Thu, Jul 3, 2025
[Archive]
Volume 20, Issue 3 (March 2022)
IJRM 2022, 20(3): 213-220 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghodsi M, Hojati V, Attaranzade A, Saifi B. Evaluation of IL-3, IL-5, and IL-6 concentration in the follicular fluid of women with Endometriosis: A cross-sectional study. IJRM 2022; 20 (3) :213-220
URL: http://ijrm.ir/article-1-1813-en.html
URL: http://ijrm.ir/article-1-1813-en.html
1- Department of Biology, Damghan Branch, Islamic Azad University, Damghan, Iran.
2- Department of Biology, Damghan Branch, Islamic Azad University, Damghan, Iran. ,Vida.hojati@gmail.com
3- Imam Reza Hospital, Mashhad University of Medical Science, Mashhad, Iran.
4- Department of Anatomy, Faculty of Medicen, Mashhad Medical Sciences, Islamic Azad University, Mashhad, Iran.
2- Department of Biology, Damghan Branch, Islamic Azad University, Damghan, Iran. ,
3- Imam Reza Hospital, Mashhad University of Medical Science, Mashhad, Iran.
4- Department of Anatomy, Faculty of Medicen, Mashhad Medical Sciences, Islamic Azad University, Mashhad, Iran.
Full-Text [PDF 626 kb]
(1102 Downloads)
| Abstract (HTML) (1654 Views)
1. Introduction
Endometriosis is a common condition in an infertile woman characterized by the growth of endometrial-like tissues with glands and stroma outside of the pelvic cavity (1). Approximately 10-15% of women in the reproductive age suffers from disease complication related to dysmenorrhea, dyspareunia, pelvic pain, and infertility (2).
On the other hand, some previous studies demonstrated differential expression of anti-inflammatory cytokines, which play a role in the endometriosis pathogenesis (3-5). Therefore, it seems that endometriosis is a pathological condition associated with a combined inflammatory and anti-inflammatory activity where pro-inflammatory type 1 T helper (Th1) cellular response dominates the Th2 anti-inflammatory response (3, 6, 7).
Most of the previous studies have been evaluated the concentration of interleukin-5 (IL-5) and IL-6 in the peritoneal fluid (PF) or serum of the endometriosis women and not in the follicular fluid (FF) (6-9). Moreover, there are conflicting results about the serum levels of IL-6 in the women with endometriosis, and there is no data on the concentration of IL-3.
Hence present study aimed to investigate the concentration of these cytokines in the FF of women with endometriosis.
2. Materials and Methods
2.1. Patient’s features
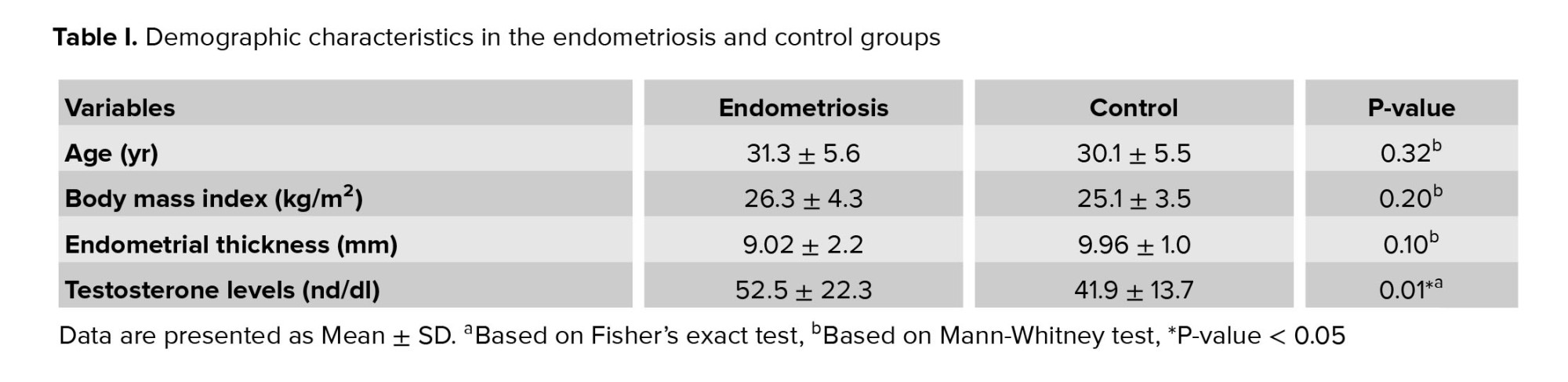
This cross-sectional study recruited a total of 68 women evaluated by laparoscopy, including 34 women with endometriosis as a endometriosis group (31.3 ± 5.6 yrs. old), and 34 healthy women as a control group (30.1 ± 5.5 yrs. old), who referred to the in vitro fertilization (IVF) center of Imam Reza hospital (reproductive medicine unit) in Mashhad, Iran during April 2018. Control groups were fertile women selected from couples referred to the IVF center for men's reproductive problems such as azoospermia.
Inclusion criteria for the control group were regular menstrual cycles, normal androgen levels, and not taking any medication. The exclusion criteria were immune system disorders, cancer, inflammatory disease, infectious diseases, history of pelvic surgery, and taking exogenous hormones three months pre-test. All participant's demographic and clinical characteristics comprising age, body mass index, endometrial thickness, menstrual cycle, galactorrhea, hirsutism, dyspareunia, and dysmenorrhea were collected and compared between the groups.
2.2. Ovarian stimulation
Ovarian stimulation was performed using a gonadotropin-releasing hormone antagonist protocol. Recombinant follicle-stimulating hormone treatment was commenced as 150-225 IU each day on day two of the menstrual cycle. A total of 0.25 mg solution contains ovulation was triggered using 10 000 IU human chorionic gonadotropin after observing at least 2 follicles with a diameter of 17 mm. After ovulation is stimulated, the best time to extract oocyte with performing a puncture through the vagina with the help of ultrasound is about 36-38 hr after the injection. Oocytes were collected separately (from 4 follicles per women). Under short-term anesthesia, the follicles were pierced with a special needle through transvaginal ultrasound to remove the oocyte with the surrounding FF.
Response to the stimulation was confirmed using laboratory tests, and the size of follicles and endometrial growth were evaluated with sonography. FF was collected and used for the measurement of interleukins concentrations.
2.3. Interleukins measurement
FF concentration of IL-3 and IL-5 were assessed by human enzyme-linked immunosorbent assay (ELISA) kit (EASTBIOPHARM, China, Hangzhou), and concentration of IL-6 was measured with human IL-6 Platinum ELISA Kit (eBioscience, an Affymetrix Company, North America and Europe). IL-3 and IL-5 assays had a sensitivity of 1.02 pg/ml and 1.52 ng/L, respectively. Both tests showed intra-assay of coefficient of variability < 10% and inter-assay of coefficient of variability < 12%. IL-6 immunoassay had a sensitivity of 0.92 pg/m, the overall intra-assay coefficient of variation of 3.4%, and the overall inter-assay coefficient of 5.2%. The normal assay range for IL-3, IL-5, and IL-6 was 2-600 pg/ml, 3-900 ng/L (EASTBIOPHARM, China, Hangzhou), and 1-100 pg/ml (eBioscience, an Affymetrix Company, North America and Europe), respectively.
2.4. Evaluation of total testosterone serum concentration
Total testosterone levels were determined by competitive immunoassay using the ADVIA Centaur kit, ADVIA Centaur XP, and ADVIA Centaur XPT systems (SIEMENS, USA). Measurement was performed in the early follicular phase (day 2-5 of the menstrual cycle) when the patient was fasting the night before.
2.5. Ethical considerations
This study was a part of a project performed under the instructions of the Ethics Committees of the Islamic Azad University, Damghan Branch, Damghan, Iran (Code: IR.IAU.REC 14230525981001). Before sample collection, participants signed informed consent.
2.6. Statistical analysis
Data were analyzed by statistical software Statistical Package for the Social Sciences (SPSS) version 16 (SPSS, Inc., Chicago, IL). The results were expressed as mean ± SD. The variables normality checked with Kolmogorov-Smirnov test. The Mann-Whitney U test analyzed non-normal distributions were used. The chi-square test was used to compare categorical variables between two groups in large samples, and in the case of small samples, Fisher’s exact test was used applied (frequency < 20%). P < 0.05 was statistically significant. To determine the diagnostic accuracy of each cytokine, the receiver operating characteristics (ROC) curve analysis was used.
3. Results
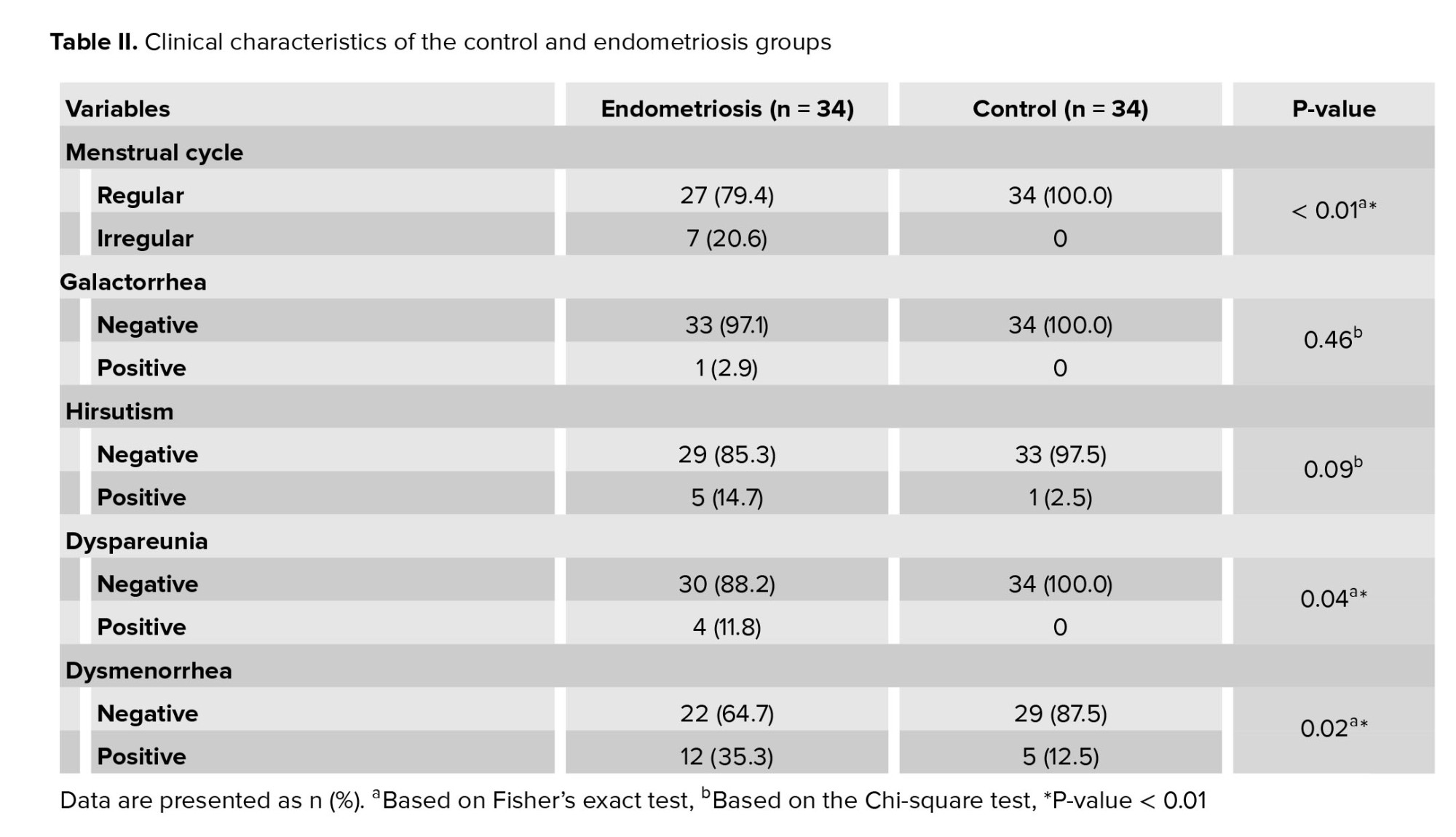
In this cross-sectional study, 34 infertile women with endometriosis were included. They were undergoing IVF at an Imam Reza hospital in Mashhad during April 2018. 34 healthy women were considered the control group who had male infertility problems (Table I). Table II shows the relationship between the menstrual cycle, hirsutism, galactorrhea, dysmenorrhea, and dyspareunia in each group.
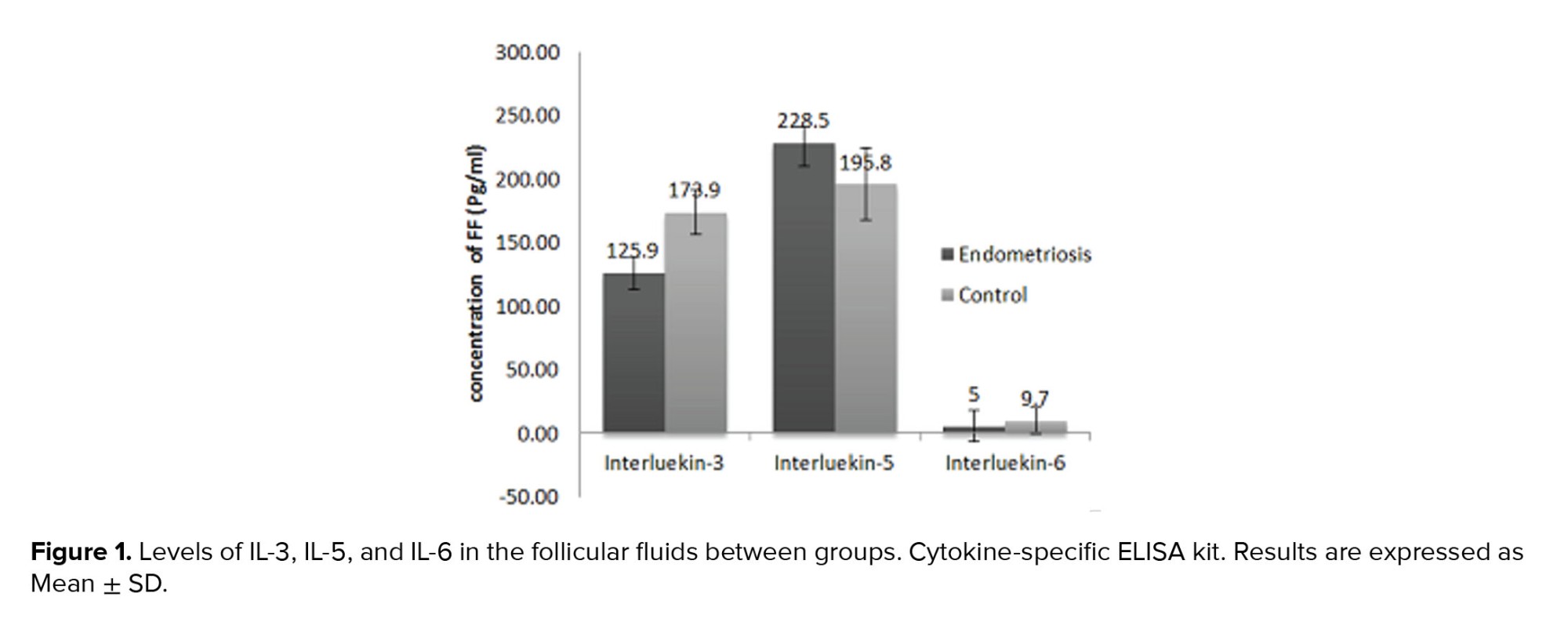
FF concentration of IL-3, IL-5, and IL-6 in women with endometriosis and controls are presented in figure 1. The mean concentration of IL-3 in the women with endometriosis was 125.9 ± 79.8 pg/ml, which was much lower than the control group (173.9 ± 109.2 pg/ml, p = 0.04). Furthermore, the mean concentration of IL-5 in the endometriosis group was lower than the control group (228.5 ± 180.7.7 ng/L vs. 195.8 ± 106.8 ng/L, p = 0.5). The mean concentration of IL-6 showed a significant increase in the endometriosis women (47.5 ± 72.8 pg/ml) compared to the control group (9.7 ± 6.5 pg/ml, p = 0.03).
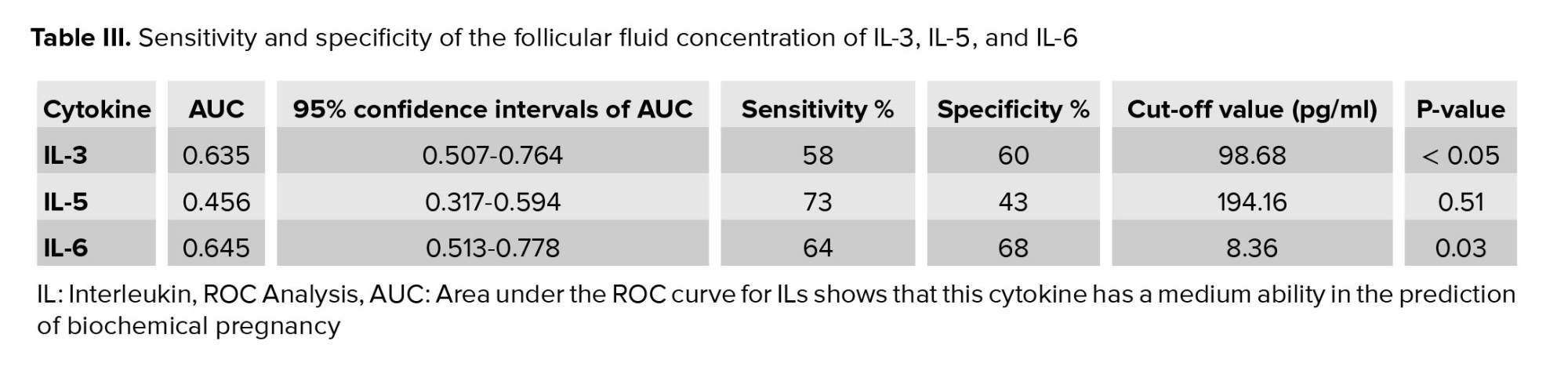
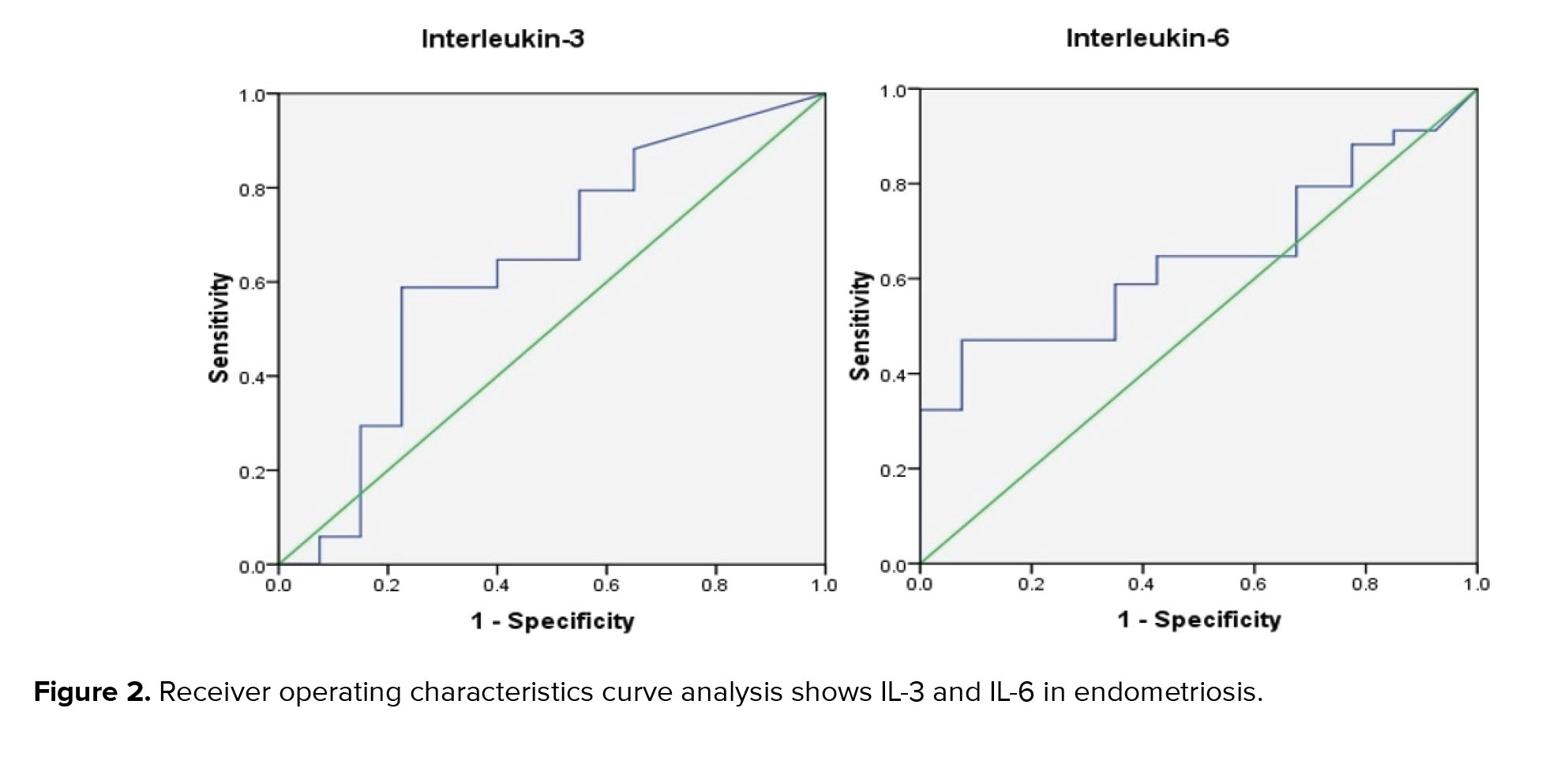
Using ROC curve analysis in the diagnosis of endometriosis, the area under the curve (AUC) for IL-3 was 0.635 (95% CI: 0.50-0.76), and the sensitivity and specificity were 58% and 60%, respectively. Furthermore, FF IL-6 achieved a low diagnostic value, with an AUC of 0.645 (95% CI: 0.51-0.78) and sensitivity and specificity of 64% and 68%, respectively (Figure 2). Table III shows the AUC, sensitivity, and specificity of IL-3, IL-5, and IL-6 for diagnosis of endometriosis with confidence intervals.
4. Discussion
In this study, IL-3, IL-5, and IL-6 were measured in endometriosis women FF. The results demonstrated significant alternations in the FF concentration of IL-6 and IL-3 in endometriosis women compared to the controls. Although the FF concentration of IL-5 reduced in endometriosis women, this change was not statistically significant.
The evidence suggests that a combination of hormonal, immunological, genetic, and environmental factors is involved in the origin and development of endometriosis. Nevertheless, the etiology of endometriosis is not fully understood (3, 10). Given the crucial role of the inflammatory immune responses in the progression and development of endometriosis, the disease is considered an immune-related chronic inflammatory disease (7). Inflammation cascade leads to the activation of the immune-related cells, which produces cytokines, chemokines, and growth factors (11).
Previous findings suggested altered cytokine secretion by Th1 and Th2 cells in women with endometriosis. Changes in the balance between Th1 and Th2 cells toward Th2 may cause failure in the immunological defense system in endometriosis women (12). Altered macrophages cause ectopic endometrial cells to move into the peritoneal cavity. These cells produce inflammatory cytokines that cause the absorption and activation of T1, T2 cells (7). As stated previously, IL-6 as a pro-inflammatory cytokine contributes to the initiation and development of the endometriosis via cytokine network (9). This cytokine is predominantly secreted by macrophages. An increased number of macrophages has been reported in the PF of the women with endometriosis, which produces more IL-6, demonstrating the involvement of IL-6 in the pathogenesis of the disease (11). The present study also indicated that IL-6 concentration significantly increases in women endometriosis. Anti-inflammatory cytokines participate in the progression of the disease through the promotion of ectopic growth, adhesion, angiogenesis, and increase in the survival of the endometrial implants (3). It seems that IL-5 elicits local inflammation presented in women endometriosis and may act at the earlier stages of this inflammatory condition to develop endometrial lesions (13).
Indeed, IL-5 causes tissue damage in the inflammatory tissues. On the other hand, tissue damage induced by the growth of endometriosis activates macrophages to produce IL-6, and as a result, inflammation occurs. IL-6 produces fibrinogen and activates other coagulating factors to form thrombin at the inflammatory sites. Such a change by IL-6 helps to improve tissue damage and inhibits systemic inflammation and tissue injury. Therefore, there is an interaction between IL-5 and IL-6 so IL-6 heals IL-5-induced tissue damage.
In inflammatory diseases, such as endometriosis, IL-5 is reduced as an anti-inflammatory factor (8). The present study demonstrated no significant reduction in the FF concentration of IL-5 between endometriosis and the controls groups. This discrepancy between present study results and previous studies might be partially due to the use of different assay methods, study design, sample size, and differences in the study sample.
IL-3 and IL-5 share a beta-receptor subunit and exert similar biological activities. These cytokines regulate inflammation and involve in the pathology of chronic inflammatory diseases (14). The current study displayed a lower concentration of IL-3 as an anti-inflammatory cytokine in the FF samples of women endometriosis, suggesting the role of IL-3 in the pathogenesis and progression of this chronic inflammatory condition.
To our knowledge, this is the first study that assesses the FF diagnostic value of IL-3 and IL-5 to predict endometriosis in women undergoing IVF. This study indicated low diagnostic accuracy for IL-3, IL-5, and IL-6 in the FF samples based on the ROC curve analysis. None of the studied cytokines achieved a cutoff point with acceptable sensitivity and specificity.
Some studies revealed that IL-6 could be a good marker for diagnosing endometriosis (15, 16). Jiang et al. found that the diagnostic accuracy of IL-6 in the serum and PF samples was high, with the AUC values of 0.905 and 0.952, respectively. The sensitivity and specificity of the serum IL-6 to predict the presence of endometriosis were 90% and 93.7%, respectively. Therefore, they concluded that the serum IL-6 could be used as an excellent marker to discriminate between the women with and without endometriosis (15). However, other studies in agreement with present study results reported that the predictive value of IL-6 is low and cannot be used as a suitable diagnostic or prognostic test for endometriosis (17, 18). The reasons for these conflicting and contradictory observations might be variations in the assay methods, patient selection, menstrual phases, endometriosis types and stages, and a relatively smaller sample size.
The present study showed that endometriosis is associated with the irregular menstrual cycle, dysmenorrhea, and dyspareunia. These data agree with the finding of the previous studies. Hadisaputra and colleagues suggested dyspareunia and dysmenorrhea as the clinical symptoms in women endometriosis (19). Moreover, a previous study identified the family history of endometriosis, galactorrhea history, pelvic surgery history, dysmenorrhea, pelvic pain, dyspareunia, fatigue, and diarrhea as risk factors associated with endometriosis (20).
Endometriosis can also affect the length of a person's menstrual cycles and how long the bleeding lasts. Some studies demonstrated that short-term menstrual periods increase the risk of endometriosis (21). Since their bodies have more tissue for shedding, they may suffer from heavy menstrual cycles, which may take longer than seven days. Their cycles may also shorten, and the menstruation begins earlier than 28 days. Pain and bleeding during ovulation are the other characteristics of these women (21).
5. Conclusion
In summary, the results demonstrated a significant elevation in the IL-6 concentration and a remarkable reduction in the IL-3 levels in the FF samples of women endometriosis compared to the control group, suggesting the involvement of these cytokines in the pathogenesis of endometriosis. However, the FF concentration of these cytokines offers little value in diagnosing endometriosis. Therefore, further investigations in larger sample sizes are necessary to elucidate and confirm the role of pro-and anti-inflammatory cytokines in the etiology of the endometriosis and to introduce an appropriate biomarker for diagnosis of the endometriosis in the early stages.
Acknowledgments
The authors wish to thank the Milad Infertility and Research Center staff, Imam Reza hospital, Mashhad University of Medical Sciences, and the Islamic Azad University, Damghan Branch for their kind assistance.
Conflicts of Interest
None declared.
Full-Text: (370 Views)
1. Introduction
Endometriosis is a common condition in an infertile woman characterized by the growth of endometrial-like tissues with glands and stroma outside of the pelvic cavity (1). Approximately 10-15% of women in the reproductive age suffers from disease complication related to dysmenorrhea, dyspareunia, pelvic pain, and infertility (2).
On the other hand, some previous studies demonstrated differential expression of anti-inflammatory cytokines, which play a role in the endometriosis pathogenesis (3-5). Therefore, it seems that endometriosis is a pathological condition associated with a combined inflammatory and anti-inflammatory activity where pro-inflammatory type 1 T helper (Th1) cellular response dominates the Th2 anti-inflammatory response (3, 6, 7).
Most of the previous studies have been evaluated the concentration of interleukin-5 (IL-5) and IL-6 in the peritoneal fluid (PF) or serum of the endometriosis women and not in the follicular fluid (FF) (6-9). Moreover, there are conflicting results about the serum levels of IL-6 in the women with endometriosis, and there is no data on the concentration of IL-3.
Hence present study aimed to investigate the concentration of these cytokines in the FF of women with endometriosis.
2. Materials and Methods
2.1. Patient’s features
This cross-sectional study recruited a total of 68 women evaluated by laparoscopy, including 34 women with endometriosis as a endometriosis group (31.3 ± 5.6 yrs. old), and 34 healthy women as a control group (30.1 ± 5.5 yrs. old), who referred to the in vitro fertilization (IVF) center of Imam Reza hospital (reproductive medicine unit) in Mashhad, Iran during April 2018. Control groups were fertile women selected from couples referred to the IVF center for men's reproductive problems such as azoospermia.
Inclusion criteria for the control group were regular menstrual cycles, normal androgen levels, and not taking any medication. The exclusion criteria were immune system disorders, cancer, inflammatory disease, infectious diseases, history of pelvic surgery, and taking exogenous hormones three months pre-test. All participant's demographic and clinical characteristics comprising age, body mass index, endometrial thickness, menstrual cycle, galactorrhea, hirsutism, dyspareunia, and dysmenorrhea were collected and compared between the groups.
2.2. Ovarian stimulation
Ovarian stimulation was performed using a gonadotropin-releasing hormone antagonist protocol. Recombinant follicle-stimulating hormone treatment was commenced as 150-225 IU each day on day two of the menstrual cycle. A total of 0.25 mg solution contains ovulation was triggered using 10 000 IU human chorionic gonadotropin after observing at least 2 follicles with a diameter of 17 mm. After ovulation is stimulated, the best time to extract oocyte with performing a puncture through the vagina with the help of ultrasound is about 36-38 hr after the injection. Oocytes were collected separately (from 4 follicles per women). Under short-term anesthesia, the follicles were pierced with a special needle through transvaginal ultrasound to remove the oocyte with the surrounding FF.
Response to the stimulation was confirmed using laboratory tests, and the size of follicles and endometrial growth were evaluated with sonography. FF was collected and used for the measurement of interleukins concentrations.
2.3. Interleukins measurement
FF concentration of IL-3 and IL-5 were assessed by human enzyme-linked immunosorbent assay (ELISA) kit (EASTBIOPHARM, China, Hangzhou), and concentration of IL-6 was measured with human IL-6 Platinum ELISA Kit (eBioscience, an Affymetrix Company, North America and Europe). IL-3 and IL-5 assays had a sensitivity of 1.02 pg/ml and 1.52 ng/L, respectively. Both tests showed intra-assay of coefficient of variability < 10% and inter-assay of coefficient of variability < 12%. IL-6 immunoassay had a sensitivity of 0.92 pg/m, the overall intra-assay coefficient of variation of 3.4%, and the overall inter-assay coefficient of 5.2%. The normal assay range for IL-3, IL-5, and IL-6 was 2-600 pg/ml, 3-900 ng/L (EASTBIOPHARM, China, Hangzhou), and 1-100 pg/ml (eBioscience, an Affymetrix Company, North America and Europe), respectively.
2.4. Evaluation of total testosterone serum concentration
Total testosterone levels were determined by competitive immunoassay using the ADVIA Centaur kit, ADVIA Centaur XP, and ADVIA Centaur XPT systems (SIEMENS, USA). Measurement was performed in the early follicular phase (day 2-5 of the menstrual cycle) when the patient was fasting the night before.
2.5. Ethical considerations
This study was a part of a project performed under the instructions of the Ethics Committees of the Islamic Azad University, Damghan Branch, Damghan, Iran (Code: IR.IAU.REC 14230525981001). Before sample collection, participants signed informed consent.
2.6. Statistical analysis
Data were analyzed by statistical software Statistical Package for the Social Sciences (SPSS) version 16 (SPSS, Inc., Chicago, IL). The results were expressed as mean ± SD. The variables normality checked with Kolmogorov-Smirnov test. The Mann-Whitney U test analyzed non-normal distributions were used. The chi-square test was used to compare categorical variables between two groups in large samples, and in the case of small samples, Fisher’s exact test was used applied (frequency < 20%). P < 0.05 was statistically significant. To determine the diagnostic accuracy of each cytokine, the receiver operating characteristics (ROC) curve analysis was used.
3. Results
In this cross-sectional study, 34 infertile women with endometriosis were included. They were undergoing IVF at an Imam Reza hospital in Mashhad during April 2018. 34 healthy women were considered the control group who had male infertility problems (Table I). Table II shows the relationship between the menstrual cycle, hirsutism, galactorrhea, dysmenorrhea, and dyspareunia in each group.
FF concentration of IL-3, IL-5, and IL-6 in women with endometriosis and controls are presented in figure 1. The mean concentration of IL-3 in the women with endometriosis was 125.9 ± 79.8 pg/ml, which was much lower than the control group (173.9 ± 109.2 pg/ml, p = 0.04). Furthermore, the mean concentration of IL-5 in the endometriosis group was lower than the control group (228.5 ± 180.7.7 ng/L vs. 195.8 ± 106.8 ng/L, p = 0.5). The mean concentration of IL-6 showed a significant increase in the endometriosis women (47.5 ± 72.8 pg/ml) compared to the control group (9.7 ± 6.5 pg/ml, p = 0.03).
Using ROC curve analysis in the diagnosis of endometriosis, the area under the curve (AUC) for IL-3 was 0.635 (95% CI: 0.50-0.76), and the sensitivity and specificity were 58% and 60%, respectively. Furthermore, FF IL-6 achieved a low diagnostic value, with an AUC of 0.645 (95% CI: 0.51-0.78) and sensitivity and specificity of 64% and 68%, respectively (Figure 2). Table III shows the AUC, sensitivity, and specificity of IL-3, IL-5, and IL-6 for diagnosis of endometriosis with confidence intervals.





4. Discussion
In this study, IL-3, IL-5, and IL-6 were measured in endometriosis women FF. The results demonstrated significant alternations in the FF concentration of IL-6 and IL-3 in endometriosis women compared to the controls. Although the FF concentration of IL-5 reduced in endometriosis women, this change was not statistically significant.
The evidence suggests that a combination of hormonal, immunological, genetic, and environmental factors is involved in the origin and development of endometriosis. Nevertheless, the etiology of endometriosis is not fully understood (3, 10). Given the crucial role of the inflammatory immune responses in the progression and development of endometriosis, the disease is considered an immune-related chronic inflammatory disease (7). Inflammation cascade leads to the activation of the immune-related cells, which produces cytokines, chemokines, and growth factors (11).
Previous findings suggested altered cytokine secretion by Th1 and Th2 cells in women with endometriosis. Changes in the balance between Th1 and Th2 cells toward Th2 may cause failure in the immunological defense system in endometriosis women (12). Altered macrophages cause ectopic endometrial cells to move into the peritoneal cavity. These cells produce inflammatory cytokines that cause the absorption and activation of T1, T2 cells (7). As stated previously, IL-6 as a pro-inflammatory cytokine contributes to the initiation and development of the endometriosis via cytokine network (9). This cytokine is predominantly secreted by macrophages. An increased number of macrophages has been reported in the PF of the women with endometriosis, which produces more IL-6, demonstrating the involvement of IL-6 in the pathogenesis of the disease (11). The present study also indicated that IL-6 concentration significantly increases in women endometriosis. Anti-inflammatory cytokines participate in the progression of the disease through the promotion of ectopic growth, adhesion, angiogenesis, and increase in the survival of the endometrial implants (3). It seems that IL-5 elicits local inflammation presented in women endometriosis and may act at the earlier stages of this inflammatory condition to develop endometrial lesions (13).
Indeed, IL-5 causes tissue damage in the inflammatory tissues. On the other hand, tissue damage induced by the growth of endometriosis activates macrophages to produce IL-6, and as a result, inflammation occurs. IL-6 produces fibrinogen and activates other coagulating factors to form thrombin at the inflammatory sites. Such a change by IL-6 helps to improve tissue damage and inhibits systemic inflammation and tissue injury. Therefore, there is an interaction between IL-5 and IL-6 so IL-6 heals IL-5-induced tissue damage.
In inflammatory diseases, such as endometriosis, IL-5 is reduced as an anti-inflammatory factor (8). The present study demonstrated no significant reduction in the FF concentration of IL-5 between endometriosis and the controls groups. This discrepancy between present study results and previous studies might be partially due to the use of different assay methods, study design, sample size, and differences in the study sample.
IL-3 and IL-5 share a beta-receptor subunit and exert similar biological activities. These cytokines regulate inflammation and involve in the pathology of chronic inflammatory diseases (14). The current study displayed a lower concentration of IL-3 as an anti-inflammatory cytokine in the FF samples of women endometriosis, suggesting the role of IL-3 in the pathogenesis and progression of this chronic inflammatory condition.
To our knowledge, this is the first study that assesses the FF diagnostic value of IL-3 and IL-5 to predict endometriosis in women undergoing IVF. This study indicated low diagnostic accuracy for IL-3, IL-5, and IL-6 in the FF samples based on the ROC curve analysis. None of the studied cytokines achieved a cutoff point with acceptable sensitivity and specificity.
Some studies revealed that IL-6 could be a good marker for diagnosing endometriosis (15, 16). Jiang et al. found that the diagnostic accuracy of IL-6 in the serum and PF samples was high, with the AUC values of 0.905 and 0.952, respectively. The sensitivity and specificity of the serum IL-6 to predict the presence of endometriosis were 90% and 93.7%, respectively. Therefore, they concluded that the serum IL-6 could be used as an excellent marker to discriminate between the women with and without endometriosis (15). However, other studies in agreement with present study results reported that the predictive value of IL-6 is low and cannot be used as a suitable diagnostic or prognostic test for endometriosis (17, 18). The reasons for these conflicting and contradictory observations might be variations in the assay methods, patient selection, menstrual phases, endometriosis types and stages, and a relatively smaller sample size.
The present study showed that endometriosis is associated with the irregular menstrual cycle, dysmenorrhea, and dyspareunia. These data agree with the finding of the previous studies. Hadisaputra and colleagues suggested dyspareunia and dysmenorrhea as the clinical symptoms in women endometriosis (19). Moreover, a previous study identified the family history of endometriosis, galactorrhea history, pelvic surgery history, dysmenorrhea, pelvic pain, dyspareunia, fatigue, and diarrhea as risk factors associated with endometriosis (20).
Endometriosis can also affect the length of a person's menstrual cycles and how long the bleeding lasts. Some studies demonstrated that short-term menstrual periods increase the risk of endometriosis (21). Since their bodies have more tissue for shedding, they may suffer from heavy menstrual cycles, which may take longer than seven days. Their cycles may also shorten, and the menstruation begins earlier than 28 days. Pain and bleeding during ovulation are the other characteristics of these women (21).
5. Conclusion
In summary, the results demonstrated a significant elevation in the IL-6 concentration and a remarkable reduction in the IL-3 levels in the FF samples of women endometriosis compared to the control group, suggesting the involvement of these cytokines in the pathogenesis of endometriosis. However, the FF concentration of these cytokines offers little value in diagnosing endometriosis. Therefore, further investigations in larger sample sizes are necessary to elucidate and confirm the role of pro-and anti-inflammatory cytokines in the etiology of the endometriosis and to introduce an appropriate biomarker for diagnosis of the endometriosis in the early stages.
Acknowledgments
The authors wish to thank the Milad Infertility and Research Center staff, Imam Reza hospital, Mashhad University of Medical Sciences, and the Islamic Azad University, Damghan Branch for their kind assistance.
Conflicts of Interest
None declared.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Parasar P, Ozcan P, Terry KL. Endometriosis: Epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep 2017; 6: 34-41. [DOI:10.1007/s13669-017-0187-1] [PMID] [PMCID]
2. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life 2014; 7: 349-357.
3. Zhou WJ, Yang HL, Shao J, Mei J, Chang KK, Zhu R, et al. Anti-inflammatory cytokines in endometriosis. Cell Mol Life Sci 2019; 76: 2111-2132. [DOI:10.1007/s00018-019-03056-x] [PMID]
4. Sharpe-Timms KL, Nabli H, Zimmer RL, Birt JA, Davis JW. Inflammatory cytokines differentially up-regulate human endometrial haptoglobin production in women with endometriosis. Hum Reprod 2010; 25: 1241-1250. [DOI:10.1093/humrep/deq032] [PMID] [PMCID]
5. Jiang L, Yan Y, Liu Zh, Wang Y. Inflammation and endometriosis. Front Biosci 2016; 21: 941-948. [DOI:10.2741/4431] [PMID]
6. Zhang T, De Carolis C, Man GChW, Wang ChCh. The link between immunity, autoimmunity and endometriosis: A literature update. Autoimmun Rev 2018; 17: 945-955. [DOI:10.1016/j.autrev.2018.03.017] [PMID]
7. da Gama Coelh Riccio L, Santulli P, Marcellin L, Abrao MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol 2018; 50: 39-49. [DOI:10.1016/j.bpobgyn.2018.01.010] [PMID]
8. Jorgensen H, Hill AS, Beste MT, Kumar MP, Chiswick E, Fedorcsak P, et al. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil Steril 2017; 107: 1191-1199. [DOI:10.1016/j.fertnstert.2017.03.013] [PMID]
9. Li Sh, Fu X, Wu T, Yang L, Hu Ch, Wu RJ. Role of interleukin-6 and its receptor in endometriosis. Med Sci Monit 2017; 23: 3801-3807. [DOI:10.12659/MSM.905226] [PMID] [PMCID]
10. Giudice LC, Kao LC. Endometriosis. Lancet 2004; 364: 1789-1799. [DOI:10.1016/S0140-6736(04)17403-5]
11. Samimi M, Pourhanifeh MH, Mehdizadehkashi A, Eftekhar T, Asemi Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J Cell Physiol 2019; 234: 19384-19392. [DOI:10.1002/jcp.28666] [PMID]
12. Harada T, Iwabe T, Terakawa N. Role of cytokines in endometriosis. Fertil Steril 2001; 76: 1-10. [DOI:10.1016/S0015-0282(01)01816-7]
13. Monsanto SP, Edwards AK, Zhou J, Nagarkatti P, Nagarkatti M, Young SL, et al. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil Steril 2016; 105: 968-977. [DOI:10.1016/j.fertnstert.2015.11.047] [PMID] [PMCID]
14. Dougan M, Dranoff G, Dougan SK. GM-CSF, IL-3, and IL-5 family of cytokines: Regulators of inflammation. Immunity 2019; 50: 796-811. [DOI:10.1016/j.immuni.2019.03.022] [PMID]
15. Jiang J, Jiang Zh, Xue M. Serum and peritoneal fluid levels of interleukin-6 and interleukin-37 as biomarkers for endometriosis. Gynecol Endocrinol 2019; 35: 571-575. [DOI:10.1080/09513590.2018.1554034] [PMID]
16. Mosbah A, Nabiel Y, Khashaba E. Interleukin-6, intracellular adhesion molecule-1, and glycodelin A levels in serum and peritoneal fluid as biomarkers for endometriosis. Int J Gynaecol Obstet 2016; 134: 247-251. [DOI:10.1016/j.ijgo.2016.01.018] [PMID]
17. Kashanian M, Sariri E, Vahdat M, Ahmari M, Moradi Y, Sheikhansari N. A comparison between serum levels of interleukin-6 and CA125 in patients with endometriosis and normal women. Med J Islam Repub Iran 2015; 29: 280.
18. AL-Tai THH, AL-Hadithi HS, Abdulsalam HS. The role of serum IL-6 changes in evaluation of endometriosis. J Fac Med Baghdad 2013; 55: 158-161.
19. Hadisaputra W. Clinical signs, symptoms and serum level of interleukin-6 and tumor necrosis factor in women with or without endometriosis. Asian Pac J Reprod 2013; 2: 142-145. [DOI:10.1016/S2305-0500(13)60135-9]
20. Ashrafi M, Jahanian Sadatmahalleh Sh, Akhoond MR, Talebi M. Evaluation of risk factors associated with endometriosis in infertile women. Int J Fertil Steril 2016; 10: 11-21.
21. Wei M, Cheng Y, Bu H, Zhao Y, Zhao W. Length of menstrual cycle and risk of endometriosis: A meta-analysis of 11 case-control studies. Medicine 2016; 95: e2922. [DOI:10.1097/MD.0000000000002922] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








