Wed, Jul 16, 2025
[Archive]
Volume 19, Issue 1 (January 2021)
IJRM 2021, 19(1): 63-74 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hajisadeghi H, Azarbayjani M A, Vafaeenasab M, Peeri M, Modares Mosala M M. Effect of regular resistance exercise, vitamin D, and calcium supplements on the bone mineral content and density in postmenopausal model of rats: An experimental study. IJRM 2021; 19 (1) :63-74
URL: http://ijrm.ir/article-1-1801-en.html
URL: http://ijrm.ir/article-1-1801-en.html
Homa Hajisadeghi1 

 , Mohammad Ali Azarbayjani *2
, Mohammad Ali Azarbayjani *2 

 , Mohammadreza Vafaeenasab3
, Mohammadreza Vafaeenasab3 

 , Maghsoud Peeri1
, Maghsoud Peeri1 

 , Mohamad Mahdi Modares Mosala4
, Mohamad Mahdi Modares Mosala4 




 , Mohammad Ali Azarbayjani *2
, Mohammad Ali Azarbayjani *2 

 , Mohammadreza Vafaeenasab3
, Mohammadreza Vafaeenasab3 

 , Maghsoud Peeri1
, Maghsoud Peeri1 

 , Mohamad Mahdi Modares Mosala4
, Mohamad Mahdi Modares Mosala4 


1- Department of Exercise Physiology, Central Tehran Branch, Islamic Azad University, Tehran, Iran.
2- Department of Exercise Physiology, Central Tehran Branch, Islamic Azad University, Tehran, Iran. ,m_azarbayjani@iauctb.ac.ir
3- Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Nuclear Medicine, Baqiyatallah University, Tehran, Iran.
2- Department of Exercise Physiology, Central Tehran Branch, Islamic Azad University, Tehran, Iran. ,
3- Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Nuclear Medicine, Baqiyatallah University, Tehran, Iran.
Full-Text [PDF 617 kb]
(1409 Downloads)
| Abstract (HTML) (2455 Views)
2.2. Ovariectomy surgery protocol
For ovariectomy operation, all animals were anesthetized intraperitoneally with a ketamine-xylazine mixture (61.5-7.6 mg/kg), and subsequently, ovaries were removed by a bilateral incision through the skin and completely excised from a dorsal approach (23). The site was sutured and all animals recovered two months after the surgical procedures.
2.3. Resistance training protocol
For Resistance training, a 1-m ladder with 2-cm grid ladder inclined at 85º was used. Prior to the resistance training, rats underwent a familiarization week with a ladder and climbing. Weights attached to their tails and the initial weight was 30% of their body weight, which was increased to 100% of their body weight gradually throughout the eight weeks. Weights were fastened to the upper portion of the tail. The resistance training included three sets of five repetitions with a 30-sec rest interval between the reps and 3 min between the sets (24).
2.4. Measurement methods
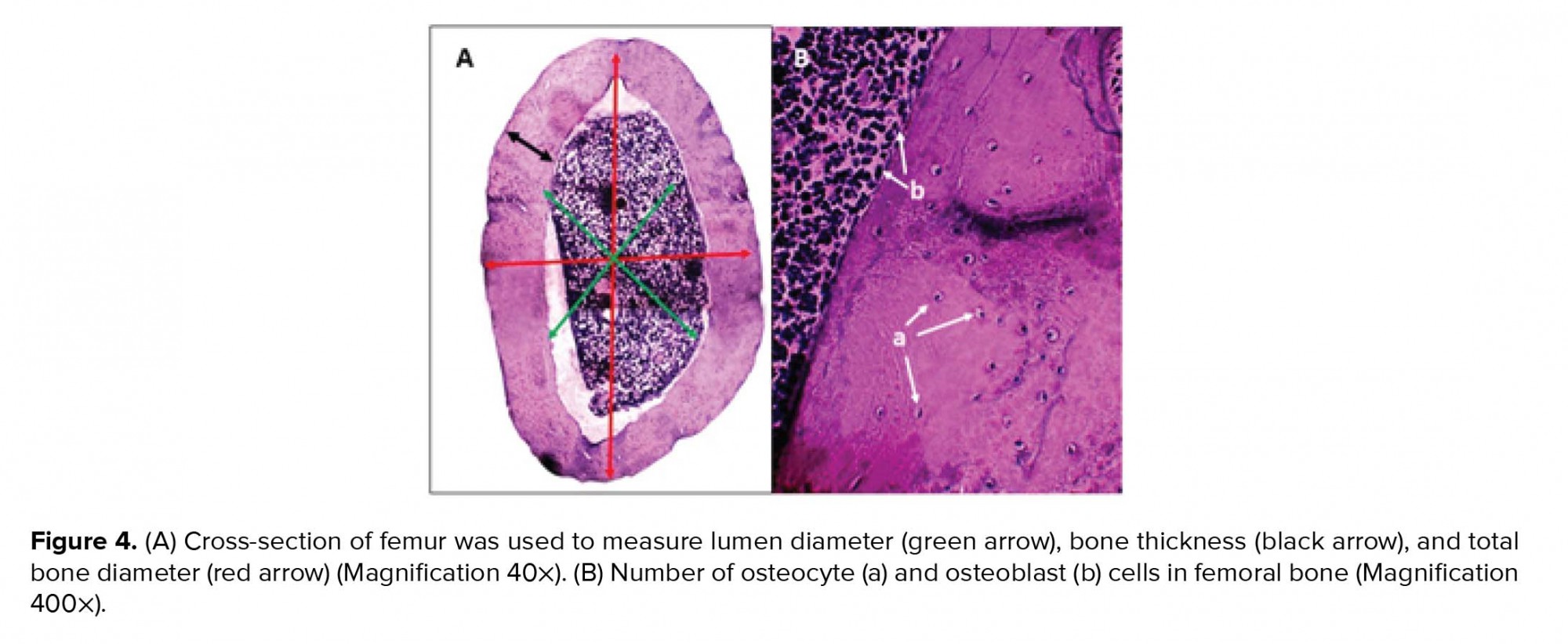
Animals were sacrificed by decapitation after the last resistance exercise sessions and after the supplement administration. Whole body was fixed in formaldehyde 10% to measure BMD (mg/cm2) and BMC (mg) of the total body, tail, hips, lumbar, and femur using dual-energy X-ray absorptiometry (387A030, Norland company, USA) (25). Femoral bone weight was measured using a digital scale (Taishi, China). Soon after, femur was retired and decalcified in hydrochloric acid/formic acid for 24 hr. Next, the samples were briefly rinsed in water and transferred to ammonia solution to neutralize acids and left for 30 min. Femurs were dehydrated in a tissue processor. After dehydration in ethanol series, the samples were embedded in paraffin and 5-µm sections were made. For histological evaluation, a standard length of 2 mm of each section of each bone was obtained to analyze the number of osteoblast, osteoclast, and osteocyte cells using a light microscope (Olympus BX51, Japan) with hematoxylin-eosin staining. Measurements were made on digitized images using the digimizer software. The total number of osteoblast, osteoclast, and osteocyte cells per square millimeter was calculated. Also, a cross-sectional area of bone tissue was used to measure the bone body thickness, bone lumen diameter, and bone total diameter. The mean bone lumen diameter (LD) and total diameter (TD) were derived by taking the average of two diameters, D1 and D2, at right angles. For each slide, the mean of 10 random fields was selected for measurements (26).
2.5. Ethical considerations
Animals had free access to water and food and at a controlled temperature of 22 ± 2ºC under 12-hr light/dark cycles. This study was approved by the Committee of Yazd Reproduction Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (IR.SSU.RSI.REC.1398.020) and all protocols were performed according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
2.6. Statistical analysis
Data were analyzed using the SPSS software, version 20 (SPSS Inc., USA) and expressed as means ± SD. The statistical analysis was done initially by a one-way analysis of variance. A significance level of p < 0.05 and p < 0.001 was used for all comparisons.
3. Results
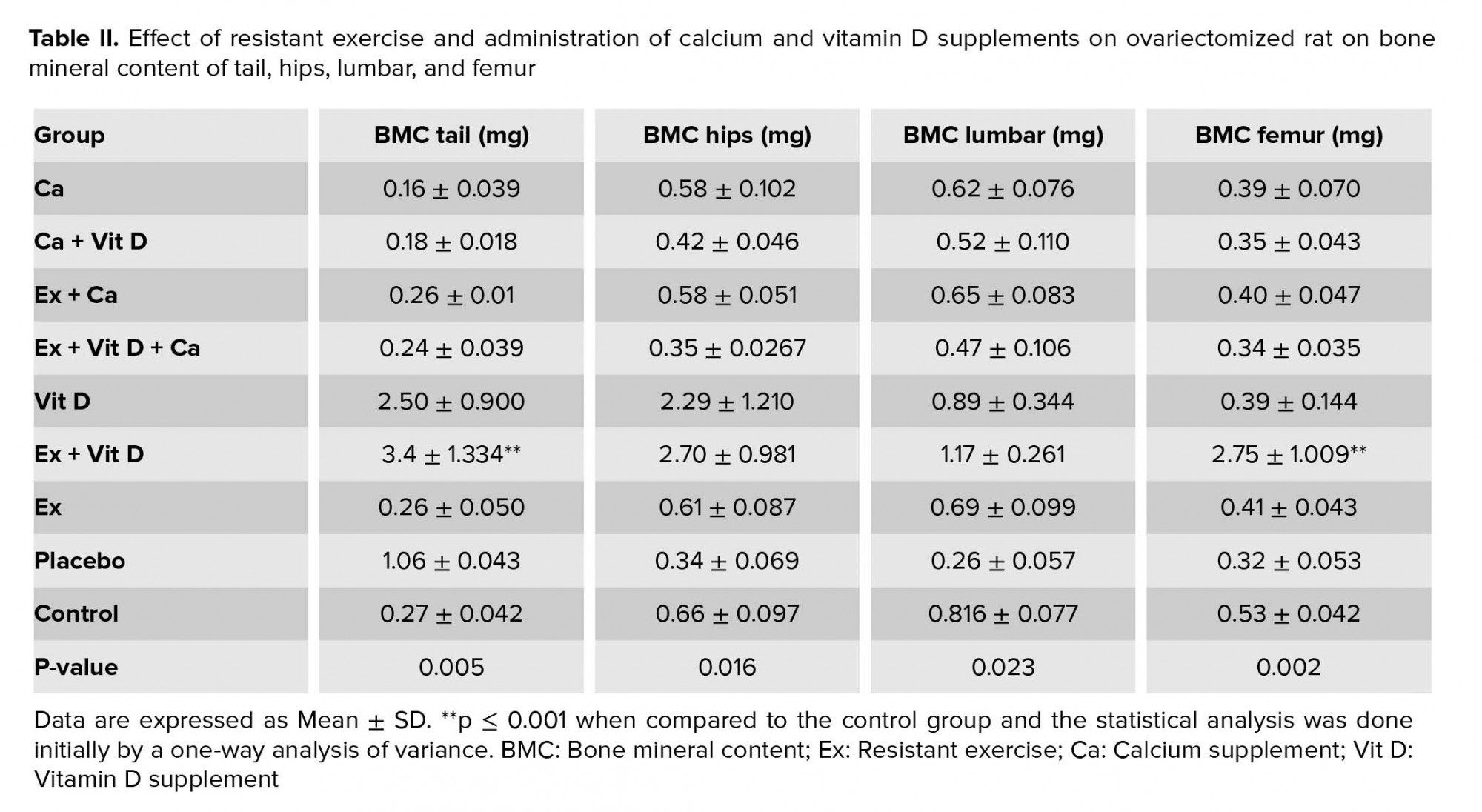
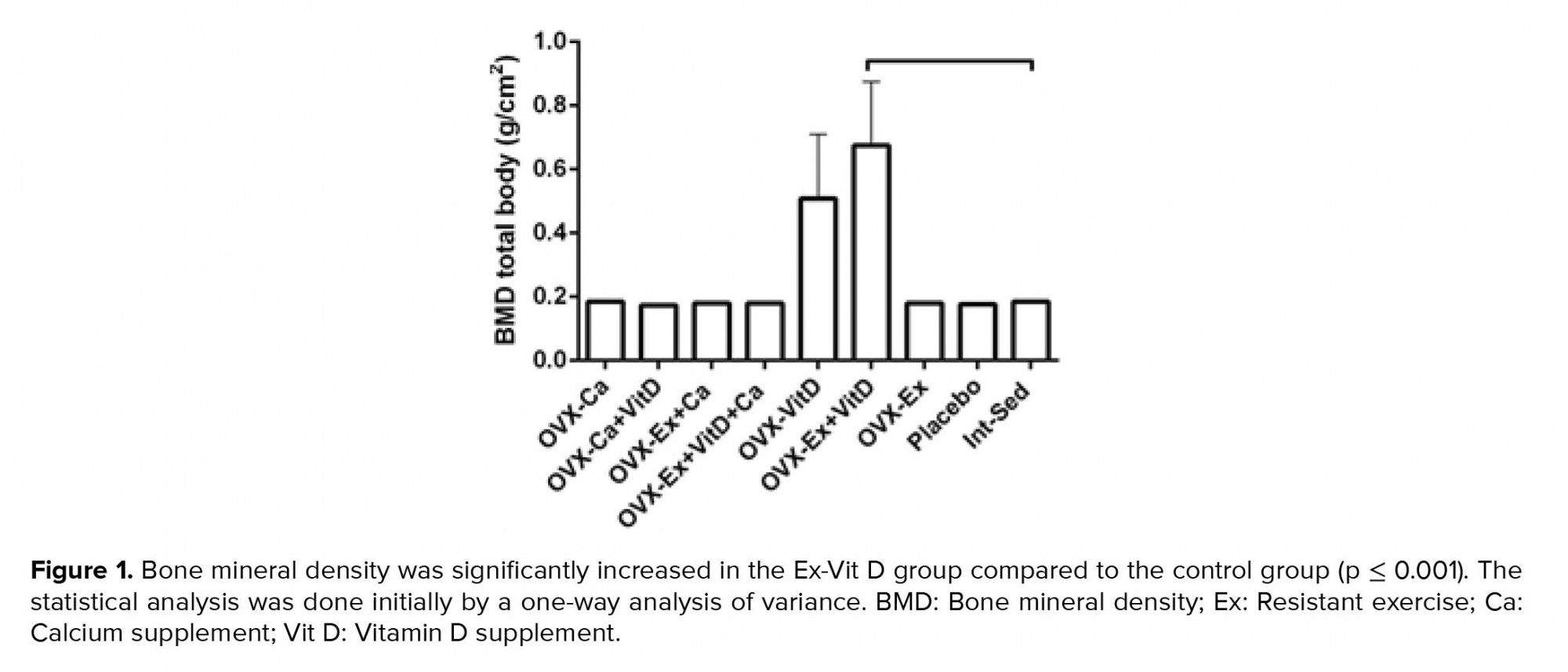
Resistant exercise with Vit D administration in ovariectomized rats significantly increased tail, hip, and lumbar BMD compared to the control group, p = 0.004, p = 0.007, p = 0.003, respectively (Table I). Also, there was an increasing trend in the femur BMD of the Ex + Vit D group in comparison with the control group (p = 0.009, Table I). The total BMD showed an increase in the Ex + Vit D group over the control group (Figure 1). However, higher tail and femur BMC were observed in the Ex + Vit D group compared to the control group (p = 0.005 and p = 0.002, respectively. No significant changes were observed in the hips and lumbar BMC compared to the control group (Table II). There was no statistically significant difference in the total BMC comparison with the control group (Figure 2).
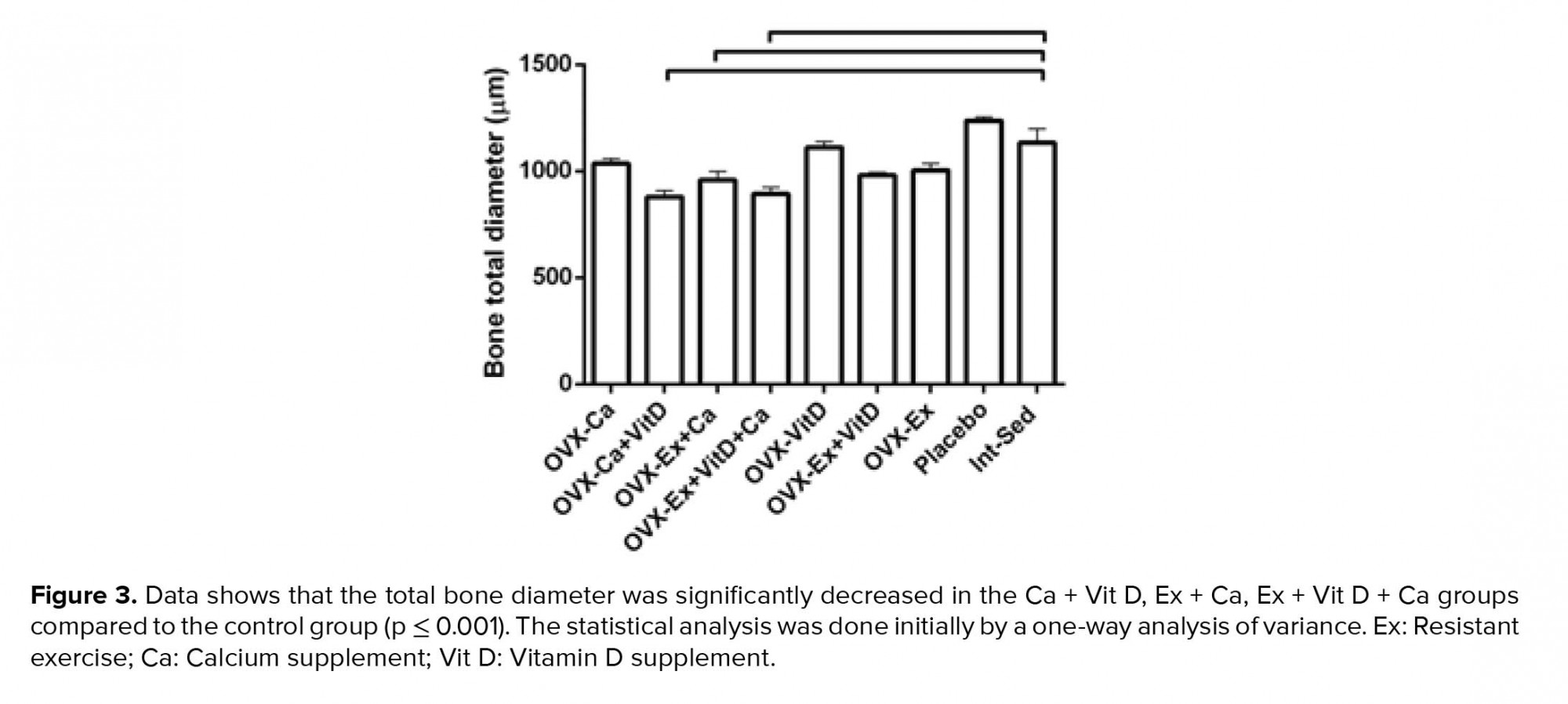
Moreover, no significant changes were observed between the groups in femoral bone weight (Table III). In addition, reduction of lumen diameter was indicated in groups that were administrated Ca supplement during the study compared to the control group. However, lumen diameter was lower in the Ex + Vit D group than the control group; a significant decrease was seen in the lumen diameter of the Ca + Vit D, Ex + Ca, and Ex + Vit D + Ca groups. Interestingle, bone thickness in the Ex + Vit D group was more than the control groups (p = 0.02; Table III). In addition, the total bone diameter was significantly decreased in the Ca + Vit D (p = 0.000), Ex + Ca (p = 0.018), and Ex + Vit D + Ca (p = 0.001) groups compared with the control group (Figure 3 and 4A).
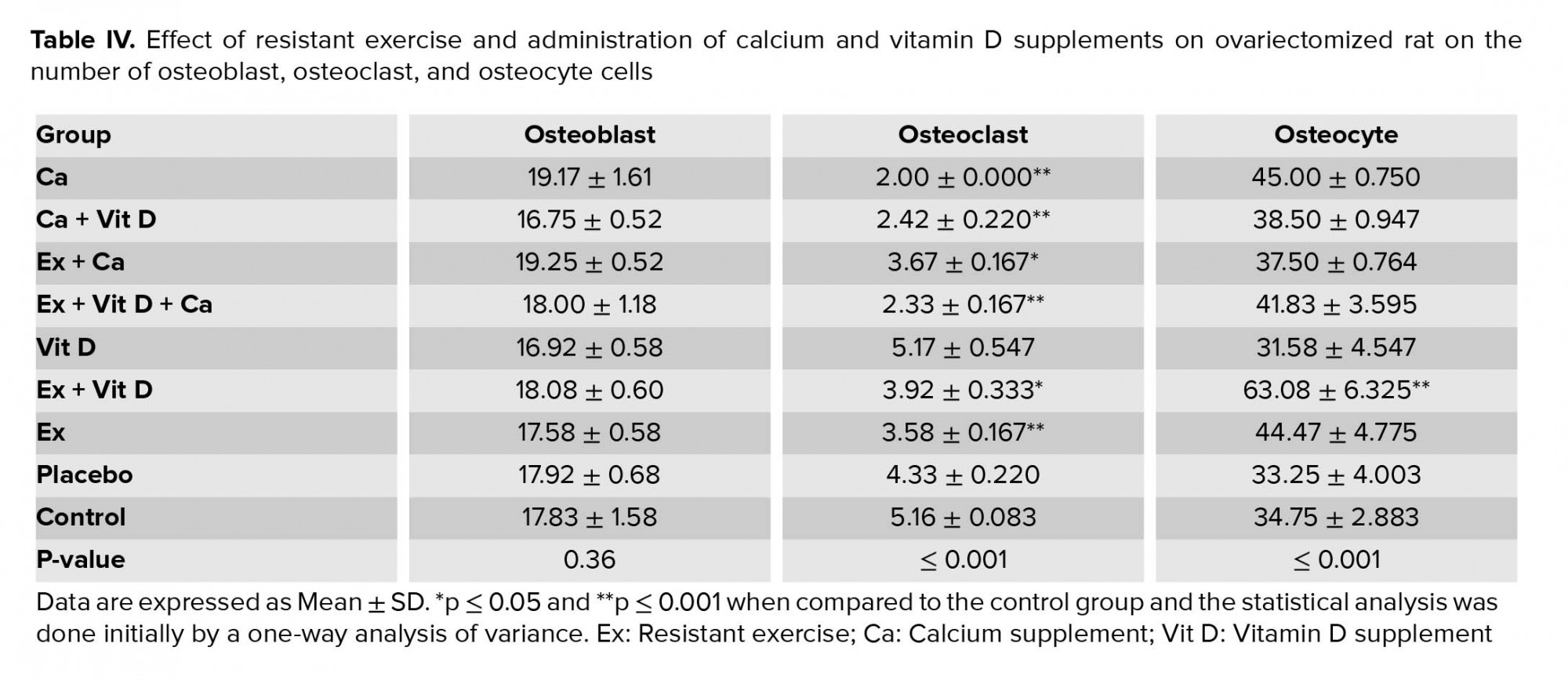
Figure 4B shows osteocyte and osteoblast cells in the cross-section of the femoral bone. Also, there were no significant differences in the number of osteoblast cells among the control group (Table IV). However, the number of osteoclast cells were decreased in the Ca + Vit D, Ex + Ca, Ex + Vit D, and Ex + Vit D + Ca groups compared to the control group; osteocyte numbers were increased only in the Ex + Vit D group.



4. Discussion
Osteoporosis is identified as a disorder affecting elderly people, especially postmenopausal women. Hence, the determination of a beneficial lifestyle for elderly women seems to be necessary in order to reduce the incidence of postmenopausal syndromes. In the current study, we assessed the effect of resistance exercise, vitamin D and calcium supplements on ovariectomized rats as a model of menopause. Our findings showed the resistance exercise in combination with vitamin D supplement had a positive effect on the maintenance of BMD and BMC in OVX rats. There was a significant difference in the BMD and BMC in Ex + Vit D group in comparison with the other groups. It was reported that the resistance exercise can lead to increasing BMD at the muscle site by enhancement of muscle size and strength (27). Also, the BMC and BMD have been reported to reveal an increase up to 20% in the loaded bone regions in athletes (28). In a recent study, Beavers evaluated the change in BMD during weight loss with various physical activity including resistance and aerobic exercise in older adults. Their result showed that the resistance exercise was osteo-protective in comparison with the caloric restriction in elderly women (29).
According to result of Khalil study in 2015, vitamin D supplement reduced the bone resorption marker in elderly women (9). Several studies reported the important role of vitamin D to prevent the decrease in the bone density and the risk of bone fracture among elderly women (30, 31). Additionally, it was mentioned that some analogs of vitamin D such as 2-Methylene-19-nor (20S)-1α25-dihydroxyvitamin D3 (2MD) contribute in the enhancement of bone mass and are supposed to be a promising treatment for osteoporosis in postmenopausal women (30). Furthermore, vitamin D contributes in regulating calcium homeostasis as a part of bone metabolism procedure that displays the importance of vitamin D presence in bone health (32). On the other hand, several investigations supposed that the dietary calcium intake or calcium supplements contribute in the improvement of BMC and BMD among postmenopausal women. Nonetheless, some others revealed that the calcium supplements alone probably cannot be adequate to decrease fracture risk and other parameters such as vitamin D is required (33). This findings were similar to our result.
Further, it has been established that exercise can increase BMD, BMC, and bone strength (33). Sport researcher believed that continuing pressure and tension to bones is a valuable factor to prevent osteoporosis (34). Although, the role of exercise alone was clear, future studies were needed to define optimal physical activity and additional supplements for preventing osteoporosis and bone fractures (35, 36). Exercise can elevate seroma calcium and vitamin D levels in body but this increase depends on the type, intensity, and time of exercise (37). Mason and colleagues in 2011 reported that vitamin D level can increase after exercise for low-weight program (38). In the current study, an increase in the BMD and BMC in the Ex + Vit D group was observed. These significant changes might be due to the elevation of vit D after exercise. On the other hand, in this group, the elevation in the vitamin D level create from two ways: (1) exogenous vitamin D by supplement intake and (2) endogenous vitamin D by exercise. So, this elevation might be responsible for BMD and BMC increase in this group in compared to other groups.
The bone thickness was morphologically compared between all study groups. There was a significant difference in the Ex + Vit D groups in comparison with the other groups. On the other hand, our finding can confirm that the combination of resistance exercise and vitamin D has a positive effect on the maintenance of bone thickness and prevention or delay of osteoporosis. In the present study, osteocytes were counted in all groups and a significant difference was observed in the Ex + Vit D group in comparison with the other groups that confirmed our previous findings and revealed the promising potential of resistance exercise together with vitamin D intake in reducing bone resorption by decreasing the rate of osteocytes apoptosis. Toumi and colleagues have illustrated that the physical activity alone does not significantly reduce the rate of osteocytes apoptosis in all sham and OVX groups (39). While it is reported that endurance exercise plays an important role in preservation of cancellous bone (40). Moreover, another study showed that exercise would be able to enhance osteogenesis in OVX rats (41). Abnormal proliferation and activity of osteoclasts lead to various bone disorders such as osteoporosis causing decreased BMD and elevate the possibility of bone fractures (7).
Besides, there was a significant increase in the number of osteocyte in the Ex + Vit D group in comparison with the other groups. Some studies have reported that exercise can elevate estrogen level in mice serum, and this elevation can prevent osteoclast activity and bone resorption (42, 43). Some researchers revealed that vitamin D level in serum can be raised in animals who were in exercise program (38, 44). In this issue, exercise might have led to the increase in the VDRs, and upsurge in the production of active vitamin D. Finally, this factor perhaps can affect osteoblasts activity and prevent osteoporosis. We also found that the osteoclast was decreased in the Ca + Vit D, Ca + Ex, and Ex + Vit D groups. In addition, our result showed that vitamin D supplementation alone may not be sufficient for the reduction of osteoclasts and decrease in bone cell apoptosis; however, the effective level of Ca requires deactivation of the osteoclasts.
5. Conclusion
Our findings demonstrated that resistance exercise in combination with vitamin D intake represents effective preventive and therapeutic strategies able to prevent or delay the onset of osteoporosis. However, additional investigations are required for the evaluation of all effective parameters on bone health, particularly from the molecular point of view.
Acknowledgements
This study has been financially supported by the Yazd Reproduction Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Full-Text: (486 Views)
1. Introduction
Menopause is an inevitable phenomenon of the general aging in female’s reproductive system (1) and is defined as the permanent termination of menstrual periods that occurs after the loss of ovarian follicle development (2). Although, the average menopausal age onset is about 51 yr, it can vary between 40 to 60 yr (3). Additionally, early menopause occurs in 1% of young women before 40 yr (4). Moreover, the menopausal estrogen loss leads to an accelerated bone loss and osteoporosis (5) that leads to reduction of bone mineral density (BMD) and subsequently the increase in osteoporotic fractures (6). Bone is defined as a mineralized connective tissue that includes four types of cells: osteoblasts, bone lining cells, osteocytes, and osteoclasts. Bone is responsible for several essential functions in the body, such as movement, support and protection of soft tissues, and calcium and phosphate storage. Despite its passive appearance, bone is an extremely dynamic organ that is continuously resorbed by osteoclasts and transformed by osteoblasts (7).
The essential role of vitamin D and its metabolites on bone absorption and formation has been identified for a long time (8). Vitamin D plays the main role in regulating bone cell proliferation and maturation, as well as bone mineralization and resorption (9). Moreover, severe vitamin D deficiency has been reported to lead to osteomalacia in adults (10). Therefore, deficiency in vitamin D can elevate the rate of bone turnover and bone loss by increasing bone resorption in postmenopausal women (11). Some studies have demonstrated that Vitamin D receptors (VDRs) are expressed in reproductive systems including ovaries, endometrium, and placenta (12, 13). Furthermore, several studies have reported the role of vitamin D + calcium supplements in maturation of the ovarian follicles (13, 14). Additionally, the efficacy of calcium intake together with Vitamin D supplement has been demonstrated as an essential intervention for preventing osteoporosis due to postmenopausal conditions (15) by increasing BMD (16). Despite the genetic efficacy on the age of menopause, it is theoretical that lifestyle factors such as diet and physical activity play a significant role in ovarian age (17). Previous studies confirmed that physical activity and, especially, physical exercise are effective in the reduction of clinical fracture in postmenopausal women (18, 19). Muir and colleagues in 2013 published a study to assess the effect of physical activity on bone density in postmenopausal woman. Their results show that, overall, a regular exercise was effective for bone density in postmenopausal women (20). In addition, several previous studies have shown that physical activity, particularly, regular exercise program is the main way to maintain the BMC and prevent bone loss in women (21, 22).
Thus, it is an interesting question - whether an exercise program in combination with calcium and vitamin D supplements can play a supporting role in the maintenance or strengthening of bone mineral content (BMC) and BMD post menopause. Accordingly, the aim of this study was to evaluate the efficacy of regular exercises in combination with vitamin D and calcium supplements on the BMD in postmenopausal rat model.
2. Materials and Methods
2.1. Animals and ethics
In this experimental study, 72 Sprague-Dawley rats, aged eight months with an initial weights of approximately 250 ± 15 gr were used. They the rats were assigned randomly into nine groups:
Menopause is an inevitable phenomenon of the general aging in female’s reproductive system (1) and is defined as the permanent termination of menstrual periods that occurs after the loss of ovarian follicle development (2). Although, the average menopausal age onset is about 51 yr, it can vary between 40 to 60 yr (3). Additionally, early menopause occurs in 1% of young women before 40 yr (4). Moreover, the menopausal estrogen loss leads to an accelerated bone loss and osteoporosis (5) that leads to reduction of bone mineral density (BMD) and subsequently the increase in osteoporotic fractures (6). Bone is defined as a mineralized connective tissue that includes four types of cells: osteoblasts, bone lining cells, osteocytes, and osteoclasts. Bone is responsible for several essential functions in the body, such as movement, support and protection of soft tissues, and calcium and phosphate storage. Despite its passive appearance, bone is an extremely dynamic organ that is continuously resorbed by osteoclasts and transformed by osteoblasts (7).
The essential role of vitamin D and its metabolites on bone absorption and formation has been identified for a long time (8). Vitamin D plays the main role in regulating bone cell proliferation and maturation, as well as bone mineralization and resorption (9). Moreover, severe vitamin D deficiency has been reported to lead to osteomalacia in adults (10). Therefore, deficiency in vitamin D can elevate the rate of bone turnover and bone loss by increasing bone resorption in postmenopausal women (11). Some studies have demonstrated that Vitamin D receptors (VDRs) are expressed in reproductive systems including ovaries, endometrium, and placenta (12, 13). Furthermore, several studies have reported the role of vitamin D + calcium supplements in maturation of the ovarian follicles (13, 14). Additionally, the efficacy of calcium intake together with Vitamin D supplement has been demonstrated as an essential intervention for preventing osteoporosis due to postmenopausal conditions (15) by increasing BMD (16). Despite the genetic efficacy on the age of menopause, it is theoretical that lifestyle factors such as diet and physical activity play a significant role in ovarian age (17). Previous studies confirmed that physical activity and, especially, physical exercise are effective in the reduction of clinical fracture in postmenopausal women (18, 19). Muir and colleagues in 2013 published a study to assess the effect of physical activity on bone density in postmenopausal woman. Their results show that, overall, a regular exercise was effective for bone density in postmenopausal women (20). In addition, several previous studies have shown that physical activity, particularly, regular exercise program is the main way to maintain the BMC and prevent bone loss in women (21, 22).
Thus, it is an interesting question - whether an exercise program in combination with calcium and vitamin D supplements can play a supporting role in the maintenance or strengthening of bone mineral content (BMC) and BMD post menopause. Accordingly, the aim of this study was to evaluate the efficacy of regular exercises in combination with vitamin D and calcium supplements on the BMD in postmenopausal rat model.
2. Materials and Methods
2.1. Animals and ethics
In this experimental study, 72 Sprague-Dawley rats, aged eight months with an initial weights of approximately 250 ± 15 gr were used. They the rats were assigned randomly into nine groups:
- Control group: underwent ovariectomy and were kept in cages for 4 months without any type of exercise.
- Ovariectomy calcium supplement group (Ca): underwent ovariectomy and after two months of housing, animals were administered 35 mg/kg calcium supplement (Calcium, Arian Salamat, Iran) by oral gavage for two months.
- Ovariectomy vitamin D supplement group (Vit D): underwent ovariectomy and after two months of housing, animals were injected 10,000 IU/wk vitamin D supplement (Vitamin D3 1000 IU, Health Aid, England) for two months.
- Ovariectomy vitamin calcium and D supplement group (Ca + Vit D): underwent ovariectomy and after two months of housing, animals were administered 35 mg/kg calcium and 10,000 IU/week vitamin D supplements for two months.
- Ovariectomy exercise group (Ex): underwent ovariectomy and performed two months of resistance training that started at the same time as the intact group.
- Ovariectomy exercise and vitamin D supplement group (Ex-Vit D): underwent ovariectomy and performed two months of resistance training. Animals were injected 10,000 IU/week vitamin D supplement that started at the same time as the intact group.
- Ovariectomy exercise and vitamin Ca supplement group (Ex-Ca): underwent ovariectomy and performed two months of resistance training. Animals were administered 35 mg/kg calcium supplement by oral gavage for two months.
- Ovariectomy exercise and calcium and vitamin D supplement group (Ex-Ca & Vit D): underwent ovariectomy and performed two months of resistance training. Animals were administered 35 mg/kg calcium and 10,000 IU/wk vitamin D supplement for two months after ovariectomy.
- Placebo group: animals were only administrated sesame oil for two months after ovariectomy.
2.2. Ovariectomy surgery protocol
For ovariectomy operation, all animals were anesthetized intraperitoneally with a ketamine-xylazine mixture (61.5-7.6 mg/kg), and subsequently, ovaries were removed by a bilateral incision through the skin and completely excised from a dorsal approach (23). The site was sutured and all animals recovered two months after the surgical procedures.
2.3. Resistance training protocol
For Resistance training, a 1-m ladder with 2-cm grid ladder inclined at 85º was used. Prior to the resistance training, rats underwent a familiarization week with a ladder and climbing. Weights attached to their tails and the initial weight was 30% of their body weight, which was increased to 100% of their body weight gradually throughout the eight weeks. Weights were fastened to the upper portion of the tail. The resistance training included three sets of five repetitions with a 30-sec rest interval between the reps and 3 min between the sets (24).
2.4. Measurement methods
Animals were sacrificed by decapitation after the last resistance exercise sessions and after the supplement administration. Whole body was fixed in formaldehyde 10% to measure BMD (mg/cm2) and BMC (mg) of the total body, tail, hips, lumbar, and femur using dual-energy X-ray absorptiometry (387A030, Norland company, USA) (25). Femoral bone weight was measured using a digital scale (Taishi, China). Soon after, femur was retired and decalcified in hydrochloric acid/formic acid for 24 hr. Next, the samples were briefly rinsed in water and transferred to ammonia solution to neutralize acids and left for 30 min. Femurs were dehydrated in a tissue processor. After dehydration in ethanol series, the samples were embedded in paraffin and 5-µm sections were made. For histological evaluation, a standard length of 2 mm of each section of each bone was obtained to analyze the number of osteoblast, osteoclast, and osteocyte cells using a light microscope (Olympus BX51, Japan) with hematoxylin-eosin staining. Measurements were made on digitized images using the digimizer software. The total number of osteoblast, osteoclast, and osteocyte cells per square millimeter was calculated. Also, a cross-sectional area of bone tissue was used to measure the bone body thickness, bone lumen diameter, and bone total diameter. The mean bone lumen diameter (LD) and total diameter (TD) were derived by taking the average of two diameters, D1 and D2, at right angles. For each slide, the mean of 10 random fields was selected for measurements (26).
2.5. Ethical considerations
Animals had free access to water and food and at a controlled temperature of 22 ± 2ºC under 12-hr light/dark cycles. This study was approved by the Committee of Yazd Reproduction Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran (IR.SSU.RSI.REC.1398.020) and all protocols were performed according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
2.6. Statistical analysis
Data were analyzed using the SPSS software, version 20 (SPSS Inc., USA) and expressed as means ± SD. The statistical analysis was done initially by a one-way analysis of variance. A significance level of p < 0.05 and p < 0.001 was used for all comparisons.
3. Results
Resistant exercise with Vit D administration in ovariectomized rats significantly increased tail, hip, and lumbar BMD compared to the control group, p = 0.004, p = 0.007, p = 0.003, respectively (Table I). Also, there was an increasing trend in the femur BMD of the Ex + Vit D group in comparison with the control group (p = 0.009, Table I). The total BMD showed an increase in the Ex + Vit D group over the control group (Figure 1). However, higher tail and femur BMC were observed in the Ex + Vit D group compared to the control group (p = 0.005 and p = 0.002, respectively. No significant changes were observed in the hips and lumbar BMC compared to the control group (Table II). There was no statistically significant difference in the total BMC comparison with the control group (Figure 2).
Moreover, no significant changes were observed between the groups in femoral bone weight (Table III). In addition, reduction of lumen diameter was indicated in groups that were administrated Ca supplement during the study compared to the control group. However, lumen diameter was lower in the Ex + Vit D group than the control group; a significant decrease was seen in the lumen diameter of the Ca + Vit D, Ex + Ca, and Ex + Vit D + Ca groups. Interestingle, bone thickness in the Ex + Vit D group was more than the control groups (p = 0.02; Table III). In addition, the total bone diameter was significantly decreased in the Ca + Vit D (p = 0.000), Ex + Ca (p = 0.018), and Ex + Vit D + Ca (p = 0.001) groups compared with the control group (Figure 3 and 4A).
Figure 4B shows osteocyte and osteoblast cells in the cross-section of the femoral bone. Also, there were no significant differences in the number of osteoblast cells among the control group (Table IV). However, the number of osteoclast cells were decreased in the Ca + Vit D, Ex + Ca, Ex + Vit D, and Ex + Vit D + Ca groups compared to the control group; osteocyte numbers were increased only in the Ex + Vit D group.








4. Discussion
Osteoporosis is identified as a disorder affecting elderly people, especially postmenopausal women. Hence, the determination of a beneficial lifestyle for elderly women seems to be necessary in order to reduce the incidence of postmenopausal syndromes. In the current study, we assessed the effect of resistance exercise, vitamin D and calcium supplements on ovariectomized rats as a model of menopause. Our findings showed the resistance exercise in combination with vitamin D supplement had a positive effect on the maintenance of BMD and BMC in OVX rats. There was a significant difference in the BMD and BMC in Ex + Vit D group in comparison with the other groups. It was reported that the resistance exercise can lead to increasing BMD at the muscle site by enhancement of muscle size and strength (27). Also, the BMC and BMD have been reported to reveal an increase up to 20% in the loaded bone regions in athletes (28). In a recent study, Beavers evaluated the change in BMD during weight loss with various physical activity including resistance and aerobic exercise in older adults. Their result showed that the resistance exercise was osteo-protective in comparison with the caloric restriction in elderly women (29).
According to result of Khalil study in 2015, vitamin D supplement reduced the bone resorption marker in elderly women (9). Several studies reported the important role of vitamin D to prevent the decrease in the bone density and the risk of bone fracture among elderly women (30, 31). Additionally, it was mentioned that some analogs of vitamin D such as 2-Methylene-19-nor (20S)-1α25-dihydroxyvitamin D3 (2MD) contribute in the enhancement of bone mass and are supposed to be a promising treatment for osteoporosis in postmenopausal women (30). Furthermore, vitamin D contributes in regulating calcium homeostasis as a part of bone metabolism procedure that displays the importance of vitamin D presence in bone health (32). On the other hand, several investigations supposed that the dietary calcium intake or calcium supplements contribute in the improvement of BMC and BMD among postmenopausal women. Nonetheless, some others revealed that the calcium supplements alone probably cannot be adequate to decrease fracture risk and other parameters such as vitamin D is required (33). This findings were similar to our result.
Further, it has been established that exercise can increase BMD, BMC, and bone strength (33). Sport researcher believed that continuing pressure and tension to bones is a valuable factor to prevent osteoporosis (34). Although, the role of exercise alone was clear, future studies were needed to define optimal physical activity and additional supplements for preventing osteoporosis and bone fractures (35, 36). Exercise can elevate seroma calcium and vitamin D levels in body but this increase depends on the type, intensity, and time of exercise (37). Mason and colleagues in 2011 reported that vitamin D level can increase after exercise for low-weight program (38). In the current study, an increase in the BMD and BMC in the Ex + Vit D group was observed. These significant changes might be due to the elevation of vit D after exercise. On the other hand, in this group, the elevation in the vitamin D level create from two ways: (1) exogenous vitamin D by supplement intake and (2) endogenous vitamin D by exercise. So, this elevation might be responsible for BMD and BMC increase in this group in compared to other groups.
The bone thickness was morphologically compared between all study groups. There was a significant difference in the Ex + Vit D groups in comparison with the other groups. On the other hand, our finding can confirm that the combination of resistance exercise and vitamin D has a positive effect on the maintenance of bone thickness and prevention or delay of osteoporosis. In the present study, osteocytes were counted in all groups and a significant difference was observed in the Ex + Vit D group in comparison with the other groups that confirmed our previous findings and revealed the promising potential of resistance exercise together with vitamin D intake in reducing bone resorption by decreasing the rate of osteocytes apoptosis. Toumi and colleagues have illustrated that the physical activity alone does not significantly reduce the rate of osteocytes apoptosis in all sham and OVX groups (39). While it is reported that endurance exercise plays an important role in preservation of cancellous bone (40). Moreover, another study showed that exercise would be able to enhance osteogenesis in OVX rats (41). Abnormal proliferation and activity of osteoclasts lead to various bone disorders such as osteoporosis causing decreased BMD and elevate the possibility of bone fractures (7).
Besides, there was a significant increase in the number of osteocyte in the Ex + Vit D group in comparison with the other groups. Some studies have reported that exercise can elevate estrogen level in mice serum, and this elevation can prevent osteoclast activity and bone resorption (42, 43). Some researchers revealed that vitamin D level in serum can be raised in animals who were in exercise program (38, 44). In this issue, exercise might have led to the increase in the VDRs, and upsurge in the production of active vitamin D. Finally, this factor perhaps can affect osteoblasts activity and prevent osteoporosis. We also found that the osteoclast was decreased in the Ca + Vit D, Ca + Ex, and Ex + Vit D groups. In addition, our result showed that vitamin D supplementation alone may not be sufficient for the reduction of osteoclasts and decrease in bone cell apoptosis; however, the effective level of Ca requires deactivation of the osteoclasts.
5. Conclusion
Our findings demonstrated that resistance exercise in combination with vitamin D intake represents effective preventive and therapeutic strategies able to prevent or delay the onset of osteoporosis. However, additional investigations are required for the evaluation of all effective parameters on bone health, particularly from the molecular point of view.
Acknowledgements
This study has been financially supported by the Yazd Reproduction Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Physiology
References
1. Bjelica A, Ćirilović VV, Todorović ST, Filipović K. Postmenopausal osteoporosis. Med Pregl 2018; 71: 201-205. [DOI:10.2298/MPNS1806201B]
2. Dalal PK, Agarwal M. Postmenopausal syndrome. Indian J Psychiatry 2015; 57 (Suppl.): S222-S232. [DOI:10.4103/0019-5545.161483] [PMID] [PMCID]
3. Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: A systematic review and meta-analysis. JAMA Cardiol 2016; 1: 767-776. [DOI:10.1001/jamacardio.2016.2415] [PMID]
4. Hernández-Angeles C, Castelo-Branco C. Early menopause: A hazard to a woman's health. Indian J Med Res 2016; 143: 420-427. [DOI:10.4103/0971-5916.184283] [PMID] [PMCID]
5. Sathyapalan Th, Aye M, Rigby AS, Fraser WD, Thatcher NJ, Kilpatrick ES, et al. Soy reduces bone turnover markers in women during early menopause: A randomized controlled trial. J Bone Miner Res 2017; 32: 157-164. [DOI:10.1002/jbmr.2927] [PMID]
6. Levin VA, Jiang X, Kagan R. Estrogen therapy for osteoporosis in the modern era. Osteoporos Int 2018; 29: 1049-1055. [DOI:10.1007/s00198-018-4414-z] [PMID]
7. Florencio-Silva R, da Silva Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res Int 2015; 2015: 1-17. [DOI:10.1155/2015/421746] [PMID] [PMCID]
8. Nakamichi Y, Udagawa N, Suda T, Takahashi N. Mechanisms involved in bone resorption regulated by vitamin D. J Steroid Biochem Mol Biol 2018; 177: 70-76. [DOI:10.1016/j.jsbmb.2017.11.005] [PMID]
9. Khalil A, Youssef GA. Effect of aerobic exercise, vitamin K and vitamin D on bone metabolism in ovariectomized adult rats. Nat Sci 2015; 13: 1-11.
10. Reid IR, Horne AM, Mihov B, Gamble GD, Al‐Abuwsi F, Singh M, et al. Effect of monthly high‐dose vitamin D on bone density in community‐dwelling older adults substudy of a randomized controlled trial. J Intern Med 2017; 282: 452-460. [DOI:10.1111/joim.12651] [PMID]
11. Eastell R, O'Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers 2016; 2: 16069. [DOI:10.1038/nrdp.2016.69] [PMID]
12. Fung JL, Hartman TJ, Schleicher RL, Goldman MB. Association of vitamin D intake and serum levels with fertility: Results from the lifestyle and fertility study. Fertil Steril 2017; 108: 302-311. [DOI:10.1016/j.fertnstert.2017.05.037] [PMID] [PMCID]
13. Gowder SJT. A Critical Evaluation of Vitamin D-Basic Overview [Internet]. UK: Intech Open; 2017. Chapter 13, Elhusseini H, Lizneva D, Gavrilova-Jordan L, Eziba N, Abdelaziz M, Brakta S, et al. Vitamin D and Female Reproduction. Available at: Https://www.intechopen.com/books/a-critical-evaluation-of-vitamin-d-basic-overview/vitamin-d-and-female-reproduction.
14. Foroozanfard F, Jamilian M, Bahmani F, Talaee R, Talaee N, Hashemi T, et al. Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin D‐deficient women with polycystic ovary syndrome: A randomized double‐blind placebo‐controlled clinical trial. Clin Endocrinol 2015; 83: 888-894. [DOI:10.1111/cen.12840] [PMID]
15. Chon SJ, Koh YK, Heo JY, Lee J, Kim MK, Yun BH, et al. Effects of vitamin D deficiency and daily calcium intake on bone mineral density and osteoporosis in Korean postmenopausal woman. Obstet Gynecol Sci 2017; 60: 53-62. [DOI:10.5468/ogs.2017.60.1.53] [PMID] [PMCID]
16. Tai V, Leung W, Grey A, Reid IR, Bolland MJ. Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ 2015; 351: h4183. [DOI:10.1136/bmj.h4183] [PMID] [PMCID]
17. Purdue-Smithe AC, Whitcomb BW, Szegda KL, Boutot ME, Manson JE, Hankinson SE, et al. Vitamin D and calcium intake and risk of early menopause. Am J Clin Nutr 2017; 105: 1493-1501. [DOI:10.3945/ajcn.116.145607] [PMID] [PMCID]
18. Kemmler W, Engelke K, von Stengel S. Long‐term exercise and bone Mineral density changes in postmenopausal women-are there periods of reduced effectiveness? J Bone Miner Res 2016; 31: 215-222. [DOI:10.1002/jbmr.2608] [PMID]
19. Segev D, Hellerstein D, Dunsky A. Physical activity-does it really increase bone density in postmenopausal women? A review of articles published between 2001-2016. Curr Aging Sci 2018; 11: 4-9. [DOI:10.2174/1874609810666170918170744] [PMID]
20. Muir JM, Ye Ch, Bhandari M, Adachi JD, Thabane L. The effect of regular physical activity on bone mineral density in post-menopausal women aged 75 and over: A retrospective analysis from the Canadian multicentre osteoporosis study. BMC Musculoskeletal Disord 2013; 14: 253-261. [DOI:10.1186/1471-2474-14-253] [PMID] [PMCID]
21. Xu J, Lombardi G, Jiao W, Banfi G. Effects of exercise on bone status in female subjects, from young girls to postmenopausal women: An overview of systematic reviews and meta-analyses. Sports Med 2016; 46: 1165-1182. [DOI:10.1007/s40279-016-0494-0] [PMID]
22. Bilek LD, Waltman NL, Lappe JM, Kupzyk KA, Mack LR, Cullen DM, et al. Protocol for a randomized controlled trial to compare bone-loading exercises with risedronate for preventing bone loss in osteopenic postmenopausal women. BMC Women's Health 2016; 16: 59-70. [DOI:10.1186/s12905-016-0339-x] [PMID] [PMCID]
23. Chen C, Noland KA, Kalu DN. Modulation of intestinal vitamin D receptor by ovariectomy, estrogen and growth hormone. Mech Ageing Dev 1997; 99: 109-122. [DOI:10.1016/S0047-6374(97)00094-8]
24. Prestes J, Leite RD, Pereira GB, Shiguemoto GE, Bernardes CF, Asano RY, et al. Resistance training and glycogen content in ovariectomized rats. Int J Sports Med 2012; 33: 550-554. [DOI:10.1055/s-0032-1304646] [PMID]
25. Khoo BCC, Beck ThJ, Qiao QH, Parakh P, Semanick L, Prince RL, et al. In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone 2005; 37: 112-121. [DOI:10.1016/j.bone.2005.03.007] [PMID]
26. Beck TJ, Ruff CB, Warden KE, Scott JW, Rao GU. Predicting femoral neck strength from bone mineral data. A structural approach. Invest Radiol 1990; 25: 6-18. [DOI:10.1097/00004424-199001000-00004] [PMID]
27. DeFina LF, Leonard D, Willis BL, Barlow CE, Finley CE, Jenkins MR, et al. High cardiorespiratory fitness is associated with reduced risk of low bone density in postmenopausal women. Journal Women's Health 2016; 25: 1073-1080. [DOI:10.1089/jwh.2014.5170] [PMID] [PMCID]
28. Willems HME, van den Heuvel EGHM, Schoemaker RJW, Klein-Nulend J, Bakker AD. Diet and exercise: A match made in bone. Current Osteoporosis Reports 2017; 15: 555-563. [DOI:10.1007/s11914-017-0406-8] [PMID] [PMCID]
29. Beavers KM, Beavers DP, Martin SB, Marsh AP, Lyles MF, Lenchik L, et al. Change in bone mineral density during weight loss with resistance versus aerobic exercise training in older adults. J Gerontol A Biol Sci Med Sci 2017; 72: 1582-1585. [DOI:10.1093/gerona/glx048] [PMID] [PMCID]
30. Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res 2016; 4: 16041-16047. [DOI:10.1038/boneres.2016.41] [PMID] [PMCID]
31. Fischer V, Haffner-Luntzer M, Prystaz K, vom Scheidt A, Busse B, Schinke Th, et al. Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci Rep 2017; 7: 7223. [DOI:10.1038/s41598-017-07511-2] [PMID] [PMCID]
32. Liguori C, Romigi A, Izzi F, Mercuri NB, Cordella A, Tarquini E, et al. Continuous positive airway pressure treatment increases serum vitamin D levels in male patients with obstructive sleep apnea. J Clin Sleep Med 2015; 11: 603-607.
https://doi.org/10.5664/jcsm.5296 [DOI:10.5664/jcsm.4766]
33. Agostini D, Donati Zeppa S, Lucertini F, Annibalini G, Gervasi M, Ferri Marini C, et al. Muscle and bone health in postmenopausal women: Role of protein and vitamin D supplementation combined with exercise training. Nutrients 2018; 10: 1103-1123. [DOI:10.3390/nu10081103] [PMID] [PMCID]
34. Tachiki T, Kouda K, Dongmei N, Tamaki J, Iki M, Kitagawa J, et al. Muscle strength is associated with bone health independently of muscle mass in postmenopausal women: the Japanese population-based osteoporosis study. J Bone Miner Metab 2019; 37: 53-59. [DOI:10.1007/s00774-017-0895-7] [PMID]
35. Kemmler W, Haberle L, von Stengel S. Effects of exercise on fracture reduction in older adults: A systematic review and meta-analysis. Osteoporos Int 2013; 24: 1937-1950. [DOI:10.1007/s00198-012-2248-7] [PMID]
36. Polidoulis I, Beyene J, Cheung AM. The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women--a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 2012; 23: 39-51. [DOI:10.1007/s00198-011-1734-7] [PMID]
37. Peterson SE, Peterson MD, Raymond G, Gilligan C, Checovich MM, Smith EL. Muscular strength and bone density with weight training in middle-aged women. Med Sci Sports Exerc 1991; 23: 499-504. [DOI:10.1249/00005768-199104000-00017] [PMID]
38. Mason C, Xiao L, Imayama I, Duggan CR, Bain C, Foster-Schubert KE, et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am J Clin Nutr 2011; 94: 95-103. [DOI:10.3945/ajcn.111.015552] [PMID] [PMCID]
39. Toumi H, Best TM, Cesaro A, Lespessailles E. Exercise and anti-osteoporotic medication combined treatment for osteoporosis. J Yoga Phys Ther 2016; 6: 1000232-1000235.
40. Huang TH, Su IH, Lewis JL, Chang MSh, Hsu AT, Perrone CE, et al. Effects of methionine restriction and endurance exercise on bones of ovariectomized rats: a study of histomorphometry, densitometry and biomechanical properties. J Appl Physiol 2015; 119: 517-526. [DOI:10.1152/japplphysiol.00395.2015] [PMID]
41. Li W, Zhang Y, Xu X, Wang K, Ding W. Relationship between osteogenesis and angiogenesis in ovariectomized osteoporotic rats after exercise training. Int J Clin Exp Pathol 2017; 10: 11438-11449.
42. Silbermann M, Bar-Shira-Maymon B, Coleman R, Reznick A, Weisman Y, Steinhagen-Thiessen E, et al. Long-term physical exercise retards trabecular bone loss in lumbar vertebrae of aging female mice. Calcif Tissue Int 1990; 46: 80-93. [DOI:10.1007/BF02556091] [PMID]
43. Wu J, Wang X, Chiba H, Higuchi M, Nakatani T, Ezaki O, et al. Combined intervention of soy isoflavone and moderate exercise prevents body fat elevation and bone loss in ovariectomized mice. Metabolism 2004; 53: 942-948. [DOI:10.1016/j.metabol.2004.01.019] [PMID]
44. Zhang L, Chen X, Wu J, Yuan Y, Guo J, Biswas S, et al. The effects of different intensities of exercise and active vitamin D on mouse bone mass and bone strength. J Bone Miner Metab 2017; 35: 265-277. [DOI:10.1007/s00774-016-0764-9] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





