Fri, Jul 11, 2025
[Archive]
Volume 19, Issue 7 (July 2021)
IJRM 2021, 19(7): 607-618 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rahimi Asl F, Khosravi M, Hajikhani R, Solati J, Fahimi H. Complementary effects of coenzyme Q10 and Lepidium sativum supplementation on the reproductive function of mice: An experimental study. IJRM 2021; 19 (7) :607-618
URL: http://ijrm.ir/article-1-1963-en.html
URL: http://ijrm.ir/article-1-1963-en.html
1- Department of Biology, Islamic Azad University North Tehran Branch, Tehran, Iran.
2- Department of Biology, Faculty of Biological Sciences, Islamic Azad University North Tehran Branch, Tehran, Iran. ,maryam.khosravi@yahoo.com
3- Department of Biology, Faculty of Biological Sciences, Islamic Azad University North Tehran Branch, Tehran, Iran.
4- Department of Biology, Karaj Branch, Islamic Azad University, Karaj, Iran.
5- Department of Genetics, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
2- Department of Biology, Faculty of Biological Sciences, Islamic Azad University North Tehran Branch, Tehran, Iran. ,
3- Department of Biology, Faculty of Biological Sciences, Islamic Azad University North Tehran Branch, Tehran, Iran.
4- Department of Biology, Karaj Branch, Islamic Azad University, Karaj, Iran.
5- Department of Genetics, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
Full-Text [PDF 8053 kb]
(1026 Downloads)
| Abstract (HTML) (1968 Views)
Full-Text: (490 Views)
- Introduction
Chemical medicines used to treat infertility problems are usually followed by side effects, however, herbal medicines have shown to improve sperm quantity and quality as well as testicular function with low side effects (1-3). Lepidium sativum (LS), sometimes referred to as garden cress is a herbal medicine with anti-infertility activity (4). It is a fast-growing annual herb from the Brassicaceae family that can reach a height of 50 cm (5). LS seeds oil contains a considerable amount of sinapic acid and sinapine, which modulate sex steroids metabolism and act on male reproductive system to improve semen parameters and sperm function (6). LS extract are used in many countries for the treatment of various medical conditions including diabetes (7), hypertension (8), and renal diseases (9). Other species of Lepidium such as Lepidium meyenii has been reported to have improving effects on sperm count and motility and on sexual behavior in male animals (10, 11). It has been shown that plants in the Brassicaceae family can elevate sexual desire in healthy menopause women by acting on the hypothalamus-pituitary-gonad axis (12). Experimental data have revealed the improving effects of LS on the epididymis morphology in a diabetic rat model (4) and gonadotropin secretion in rabbits (10). Other key factors affecting male fertility are reactive oxygen species (ROS). Under normal physiologic conditions, a slight amount of ROS is produced by sperm cells. However, the excess production of ROS by sperms can cause DNA damage, motility decrease, and sperm membrane dysfunction (13). The balance between production and destruction of ROS is essential for sperm motility and male fertility (14) and hence, the medicines and nutrients that are able to improve this balance can improve male fertility. Antioxidants such as coenzyme Q10 (CoQ10) have been shown to inhibit ROS and prevent oxidative DNA damage (15, 16).
Although a large body of experimental studies have been carried out to investigate the antioxidant, antimicrobial, and anti-inflammatory characteristics of oil extracted from the seeds of LS, few studies have reported the anti-infertility effects of LS seed oil. There are also very few, if any, reports on the synergistic effects of LS seed extract and CoQ10 on improving the male reproductive system function. Therefore, this study was designed to determine the combined effects of LS and CoQ10 on the reproductive function in adult male NMRI mice and the findings will add new approach in the treatment of male infertility by using combined LS and CoQ10 administration.
Although a large body of experimental studies have been carried out to investigate the antioxidant, antimicrobial, and anti-inflammatory characteristics of oil extracted from the seeds of LS, few studies have reported the anti-infertility effects of LS seed oil. There are also very few, if any, reports on the synergistic effects of LS seed extract and CoQ10 on improving the male reproductive system function. Therefore, this study was designed to determine the combined effects of LS and CoQ10 on the reproductive function in adult male NMRI mice and the findings will add new approach in the treatment of male infertility by using combined LS and CoQ10 administration.
- Materials and Methods
- 1. Preparation of herbal extract
In this laboratory experimental study, to prepare the aqueous LS seed extract, the Moroccan traditional method was followed (17). Briefly, LS seeds were obtained from regional botanical shops, dried in the shade, and kept away from light (to prevent light-dependent reactions in seed cells) in closed containers until use. A mixture of 100-ml distilled water and 1 gr of powdered seeds was simmered for 10 min and left to be cooled for 15 min. It was then percolated by using a Millipore filter (Millipore 0.2, St. Quentin en Yvelines, France). The filtered extract was freeze-dried and the desired concentrations were prepared as fresh daily samples just before the treatment.
- 2. Drug
Purified CoQ10 powder (Pure bulk, US) was used in this study.
- 3. Animals and experimental design
Eighty 3-months-old NMRI male mice (30-35 gr) were obtained from the Pasture Institute (Tehran, Iran). The animals were maintained in standard cages under controlled temperature (20-22ºC), humidity (25-30%), and a 12-hr light/dark cycle, with ad libitum access to standard commercial food and freshwater. Mice were handled with humane care according to the guidelines for the care and use of laboratory animals. Anesthetized animals underwent surgery and were sacrificed after the period of treatment. According to the previous studies (18, 19), the animals were divided into the following groups of 10 mice in each group: control group (treated with water, 0.2 ml via gavage), CoQ10-treated groups (treated with 200, 300, and 400 mg/kg/body weight of CoQ10 via gavage), and LS-treated groups (treated with 200, 400, 600 mg/kg/body weight of LS seed extract via gavage). The treatments were performed daily for two wk and targeted second half and late phase of spermatogenesis. After obtaining the results, at this step, 600 mg/kg/body weight of LS seed extract and 200 mg/kg/body weight of CoQ10 exhibited the highest improving effects on studied indices, therefore, a group (treated with 600 mg/kg/body weight of LS seed extract + 200 mg/kg/body weight CoQ10 via gavage) was designed as combined group which received the treatment daily for two wk.
The evaluation of male sexual behavior was carried out using the method described by Schrader and Lemasters (20). In brief, sexual behavior (sniffing, following, mounting, and coupling) of male mice was evaluated two wk after the treatment by using receptive female mice in the male's home cage. The cage contained wood chips, food, and water. To prepare the host for male sexual behavior examination, the female mice were injected with estradiol benzoate (50 µg/kg) and progesterone (500 µg/kg) for three consecutive days and 6 hr before the test, respectively. For the study of sexual behavior, the control and the experimental groups were separately placed in a cage with a sexually experienced male and a receptive female. For each behavior, the delay in the onset of behavior and the number of sniffing, following, mounting, and coupling behaviors were measured for 60 min, and the results of the treatment group were compared with the control group. Behavioral parameters were videotaped at the time and analyzed after the experimental procedure had been completed.
- 5. Hormone assay
Serum levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone were determined in duplicate by using solid-phase enzyme-linked immunosorbent assay (ELISA) kits (Fine Test, China) with sensitivities of < 1.4 ml U/ml, < 0.3 ng/ml, and < 1.8 ng/ml, respectively. Intra-assay and inter-assay coefficients of variation were < 10%, according to the competitive binding principle and the manufacturer’s instructions.
- 6. Evaluation of epididymal sperm count
The animals were anesthetized with a combination of ketamine and xylazine, then the testis and epididymis were removed and analyzed under the light microscope (Olympus IX70, Tokyo). Epididymis spermatozoa were retrieved, diluted in 1 ml of normal saline, and incubated for 15 min (37ºC). The sperm quality was determined by Sperm Class Analyzer® (SCA) CASA system (HTF, Tehran, Iran) containing a bright field microscope (Nikon E-200, Japan), a digital camera (Canon, Japan), and a computer with SCA® software (Canon, USA). At least 400 sperm cells were captured and analyzed for each sample (21).
Mouse hypothalamus total RNA was extracted according to the manufacturer's protocol from freshly harvested tissue using the TRIzol reagent (Invitrogen, USA). Next, after being crushed physically by the sampler and chemically by 1 ml of TRIzol, the sample was mixed with 200 ul of chloroform, shaken vigorously for 30 sec, and then centrifuged at 15,000 g for 5 min at -10 to -7οC.
The upper aqueous phase containing DNA and RNA and with 200 ul of the volume was transferred to a sterile RNase-free tube. The total RNA was then precipitated by adding 500 ul of cold isopropanol, shaking for 5 min, and centrifuging at 1,200 g for 10 min at 4 to 8ºC, followed by two washing steps by 75% ethanol. After the evaporation of the alcohol, the pellet containing RNA was dissolved in distilled water. The RNA product and its purity were evaluated by spectrometry analysis. RNA electrophoresis on a 1% agarose gel was done to assure the absence of RNA degradation. After DNase treatment to remove genomic contamination, 2.0 μg of total RNA from each sample was reverse transcribed by using the First Strand cDNA Synthesis Kit (Fermentas, USA) according to manufacturer's instructions using random hexamer primers.
The mRNA expression of the GnRH gene was evaluated using real-time polymerase chain reaction (PCR) by SYBR Green Master Mix (Qiagen Fast Start Universal, SYBR Green Master Mix) under the following conditions: denaturation at 95°C for 10 sec, annealing at 60°C for 10 sec, and extension at 72°C for 20 sec with 40 repeated thermal cycles. PCR reactions were carried out in triplicate and the expression of all mRNAs was calculated using the LinReg PCR software to see relative changes in gene expression by using the β2-microglobulin (B2M) gene as an internal control because of its high stability. Table I shows the cDNA primers used in this experiment. The primers used in this study were synthesized by Sigma-Aldrich (St. Louis, MO, USA). The primers were evaluated using primer BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).

2. 8. Ethical considerations
This study was conducted in accordance with the guidelines of the Medical Ethics Committee on the use and care of laboratory animals. All investigations conformed to the ethical and humane principles of research and were approved by the research ethics committee of the Tehran Islamic Azad University of Medical Sciences (Code: IR.IAU.TMU.REC.1398.095).
2. 9. Statistical analysis
Data are presented as mean ± SEM and were analyzed using the unpaired t test and one-way analysis of variance followed by Tukey’s post hoc test. P-values < 0.05 were considered as statistically significant. Statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software Inc., San Diego, USA) and SPSS20 (SPSS Inc., Chicago, USA).
3. Results
3.1. Effect of LS on sexual behavior
To evaluate the sexual behavior of the experimental and control groups of mice, receptive female mice were placed in the males’ home cages. Our observation revealed that the numbers of sniffing (p = 0.0007, figure 1A) and following (p = 0.0010, figure 1B) behaviors were significantly higher in the LS-treated groups (400 and 600 mg/kg/body weight) than the control group, while there was no significant difference in the numbers of mounting (p = 0.3635, figure 1C) and coupling (p = 0.4178, Figure 1D) behaviors between the experimental and control groups.
3.2. Effect of CoQ10 on sexual behavior
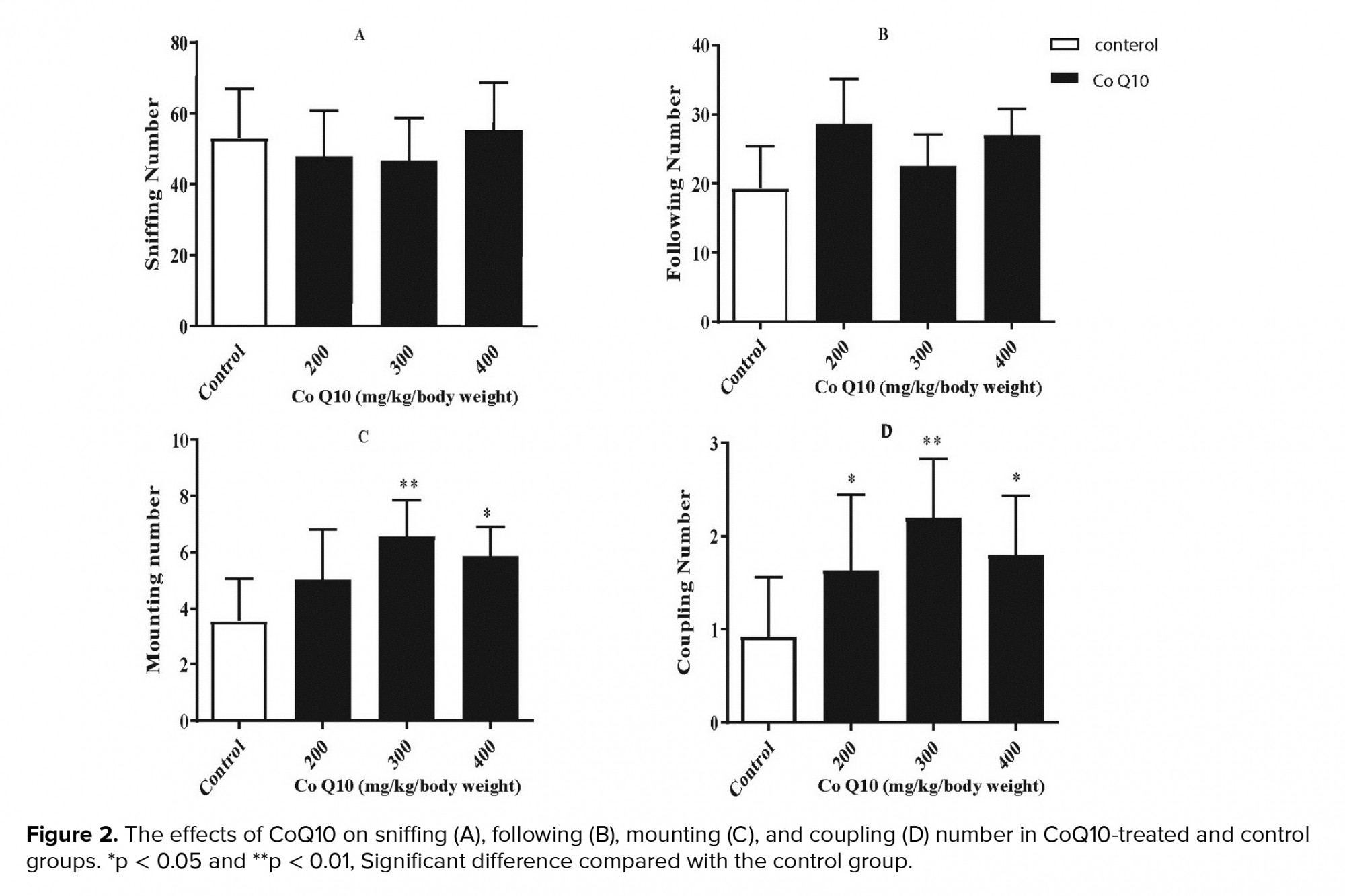
The numbers of sniffing (p = 0.6003, figure 2A) and following (p = 0.7602, figure 2B) behaviors did not show a significant difference compared to the control group. The monitoring of sexual behavior in the experimental and control groups showed that CoQ10-treated groups (300 and 400 mg/kg/body weight) exhibited significantly more frequent mounting (p = 0.017, figure 2C) and coupling (p = 0.0006, figure 2D) behaviors than the control group.
3.3. Combination effect of CoQ10 and LS on sexual behavior
Compared to the data obtained from the control group, the co-treatment of CoQ10 (200 mg/kg/body weight) and LS (600 mg/kg/body weight) caused a significant increase in all aspects of sexual behaviors: sniffing (p = 0.0022, figure 3A), following (p = 0.0082, figure 3B), mounting (p = 0.0124, figure 3C), and coupling (p = 0.0267, figure 3D) behaviors.
3.4. Co-treatment effect of CoQ10 and LS on the serum levels of testosterone, LH, and FSH
The mean values of serum testosterone (p = 0.0011), LH (p = 0.0062), and FSH (p = 0.0001) were revealed to be significantly higher for the co-treatment group than the control group (Figure 4).
3.5. Co-treatment effect of CoQ10 and LS on semen parameters
The co-treatment of CoQ10 and LS intensified sperm parameters. The percentage of sperm viability increased significantly compared to the pre-treatment sperm viability (p = 0.03). The total sperm motility increased significantly by 5% (p = 0.001) after the co-treatment. There was no significant difference in the total sperm count (p = 0.6532) and the number of healthy sperms between the control and co-treated groups (p = 0.7322) (Table II).
3.6. Expression analysis of GnRH gene
Based on the results obtained from real-time PCR data analysis, the co-treatment of CoQ10 (200 mg/kg/body weight) and LS (600 mg/kg/body weight) significantly increased the relative expression level of GnRH gene compared with the control group (p = 0.0016) (Figure 5).





4. Discussion
Our findings indicate that while LS treatment increases the sniffing and following behaviors, CoQ10 treatment enhances the mounting and coupling behaviors in mice. Interestingly, a co-treatment of CoQ10 and LS led to enhancement in all aspects of sexual behaviors, that is, sniffing, following, mounting, and coupling behaviors, as well as sperm quantity and quality accompanied by an increase in serum testosterone, LH, and FSH levels which was partly caused by increased GnRH gene expression level. The findings clearly show that CoQ10 with LS can prevent infertility or play a big role in male infertility treatment.
Indeed, male infertility accounts for 30-50% of infertility cases. Generally, male fertility depends on the quality, motility, and sperms morphology, therefore any disorder in these factors can lead to a dysfunction in reproductive system (2). In line with our findings, it has been shown that reduced antioxidant capacity caused by decreased cellular CoQ10 function or content can lead to an increase in free radicals’ adverse effects on sexual behavior and fertility (22). CoQ10 is an androgenic-soluble benzoquinone compound found throughout the body in cell membranes, especially in the mitochondrial membranes, and is abundant in the heart, lungs, and adrenal glands and has improving effects on male reproductive organs (23). CoQ10 induces antioxidant activity in rat testes (24). In addition, it has been reported that CoQ10 supplementation can boost fertility factors such as sperm count and motility (16) and elevates the sexual hormones levels and spermatogenesis in rats (25).
Many medicinal herbs have been traditionally administrated to treat infertility due to their potential beneficial effects on male fertility parameters (2). One strategy for the evaluation of couples with male infertility is the identification of sexual behavior parameters. LS is one of the most popular herbs used to elevate sexual pleasure and sexual behaviors (11). It has been reported to increase dopamine and acetylcholine levels and regulate gamma-aminobutyric acid (GABA) secretion in rats, both of which are related to sexual behavior (26). Moreover, LS increases the blood supply and consequently erection via nitric oxide synthesis induction in the endothelial cells (27). LS can also enhance sex steroid levels in male and female reproductive system (28). Other therapeutic effects of LS on the rat reproductive system such as spermatogenesis and serum LH level enhancement as well as its antioxidant activity in mice have been identified by recent investigations (11). In accordance with previous studies, our results showed that the treatment of mice with 600 mg/kg/body of LS significantly improves some aspects of sexual behavior in male mice.
We have shown that a co-administration of LS and CoQ10 has a complementary effect on all aspects of sexual behavior compared to LS (or CoQ10) alone which improved only some aspects - not all - of sexual behavior. The hypothalamus secretory-neural axis and hypothalamus-pituitary-gonads (HPG) axis play important roles in sexual behavior control and gametogenesis through GnRH, LH, FSH, and sexual steroid hormone production. Any dysfunctions in these axes can result in sexual desire disorder or infertility (29). Interestingly, our results revealed that co-treatment with CoQ10 and LS significantly induces the GnRH gene overexpression, which, in turn, elevates the FSH and LH levels. The elevated FSH and LH levels in turn causes an increase in serum testosterone level and sperm count, respectively. Our findings suggest that CoQ10 + LS supplementation has a strong regulatory influence on the HPG axis and stimulate the release of GnRH and LH from hypothalamus and pituitary gland, respectively. The results of the current study also suggest that the increases in motility and viability of sperms as well as sexual activity that followed co-treatment with CoQ10 and LS could be related to the changes in sex hormone levels. It has been shown that CoQ10 has beneficial effects on the reproductive hormones metabolism and secretion (30).
The fundamental effect of CoQ10 on sperm parameters may be mediated through multiple pathways. CoQ10 is concentrated within the mitochondrial midpiece of sperm and provides the energy for sperm movement (31). It can also have additive neuroprotective effects and reduces lipid peroxidation of the sperm membrane, which increases the membrane fluidity and motility (32). On the other hand, LS has antioxidant properties (8) and a positive impact on sexual behaviors and fertility (4, 12). Taken together, these facts suggest that LS and CoQ10 can synergistically increase spermatogenesis, sex hormones production and release, and antioxidant activity. Accordingly, all aspects of sexual behavior in male mice can be enhanced by co-administration of LS and CoQ10 via inducing sexual hormones secretions. However, the exact cellular and molecular basis for the improving effects of LS and CoQ10 on male reproductive system of male mice remains unknown and further research focusing on LS and CoQ10 effects on HPG axis and investigating the cellular and molecular mechanisms involved in LS and CoQ10 effects on testicular tissue as well as sperm cells will reveal many mysteries behind their action on target cells and tissues.
5. Conclusion
Our results demonstrate the synergistic effects of CoQ10 and LS supplementation on the semen parameters, sperm function, and sexual behaviors. The improving effects of LS and CoQ10 on male reproductive system are at least in part mediated by their regulatory effects on HPG axis via inducing the GnRH gene overexpression and increasing of LH, FSH, and testosterone production or secretion.
Acknowledgements
This research was supported by the Science and Research Branch, Islamic Azad University, Tehran, Iran.
Conflict of interest
The authors declare that they have no conflict of interest.
The upper aqueous phase containing DNA and RNA and with 200 ul of the volume was transferred to a sterile RNase-free tube. The total RNA was then precipitated by adding 500 ul of cold isopropanol, shaking for 5 min, and centrifuging at 1,200 g for 10 min at 4 to 8ºC, followed by two washing steps by 75% ethanol. After the evaporation of the alcohol, the pellet containing RNA was dissolved in distilled water. The RNA product and its purity were evaluated by spectrometry analysis. RNA electrophoresis on a 1% agarose gel was done to assure the absence of RNA degradation. After DNase treatment to remove genomic contamination, 2.0 μg of total RNA from each sample was reverse transcribed by using the First Strand cDNA Synthesis Kit (Fermentas, USA) according to manufacturer's instructions using random hexamer primers.
The mRNA expression of the GnRH gene was evaluated using real-time polymerase chain reaction (PCR) by SYBR Green Master Mix (Qiagen Fast Start Universal, SYBR Green Master Mix) under the following conditions: denaturation at 95°C for 10 sec, annealing at 60°C for 10 sec, and extension at 72°C for 20 sec with 40 repeated thermal cycles. PCR reactions were carried out in triplicate and the expression of all mRNAs was calculated using the LinReg PCR software to see relative changes in gene expression by using the β2-microglobulin (B2M) gene as an internal control because of its high stability. Table I shows the cDNA primers used in this experiment. The primers used in this study were synthesized by Sigma-Aldrich (St. Louis, MO, USA). The primers were evaluated using primer BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).

2. 8. Ethical considerations
This study was conducted in accordance with the guidelines of the Medical Ethics Committee on the use and care of laboratory animals. All investigations conformed to the ethical and humane principles of research and were approved by the research ethics committee of the Tehran Islamic Azad University of Medical Sciences (Code: IR.IAU.TMU.REC.1398.095).
2. 9. Statistical analysis
Data are presented as mean ± SEM and were analyzed using the unpaired t test and one-way analysis of variance followed by Tukey’s post hoc test. P-values < 0.05 were considered as statistically significant. Statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software Inc., San Diego, USA) and SPSS20 (SPSS Inc., Chicago, USA).
3. Results
3.1. Effect of LS on sexual behavior
To evaluate the sexual behavior of the experimental and control groups of mice, receptive female mice were placed in the males’ home cages. Our observation revealed that the numbers of sniffing (p = 0.0007, figure 1A) and following (p = 0.0010, figure 1B) behaviors were significantly higher in the LS-treated groups (400 and 600 mg/kg/body weight) than the control group, while there was no significant difference in the numbers of mounting (p = 0.3635, figure 1C) and coupling (p = 0.4178, Figure 1D) behaviors between the experimental and control groups.
3.2. Effect of CoQ10 on sexual behavior
The numbers of sniffing (p = 0.6003, figure 2A) and following (p = 0.7602, figure 2B) behaviors did not show a significant difference compared to the control group. The monitoring of sexual behavior in the experimental and control groups showed that CoQ10-treated groups (300 and 400 mg/kg/body weight) exhibited significantly more frequent mounting (p = 0.017, figure 2C) and coupling (p = 0.0006, figure 2D) behaviors than the control group.
3.3. Combination effect of CoQ10 and LS on sexual behavior
Compared to the data obtained from the control group, the co-treatment of CoQ10 (200 mg/kg/body weight) and LS (600 mg/kg/body weight) caused a significant increase in all aspects of sexual behaviors: sniffing (p = 0.0022, figure 3A), following (p = 0.0082, figure 3B), mounting (p = 0.0124, figure 3C), and coupling (p = 0.0267, figure 3D) behaviors.
3.4. Co-treatment effect of CoQ10 and LS on the serum levels of testosterone, LH, and FSH
The mean values of serum testosterone (p = 0.0011), LH (p = 0.0062), and FSH (p = 0.0001) were revealed to be significantly higher for the co-treatment group than the control group (Figure 4).
3.5. Co-treatment effect of CoQ10 and LS on semen parameters
The co-treatment of CoQ10 and LS intensified sperm parameters. The percentage of sperm viability increased significantly compared to the pre-treatment sperm viability (p = 0.03). The total sperm motility increased significantly by 5% (p = 0.001) after the co-treatment. There was no significant difference in the total sperm count (p = 0.6532) and the number of healthy sperms between the control and co-treated groups (p = 0.7322) (Table II).
3.6. Expression analysis of GnRH gene
Based on the results obtained from real-time PCR data analysis, the co-treatment of CoQ10 (200 mg/kg/body weight) and LS (600 mg/kg/body weight) significantly increased the relative expression level of GnRH gene compared with the control group (p = 0.0016) (Figure 5).






4. Discussion
Our findings indicate that while LS treatment increases the sniffing and following behaviors, CoQ10 treatment enhances the mounting and coupling behaviors in mice. Interestingly, a co-treatment of CoQ10 and LS led to enhancement in all aspects of sexual behaviors, that is, sniffing, following, mounting, and coupling behaviors, as well as sperm quantity and quality accompanied by an increase in serum testosterone, LH, and FSH levels which was partly caused by increased GnRH gene expression level. The findings clearly show that CoQ10 with LS can prevent infertility or play a big role in male infertility treatment.
Indeed, male infertility accounts for 30-50% of infertility cases. Generally, male fertility depends on the quality, motility, and sperms morphology, therefore any disorder in these factors can lead to a dysfunction in reproductive system (2). In line with our findings, it has been shown that reduced antioxidant capacity caused by decreased cellular CoQ10 function or content can lead to an increase in free radicals’ adverse effects on sexual behavior and fertility (22). CoQ10 is an androgenic-soluble benzoquinone compound found throughout the body in cell membranes, especially in the mitochondrial membranes, and is abundant in the heart, lungs, and adrenal glands and has improving effects on male reproductive organs (23). CoQ10 induces antioxidant activity in rat testes (24). In addition, it has been reported that CoQ10 supplementation can boost fertility factors such as sperm count and motility (16) and elevates the sexual hormones levels and spermatogenesis in rats (25).
Many medicinal herbs have been traditionally administrated to treat infertility due to their potential beneficial effects on male fertility parameters (2). One strategy for the evaluation of couples with male infertility is the identification of sexual behavior parameters. LS is one of the most popular herbs used to elevate sexual pleasure and sexual behaviors (11). It has been reported to increase dopamine and acetylcholine levels and regulate gamma-aminobutyric acid (GABA) secretion in rats, both of which are related to sexual behavior (26). Moreover, LS increases the blood supply and consequently erection via nitric oxide synthesis induction in the endothelial cells (27). LS can also enhance sex steroid levels in male and female reproductive system (28). Other therapeutic effects of LS on the rat reproductive system such as spermatogenesis and serum LH level enhancement as well as its antioxidant activity in mice have been identified by recent investigations (11). In accordance with previous studies, our results showed that the treatment of mice with 600 mg/kg/body of LS significantly improves some aspects of sexual behavior in male mice.
We have shown that a co-administration of LS and CoQ10 has a complementary effect on all aspects of sexual behavior compared to LS (or CoQ10) alone which improved only some aspects - not all - of sexual behavior. The hypothalamus secretory-neural axis and hypothalamus-pituitary-gonads (HPG) axis play important roles in sexual behavior control and gametogenesis through GnRH, LH, FSH, and sexual steroid hormone production. Any dysfunctions in these axes can result in sexual desire disorder or infertility (29). Interestingly, our results revealed that co-treatment with CoQ10 and LS significantly induces the GnRH gene overexpression, which, in turn, elevates the FSH and LH levels. The elevated FSH and LH levels in turn causes an increase in serum testosterone level and sperm count, respectively. Our findings suggest that CoQ10 + LS supplementation has a strong regulatory influence on the HPG axis and stimulate the release of GnRH and LH from hypothalamus and pituitary gland, respectively. The results of the current study also suggest that the increases in motility and viability of sperms as well as sexual activity that followed co-treatment with CoQ10 and LS could be related to the changes in sex hormone levels. It has been shown that CoQ10 has beneficial effects on the reproductive hormones metabolism and secretion (30).
The fundamental effect of CoQ10 on sperm parameters may be mediated through multiple pathways. CoQ10 is concentrated within the mitochondrial midpiece of sperm and provides the energy for sperm movement (31). It can also have additive neuroprotective effects and reduces lipid peroxidation of the sperm membrane, which increases the membrane fluidity and motility (32). On the other hand, LS has antioxidant properties (8) and a positive impact on sexual behaviors and fertility (4, 12). Taken together, these facts suggest that LS and CoQ10 can synergistically increase spermatogenesis, sex hormones production and release, and antioxidant activity. Accordingly, all aspects of sexual behavior in male mice can be enhanced by co-administration of LS and CoQ10 via inducing sexual hormones secretions. However, the exact cellular and molecular basis for the improving effects of LS and CoQ10 on male reproductive system of male mice remains unknown and further research focusing on LS and CoQ10 effects on HPG axis and investigating the cellular and molecular mechanisms involved in LS and CoQ10 effects on testicular tissue as well as sperm cells will reveal many mysteries behind their action on target cells and tissues.
5. Conclusion
Our results demonstrate the synergistic effects of CoQ10 and LS supplementation on the semen parameters, sperm function, and sexual behaviors. The improving effects of LS and CoQ10 on male reproductive system are at least in part mediated by their regulatory effects on HPG axis via inducing the GnRH gene overexpression and increasing of LH, FSH, and testosterone production or secretion.
Acknowledgements
This research was supported by the Science and Research Branch, Islamic Azad University, Tehran, Iran.
Conflict of interest
The authors declare that they have no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Biology
References
1. Choy JT, Eisenberg ML. Male infertility as a window to health. Fertil Steril 2018; 110: 810-814. [DOI:10.1016/j.fertnstert.2018.08.015] [PMID]
2. Barratt ChLR, Björndahl L, De Jonge ChJ, Lamb DJ, Osorio Martini F, McLachlan R, et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum Reprod Update 2017; 23: 660-680. [DOI:10.1093/humupd/dmx021] [PMID] [PMCID]
3. Oyeyemi MO, Olukole SG, Esan O. Sperm morphological studies of West African Dwarf Bucks treated with pumpkin plant (Cucurbita pepo). Int J Morphol 2008; 26: 121-126. [DOI:10.4067/S0717-95022008000100020]
4. Imade OV, Erinfolami WA, Ajadi RA, Abioja MO, Rahman SA, Smith OF, et al. Effects of Lepidium sativum supplementation on growth and gonadotropins secretion in ovariectomized, estrogen-implanted rabbits. Asian Pac J Reprod 2018; 7: 155-160. [DOI:10.4103/2305-0500.237052]
5. Kamani M, Seyed Hosseini E, Haddad Kashani H, Atlasi MA, Nikzad H. Protective effect of Lepidium sativum seed extract on histopathology and morphology of epididymis in the diabetic rat model. Int J Morphol 2017; 35: 603-610. [DOI:10.4067/S0717-95022017000200036]
6. Gonzales GF, Cordova A, Gonzales C, Chung A, Vega K, Villena A. Lepidium meyenii (Maca) improved semen parameters in adult men. Asian J Androl 2001; 3: 301-303.
7. Rehman NU, Khan A, Alkharfy KhM, Gilani AH. Pharmacological basis for the medicinal use of Lepidium sativum in airways disorders. J Evidence-Based Complement Altern Med 2012; 2012: 596524. [DOI:10.1155/2012/596524] [PMID] [PMCID]
8. Patel U, Kulkarni M, Undale V, Bhosale A. Evaluation of diuretic activity of aqueous and methanol extracts of Lepidium sativum garden cress (Cruciferae) in rats. Trop J Pharm Res 2009; 8: 215-219. [DOI:10.4314/tjpr.v8i3.44536]
9. Gonzales GF, Ruiz A, Gonzales C, Villegas L, Cordova A. Effect of Lepidium meyenii (maca) roots on spermatogenesis of male rats. Asian J Androl 2001; 3: 231-233.
10. Abdallah HM, Farag MA, Algandaby MM, Nasrullah MZ, Abdel-Naim AB, Eid BG, et al. Osteoprotective activity and metabolite fingerprint via UPLC/MS and GC/MS of Lepidium sativum in ovariectomized rats. Nutrients 2020; 12: 2075. 1-20. [DOI:10.3390/nu12072075] [PMID] [PMCID]
11. Uchiyama F, Jikyo T, Takeda R, Ogata M. Lepidium meyenii (Maca) enhances the serum levels of luteinising hormone in female rats. J Ethnopharmacol 2014; 151: 897-902. [DOI:10.1016/j.jep.2013.11.058] [PMID]
12. Aoki Y, Tsujimura A, Nagashima Y, Hiramatsu I, Uesaka Y, Nozaki T, et al. Effect of Lepidium meyenii on in vitro fertilization via improvement in acrosome reaction and motility of mouse and human sperm. Reprod Med Biol 2018; 18: 57-64. [DOI:10.1002/rmb2.12251] [PMID] [PMCID]
13. Duru NK, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril 2000; 74: 1200-1207. [DOI:10.1016/S0015-0282(00)01591-0]
14. Henkel RR. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J Androl 2011; 13: 43-52. [DOI:10.1038/aja.2010.76] [PMID] [PMCID]
15. Asgari S, Ansari Samani R, Deris F, Shahinfard N, Salimi M, Mortazaei S, et al. Antioxidant activity and the lowering effect of hydroalcoholic extract of Allium hirtifolium boisson some haemostatic factors in hypercholesterolemic rabbits. J Mazandaran Univ Med Sci 2012; 22: 40-48.
16. Lafuente R, González-Comadrán M, Solà I, López G, Brassesco M, Carreras R, et al. Coenzyme Q10 and male infertility: a meta-analysis. J Assist Reprod Genet 2013; 30: 1147-1156. [DOI:10.1007/s10815-013-0047-5] [PMID] [PMCID]
17. Eddouks M, Maghrani M, Zeggwagh NA, Michel J. Study of the hypoglycaemic activity of Lepidium sativum L. aqueous extract in normal and diabetic rats. J Ethnopharmacol 2005; 97: 391-395. [DOI:10.1016/j.jep.2004.11.030] [PMID]
18. Victoria IO, Fedrick SO, Oladele G, Oluwafemi AE, Atiang BJ. Effects of Lepidium sativum seed on reproductive characteristics in rabbit bucks. J Phytopharmacol 2020; 9: 89-95. [DOI:10.31254/phyto.2020.9203]
19. Abdulidha NA, Jaccob AA, AL-Moziel MS. Protective effects of Co-Q10, Ginkgo biloba, and l-carnitine on brain, kidney, liver, and endocrine system against sub-acute heavy metals toxicity in male rats. Toxicol Environ Health Sci 2020; 12: 331-341. [DOI:10.1007/s13530-020-00061-7]
20. Working PK. Male reproductive toxicology: Comparison of the human to animal models. Environmental Health Perspectives 1988; 77: 37-44. [DOI:10.1289/ehp.887737] [PMID] [PMCID]
21. Stachecki JJ, Ginsburg KA, Leach RE, Armant DR. Computer-assisted semen analysis (CASA) of epididymal sperm from the domestic cat. Asian J Androl 1993; 14: 60-65.
22. Jovanović Z. [Oxidative stress in the pathogenesis of neurodegenerative diseases]. Med Čas 2011; 45: 16-23. (in Bosnian) [DOI:10.5937/medcas1101016J]
23. Aly NH. Reno-protective efficiency of coenzyme Q10 on adriamycin-induced nephrotoxicity in rats. J Appl Sci Res 2012; 8: 589-597.
24. Palmeira CM, Santos DL, Seiça R, Moreno AJ, Santos MS. Enhanced mitochondrial testicular antioxidant capacity in Goto-Kakizaki diabetic rats: Role of coenzyme Q. Am J Physiol Cell Physiol 2001; 281: C1023-C1028. [DOI:10.1152/ajpcell.2001.281.3.C1023] [PMID]
25. Ghanbarzadeh S, Garjani A, Ziaee M, Khorrami A. Effects of L-carnitine and coenzyme q10 on impaired spermatogenesis caused by isoproterenol in male rats. Drug Res 2014; 64: 449-453. [DOI:10.1055/s-0033-1361103] [PMID]
26. Mali RG, Mahajan SG, Mehta AA. Lepidium sativum (Garden cress): A review of contemporary literature and medicinal properties. Oriental Pharm Exp Med 2007; 7: 331-335. [DOI:10.3742/OPEM.2007.7.4.331]
27. Chauhan NS, Sharma V, Dixit VK, Thakur M. A review on plants used for improvement of sexual performance and virility. BioMed Res Int 2014; 2014: 868062. 1-20. [DOI:10.1155/2014/868062] [PMID] [PMCID]
28. Gonzales GF. Ethnobiology and ethnopharmacology of Lepidium meyenii (Maca), a plant from the Peruvian highlands. Evid Based Complement Altern Med 2012; 2012: 193496. 1-10. [DOI:10.1155/2012/193496] [PMID] [PMCID]
29. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002; 53: 865-871. [DOI:10.1016/S0022-3999(02)00429-4]
30. Banihani SA. Effect of coenzyme q10 supplementation on testosterone. Biomolecules 2018; 8: 172. 1-9. [DOI:10.3390/biom8040172] [PMID] [PMCID]
31. Tiseo BC, Gaskins AJ, Hauser R, Chavarro JE, Tanrikut C, Team ES. Coenzyme Q10 intake from food and semen parameters in a subfertile population. Urology 2017; 102: 100-105. [DOI:10.1016/j.urology.2016.11.022] [PMID] [PMCID]
32. Xu Y, Nisenblat V, Lu C, Li R, Qiao J, Zhen X, et al. Pretreatment with coenzyme Q10 improves ovarian response and embryo quality in low-prognosis young women with decreased ovarian reserve: A randomized controlled trial. Reprod Biol Endocrinol 2018; 16: 29. 1-11. [DOI:10.1186/s12958-018-0343-0] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








