Sat, Jul 12, 2025
[Archive]
Volume 19, Issue 10 (October 2021)
IJRM 2021, 19(10): 889-898 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Doroudi R, Changizi Z, Nematollahi-Mahani S N. Effects of melatonin and human follicular fluid supplementation of in vitro maturation medium on mouse vitrified germinal vesicle oocytes: A laboratory study. IJRM 2021; 19 (10) :889-898
URL: http://ijrm.ir/article-1-1899-en.html
URL: http://ijrm.ir/article-1-1899-en.html
1- Department of Anatomy, Afzalipour School of Medicine, Kerman University of Medical Sciences, Kerman, Iran.
2- Leishmaniasis Research Center, Kerman University of Medical Sciences, Kerman, Iran.
3- Kerman Physiology Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran. ,nnematollahi@kmu.ac.ir
2- Leishmaniasis Research Center, Kerman University of Medical Sciences, Kerman, Iran.
3- Kerman Physiology Research Center, Institute of Neuropharmacology, Kerman University of Medical Sciences, Kerman, Iran. ,
Full-Text [PDF 313 kb]
(1130 Downloads)
| Abstract (HTML) (1823 Views)
Full-Text: (427 Views)
- Introduction
Obtaining qualified oocytes from ovarian stimulation procedures has remained a challenge in common assisted reproductive technologies (ART) (1).
Some harvested immature oocytes may not respond to in vivo gonadotropin stimulus and ART programs, but many are still capable of undergoing spontaneous in vitro maturation (IVM) and in vitro fertilization (IVF) in suitable culture conditions; therefore, the IVM technique was introduced (2). Cryopreservation of immature oocytes and IVM may preserve fecundity in some cases including ovarian cancer. Vitrification, which has the lowest rate of cryoinjuries, is considered the most appropriate method of cryopreservation in oocytes derived from humans and other species. Surveillance of human oocytes following vitrification, warming, and the IVM processes widely varies based on the exposure time, concentration of cryoprotectant solutions, rate of ice-crystal formation, and IVM protocol (3). Various problems are associated with the freezing of oocytes, including most commonly, spindle disorganization, microtubule disturbances, and increased risk of polyploidy at fertilization (4).
Follicular fluid (FF) contains various hormones, growth factors, glycosaminoglycans, and steroids (5). It is also rich in components crucial to oocyte maturation and embryo development including nutrients, gonadotropins, apoptosis inhibitors, meiosis-activating sterol, etc. IVM outcomes with autologous or heterologous FF are not consistent, and depend on the size of the follicles, the FF resource, and the concentration of FF components (6).
Melatonin is an indolamine derived from the amino acid tryptophan. The pineal gland is the main source of melatonin production, but it is also secreted in other parts of the body such as in the lacrimal gland, gastrointestinal tract, skin, ovary, testes, and liver. This indolamine is responsible for controlling the circadian rhythm and seasonal reproductive regulation; however, recent findings suggest melatonin also has an immunomodulatory function and cytoprotective role. As an antioxidant, melatonin induces DNA repair mechanisms, and regulates the action of other antioxidant agents and cell metabolism (7). Melatonin also plays a role in ovarian function and female reproduction. Supplementation of the culture medium with melatonin increases the rate of fertilization and enhances the earlier stages of embryonic development (8). Embryo culture medium supplemented with melatonin has improved blastocyst formation, the hatched blastocyst rate and the total blastomere number (9). In humans, melatonin could alter oxidative stress in FF and improve fertilization outcomes following oral consumption (10). Indolamine receptors have been detected in cumulus cells and oocytes (11), suggesting they have a role in oocyte development. Some in vitro studies have also demonstrated positive effects of melatonin use in oocyte maturation protocols and embryo culture. However, the use of melatonin in IVM may not affect (12), inhibit (13), or enhance nuclear maturation (11), and may also have no beneficial effect on embryo development (12).
Considering the controversial impact of FF and melatonin, the aim of the present study was to determine whether supplementation of IVM culture medium with a defined concentration of exogenous melatonin and FF could have any effect on nuclear and cytoplasmic maturation of vitrified oocytes.
2. Materials and Methods
2.1. Animals
Thirty 6-8-wk-old female NMRI mice were maintained in a 12-hr light/dark cycle at 22-24ºC with unrestricted access to food and tap water.
2.2. Chemicals
All materials used in the study were purchased from Sigma Chemical Company (St Louis, MO, USA) unless stated otherwise.
2.3. Study design and treatment interventions
This study was conducted between June 2017 and November 2018, at Afzalipour Medical School of Kerman University of Medical Sciences, Kerman, Iran. In vivo matured MII (Vivo-MII) oocytes were used as a control for the IVM conditions following ionomycin oocyte activation. The development of Vivo-MII oocytes to two-cell-stage embryos was compared with that of the in vitro matured GV oocytes (non-Vit-GV) group after ionomycin oocyte activation.
Vitrified GV (Vit-GV) oocytes were randomly assigned to control and treatment groups. The control group (C-Vit-GV) received no treatment, and its competence to undergo IVM and IVF was compared with the non-Vit-GV oocytes in order to estimate probable vitrification cryoinjuries.
The remaining vitrified-warmed oocytes were randomly allocated to the following groups: melatonin-treated Vit-GV (M-Vit-GV) oocytes, which were supplemented with 10 µM melatonin in the culture medium; HFF-treated Vit-GV (HFF-Vit-GV) oocytes, which were cultured in 40% human follicular fluid (HFF) in the culture medium; and melatonin + HFF treated Vit-GV (M + HFF-Vit-GV) oocytes, which were supplemented with 10 µM melatonin and 40% HFF. IVM and IVF competence of the different treated groups were compared with that of the C-Vit-GV group.
2.4. HFF
Samples of FF were collected from women aged < 35 yr, with follicles > 14 mm diameter at day 11-12 of their menstrual cycle, and without polycystic ovaries. Aspirated FF containing mature oocytes was centrifuged at 300 × g for 10 min. The supernatant was collected in a new clean tube, inactivated at 56ºC for 30 min, double filtered with 1.2 and 0.22 µm filters (Millipore Corporation, Bedford, MA), and stored at -20ºC for further use.
2.5. GV and MII oocyte collection
In order to collect GV oocytes, the animals were sacrificed, and the ovaries were removed and immediately placed in an α-MEM culture medium. Cumulus-oocyte complexes (COCs) were harvested from antral follicles using a 28 G syringe needle. COCs were transferred into a pre-warmed culture medium and washed twice, then denuded by a 28 G needle and repeated pipetting, washed three times in the culture medium, and assessed for the presence of a GV, under an inverted microscope (Nikon TS100, Japan). The GV oocytes were relocated into drops of culture medium under mineral oil and incubated in a humid atmosphere of 5% CO2 at 37ºC. In order to collect MII oocytes, the female mice were superovulated by intraperitoneal injection of 10 IU of pregnant mare’s serum gonadotropin, at 12-15 pm, followed by 10 IU of human chorionic gonadotropin (hCG, Serono, the Netherlands) 48 hr later. MII oocytes were collected from the fallopian tubes’ ampulla 13-15 hr after the hCG treatment and transferred to drops of the medium under mineral oil.
2.6. GV oocyte vitrification and warming
GV oocytes were exposed to an equilibration solution containing 20% human serum + 7.5% ethylene glycol (Merck, Germany) + 7.5% dimethyl sulfoxide (DMSO, Merck, Germany) in the culture medium for 10 min at room temperature, and a vitrification solution containing 20% human serum + 15% ethylene glycol + 15% DMSO + 0.5 M sucrose in the culture medium for 45 sec at room temperature.
The cryotop method was employed to transfer the oocytes to liquid nitrogen. Vitrified oocytes were removed from the liquid nitrogen, warmed rapidly, and transferred to an equilibration solution (α-MEM supplemented with 20% human serum and 1-mole sucrose) for 1 min. The vitrified oocytes were then transferred to a decreasing concentration of sucrose (1, 0.5, and 0.25-mole sucrose) for 3-5 min. Finally, the oocytes were washed three-five times in α-MEM with 20% human serum. The oocytes were examined under an inverted microscope and the viable oocytes were collected in drops of culture medium under mineral oil for further experiments.
2.7. Oocyte viability assessment and IVM
After incubation of the oocytes for 1 hr in 5% CO2 in humidified air at 37ºC, the oocytes were assessed for viability and the ones with dark and highly granulated cytoplasm were excluded. The surviving oocytes were allocated to different treatment groups as noted in the study design, cultured in IVM medium containing α-MEM supplemented with 100 U FSH, 10 U hCG, 100 mL FBS, and 10 mL P/S per liter, and maintained for 48 hr in an atmosphere of 5% CO2 in humidified air at 37ºC. The oocytes were then observed every 24 hr by an inverted microscope to assess the oocyte maturity indicated by germinal vesicle breakdown (GVBD) and extrusion of the first polar body to the perivitelline space (MII oocytes).
2.8. Artificial oocyte activation
In order to determine the effects of melatonin and HFF treatments on the fertilization capacity of in vitro matured MII oocytes, the GV oocytes were transferred to 10 µmol of ionomycin and 2 mmol of 6-dimethyl-aminopurine in α-MEM supplemented with 5% human serum for 6 min and 3 hr, respectively. The MII oocytes were then transferred to α-MEM with 20% human serum and assessed for the development to two-cell-stage embryos 24 and 48 hr later using an inverted phase-contrast microscope (Olympus, IX71, Japan).
2.9. Ethical considerations
The experiments and animal use were approved by the Ethics Committee of Kerman University of Medical Sciences, Kerman, Iran (Code: IR.KMU.REC.1394.103) and were conducted according to the guidelines for the care and use of laboratory animals. A written informed consent was obtained from all women whose FF was used in the study.
2.10. Statistical analysis
A Chi-square test was performed to compare the maturation and fertilization rates. Statistical analysis between the treatment groups was performed using Statistical Package for the Social Sciences software version 16.0 (SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered significant.
3. Results
The outcomes related to the effect of melatonin, HFF, and their combination on IVM (development to GVBD and MII) and IVF (development to two-cell embryo) of 500 vitrified mouse oocytes are presented below.
3.1. Competence of the IVM medium
The developmental rate of non-Vit-GV and Vivo-MII oocytes to two-cell embryos was compared to assess the competence of the IVM conditions. Following artificial oocyte activation, 94% of Vivo-MII oocytes and 93% of non-Vit-GV oocytes developed to two-cell stage embryos (Figure 1), so the results were comparable between the groups.
After one hr incubation of warmed Vit-GV oocytes in the culture medium, the oocytes were classified as alive oocytes (75.5%) with homogeneous clear cytoplasm and dead oocytes (24.5%) with dark and granulated cytoplasm; the latter oocytes were excluded. The competence of the IVM conditions was assessed by counting GVBD and MII oocytes in the non-Vit-GV and C-Vit-GV groups after 24 and 48 hr of IVM respectively.
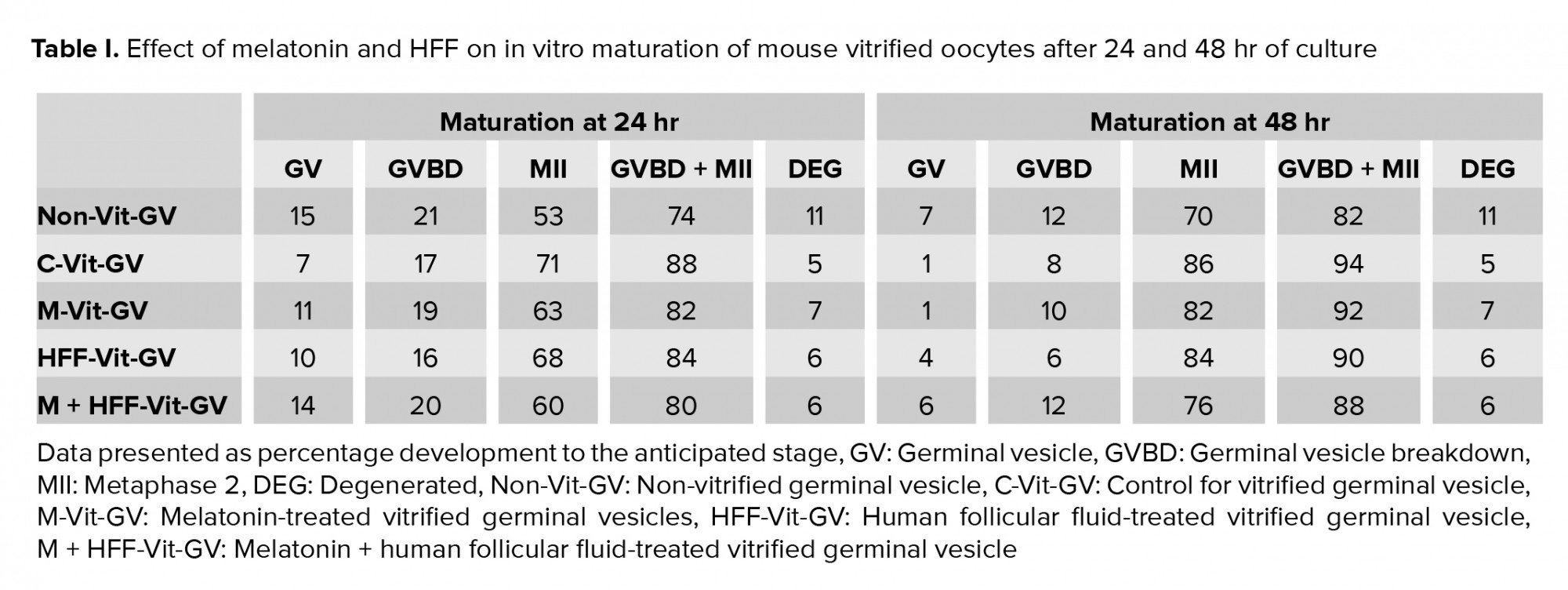
74% of non-Vit-GV oocytes underwent GVBD/MII maturation after 24 hr (21% GVBD and 53% MII) and 88% of Vit-GV oocytes did so (17% GVBD and 71% MII). These values changed to 82% (12% GVBD and 70% MII) and 94% (8% GVBD and 86% MII), respectively, 48 hr after the incubation (Tables I). The difference was not statistically significant between the groups, indicating no deleterious effects of the vitrification protocol.
Table I presents the developmental competence of the 400 surviving oocytes distributed in the C-Vit-GV and three treatment groups (M-Vit-GV, HFF-Vit-GV, and M + HFF-Vit-GV) after 24 and 48 hr of IVM. No significant difference was detected among the groups.
3.2. Oocyte activation and development to two-cell embryos
Observing the pronucleus in any of the matured oocytes (MII) was considered the initial sign of oocyte activation to undergo development to the two-cell stage. Data shown in figure 1 suggest that (i) in vivo matured oocytes underwent the two-cell stage at a rate comparable with the non-vitrified in vitro matured GV oocytes (non-Vit-GV); (ii) vitrified in vitro matured GV oocytes (C-Vit-GV) underwent two-cell stage at a comparable rate as the non-Vit-GV oocytes; and (iii) in-vitro development of vitrified in vitro matured oocytes following artificial oocyte activation by ionomycin was higher when the culture media were enriched by melatonin, HFF and especially their combination. However, none of the treatments resulted in a significant difference compared with the C-Vit-GV group of oocytes.

4. Discussion
In the present study, vitrified mouse GV oocytes underwent IVM and their viability and maturation were assessed to determine whether the vitrification process had any harmful effects. We found that vitrified GV oocytes could reach a maturation rate comparable with that of non-vitrified oocytes and therefore vitrification had no negative impact on the maturation of GV oocytes, evaluated 24 and 48 hr post warming. Theoretically, GV oocytes, due to the presence of a nuclear membrane and diffused chromatins, are more resistant to cryoinjuries and chromosomal disorders (3); however, the maturation potential of oocytes aspirated from stimulated ovaries following cryopreservation is controversial due to factors such as the presence of cumulus cells, culturing duration, oocyte maturation level, and IVM media. A decrease in the maturation and viability rate of vitrified human oocysts was reported when compared with non-vitrified human oocytes (14). However, no differences in the incidence of diploid metaphase II bovine oocytes among control, vitrified, and non-vitrified groups has been detected (15). Similar survival rate in both mature and immature vitrified oocytes after warming was also reported. However, they found a significantly higher oocyte maturation rate in the oocytes that had undergone IVM before vitrification than in the oocytes vitrified after IVM (16). Our results also revealed that the employed IVM protocol could support the maturation of vitrified GV oocytes as no significant difference was found regarding the competence to undergo GVBD and MII stage.
FF has a crucial role in nutritional and hormonal support of oocytes particularly before ovulation (17). However, findings from other studies regarding the positive impact of FF on in vitro conditions are inconsistent. When GV oocytes were cultured in pure FF derived from small follicles 1-5 mm in diameter, the maturation rate was significantly low (18). In contrast, a higher rate of pronucleus formation was detected when the culture medium was enriched with porcine FF (19). Other studies have also reported that supplementation of maturation medium with 10% FF obtained from small, medium, large, and pre-ovulatory follicles (18), and also 20% FF (20) improved the maturation potential of bovine oocytes. In the present study, addition of FF to the oocyte maturation medium resulted in a slight difference in the maturation and fertilization rate between the C-Vit-GV and HFF-Vit-GV groups. The low inhibitory effect of FF in our experiment could be due to the addition of FSH which is known to be a functional blocker of oocyte maturation inhibitors and also meiosis arrest substances in FF, such as coagulants and inhibitory and fibrinolytic factors. Also, the absence of related promotional effects of FF on the maturation of oocytes and embryo development could be explained by protein denaturation and formation of toxic levels of ammonia in the culture medium. Of note, none of the aforementioned studies addressed the effects of active species of oxygen on the maturity potential of GV oocytes.
Some studies have demonstrated the beneficial effects of ascorbate, tocopherol, and melatonin as the most well-known antioxidants that may aid in oocyte recovery after vitrification (21, 22). Melatonin has been used in egg, sperm and embryo vitrification procedures to assist with metabolic regulation and ROS level control (23), which has led to decreased two-cell block, apoptosis and embryonic fragmentation, the promotion of sex steroid hormone production, facilitation of nuclear and cytoplasmic maturation, and enhancement of the blastulation rate, blastocyst cell numbers, and embryo implementation (8). Although the majority of reports claim the beneficial effect of melatonin administration in post warming procedures of IVM and IVF of oocytes at various stages of maturation (24), evidence suggests that melatonin effectiveness widely varies based on the experimental model, exposure duration, supplementation dose, indolamine origin, and maturation stage of the oocyte (25).
We observed that treatment of IVM and IVF media with melatonin seemed to have largely unaffected the vitrified GV oocytes in their completion of meiosis and embryo development, as there was an insignificant difference in MII and two-cell parameters between the treatment and control groups. This phenomenon might have been a result of vitrification prior to maturation, as GV oocytes are less sensitive to vitrification side effects compared with MII ones, even in the case of melatonin supplementation. It has been shown that early-stage embryos have little response to melatonin supplementation; accordingly, Bahadori and colleagues in 2013 observed decreased melatonin receptor expression in the mouse embryos that they studied (26). On the other hand, the dose-dependent effect of melatonin on vitrified MII oocytes revealed that not only did the addition of melatonin at 10-9 M and higher concentrations result in significant beneficial effects, but also it was cytotoxic at 10-3 M (27). In our study, neither the recommended dose of melatonin (10-5 M) (8) nor 40% HFF, nor their combination, significantly improved the maturation and developmental rate of vitrified GV oocytes. As the maturation rate of in vivo matured and non-vitrified GV oocytes were comparable with the vitrified oocytes, it can be deduced that the capability of the vitrification procedure and the maturation and fertilization media were adequate to support the procedure.
To the best of the authors’ knowledge, the present study is the first to investigate the effect of HFF and melatonin separately and in combination on vitrified mouse immature oocytes and on different maturation stages continuously in the same media. We used the ionomycin oocyte activation protocol which is more consistent than sperm-mediated oocyte activation, in which the results are prone to variations in the sperm quality. However, supplementing media with melatonin, HFF, and their combination through a prolonged culture may lead to different outcomes of embryo development, which cannot be examined from our study and so warrants further investigation.
5. Conclusion
It can be concluded that GV oocyte vitrification is a promising method of cryopreservation that can be used for patients with ovarian failure and other related health conditions. When maturation and fertilization media are efficient, supplementation of culture media with enhancing factors such as FF, melatonin, etc. could exert little/if any positive impact on IVM/IVF outcomes in vitrified GV oocytes. However, further studies are required to identify the vitrified GV oocytes’ needs and the extent to which different culture supplements can influence ART outcomes.
Acknowledgements
This study has been extracted from the M.Sc. thesis of R. Doroudi. Vice Chancellor in research affairs of Kerman University of Medical Sciences, Kerman, Iran provided the financial support which is acknowledged.
Conflict of Interest
The authors declare that they have no conflict of interest.
Some harvested immature oocytes may not respond to in vivo gonadotropin stimulus and ART programs, but many are still capable of undergoing spontaneous in vitro maturation (IVM) and in vitro fertilization (IVF) in suitable culture conditions; therefore, the IVM technique was introduced (2). Cryopreservation of immature oocytes and IVM may preserve fecundity in some cases including ovarian cancer. Vitrification, which has the lowest rate of cryoinjuries, is considered the most appropriate method of cryopreservation in oocytes derived from humans and other species. Surveillance of human oocytes following vitrification, warming, and the IVM processes widely varies based on the exposure time, concentration of cryoprotectant solutions, rate of ice-crystal formation, and IVM protocol (3). Various problems are associated with the freezing of oocytes, including most commonly, spindle disorganization, microtubule disturbances, and increased risk of polyploidy at fertilization (4).
Follicular fluid (FF) contains various hormones, growth factors, glycosaminoglycans, and steroids (5). It is also rich in components crucial to oocyte maturation and embryo development including nutrients, gonadotropins, apoptosis inhibitors, meiosis-activating sterol, etc. IVM outcomes with autologous or heterologous FF are not consistent, and depend on the size of the follicles, the FF resource, and the concentration of FF components (6).
Melatonin is an indolamine derived from the amino acid tryptophan. The pineal gland is the main source of melatonin production, but it is also secreted in other parts of the body such as in the lacrimal gland, gastrointestinal tract, skin, ovary, testes, and liver. This indolamine is responsible for controlling the circadian rhythm and seasonal reproductive regulation; however, recent findings suggest melatonin also has an immunomodulatory function and cytoprotective role. As an antioxidant, melatonin induces DNA repair mechanisms, and regulates the action of other antioxidant agents and cell metabolism (7). Melatonin also plays a role in ovarian function and female reproduction. Supplementation of the culture medium with melatonin increases the rate of fertilization and enhances the earlier stages of embryonic development (8). Embryo culture medium supplemented with melatonin has improved blastocyst formation, the hatched blastocyst rate and the total blastomere number (9). In humans, melatonin could alter oxidative stress in FF and improve fertilization outcomes following oral consumption (10). Indolamine receptors have been detected in cumulus cells and oocytes (11), suggesting they have a role in oocyte development. Some in vitro studies have also demonstrated positive effects of melatonin use in oocyte maturation protocols and embryo culture. However, the use of melatonin in IVM may not affect (12), inhibit (13), or enhance nuclear maturation (11), and may also have no beneficial effect on embryo development (12).
Considering the controversial impact of FF and melatonin, the aim of the present study was to determine whether supplementation of IVM culture medium with a defined concentration of exogenous melatonin and FF could have any effect on nuclear and cytoplasmic maturation of vitrified oocytes.
2. Materials and Methods
2.1. Animals
Thirty 6-8-wk-old female NMRI mice were maintained in a 12-hr light/dark cycle at 22-24ºC with unrestricted access to food and tap water.
2.2. Chemicals
All materials used in the study were purchased from Sigma Chemical Company (St Louis, MO, USA) unless stated otherwise.
2.3. Study design and treatment interventions
This study was conducted between June 2017 and November 2018, at Afzalipour Medical School of Kerman University of Medical Sciences, Kerman, Iran. In vivo matured MII (Vivo-MII) oocytes were used as a control for the IVM conditions following ionomycin oocyte activation. The development of Vivo-MII oocytes to two-cell-stage embryos was compared with that of the in vitro matured GV oocytes (non-Vit-GV) group after ionomycin oocyte activation.
Vitrified GV (Vit-GV) oocytes were randomly assigned to control and treatment groups. The control group (C-Vit-GV) received no treatment, and its competence to undergo IVM and IVF was compared with the non-Vit-GV oocytes in order to estimate probable vitrification cryoinjuries.
The remaining vitrified-warmed oocytes were randomly allocated to the following groups: melatonin-treated Vit-GV (M-Vit-GV) oocytes, which were supplemented with 10 µM melatonin in the culture medium; HFF-treated Vit-GV (HFF-Vit-GV) oocytes, which were cultured in 40% human follicular fluid (HFF) in the culture medium; and melatonin + HFF treated Vit-GV (M + HFF-Vit-GV) oocytes, which were supplemented with 10 µM melatonin and 40% HFF. IVM and IVF competence of the different treated groups were compared with that of the C-Vit-GV group.
2.4. HFF
Samples of FF were collected from women aged < 35 yr, with follicles > 14 mm diameter at day 11-12 of their menstrual cycle, and without polycystic ovaries. Aspirated FF containing mature oocytes was centrifuged at 300 × g for 10 min. The supernatant was collected in a new clean tube, inactivated at 56ºC for 30 min, double filtered with 1.2 and 0.22 µm filters (Millipore Corporation, Bedford, MA), and stored at -20ºC for further use.
2.5. GV and MII oocyte collection
In order to collect GV oocytes, the animals were sacrificed, and the ovaries were removed and immediately placed in an α-MEM culture medium. Cumulus-oocyte complexes (COCs) were harvested from antral follicles using a 28 G syringe needle. COCs were transferred into a pre-warmed culture medium and washed twice, then denuded by a 28 G needle and repeated pipetting, washed three times in the culture medium, and assessed for the presence of a GV, under an inverted microscope (Nikon TS100, Japan). The GV oocytes were relocated into drops of culture medium under mineral oil and incubated in a humid atmosphere of 5% CO2 at 37ºC. In order to collect MII oocytes, the female mice were superovulated by intraperitoneal injection of 10 IU of pregnant mare’s serum gonadotropin, at 12-15 pm, followed by 10 IU of human chorionic gonadotropin (hCG, Serono, the Netherlands) 48 hr later. MII oocytes were collected from the fallopian tubes’ ampulla 13-15 hr after the hCG treatment and transferred to drops of the medium under mineral oil.
2.6. GV oocyte vitrification and warming
GV oocytes were exposed to an equilibration solution containing 20% human serum + 7.5% ethylene glycol (Merck, Germany) + 7.5% dimethyl sulfoxide (DMSO, Merck, Germany) in the culture medium for 10 min at room temperature, and a vitrification solution containing 20% human serum + 15% ethylene glycol + 15% DMSO + 0.5 M sucrose in the culture medium for 45 sec at room temperature.
The cryotop method was employed to transfer the oocytes to liquid nitrogen. Vitrified oocytes were removed from the liquid nitrogen, warmed rapidly, and transferred to an equilibration solution (α-MEM supplemented with 20% human serum and 1-mole sucrose) for 1 min. The vitrified oocytes were then transferred to a decreasing concentration of sucrose (1, 0.5, and 0.25-mole sucrose) for 3-5 min. Finally, the oocytes were washed three-five times in α-MEM with 20% human serum. The oocytes were examined under an inverted microscope and the viable oocytes were collected in drops of culture medium under mineral oil for further experiments.
2.7. Oocyte viability assessment and IVM
After incubation of the oocytes for 1 hr in 5% CO2 in humidified air at 37ºC, the oocytes were assessed for viability and the ones with dark and highly granulated cytoplasm were excluded. The surviving oocytes were allocated to different treatment groups as noted in the study design, cultured in IVM medium containing α-MEM supplemented with 100 U FSH, 10 U hCG, 100 mL FBS, and 10 mL P/S per liter, and maintained for 48 hr in an atmosphere of 5% CO2 in humidified air at 37ºC. The oocytes were then observed every 24 hr by an inverted microscope to assess the oocyte maturity indicated by germinal vesicle breakdown (GVBD) and extrusion of the first polar body to the perivitelline space (MII oocytes).
2.8. Artificial oocyte activation
In order to determine the effects of melatonin and HFF treatments on the fertilization capacity of in vitro matured MII oocytes, the GV oocytes were transferred to 10 µmol of ionomycin and 2 mmol of 6-dimethyl-aminopurine in α-MEM supplemented with 5% human serum for 6 min and 3 hr, respectively. The MII oocytes were then transferred to α-MEM with 20% human serum and assessed for the development to two-cell-stage embryos 24 and 48 hr later using an inverted phase-contrast microscope (Olympus, IX71, Japan).
2.9. Ethical considerations
The experiments and animal use were approved by the Ethics Committee of Kerman University of Medical Sciences, Kerman, Iran (Code: IR.KMU.REC.1394.103) and were conducted according to the guidelines for the care and use of laboratory animals. A written informed consent was obtained from all women whose FF was used in the study.
2.10. Statistical analysis
A Chi-square test was performed to compare the maturation and fertilization rates. Statistical analysis between the treatment groups was performed using Statistical Package for the Social Sciences software version 16.0 (SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered significant.
3. Results
The outcomes related to the effect of melatonin, HFF, and their combination on IVM (development to GVBD and MII) and IVF (development to two-cell embryo) of 500 vitrified mouse oocytes are presented below.
3.1. Competence of the IVM medium
The developmental rate of non-Vit-GV and Vivo-MII oocytes to two-cell embryos was compared to assess the competence of the IVM conditions. Following artificial oocyte activation, 94% of Vivo-MII oocytes and 93% of non-Vit-GV oocytes developed to two-cell stage embryos (Figure 1), so the results were comparable between the groups.
After one hr incubation of warmed Vit-GV oocytes in the culture medium, the oocytes were classified as alive oocytes (75.5%) with homogeneous clear cytoplasm and dead oocytes (24.5%) with dark and granulated cytoplasm; the latter oocytes were excluded. The competence of the IVM conditions was assessed by counting GVBD and MII oocytes in the non-Vit-GV and C-Vit-GV groups after 24 and 48 hr of IVM respectively.
74% of non-Vit-GV oocytes underwent GVBD/MII maturation after 24 hr (21% GVBD and 53% MII) and 88% of Vit-GV oocytes did so (17% GVBD and 71% MII). These values changed to 82% (12% GVBD and 70% MII) and 94% (8% GVBD and 86% MII), respectively, 48 hr after the incubation (Tables I). The difference was not statistically significant between the groups, indicating no deleterious effects of the vitrification protocol.
Table I presents the developmental competence of the 400 surviving oocytes distributed in the C-Vit-GV and three treatment groups (M-Vit-GV, HFF-Vit-GV, and M + HFF-Vit-GV) after 24 and 48 hr of IVM. No significant difference was detected among the groups.
3.2. Oocyte activation and development to two-cell embryos
Observing the pronucleus in any of the matured oocytes (MII) was considered the initial sign of oocyte activation to undergo development to the two-cell stage. Data shown in figure 1 suggest that (i) in vivo matured oocytes underwent the two-cell stage at a rate comparable with the non-vitrified in vitro matured GV oocytes (non-Vit-GV); (ii) vitrified in vitro matured GV oocytes (C-Vit-GV) underwent two-cell stage at a comparable rate as the non-Vit-GV oocytes; and (iii) in-vitro development of vitrified in vitro matured oocytes following artificial oocyte activation by ionomycin was higher when the culture media were enriched by melatonin, HFF and especially their combination. However, none of the treatments resulted in a significant difference compared with the C-Vit-GV group of oocytes.


4. Discussion
In the present study, vitrified mouse GV oocytes underwent IVM and their viability and maturation were assessed to determine whether the vitrification process had any harmful effects. We found that vitrified GV oocytes could reach a maturation rate comparable with that of non-vitrified oocytes and therefore vitrification had no negative impact on the maturation of GV oocytes, evaluated 24 and 48 hr post warming. Theoretically, GV oocytes, due to the presence of a nuclear membrane and diffused chromatins, are more resistant to cryoinjuries and chromosomal disorders (3); however, the maturation potential of oocytes aspirated from stimulated ovaries following cryopreservation is controversial due to factors such as the presence of cumulus cells, culturing duration, oocyte maturation level, and IVM media. A decrease in the maturation and viability rate of vitrified human oocysts was reported when compared with non-vitrified human oocytes (14). However, no differences in the incidence of diploid metaphase II bovine oocytes among control, vitrified, and non-vitrified groups has been detected (15). Similar survival rate in both mature and immature vitrified oocytes after warming was also reported. However, they found a significantly higher oocyte maturation rate in the oocytes that had undergone IVM before vitrification than in the oocytes vitrified after IVM (16). Our results also revealed that the employed IVM protocol could support the maturation of vitrified GV oocytes as no significant difference was found regarding the competence to undergo GVBD and MII stage.
FF has a crucial role in nutritional and hormonal support of oocytes particularly before ovulation (17). However, findings from other studies regarding the positive impact of FF on in vitro conditions are inconsistent. When GV oocytes were cultured in pure FF derived from small follicles 1-5 mm in diameter, the maturation rate was significantly low (18). In contrast, a higher rate of pronucleus formation was detected when the culture medium was enriched with porcine FF (19). Other studies have also reported that supplementation of maturation medium with 10% FF obtained from small, medium, large, and pre-ovulatory follicles (18), and also 20% FF (20) improved the maturation potential of bovine oocytes. In the present study, addition of FF to the oocyte maturation medium resulted in a slight difference in the maturation and fertilization rate between the C-Vit-GV and HFF-Vit-GV groups. The low inhibitory effect of FF in our experiment could be due to the addition of FSH which is known to be a functional blocker of oocyte maturation inhibitors and also meiosis arrest substances in FF, such as coagulants and inhibitory and fibrinolytic factors. Also, the absence of related promotional effects of FF on the maturation of oocytes and embryo development could be explained by protein denaturation and formation of toxic levels of ammonia in the culture medium. Of note, none of the aforementioned studies addressed the effects of active species of oxygen on the maturity potential of GV oocytes.
Some studies have demonstrated the beneficial effects of ascorbate, tocopherol, and melatonin as the most well-known antioxidants that may aid in oocyte recovery after vitrification (21, 22). Melatonin has been used in egg, sperm and embryo vitrification procedures to assist with metabolic regulation and ROS level control (23), which has led to decreased two-cell block, apoptosis and embryonic fragmentation, the promotion of sex steroid hormone production, facilitation of nuclear and cytoplasmic maturation, and enhancement of the blastulation rate, blastocyst cell numbers, and embryo implementation (8). Although the majority of reports claim the beneficial effect of melatonin administration in post warming procedures of IVM and IVF of oocytes at various stages of maturation (24), evidence suggests that melatonin effectiveness widely varies based on the experimental model, exposure duration, supplementation dose, indolamine origin, and maturation stage of the oocyte (25).
We observed that treatment of IVM and IVF media with melatonin seemed to have largely unaffected the vitrified GV oocytes in their completion of meiosis and embryo development, as there was an insignificant difference in MII and two-cell parameters between the treatment and control groups. This phenomenon might have been a result of vitrification prior to maturation, as GV oocytes are less sensitive to vitrification side effects compared with MII ones, even in the case of melatonin supplementation. It has been shown that early-stage embryos have little response to melatonin supplementation; accordingly, Bahadori and colleagues in 2013 observed decreased melatonin receptor expression in the mouse embryos that they studied (26). On the other hand, the dose-dependent effect of melatonin on vitrified MII oocytes revealed that not only did the addition of melatonin at 10-9 M and higher concentrations result in significant beneficial effects, but also it was cytotoxic at 10-3 M (27). In our study, neither the recommended dose of melatonin (10-5 M) (8) nor 40% HFF, nor their combination, significantly improved the maturation and developmental rate of vitrified GV oocytes. As the maturation rate of in vivo matured and non-vitrified GV oocytes were comparable with the vitrified oocytes, it can be deduced that the capability of the vitrification procedure and the maturation and fertilization media were adequate to support the procedure.
To the best of the authors’ knowledge, the present study is the first to investigate the effect of HFF and melatonin separately and in combination on vitrified mouse immature oocytes and on different maturation stages continuously in the same media. We used the ionomycin oocyte activation protocol which is more consistent than sperm-mediated oocyte activation, in which the results are prone to variations in the sperm quality. However, supplementing media with melatonin, HFF, and their combination through a prolonged culture may lead to different outcomes of embryo development, which cannot be examined from our study and so warrants further investigation.
5. Conclusion
It can be concluded that GV oocyte vitrification is a promising method of cryopreservation that can be used for patients with ovarian failure and other related health conditions. When maturation and fertilization media are efficient, supplementation of culture media with enhancing factors such as FF, melatonin, etc. could exert little/if any positive impact on IVM/IVF outcomes in vitrified GV oocytes. However, further studies are required to identify the vitrified GV oocytes’ needs and the extent to which different culture supplements can influence ART outcomes.
Acknowledgements
This study has been extracted from the M.Sc. thesis of R. Doroudi. Vice Chancellor in research affairs of Kerman University of Medical Sciences, Kerman, Iran provided the financial support which is acknowledged.
Conflict of Interest
The authors declare that they have no conflict of interest.
Type of Study: Original Article |
Subject:
Assisted Reproductive Technologies
References
1. Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil Steril 2012; 97: 1078-1084. [DOI:10.1016/j.fertnstert.2012.01.122] [PMID]
2. Hatırnaz Ş, Ata B, Hatırnaz ES, Dahan MH, Tannus S, Tan J, et al. Oocyte in vitro maturation: A sytematic review. Turk J Obstet Gynecol 2018; 15: 112-125. [DOI:10.4274/tjod.23911] [PMID] [PMCID]
3. Sciorio R. Cryopreservation of human embryos and oocytes for fertility preservation in cancer and non cancer patients: A mini review. Gynecol Endocrinol 2020; 36: 381-388. [DOI:10.1080/09513590.2020.1719402] [PMID]
4. Al-Hasani S, Ozmen B, Koutlaki N, Schoepper B, Diedrich K, Schultze-Mosgau A. Three years of routine vitrification of human zygotes: Is it still fair to advocate slow-rate freezing? Reprod BioMed Online 2007; 14: 288-293. [DOI:10.1016/S1472-6483(10)60869-3]
5. Krisher RL. In vivo and in vitro environmental effects on mammalian oocyte quality. Annu Rev Anim Biosci 2013; 1: 393-417. [DOI:10.1146/annurev-animal-031412-103647] [PMID]
6. Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus-oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update 2003; 9: 35-48. [DOI:10.1093/humupd/dmg009] [PMID]
7. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, et al. Extrapineal melatonin: Sources, regulation, and potential functions. Cell Mol Life Sci 2014; 71: 2997-3025. [DOI:10.1007/s00018-014-1579-2] [PMID]
8. Ishizuka B, Kuribayashi Y, Murai K, Amemiya A, Itoh MT. The effect of melatonin on in vitro fertilization and embryo development in mice. J Pineal Res 2000; 28: 48-51. [DOI:10.1034/j.1600-079x.2000.280107.x] [PMID]
9. Gao C, Han HB, Tian XZ, Tan DX, Wang L, Zhou GB, et al. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J Pineal Res 2012; 52: 305-311. [DOI:10.1111/j.1600-079X.2011.00944.x] [PMID]
10. Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 2008; 44: 280-287. [DOI:10.1111/j.1600-079X.2007.00524.x] [PMID]
11. El-Raey M, Geshi M, Somfai T, Kaneda M, Hirako M, Abdel-Ghaffar AE, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol Reprod Dev 2011; 78: 250-262. [DOI:10.1002/mrd.21295] [PMID]
12. Takada L, Martins Junior A, Mingoti GZ, Balieiro JCC, Coelho LA. Melatonin in maturation media fails to improve oocyte maturation, embryo development rates and DNA damage of bovine embryos. Sci Agric (Piracicaba, Braz) 2010; 67: 393-398. [DOI:10.1590/S0103-90162010000400003]
13. Farahavar A, Shahne AZ, Kohram H, Vahedi V. Effect of melatonin on in vitro maturation of bovine oocytes. Afr J Biotechnol 2010; 9: 2579-2583.
14. Yazdanpanah F, Khalili MA, Eftekhar M, Karimi H. The effect of vitrification on maturation and viability capacities of immature human oocytes. Arch Gynecol Obstet 2013; 288: 439-444. [DOI:10.1007/s00404-013-2777-0] [PMID]
15. Luna HS, Ferrari I, Rumpf R. Influence of stage of maturation of bovine oocytes at time of vitrification on the incidence of diploid metaphase II at completion of maturation. Anim Reprod Sci 2001; 68: 23-28. [DOI:10.1016/S0378-4320(01)00136-1]
16. Fasano G, Demeestere I, Englert Y. In-vitro maturation of human oocytes: Before or after vitrification? J Assist Reprod Genet 2012; 29: 507-512. [DOI:10.1007/s10815-012-9751-9] [PMID] [PMCID]
17. Annes K, Müller DB, Vilela JA, Valente RS, Caetano DP, Cibin FW, et al. Influence of follicle size on bovine oocyte lipid composition, follicular metabolic and stress markers, embryo development and blastocyst lipid content. Reprod Fertil Dev 2019; 31: 462-472. [DOI:10.1071/RD18109] [PMID]
18. Sirard MA, Roy F, Patrick B, Mermillod P, Guilbault LA. Origin of the follicular fluid added to the media during bovine IVM influences embryonic development. Theriogenology 1995; 44: 85-94. [DOI:10.1016/0093-691X(95)00150-7]
19. Funahashi H, Day BN. Effects of different serum supplements in maturation medium on meiotic and cytoplasmic maturation of pig oocytes. Theriogenology 1993; 39: 965-973. [DOI:10.1016/0093-691X(93)90433-6]
20. Sun FJ, Holm P, Irvine B, Seamark RF. Effect of sheep and human follicular fluid on the maturation of sheep oocytes in vitro. Theriogenology 1994; 41: 981-988. [DOI:10.1016/0093-691X(94)90513-I]
21. Méndez M, Argudo D, Soria M, Galarza L, Perea F. [Effect of the addition of melatonin in the oocyte maturation and/or vitrification medium on in vitro production of bovine embryos]. Rev Inv Vet Perú 2020; 31: e17557. (in Spanish) [DOI:10.15381/rivep.v31i1.17557]
22. Tian H, Qi Q, Yan F, Wang Ch, Hou F, Ren W, et al. Enhancing the developmental competence of prepubertal lamb oocytes by supplementing the in vitro maturation medium with sericin and the fibroblast growth factor 2 -leukemia inhibitory factor -insulin-like growth factor 1 combination. Theriogenology 2020; 159: 13-19. [DOI:10.1016/j.theriogenology.2020.10.019] [PMID]
23. Tamura H, Tanabe M, Jozaki M, Taketani T, Sugino N. Antioxidative action of melatonin and reproduction. Glycative Stress Res 2019; 6: 192-197.
24. Gutiérrez-Añez JC, Lucas-Hahn A, Hadeler KG, Aldag P, Niemann H. Melatonin enhances in vitro developmental competence of cumulus-oocyte complexes collected by ovum pick-up in prepubertal and adult dairy cattle. Theriogenology 2021; 161: 285-293. [DOI:10.1016/j.theriogenology.2020.12.011] [PMID]
25. Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, et al. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil Steril 2009; 92: 328-343. [DOI:10.1016/j.fertnstert.2008.05.016] [PMID]
26. Bahadori MH, Ghasemian F, Ramezani M, Asgari Z. Melatonin effect during different maturation stages of oocyte and subsequent embryo development in mice. Iran J Reprod Med 2013; 11: 11-18.
27. Succu S, Pasciu V, Manca ME, Chelucci S, Torres-Rovira L, Leoni GG, et al. Dose-dependent effect of melatonin on postwarming development of vitrified ovine embryos. Theriogenology 2014; 81: 1058-1066. [DOI:10.1016/j.theriogenology.2014.01.032] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








