Fri, Apr 26, 2024
[Archive]
Volume 19, Issue 11 (November 2021)
IJRM 2021, 19(11): 979-986 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Singh V, Tamar N, Lone Z, Das E, Sahu R, Majumdar S. Association between serum 25-hydroxy vitamin D level and menstrual cycle length and regularity: A cross-sectional observational study. IJRM 2021; 19 (11) :979-986
URL: http://ijrm.ir/article-1-1948-en.html
URL: http://ijrm.ir/article-1-1948-en.html
1- Obstetrics and Gynecology Department, Aiims, Raipur, India. , ddvinitasingh@gmail.com
2- Obstetrics and Gynecology Department, Aiims, Raipur, India.
2- Obstetrics and Gynecology Department, Aiims, Raipur, India.
Keywords: Menstrual disturbances, Ovulation, Reproductive period, Vitamin D deficiency, Dietary supplements, Polycystic ovarian syndrome.
Full-Text [PDF 486 kb]
(937 Downloads)
| Abstract (HTML) (1522 Views)
Full-Text: (397 Views)
- Introduction
It is already well known that vitamin D plays an important role in maintaining bone strength by controlling the mineralization of bones. There is growing evidence of vitamin D also being involved in cell proliferation and immune regulation (1, 2). Vitamin D tends to exert its diversified role in influencing reproductive physiology through vitamin D receptors, which are found in the female reproductive tract, such as in the ovaries (particularly in the granulosa cells), uterus, and placenta (3). Vitamin D has been documented in patients with polycystic ovarian syndrome (PCOS), hyperandrogenism and infertility (4, 5). Vitamin D likely executes its control on ovarian function and hence the menstrual cycle through anti- mullerian hormone (AMH) receptors, which also share a domain for the vitamin D response element (3, 6). Vitamin D supplementation in a high dose can correct metabolic disorders associated with PCOS (7, 8). Studies have shown that vitamin D can play a role in ovarian reserve and dysmenorrhoea (9, 10). Vitamin D and calcium supplementation in PCOS patients has also been shown to influence follicle maturation and menstruation (11, 12). Some researchers question the role of vitamin D (in combination with calcium) in any kind of menstrual problems in females (13). Altered calcium homeostasis and parathormone PTH levels secondary to vitamin D deficiency could also be the cause of the dysregulated follicular development causing menstrual dysfunction in women with PCOS (4, 14).
This study was done to find whether any association exists between deficient vitamin D levels and menstrual irregularities. Vitamin D is inexpensive and easily available with minimal side effects, so it can be considered for supplementation in women with menstrual irregularities if any positive association is found.
This study was done to find whether any association exists between deficient vitamin D levels and menstrual irregularities. Vitamin D is inexpensive and easily available with minimal side effects, so it can be considered for supplementation in women with menstrual irregularities if any positive association is found.
- Materials and Methods
We conducted this cross-sectional observational study in a tertiary care teaching hospital located in central India over three months from April 2019 to June 2019. Female patients of reproductive age attending Gynecology out patient department. Aiims Raipur with complaints of menstrual irregularities were included. The sample size was calculated based on standard deviation and minimum difference detected for the mean vitamin D levels between irregular and regular cycles taken from a previous study (7), with 80% power and a 5% level of significance. The necessary sample size was calculated as 166 (83 in each group).
The inclusion criteria were: female patients of reproductive age attending Gynecology OPD, Aiims Raipur with complaints of menstrual irregularity (defined as a shortest to longest cycle variation of ≥ 8-10 days). Participants were divided into two groups: (i) frequent cycle (< 24 days interval); (ii) infrequent cycle (> 38 days). The exclusion criteria were: 1) known etiology for irregular menstruation like thyroid disorder, hyper prolactinoma, uterine pathology, or ovarian tumor; 2) patients on hormonal therapy, insulin sensitizers, vitamin D, calcium, or glucocorticoids. Women with similar profiles attending GOPD with complaints other than menstrual irregularities were recruited as controls.
The case records form was filled for all the participants, which included demographic details of the recruited patients. A detailed history was taken regarding their menstrual cycle length, amount of flow, pain associated with menses or any other associated pathology. A detailed medical, surgical, family, and personal history was taken, along with a dietary history (with special reference to the intake of calcium-rich food such as milk, cheese, paneer and dark green leafy vegetables), and drug history (e.g. hormonal supplementation, vitamin supplementation, metformin, glucocorticoids, beta-blockers).
A detailed general examination including body mass index (BMI) was calculated for each participant. About 5 ml of venous blood was withdrawn from the antecubital vein of participants in a plain red vacutainer and serum 25-hydroxy Vitamin D (25(OH)D) levels were measured. Vitamin D in the serum was measured using the ADVIA centaur vitamin D assay on the ADVIA Centaur XP system by competitive immunoassay, which used an anti-fluorescein monoclonal mouse antibody of 25(OH)D.
The inclusion criteria were: female patients of reproductive age attending Gynecology OPD, Aiims Raipur with complaints of menstrual irregularity (defined as a shortest to longest cycle variation of ≥ 8-10 days). Participants were divided into two groups: (i) frequent cycle (< 24 days interval); (ii) infrequent cycle (> 38 days). The exclusion criteria were: 1) known etiology for irregular menstruation like thyroid disorder, hyper prolactinoma, uterine pathology, or ovarian tumor; 2) patients on hormonal therapy, insulin sensitizers, vitamin D, calcium, or glucocorticoids. Women with similar profiles attending GOPD with complaints other than menstrual irregularities were recruited as controls.
The case records form was filled for all the participants, which included demographic details of the recruited patients. A detailed history was taken regarding their menstrual cycle length, amount of flow, pain associated with menses or any other associated pathology. A detailed medical, surgical, family, and personal history was taken, along with a dietary history (with special reference to the intake of calcium-rich food such as milk, cheese, paneer and dark green leafy vegetables), and drug history (e.g. hormonal supplementation, vitamin supplementation, metformin, glucocorticoids, beta-blockers).
A detailed general examination including body mass index (BMI) was calculated for each participant. About 5 ml of venous blood was withdrawn from the antecubital vein of participants in a plain red vacutainer and serum 25-hydroxy Vitamin D (25(OH)D) levels were measured. Vitamin D in the serum was measured using the ADVIA centaur vitamin D assay on the ADVIA Centaur XP system by competitive immunoassay, which used an anti-fluorescein monoclonal mouse antibody of 25(OH)D.
- 1. Ethical considerations
The study was started only after obtaining ethical approval from the Aiims Ethical Committee (Code: AIIMSRPR/IEC/2018/155) and obtaining informed consent from all the participants.
- 2. Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS, version 16.0 for Windows, SPSS Inc., Chicago, IL, USA). Continuous and categorical variables were expressed as mean (standard deviation) and frequency (%), respectively. 25(OH)D was structured both as a continuous linear variable and as a dichotomous variable of “insufficient” vs. “sufficient”, based on the World Health Organization’s cutoff of 20 ng/ml (15). Chi-square test was done to determine the difference in proportions of demographic data such as age, BMI, marital history, and parity. Student's t test was applied to compare the mean vitamin D levels between women with irregular vs. regular cycles and long vs. short cycles. Associations between 25(OH)D and regular and irregular cycles, age and BMI were estimated through logistic regression. Two-sided p-values were considered significant at p < 0.05.
- Results
Table I reflects the sociodemographic characteristics of the females in the two study group and it was observed that there no significant difference between the two groups in terms of age, BMI and marital status but there was significant difference in terms of parity in two groups (p = 0.02). Table II and figure 1 show that there was a significant difference (p < 0.001) in mean vitamin D level between those that had irregular cycles vs. regular cycles. The women who had regular cycles had significantly higher vitamin D levels than those with irregular cycles. There was no significant difference (p > 0.05) in the mean vitamin D level between the females who had infrequent cycles vs. those with frequent cycles.
Table III shows the associations between vitamin D levels and cycle regularity, age, and BMI. 86.7% of patients with menstrual irregularities were vitamin D deficient, i.e. they had a vitamin D level < 20 ng/ml, whereas only 31.0% of patients with normal menstruation were found to have vitamin D deficiency. Among patients with menstrual disorders who were vitamin D deficient, 83.1% of them presented with infrequent cycles, 16.9% had frequent cycles and 25.0% suffered from dysmenorrhoea. Binary logistic regression was applied. Decreased levels of vitamin D were associated with a 13.3 times higher odds of irregular cycles (odds ratio: 13.3 (confidence interval: 5.79, 30.6), p < 0.001). 25(OH)D levels were not associated with age or BMI.
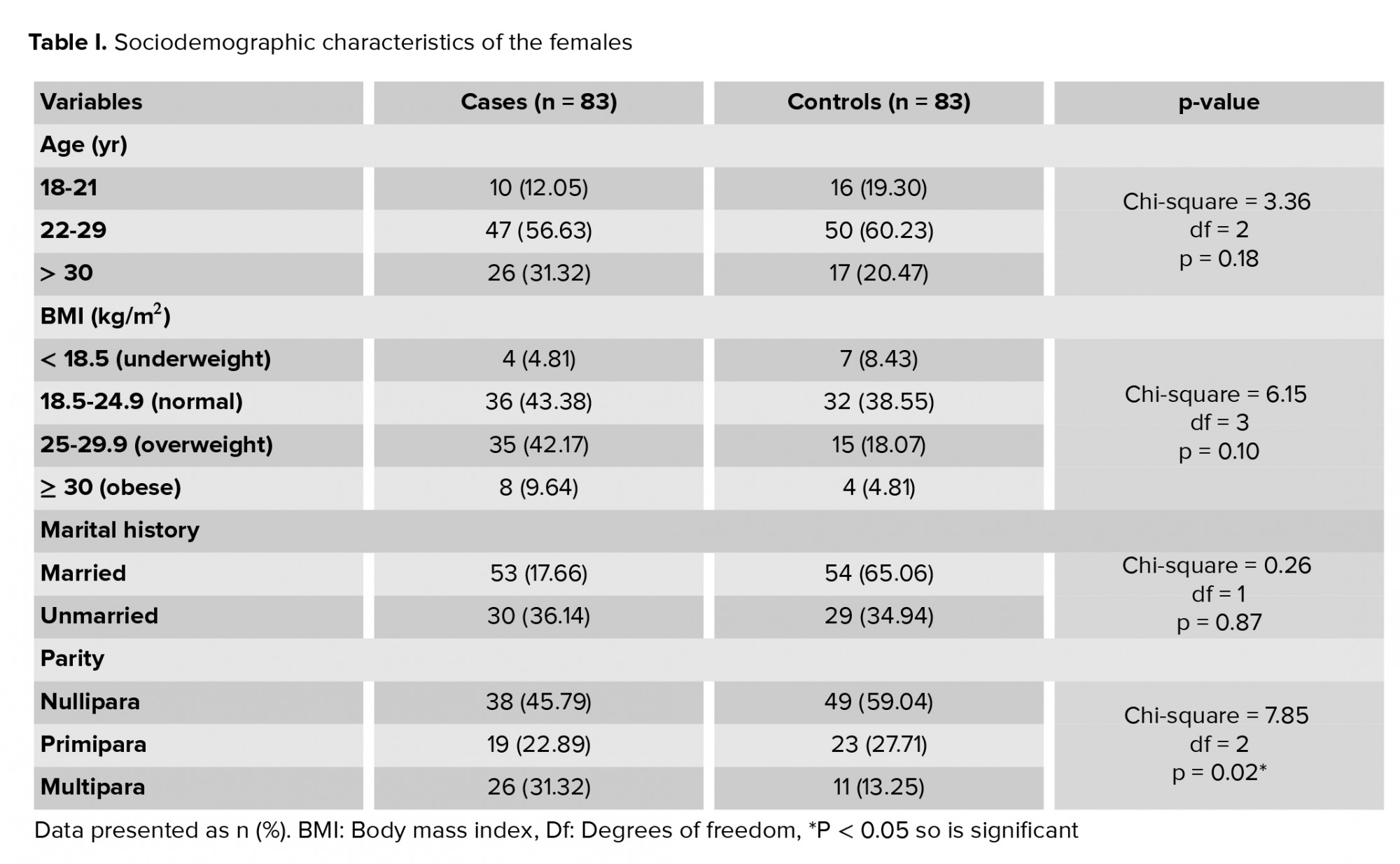
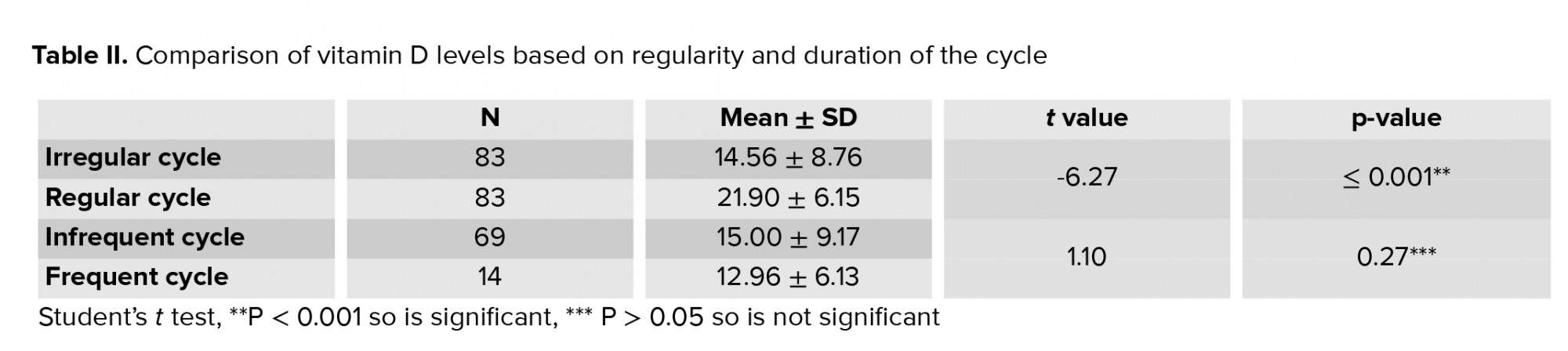
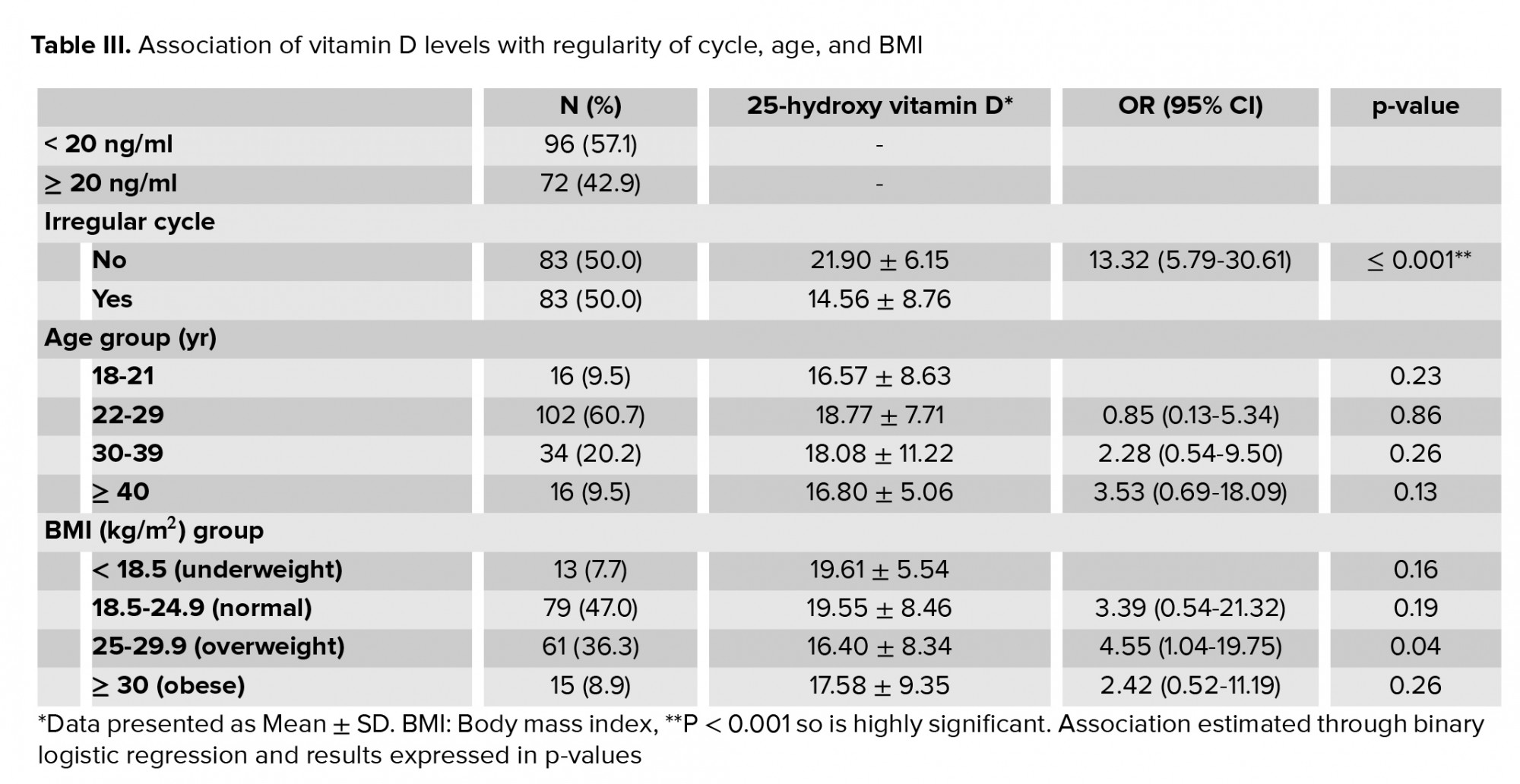

Table III shows the associations between vitamin D levels and cycle regularity, age, and BMI. 86.7% of patients with menstrual irregularities were vitamin D deficient, i.e. they had a vitamin D level < 20 ng/ml, whereas only 31.0% of patients with normal menstruation were found to have vitamin D deficiency. Among patients with menstrual disorders who were vitamin D deficient, 83.1% of them presented with infrequent cycles, 16.9% had frequent cycles and 25.0% suffered from dysmenorrhoea. Binary logistic regression was applied. Decreased levels of vitamin D were associated with a 13.3 times higher odds of irregular cycles (odds ratio: 13.3 (confidence interval: 5.79, 30.6), p < 0.001). 25(OH)D levels were not associated with age or BMI.




- Discussions
Our study’s results demonstrated a significant difference (p < 0.001) in mean vitamin D levels in females with irregular cycles vs. in those with regular cycles. The women with regular cycles had significantly higher vitamin D levels. A lower level of vitamin D was associated with 13.3 times odds of having an irregular cycle (OR (95% CI): 13.3 (5.79-30.6), p < 0.001).
Several other studies have similarly found that patients with PCOS were vitamin D deficient (4, 5, 16). In our study, there was no significant difference (p > 0.05) in mean vitamin D levels among the females with long cycles vs. those with short cycles. Previous similar studies have reported associations between lower levels of 25(OH)D and an increased odds of either irregular or long cycles (9).
Lower levels of 25(OH)D were associated with both long menstrual cycles and long follicular phases, and with a tendency towards short luteal phases. The strongest associations were for vitamin D deficiency (< 20 ng/ml). The probability of a long follicular phase and the probability of a short luteal phase both increased with decreasing 25(OH)D (17).
No difference was found between the PCOS group and control group, i.e. 80% vs. 70%, respectively, had vitamin D deficiency (p = 0.14) (18). In a cross-sectional study on reproductive age African American women it has been concluded that those with insufficient 25(OH)D levels were associated with a prolonged follicular phase leading to delayed ovulation and hence long menstrual cycles (but not short or irregular cycles) (9). Other studies have focused on supplementing vitamin D and calcium along with other drugs like metformin to improve ovulation and menstrual disorders in PCOS patients (19, 20). In our study, no association was seen between age or BMI and vitamin D levels, but there are studies in which subjects diagnosed with PCOS showed improvement in their complaints of infertility, BMI, and biochemical and metabolic disorders after vitamin D and calcium supplementation (13, 21). Several other studies along with one randomized, single-blind, placebo-controlled intervention study have found evidence of improvement in menstrual regularity after calcium and vitamin D supplementation along with metformin (p = 0.002) (4, 22). Dietary supplementation with vitamin D through fortified foods or intake of vitamin D sachets containing 60,000 units along with calcium in females with vitamin D deficiency could be an important step in improving their reproductive health (23, 24). Vitamin D deficiency is very common in women of reproductive age which may affect menstrual cycle length due to a pronged follicular phase causing delayed ovulation. This is an emerging field of research and hence further prospective studies with larger sample sizes and longer duration are needed to examine the role of vitamin D in reproduction specific to the menstrual cycle and fertility of reproductive age women, the underlying mechanisms, effective cutoff values, and exact dose of supplementation required (25). The current study had the limitation of a small sample size; low levels of vitamin D are generally found in the local population so to reach any conclusive evidence and provide recommendations we would require a large sample size with properly matched cases and controls from the population.
Several other studies have similarly found that patients with PCOS were vitamin D deficient (4, 5, 16). In our study, there was no significant difference (p > 0.05) in mean vitamin D levels among the females with long cycles vs. those with short cycles. Previous similar studies have reported associations between lower levels of 25(OH)D and an increased odds of either irregular or long cycles (9).
Lower levels of 25(OH)D were associated with both long menstrual cycles and long follicular phases, and with a tendency towards short luteal phases. The strongest associations were for vitamin D deficiency (< 20 ng/ml). The probability of a long follicular phase and the probability of a short luteal phase both increased with decreasing 25(OH)D (17).
No difference was found between the PCOS group and control group, i.e. 80% vs. 70%, respectively, had vitamin D deficiency (p = 0.14) (18). In a cross-sectional study on reproductive age African American women it has been concluded that those with insufficient 25(OH)D levels were associated with a prolonged follicular phase leading to delayed ovulation and hence long menstrual cycles (but not short or irregular cycles) (9). Other studies have focused on supplementing vitamin D and calcium along with other drugs like metformin to improve ovulation and menstrual disorders in PCOS patients (19, 20). In our study, no association was seen between age or BMI and vitamin D levels, but there are studies in which subjects diagnosed with PCOS showed improvement in their complaints of infertility, BMI, and biochemical and metabolic disorders after vitamin D and calcium supplementation (13, 21). Several other studies along with one randomized, single-blind, placebo-controlled intervention study have found evidence of improvement in menstrual regularity after calcium and vitamin D supplementation along with metformin (p = 0.002) (4, 22). Dietary supplementation with vitamin D through fortified foods or intake of vitamin D sachets containing 60,000 units along with calcium in females with vitamin D deficiency could be an important step in improving their reproductive health (23, 24). Vitamin D deficiency is very common in women of reproductive age which may affect menstrual cycle length due to a pronged follicular phase causing delayed ovulation. This is an emerging field of research and hence further prospective studies with larger sample sizes and longer duration are needed to examine the role of vitamin D in reproduction specific to the menstrual cycle and fertility of reproductive age women, the underlying mechanisms, effective cutoff values, and exact dose of supplementation required (25). The current study had the limitation of a small sample size; low levels of vitamin D are generally found in the local population so to reach any conclusive evidence and provide recommendations we would require a large sample size with properly matched cases and controls from the population.
- Conclusion
Menstrual irregularities in reproductive age women is a very common problem in our society and vitamin D deficiency is also highly prevalent. Other studies have concluded that the optimum blood level of vitamin D has a role in folliculogenesis, normal ovulation function and regular menstruation. This study was conducted to examine whether any association exists between deficient vitamin D levels and menstrual irregularities. Vitamin D is inexpensive and easily available with minimal side effects, so it can be considered for supplementation in women with menstrual irregularities. Further studies are recommended for reaching more conclusive results regarding the effectiveness of vitamin D supplementation for the correction of menstrual irregularities and in treatment of infertility, and its other benefits.
Acknowledgments
The authors sincerely acknowledge all the patients who consented, participated, and cooperated in this study. We also acknowledge our colleagues and juniors who helped in patient recruitment and data collection. There was no source of financial assistance for this study.
Conflict of Interest
There is no conflict of interest in the present study.
Acknowledgments
The authors sincerely acknowledge all the patients who consented, participated, and cooperated in this study. We also acknowledge our colleagues and juniors who helped in patient recruitment and data collection. There was no source of financial assistance for this study.
Conflict of Interest
There is no conflict of interest in the present study.
Type of Study: Original Article |
Subject:
Cellular and Molecular Biology of Reproduction
References
1. Wang H, Chen W, Li D, Yin X, Zhang X, Olsen N, et al. Vitamin D and chronic diseases. Aging Dis 2017; 8: 346-353. [DOI:10.14336/AD.2016.1021] [PMID] [PMCID]
2. de Almeida LF, Coimbra TM. Vitamin D actions on cell differentiation, proliferation and inflammation. Int J Complement Alt Med 2017; 6: 00201. [DOI:10.15406/ijcam.2017.06.00201]
3. Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil Steril 2014; 102: 460-468. [DOI:10.1016/j.fertnstert.2014.04.046] [PMID]
4. Li HWR, Brereton RE, Anderson RA, Wallace AM, Ho CKM. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism 2011; 60: 1475-1481. [DOI:10.1016/j.metabol.2011.03.002] [PMID]
5. Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: Secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab 2016; 101: 3027-3035. [DOI:10.1210/jc.2015-4352] [PMID] [PMCID]
6. Luk J, Torrealday S, Neal Perry G, Pal L. Relevance of vitamin D in reproduction. Hum Reprod 2012; 27: 3015-3027. [DOI:10.1093/humrep/des248] [PMID] [PMCID]
7. Jukic AMZ, Steiner AZ, Baird DD. Lower plasma 25-hydroxyvitamin D is associated with irregular menstrual cycles in a cross-sectional study. Reprod Biol Endocrinol 2015; 13: 20. [DOI:10.1186/s12958-015-0012-5] [PMID] [PMCID]
8. Moini A, Ebrahimi T, Shirzad N, Hosseini R, Radfar M, Bandarian F, et al. The effect of vitamin D on primary dysmenorrhea with vitamin D deficiency: A randomized doubleblind controlled clinical trial. Gynecol Endocrinol 2016; 32: 502-505. [DOI:10.3109/09513590.2015.1136617] [PMID]
9. Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: Results of a randomized, double-blind, placebo-controlled study. Arch Intern Med 2012; 172: 366-367. [DOI:10.1001/archinternmed.2011.715] [PMID]
10. Drakopoulos P, van de Vijver A, Schutyser V, Milatovic S, Anckaert E, Schiettecatte J, et al. The effect of serum vitamin D levels on ovarian reserve markers: A prospective cross-sectional study. Hum Reprod 2017; 32: 208-214. [DOI:10.1093/humrep/dew304]
11. Ghasemi Tehrani H, Mostajeran F, Shahsavari S. The effect of calcium and vitamin D supplementation on menstrual cycle, body mass index and hyperandrogenism state of women with poly cystic ovarian syndrome. J Res Med Sci 2014; 19: 875-880.
12. Dehghani Firouzabadi R, Aflatoonian A, Modarresi S, Sekhavat L, MohammadTaheri S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complement Ther Clin Pract 2012; 18: 85-88. [DOI:10.1016/j.ctcp.2012.01.005] [PMID]
13. Zarei S, Mohammad-Alizadeh-Charandabi S, Mirghafourvand M, Javadzadeh Y, Effati-Daryani F. Effect of calcium-vitamin D and calcium-alone on pain intensity and menstrual blood loss in women with primary dysmenorrhea: A randomized controlled trial. Pain Med 2016; 18: 3-13. [DOI:10.1093/pm/pnw121] [PMID]
14. Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab 2014; 99: E1137-E1145. [DOI:10.1210/jc.2013-4161] [PMID] [PMCID]
15. Anık A, Akbaba Ö. Vitamin D deficiency and insufficiency according to current criteria for children: Vitamin D status of elementary school children in Turkey. J Clin Res Pediatr Endocrinol 2019; 11: 327-328. [DOI:10.4274/jcrpe.galenos.2019.2019.0103] [PMID] [PMCID]
16. Chauhan R, Sahani S, Garg A. Evaluation of vitamin D3 in patients of polycystic ovary syndrome and their correlation. Int J Reprod Contracept Obstet Gynecol 2017; 6: 2010-2016. [DOI:10.18203/2320-1770.ijrcog20171967]
17. Jukic AMZ, Wilcox AJ, McConnaughey DR, Weinberg CR, Steiner AZ. 25-hydroxyvitamin D and long menstrual cycles in a prospective cohort study. Epidemiology 2018; 29: 388-396. [DOI:10.1097/EDE.0000000000000804] [PMID] [PMCID]
18. Figurová J, Dravecká I, Javorský M, Petríková J, Lazúrová I. Prevalence of vitamin D deficiency in Slovak women with polycystic ovary syndrome and its relation to metabolic and reproductive abnormalities. Wiener Klin Wochenschr 2016; 128: 641-648. [DOI:10.1007/s00508-015-0768-9] [PMID]
19. Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: A narrative review. Int J Clin Pract 2013; 67: 225-235. [DOI:10.1111/ijcp.12031] [PMID]
20. Thomson RL, Spedding S, Buckley JD. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2012; 77: 343-350. [DOI:10.1111/j.1365-2265.2012.04434.x] [PMID]
21. Showell MG, Brown J, Clarke J, Hart RJ. Antioxidants for female subfertility. Cochrane Database Syst Rev 2013; 8: CD007807. [DOI:10.1002/14651858.CD007807.pub2] [PMCID]
22. Gupta T, Rawat M, Gupta N, Arora S. Study of effect of vitamin D supplementation on the clinical, hormonal and metabolic profile of the PCOS women. J Obstet Gynecol India 2017; 67: 349-355. [DOI:10.1007/s13224-017-1008-1] [PMID] [PMCID]
23. Kadoura S, Alhalabi M, Nattouf AH. Effect of calcium and vitamin D supplements as an adjuvant therapy to metformin on menstrual cycle abnormalities, hormonal profile, and IGF-1 system in polycystic ovary syndrome patients: A randomized, placebo-controlled clinical trial. Adv Pharmacol Sci 2019; 2019: 9680390. [DOI:10.1155/2019/9680390] [PMID] [PMCID]
24. Lhamo Y, Chugh PK, Tripathi CD. Vitamin D supplements in the Indian market. Indian J Pharm Sci 2016; 78: 41-47. [DOI:10.4103/0250-474X.180248] [PMID] [PMCID]
25. Moridi I, Chen A, Tal O, Tal R. The association between vitamin D and anti-müllerian hormone: A systematic review and meta-analysis. Nutrients 2020; 12: 1567. [DOI:10.3390/nu12061567] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








