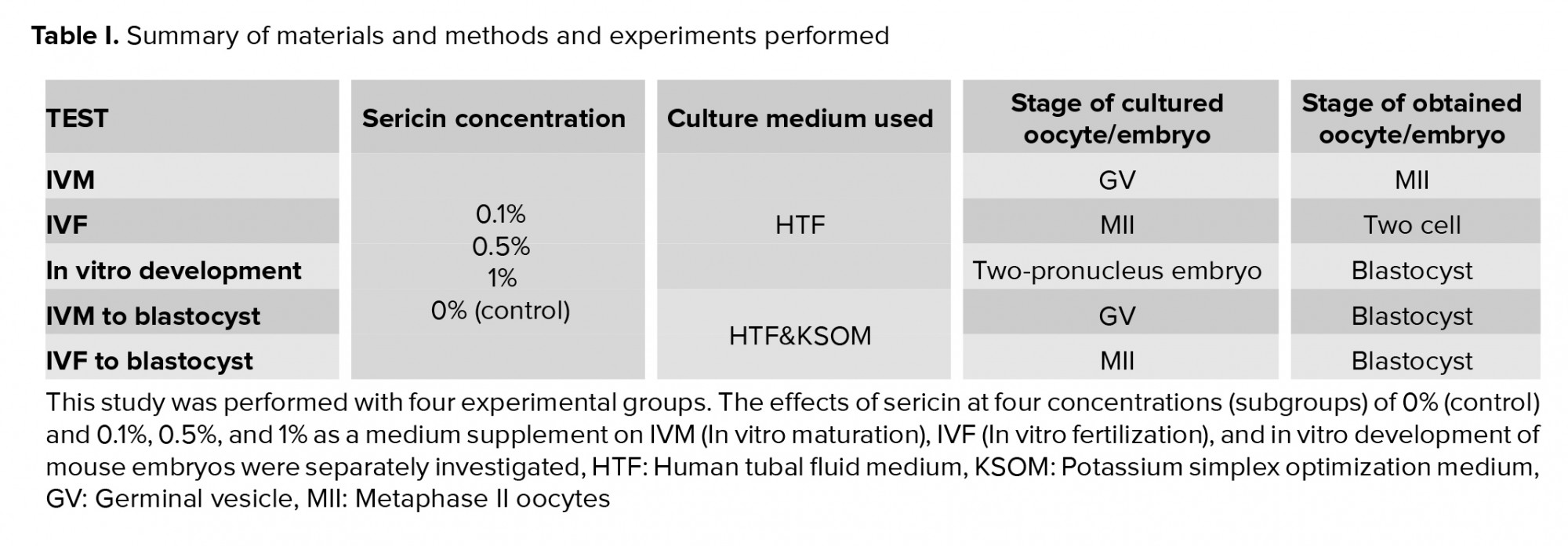
2. Materials and Methods
2.1. Sericin preparation and study groups
Sericin and the other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), and the culture-related plastics were purchased from Falcon (Paignton, UK). Sericin was diluted in a human tubal fluid medium (HTF). Sericin was considered as a supplement in the embryo culture medium at 0.1%, 0.5% and 1% concentrations (subgroups I, II, and III, respectively) in comparison with a sericin-free group as a control group. Four study subgroups were created within each experimental group: IVM, IVF, in vitro development, and IVM+ in vitro development.
2.2. Animals
32 NMRI mice were used to extract germinal vesicle (GV) oocytes, metaphase II (MII) oocytes, and sperm. Then, IVM, IVF, and evaluation of mouse embryo development in vitro were performed. The mice had free access to water and food and were preserved at a temperature of 23 ± 2ºC and a 12-hr light/dark cycle. All processes were carried out at the Kurdistan University of Medical Science in 2018.
2.3. Collection of immature GV oocytes and IVM GV oocytes and IVM
A total of 5 IU of pregnant mare serum gonadotropin (PMSG) was injected into each 4-6-wk-old female mouse. Then, 48 hr post injection, immature GV oocytes were released from the ovaries by puncturing the follicles as visualized under a stereomicroscope. The GV oocytes were collected and randomly classified into four subgroups: 0% (control) and 0.1%, 0.5%, and 1% HTF containing sericin (4). The GV oocytes were incubated at 37ºC in a humidified atmosphere of 5% CO2 in the air for 24 hr. Next, the oocytes displaying the first polar bodies by a stereomicroscope were considered as MII oocytes. The MII oocytes were then recruited for the IVM+ in vitro development follow-up group (Table I).
2.4. Collection of MII oocytes and IVF
IVF was performed as described previously with minor modifications (12). PMSG was injected into female mice that were 4-6 wk old. After 48 hr, human Chorionic Gonadotropin (hCG) was injected, and oocytes were collected from mouse oviducts 14 hr post the hCG injection. The mature oocytes (metaphase meiosis II) were transferred to 100-µl IVF medium droplets, and 1×106 sperms/ml was added to the IVF droplets supplemented with sericin: 0% (control) and 0.1%, 0.5%, and 1% as test subgroups for 24 hr. Then, the zygotes were monitored by a reverse microscope and the percentage of two-cell formation was recorded to assess the rate of fertilization. (Table I).
2.5 Collection of zygotes and in vitro development
After the PMSG and hCG injections, the injected female mice mated with male mice. 18 hr post hCG injection; female mice with a vaginal plaque were selected and euthanized with cervical dislocation. The two-pronucleus-stage embryos were collected from the mouse oviducts, observed, and screened at ×100 magnification on a warmed microscope stage (37ºC); they were not fragmented or degenerated and were classified into three subgroups of 0.1%, 0.5%, and 1% sericin added potassium-supplemented simplex optimized medium (Ksom) and non-added sericin as a control group (with KSOMAA). Each plate contained 200 ul of KSOMAA covered by preincubated mineral oil used for two-cell-stage embryo culture and development at 37ºC in 5% CO2 in the air inside a humidified incubator (10). The number of zygotes developing to early blastocysts was calculated in each sericin subgroup.
2.6. Follow-up from IVM to the blastocyst stage
For assessing the cumulative effect of sericin as an embryo culture supplement, we used the MII oocytes obtained from the IVM study group for IVF and subsequent embryo development. During all three stages of the experiment, the culture media used were supplemented with three different concentrations of sericin (13). The number of obtained blastocysts was considered as a complete process of oocyte maturation, fertilization, and in vitro embryo development in the presence of sericin. It was then compared to the control group.
2.7. Follow-up from IVF to the blastocyst stage
To assess the cumulative effect of sericin as an embryo culture supplement in addition to IVF, the medium for the culture of the subsequent embryo from IVF was supplemented with three different concentrations of sericin. The number of blastocysts was considered as a complete process of IVF of MII oocytes and development to blastocyst in the presence of sericin and compared to the control group.
2.8. Ethical considerations
All procedures were carried out based on the guidelines of the Ethics Committee of the Kurdistan University of Medical Science, Sanandaj, Iran (Code: IR.MUK.REC.1395/215). According to the University Ethics Committee, working with laboratory animals in this study did not violate any rules.
2.9. Statistical analysis
In this experimental study, the IVM and IVF rates and the percentage of development to the blastocyst stage (in vitro development) in mouse oocytes and embryos were calculated for each sericin group and subgroup, and these values were then compared with those of the control group. One-way ANOVA was run to assess group differences. Data were analyzed by Stata 14 (Stata Corp. 2015. Stata Statistical Software: Release 14. College Station, TX: Stata Corp LP). The significance level in this study was set at p < 0.05.
3. Results
3.1. The effect of Sericin as an embryo culture supplement on IVM, IVF, and the in vitro development rate
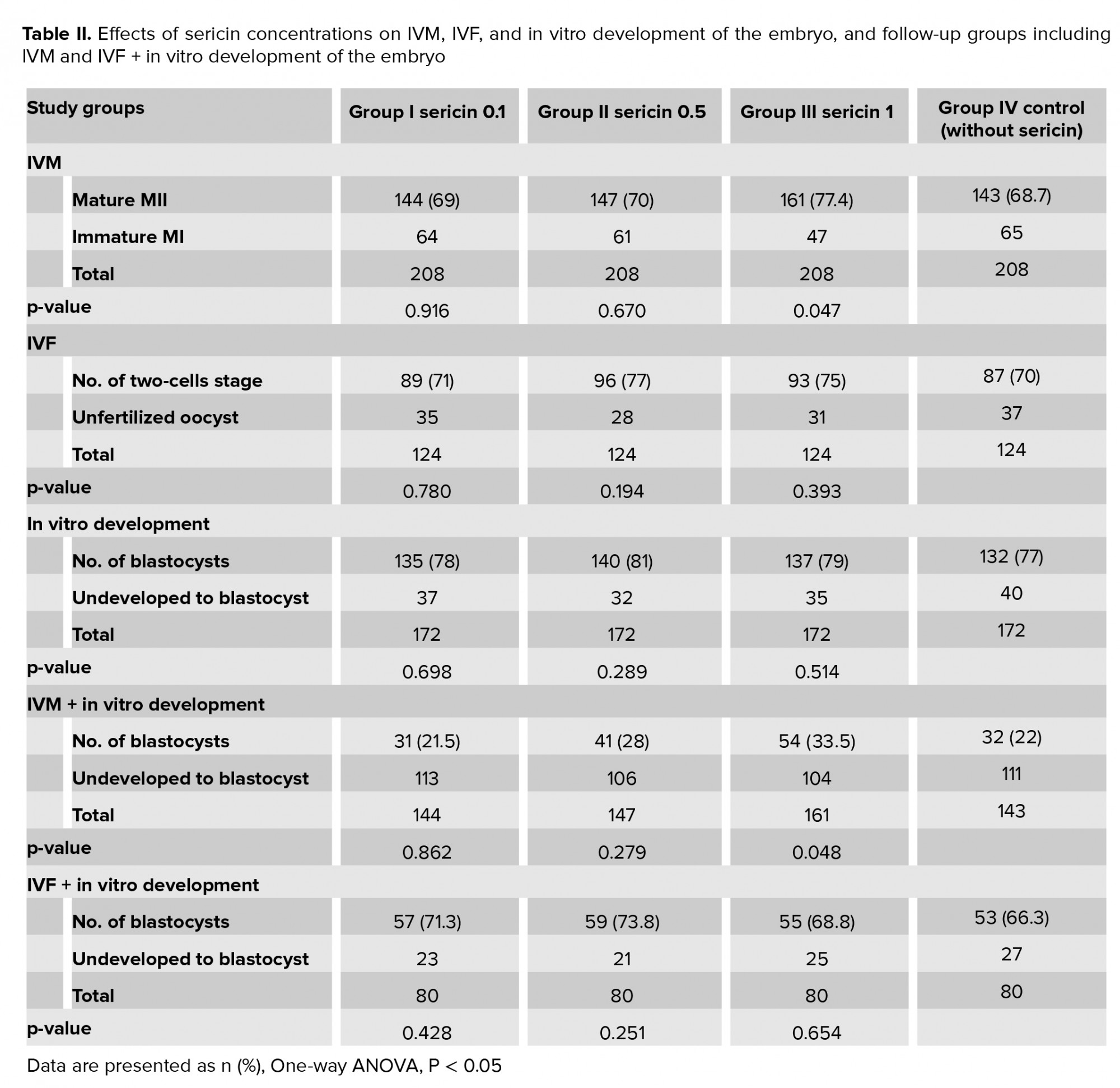
Initially, 832 GV oocytes were classified into 208 GV oocytes for each sericin subgroup and control group. Among the three sericin subgroups, the effect of 1% sericin (161/208 = 77.4%) was significant in comparison with the control sericin-free group (143/208 = 68.7%). On the other hand, the 0.1 and 0.5% subgroups did not show any significant relationship.
For IVF, a total of 124 oocytes were recruited for each sericin subgroup of 0.1, 0.5, 1% sericin, and the sericin-free control group for testing the effect of sericin on IVF. Notably, the one-way ANOVA analysis of the three sericin subgroups showed no significant relationship in comparison with the control group.
Regarding the in vitro development rate, after collecting the 688 two-pro nucleus-stage embryos from the oviducts and dividing them into three subgroups based on sericin concentration and a control group (without sericin), the number of zygotes that developed into early blastocysts was calculated. One-way ANOVA analyses revealed that the three concentrations of sericin as an embryo culture supplement had no significant effect on the in vitro development of mice zygotes (Table II).
3.2. The effect of sericin as an embryo culture supplement on mice embryo culture from GV oocytes to the blastocyst stage (IVM + in vitro development)
In this study, to assess the cumulative effect of sericin as an embryo culture supplement, the effect of 0.1%, 0.5%, and 1% sericin on the development of MII oocytes from the IVM study group to two-cell embryos (after IVF), and finally to the blastocyst stage was evaluated. The one-way ANOVA results revealed a significant relationship between HTF in which 1% sericin was added as a supplement and the follow-up of GV oocytes to blastocysts. Importantly, 0.1% and 0.5% sericin did not have significant effects (Table II).
3.3. The effect of sericin as an embryo culture supplement on IVF of MII oocytes to the blastocyst stage (IVF + in vitro development rate)
The cumulative effect of sericin as an embryo culture supplement on IVM and then on the development to blastocysts was examined in the IVF+ in vitro development study group. It was found that the rate of in vitro-fertilized MII oocytes reaching the blastocyst stage was not significantly different in the 0.1%, 0.5%, or 1% sericin subgroups than in the control group (Table II). The results showed that in the IVM group, the number of oocytes that reached stage MII was significantly higher in the 1% sericin subgroup (161/208 = 77.4%) compared to the control group. No significant difference was observed between the IVF and in vitro development of embryo groups with different concentrations of sericin compared to the control group (p = 0.047). In the IVM+ in vitro development of embryo group, the number of oocytes was higher after passing IVM and IVF and reaching the blastocyst stage when 1% sericin was used compared to the other sericin subgroups. There was also a significant difference compared to the control group (p = 0.048). However, in the IVF + in vitro development of the embryo group, no significant relationship was found between the different subgroups.
4. Discussion
In the present study, the effect of sericin on IVM, IVF, and in vitro development was determined separately. Further, its cumulative effect from immature oocytes to blastocyst development was assessed.
Our findings show that, while the IVM rate was higher in the group of supplementation with 1% sericin in comparison with the sericin-free group, adding 0.1% or 0.5% sericin to the medium did not show any significant effect on the maturation rate. The current study is the first to report the effects of sericin supplementation on mice oocyte maturation in culture. In line with our study, Do and colleagues were shown that the addition of IVM medium with 1% sericin may improve the meiotic capacity of pig oocytes and the quality of blastocysts, which is determined by the DNA fragmentation index. (14). Inconsistent with our results, a study showed that the addition of 0.5% sericin to an in vitro culture medium improved the full cumulus expansion rate and the percentage of oocytes that reached the metaphase-II stage in Sanjabi ewes. Also, they reported that supplementation of 0.1% sericin in the maturation culture had a significant effect on nuclear and cytoplasmic maturation, thereby increasing preimplantation development of in vitro-cultured embryos (10). In another study reported that using 0.5% sericin as an alternative protein supplement enabled the feasibility of IVM of bovine oocytes to be demonstrated (13).
In the current study, sericin was associated with a higher total formation rate of blastocysts from MII-stage embryos. It has been reported that sericin, by preventing oxidative stress during bovine embryo culture, helps to improve the embryo quality and increase embryonic development (15). The results of this study showed that adding 1% sericin to the culture medium had a significant positive effect on pre-implantation growth of embryos compared to other concentrations of sericin. Moreover, these results revealed that the percentages of MII oocyte maturation and development to blastocyst were lower at the low concentration (0.1%) of sericin compared to the control group. It seems that the sericin effect on the MII oocyte maturation and development to blastocyst is concentration-dependent. MII maturation and development to blastocyst in rats increased with higher sericin concentration. In another study, contrary to our results, the percentages of cleavage and blastocyst rates in ewe embryos were significantly lower at high concentrations (2.5%) of sericin treatments compared with the control group (16).
In another study, reported that adding 0.5% sericin to the culture medium increased the blastocyst rate compared to the control group without sericin and acted as an alternative protein supplement for IVM of bovine oocytes and zygotes (4). In addition, some researchers have found that adding 0.5% sericin to an in vitro culture medium improves oxidative stress, pre-implantation progression, and the quality of embryos (7). In another study, it was founded that supplementation with sericin during embryo culture in vitro enhanced the development rates of ovum pick- up-derived embryos cultured separately, and improved the quality of the bovine embryos specifically (15). However, our results showed that supplementation of the medium with different concentrations of sericin had no significant effect on the development of the embryo in vitro. The highest maturation and development rate was observed in the oocytes that matured in a medium supplemented with 1% sericin.
5. Conclusion
It can be concluded that the medium supplemented with 1% sericin improved the IVM and development of MII oocyte-derived embryos to blastocysts in the mouse model. To ensure optimal concentration, more investigations are needed.
Acknowledgements
The results of this article are part of the research project number: IR.MUK.1395/215 which was financially supported by the School of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Sericin is a water-soluble silk protein extracted from cocoons and it is the second major protein component (besides fibroin) of silk used in biological materials due to their anti-UV and antibacterial properties. Sericin is composed of 18 types of amino acids as well as polar side groups such as carboxyl, hydroxyl, and amino groups and it is rich in serine and aspartic acid (6). Izobe et al. Found that culturing two-cell embryos separately for seven days in an environment with 0.5% sericin resulted in the highest rate of blastocyst formation and development into expanded blastocysts. It has been shown that preimplantation development and the quality of cultured bovine embryos increased following the addition of sericin to an in vitro medium, which prevented oxidative stress (7). It was reported that supplementation of a medium with 0.1% sericin during in vitro maturation (IVM) improved the nuclear maturation and fertilizability of sheep oocytes. Also, because of its antibacterial and UV-resistant properties, as a biomaterial. It may replace bovine serum albumin with Sericin in chemical environments without risk of disease transmission. Several studies have suggested the benefits of using sericin as a protein replacement (8). Also, some studies have suggested that sericin can have a role in cryoprotection in the freezing of mice, human, and buffalo sperm (9-11).
The present study is the first to investigate the effect of 0.1%, 0.5%, and 1% sericin as a medium supplement on IVM, the rate of in vitro fertilization (IVF), and in vitro development of mouse embryos.
2. Materials and Methods
2.1. Sericin preparation and study groups
Sericin and the other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA), and the culture-related plastics were purchased from Falcon (Paignton, UK). Sericin was diluted in a human tubal fluid medium (HTF). Sericin was considered as a supplement in the embryo culture medium at 0.1%, 0.5% and 1% concentrations (subgroups I, II, and III, respectively) in comparison with a sericin-free group as a control group. Four study subgroups were created within each experimental group: IVM, IVF, in vitro development, and IVM+ in vitro development.
2.2. Animals
32 NMRI mice were used to extract germinal vesicle (GV) oocytes, metaphase II (MII) oocytes, and sperm. Then, IVM, IVF, and evaluation of mouse embryo development in vitro were performed. The mice had free access to water and food and were preserved at a temperature of 23 ± 2ºC and a 12-hr light/dark cycle. All processes were carried out at the Kurdistan University of Medical Science in 2018.
2.3. Collection of immature GV oocytes and IVM GV oocytes and IVM
A total of 5 IU of pregnant mare serum gonadotropin (PMSG) was injected into each 4-6-wk-old female mouse. Then, 48 hr post injection, immature GV oocytes were released from the ovaries by puncturing the follicles as visualized under a stereomicroscope. The GV oocytes were collected and randomly classified into four subgroups: 0% (control) and 0.1%, 0.5%, and 1% HTF containing sericin (4). The GV oocytes were incubated at 37ºC in a humidified atmosphere of 5% CO2 in the air for 24 hr. Next, the oocytes displaying the first polar bodies by a stereomicroscope were considered as MII oocytes. The MII oocytes were then recruited for the IVM+ in vitro development follow-up group (Table I).
2.4. Collection of MII oocytes and IVF
IVF was performed as described previously with minor modifications (12). PMSG was injected into female mice that were 4-6 wk old. After 48 hr, human Chorionic Gonadotropin (hCG) was injected, and oocytes were collected from mouse oviducts 14 hr post the hCG injection. The mature oocytes (metaphase meiosis II) were transferred to 100-µl IVF medium droplets, and 1×106 sperms/ml was added to the IVF droplets supplemented with sericin: 0% (control) and 0.1%, 0.5%, and 1% as test subgroups for 24 hr. Then, the zygotes were monitored by a reverse microscope and the percentage of two-cell formation was recorded to assess the rate of fertilization. (Table I).
2.5 Collection of zygotes and in vitro development
After the PMSG and hCG injections, the injected female mice mated with male mice. 18 hr post hCG injection; female mice with a vaginal plaque were selected and euthanized with cervical dislocation. The two-pronucleus-stage embryos were collected from the mouse oviducts, observed, and screened at ×100 magnification on a warmed microscope stage (37ºC); they were not fragmented or degenerated and were classified into three subgroups of 0.1%, 0.5%, and 1% sericin added potassium-supplemented simplex optimized medium (Ksom) and non-added sericin as a control group (with KSOMAA). Each plate contained 200 ul of KSOMAA covered by preincubated mineral oil used for two-cell-stage embryo culture and development at 37ºC in 5% CO2 in the air inside a humidified incubator (10). The number of zygotes developing to early blastocysts was calculated in each sericin subgroup.
2.6. Follow-up from IVM to the blastocyst stage
For assessing the cumulative effect of sericin as an embryo culture supplement, we used the MII oocytes obtained from the IVM study group for IVF and subsequent embryo development. During all three stages of the experiment, the culture media used were supplemented with three different concentrations of sericin (13). The number of obtained blastocysts was considered as a complete process of oocyte maturation, fertilization, and in vitro embryo development in the presence of sericin. It was then compared to the control group.
2.7. Follow-up from IVF to the blastocyst stage
To assess the cumulative effect of sericin as an embryo culture supplement in addition to IVF, the medium for the culture of the subsequent embryo from IVF was supplemented with three different concentrations of sericin. The number of blastocysts was considered as a complete process of IVF of MII oocytes and development to blastocyst in the presence of sericin and compared to the control group.

2.8. Ethical considerations
All procedures were carried out based on the guidelines of the Ethics Committee of the Kurdistan University of Medical Science, Sanandaj, Iran (Code: IR.MUK.REC.1395/215). According to the University Ethics Committee, working with laboratory animals in this study did not violate any rules.
2.9. Statistical analysis
In this experimental study, the IVM and IVF rates and the percentage of development to the blastocyst stage (in vitro development) in mouse oocytes and embryos were calculated for each sericin group and subgroup, and these values were then compared with those of the control group. One-way ANOVA was run to assess group differences. Data were analyzed by Stata 14 (Stata Corp. 2015. Stata Statistical Software: Release 14. College Station, TX: Stata Corp LP). The significance level in this study was set at p < 0.05.
3. Results
3.1. The effect of Sericin as an embryo culture supplement on IVM, IVF, and the in vitro development rate
Initially, 832 GV oocytes were classified into 208 GV oocytes for each sericin subgroup and control group. Among the three sericin subgroups, the effect of 1% sericin (161/208 = 77.4%) was significant in comparison with the control sericin-free group (143/208 = 68.7%). On the other hand, the 0.1 and 0.5% subgroups did not show any significant relationship.
For IVF, a total of 124 oocytes were recruited for each sericin subgroup of 0.1, 0.5, 1% sericin, and the sericin-free control group for testing the effect of sericin on IVF. Notably, the one-way ANOVA analysis of the three sericin subgroups showed no significant relationship in comparison with the control group.
Regarding the in vitro development rate, after collecting the 688 two-pro nucleus-stage embryos from the oviducts and dividing them into three subgroups based on sericin concentration and a control group (without sericin), the number of zygotes that developed into early blastocysts was calculated. One-way ANOVA analyses revealed that the three concentrations of sericin as an embryo culture supplement had no significant effect on the in vitro development of mice zygotes (Table II).
3.2. The effect of sericin as an embryo culture supplement on mice embryo culture from GV oocytes to the blastocyst stage (IVM + in vitro development)
In this study, to assess the cumulative effect of sericin as an embryo culture supplement, the effect of 0.1%, 0.5%, and 1% sericin on the development of MII oocytes from the IVM study group to two-cell embryos (after IVF), and finally to the blastocyst stage was evaluated. The one-way ANOVA results revealed a significant relationship between HTF in which 1% sericin was added as a supplement and the follow-up of GV oocytes to blastocysts. Importantly, 0.1% and 0.5% sericin did not have significant effects (Table II).
3.3. The effect of sericin as an embryo culture supplement on IVF of MII oocytes to the blastocyst stage (IVF + in vitro development rate)
The cumulative effect of sericin as an embryo culture supplement on IVM and then on the development to blastocysts was examined in the IVF+ in vitro development study group. It was found that the rate of in vitro-fertilized MII oocytes reaching the blastocyst stage was not significantly different in the 0.1%, 0.5%, or 1% sericin subgroups than in the control group (Table II). The results showed that in the IVM group, the number of oocytes that reached stage MII was significantly higher in the 1% sericin subgroup (161/208 = 77.4%) compared to the control group. No significant difference was observed between the IVF and in vitro development of embryo groups with different concentrations of sericin compared to the control group (p = 0.047). In the IVM+ in vitro development of embryo group, the number of oocytes was higher after passing IVM and IVF and reaching the blastocyst stage when 1% sericin was used compared to the other sericin subgroups. There was also a significant difference compared to the control group (p = 0.048). However, in the IVF + in vitro development of the embryo group, no significant relationship was found between the different subgroups.

4. Discussion
In the present study, the effect of sericin on IVM, IVF, and in vitro development was determined separately. Further, its cumulative effect from immature oocytes to blastocyst development was assessed.
Our findings show that, while the IVM rate was higher in the group of supplementation with 1% sericin in comparison with the sericin-free group, adding 0.1% or 0.5% sericin to the medium did not show any significant effect on the maturation rate. The current study is the first to report the effects of sericin supplementation on mice oocyte maturation in culture. In line with our study, Do and colleagues were shown that the addition of IVM medium with 1% sericin may improve the meiotic capacity of pig oocytes and the quality of blastocysts, which is determined by the DNA fragmentation index. (14). Inconsistent with our results, a study showed that the addition of 0.5% sericin to an in vitro culture medium improved the full cumulus expansion rate and the percentage of oocytes that reached the metaphase-II stage in Sanjabi ewes. Also, they reported that supplementation of 0.1% sericin in the maturation culture had a significant effect on nuclear and cytoplasmic maturation, thereby increasing preimplantation development of in vitro-cultured embryos (10). In another study reported that using 0.5% sericin as an alternative protein supplement enabled the feasibility of IVM of bovine oocytes to be demonstrated (13).
In the current study, sericin was associated with a higher total formation rate of blastocysts from MII-stage embryos. It has been reported that sericin, by preventing oxidative stress during bovine embryo culture, helps to improve the embryo quality and increase embryonic development (15). The results of this study showed that adding 1% sericin to the culture medium had a significant positive effect on pre-implantation growth of embryos compared to other concentrations of sericin. Moreover, these results revealed that the percentages of MII oocyte maturation and development to blastocyst were lower at the low concentration (0.1%) of sericin compared to the control group. It seems that the sericin effect on the MII oocyte maturation and development to blastocyst is concentration-dependent. MII maturation and development to blastocyst in rats increased with higher sericin concentration. In another study, contrary to our results, the percentages of cleavage and blastocyst rates in ewe embryos were significantly lower at high concentrations (2.5%) of sericin treatments compared with the control group (16).
In another study, reported that adding 0.5% sericin to the culture medium increased the blastocyst rate compared to the control group without sericin and acted as an alternative protein supplement for IVM of bovine oocytes and zygotes (4). In addition, some researchers have found that adding 0.5% sericin to an in vitro culture medium improves oxidative stress, pre-implantation progression, and the quality of embryos (7). In another study, it was founded that supplementation with sericin during embryo culture in vitro enhanced the development rates of ovum pick- up-derived embryos cultured separately, and improved the quality of the bovine embryos specifically (15). However, our results showed that supplementation of the medium with different concentrations of sericin had no significant effect on the development of the embryo in vitro. The highest maturation and development rate was observed in the oocytes that matured in a medium supplemented with 1% sericin.
5. Conclusion
It can be concluded that the medium supplemented with 1% sericin improved the IVM and development of MII oocyte-derived embryos to blastocysts in the mouse model. To ensure optimal concentration, more investigations are needed.
Acknowledgements
The results of this article are part of the research project number: IR.MUK.1395/215 which was financially supported by the School of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Assisted Reproductive Technologies
References
1. Wrenzycki C. In vitro production of (Farm) animal embryos. In: Nienann H, Wrenzycki Ch. Animal biotechnology. USA, New York: Springer; 2018. 269-304. [DOI:10.1007/978-3-319-92327-7_12]
2. Moawad AR, Ghoneim IM, Darwish GM, Badr MR, El-Badry DA, EL-Wishy ABA. Factors affecting in vitro embryo production: Insights into dromedary camel. J Anim Reprod Biotechnol 2020; 35: 119-141. [DOI:10.12750/JARB.35.2.119]
3. Joaquim DC, Borges ED, Viana IGR, Navarro PA, Vireque AA. Risk of contamination of gametes and embryos during cryopreservation and measures to prevent cross-contamination. BioMed Res Int 2017; 2017: 1840417. 1-11. [DOI:10.1155/2017/1840417] [PMID] [PMCID]
4. Hajarian H, Aghaz F, Karami Shabankareh H. Replacement of serum with sericin in in vitro maturation and culture media: Effects on embryonic developmental competence of Sanjabi sheep embryo during breeding season. Theriogenology 2017; 92: 144-148. [DOI:10.1016/j.theriogenology.2016.12.027] [PMID]
5. Dash R, Acharya C, Bindu PC, Kundu SC. Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Reports 2008; 41: 236-241. [DOI:10.5483/BMBRep.2008.41.3.236] [PMID]
6. Kunz RI, Brancalhao RMC, Ribeiro LF, Natali MR. Silkworm sericin: Properties and biomedical applications. BioMed Res Int 2016; 2016: 8175701. [DOI:10.1155/2016/8175701] [PMID] [PMCID]
7. Isobe T, Ikebata Y, Onitsuka T, Wittayarat M, Sato Y, Taniguchi M, et al. Effect of sericin on preimplantation development of bovine embryos cultured individually. Theriogenology 2012; 78: 747-752. [DOI:10.1016/j.theriogenology.2012.03.021] [PMID]
8. Yasmin C, Otoi T, Setiadi MA, Karja NWK. Maturation and fertilisation of sheep oocytes cultured in serum-free medium containing silk protein sericin. Acta Vet Hung 2015; 63: 110-117. [DOI:10.1556/avet.2015.009] [PMID]
9. Ghasemi M, Farshad A, Hajarian H, Banafshi O, Asadollahi V, Fathi F. The effects of sericin on cryopreserved sperm cells and subsequent embryo development in mice. Int J Reprod BioMed 2018; 16: 405-412. [DOI:10.29252/ijrm.16.6.405] [PMID] [PMCID]
10. Aghaz F, Khazaei M, Vaisi-Raygani A, Bakhtiyari M. Cryoprotective effect of sericin supplementation in freezing and thawing media on the outcome of cryopreservation in human sperm. Aging Male 2018; 23: 1-8. [DOI:10.1080/13685538.2018.1529156] [PMID]
11. Kumar P, Kumar D, Sikka P, Singh P. Sericin supplementation improves semen freezability of buffalo bulls by minimizing oxidative stress during cryopreservation. Anim Reprod Sci 2015; 152: 26-31. [DOI:10.1016/j.anireprosci.2014.11.015] [PMID]
12. Ahmadi H, Fathi F, Moeini A, Amidi F, Sobhani A. Evaluation of prooxidant-antioxidant balance in in vitro fertilization-conceived mice. Clin Exp Reprod Med 2018; 45: 82-87. [DOI:10.5653/cerm.2018.45.2.82] [PMID] [PMCID]
13. Hosoe M, Yoshida N, Hashiyada Y, Teramoto H, Takahashi T, Niimura S. Sericin accelerates the production of hyaluronan and decreases the incidence of polyspermy fertilization in bovine oocytes during in vitro maturation. J Reprod Dev 2014; 60: 268-273. [DOI:10.1262/jrd.2013-110] [PMID] [PMCID]
14. Do LTK, Namula Z, Luu VV, Sato Y, Taniguchi M, Isobe T, et al. Effect of sericin supplementation during in vitro maturation on the maturation, fertilization and development of porcine oocytes. Reprod Dom Anim 2014; 49: e17-e20. [DOI:10.1111/rda.12274] [PMID]
15. Isobe T, Ikebata Y, Do LTK, Tanihara F, Taniguchi M, Otoi T. In vitro development of OPU-derived bovine embryos cultured either individually or in groups with the silk protein sericin and the viability of frozen-thawed embryos after transfer. Anim Sci J 2015; 86: 661-665. [DOI:10.1111/asj.12341] [PMID]
16. Aghaz F, Hajarian H, Shabankareh HK, Abdolmohammadi A. Effect of sericin supplementation in maturation medium on cumulus cell expansion, oocyte nuclear maturation, and subsequent embryo development in Sanjabi ewes during the breeding season. Theriogenology 2015; 84: 1631-1635. [DOI:10.1016/j.theriogenology.2015.08.013] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





