Thu, Feb 19, 2026
[Archive]
Volume 20, Issue 1 (January 2022)
IJRM 2022, 20(1): 29-36 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Keikha F, Shahrokh Tehraninejad E, Rakhshkhorshid M, Afiat M, Hagholahi F, Ghasemi F. The association of the betatrophin level with metabolic and inflammatory parameters in infertile women with polycystic ovary syndrome: A case-control study. IJRM 2022; 20 (1) :29-36
URL: http://ijrm.ir/article-1-2045-en.html
URL: http://ijrm.ir/article-1-2045-en.html
Fatemeh Keikha1 

 , Ensieh Shahrokh Tehraninejad1
, Ensieh Shahrokh Tehraninejad1 

 , Marzieh Rakhshkhorshid2
, Marzieh Rakhshkhorshid2 

 , Malihe Afiat *3
, Malihe Afiat *3 

 , Fedyeh Hagholahi1
, Fedyeh Hagholahi1 

 , Fatemeh Ghasemi1
, Fatemeh Ghasemi1 




 , Ensieh Shahrokh Tehraninejad1
, Ensieh Shahrokh Tehraninejad1 

 , Marzieh Rakhshkhorshid2
, Marzieh Rakhshkhorshid2 

 , Malihe Afiat *3
, Malihe Afiat *3 

 , Fedyeh Hagholahi1
, Fedyeh Hagholahi1 

 , Fatemeh Ghasemi1
, Fatemeh Ghasemi1 


1- Health Reproductive Research Center, Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Midwifery, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Milad Infertility Center, Department of Obstetrics and Gynecology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. ,AfiatM@mums.ac.ir
2- Department of Midwifery, School of Nursing and Midwifery, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Milad Infertility Center, Department of Obstetrics and Gynecology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. ,
Full-Text [PDF 286 kb]
(1559 Downloads)
| Abstract (HTML) (2200 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is a common hormonal health problem that occurs in 5-10% of women of reproductive age (1). Ovulatory dysfunction and hyperandrogenism are two features of PCOS (2). It is hypothesized that the syndrome can be due to several metabolic disorders including low-grade chronic inflammation, resistance to insulin, metabolic syndrome, obesity, hyperlipidemia, glucose intolerance, hypertension, and/or an elevation of the risk of Type 2 diabetes (3).
Two of the most significant and highly prevalent parameters among women with PCOS are insulin resistance and metabolic disorders (4). As a consequence of resistance to insulin, betatrophin levels are expected to vary in the serum as well as in other biological compartments (5). Betatrophin is a peptide hormone derived from liver and adipose tissue (3). This protein regulates glucose homeostasis and lipid metabolism (6). An association between circulating betatrophin levels, Type 1 diabetes, and PCOS has been reported (5); however, the pathogenesis effects of betatrophin on the secretion of insulin and homeostasis of glucose are not fully known (2).
Recent clinical studies have confirmed a positive association between circulating betatrophin levels and serum lipid levels in participants with impaired glucose tolerance, participants with Type 2 diabetes, and healthy individuals. Furthermore, “it has been suggested that betatrophin may be associated with circulating low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, and triglycerides (TG), probably via different mechanisms” (7). A study was conducted to find the relationship between betatrophin levels and anthropometric, hormonal, and metabolic parameters in women with PCOS with a body mass index (BMI) < 25 kg/m2 who were glucose tolerant. The researchers studied 50 of such women using the Rotterdam criteria, and 60 healthy controls who were age and BMI matched and did not present any feature of clinical or biochemical hyperandrogenism. The study confirmed that serum betatrophin levels were significantly higher in glucose tolerant women with PCOS who had a BMI < 25 kg/m2 than in the control group (8).
The results of another study with the aim of investigating betatrophin serum levels in participants with newly-diagnosed PCOS and their influence demonstrated a positive correlation between the serum betatrophin levels in women with PCOS and their fasting blood glucose, TG, BMI, and waist-to-hip ratio (p = 0.01). However, serum betatrophin levels were uncorrelated with the fasting insulin and homeostasis model assessment of insulin resistance (HOMA-IR) (p = 0.05). Multiple stepwise regression analysis showed that the TG level was an independent influence of the serum betatrophin level. However, correlations mentioned above were not found in the control group (9). Many studies have examined the association between betatrophin and metabolic and inflammatory parameters and how they are linked to metabolic diseases (2, 3, 10).
In this study, we aimed to investigate the betatrophin level and its association with metabolic and inflammatory parameters in infertile women with and without PCOS during the intrauterine insemination (IUI) cycle.
2. Materials and Methods
This case-control study was conducted in the infertility clinic of Imam Khomeini Hospital in Tehran, Iran from September 2017 to November 2018.
45 infertile women with a diagnosis of PCOS and 45 infertile women with regular menstruation and with other causes of infertility (idiopathic or male factor) chosen by convenience sampling were included in the study. The participants entered the course of IUI. PCOS diagnosis was determined according to the Rotterdam PCOS Consensus criteria that require at least the presence of two of the following criteria: 1) ovulation dysfunction (anovulation or sporadic ovulation); 2) hyperandrogenism (clinical or biochemical); and 3) polycystic ovaries present on ultrasound (15 follicles 2-10 mm in diameter in either ovary) (11). The other inclusion criteria were: age of 20-40 yr; BMI < 35 kg/m2; at least one tubal patency; and normal levels of thyroid stimulating hormone and prolactin. Women with any underlying disease (e.g., diabetes, hypertension, cardiac or hepatorenal disease, chronic inflammatory disease, etc.), women who had had an infection in the previous two weeks, women taking oral contraceptive pills, antiandrogens or other hormonal drugs in the last month, and women who use recreational drugs, smoke or drink alcohol were excluded.
Participants were interviewed to obtain age and reproductive history, including gravidity, parity, abortion, ectopic pregnancy, menstruation pattern, infertility duration, and stimulation duration. The height and weight of each participant were measured using a digital bathroom scale (Omron Company, Japan) and a SECA stadiometer (SECA Instruments Ltd, Germany). The measurements were recorded to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated by dividing weight (in kilograms) by height squared (in meters) while the participants were weighted barefooted and in light clothing. Fasting brachial venous blood samples were taken to measure the levels of betatrophin, fasting blood sugar (FBS), insulin, luteinizing hormone (LH), follicle-stimulating hormone (FSH), LDL-C, estradiol, and high-sensitivity C-reactive protein (hs-CRP). The participants were on the 3rd day of their menstrual cycle at the time of the blood test. The collected blood was centrifuged at 4000 × g for 10 min right after the test and stored at -80°C until the analysis. To find the amounts of LH, FSH, hs-CRP, FBS, and estradiol, automated chemiluminescence immunoassay systems were used (BT 1500 Biotecnica instruments SPA, Italy). The Friedewald formula (12) and the enzyme-linked immunosorbent assay (ELISA) technique were used for the calculation of LDL-C and hs-CRP, respectively. The intra-assay coefficient of about 5% and inter-assay coefficient of about 10% were determined for each factor. To evaluate the HOMA index, fasting insulin and glucose were used. Insulin resistance was calculated by glucose (mmol/L) multiplied by fasting insulin (mU/L)/22.5 (HOMA-IR formula). The chemiluminescence immunometric assay and a commercial kit (Immulite 2000 Analyzer; CPC, USA) were used to measure fasting insulin. To measure fasting glucose, a glucose oxidase assay (Tosoh Corp., Tosoh, Japan) was used.
ELISA kits (EIAab Science, Wuhan, China; Catalogue No. E11644h) were used to assess fasting serum betatrophin levels following the manufacturer’s instructions. The tests were performed in duplicate, and samples with more than 5% coefficient of variation were excluded.
Every participant in the case or control group was stimulated to ovulate with two clomiphene citrate tablets (Ovumid 50, Iran Hormone Pharmaceutical Co, Tehran, Iran) starting from the 3rd day of their menstrual cycle. On the 8th day of the cycle, 150 units of human menopausal gonadotropin (karma-HMG 75 u, Pharmatech GmbH, Germany) were administered. Following that, serial transvaginal sonography (Philips ultrasound, affinity 70, Japan) was performed until the dominant follicle reached a diameter of 18-20 mm. Subsequently, 10,000 units of human chorionic gonadotropin (karma-HCG 5000 u, Pharmatech GmbH, Germany) were prescribed and 36 hr later, participants were subjected to IUI. Two wk later, the level of beta-human chorionic gonadotropin was checked (MPR-404, ELISAMicroplate Reader, Roedermark, Germany) and, if positive, an ultrasound was performed to evaluate the pregnancy and observe the fetal heart rate.
2.1. Ethical considerations
The study was approved by the Ethics Committee of the Tehran University of Medical Sciences (Code: IR.TUMS.VCR.REC.1396.2259). Written informed consent was obtained from all participants before the initiation of the study.
2.2. Statistical analysis
SPSS (version 25, Armonk, NY, IBM Corp.), Statistical Package for the Social Sciences, were used to analyse the data. The Shapiro-Wilk test was used to test the distribution of the data. Data are presented as mean ± SD or median (interquartile range). An independent t test and a Chi-square test were used to assess between-group differences for continuous variables and categorical variables, respectively. In order to compare group medians for data with non-normal distributions the Mann-Whitney U test was used. Spearman’s Rank Correlation Coefficient was used to analyse Bivariate relations between betatrophin levels and covariates. The determinants of betatrophin (independent variables: BMI, FSH, LH, LDL-C, hs-CRP, FBS, insulin, and HOMA-IR) were evaluated separately using multiple linear regression. Furthermore, the effect of betatrophin and HOMA-IR on pregnancy was investigated using logistic regression.
3. Results
There were 45 participants in each group. Four participants from the control group withdrew from the study before its completion; they did not return after administration of clomiphene citrate for treatment. Age, reproductive history, FSH, LH, LDL-C, hs-CRP, FBS, and HOMA-IR were similar between the groups. As expected, women with PCOS had a higher mean BMI than the control group. Clinical and hormonal details of the participants in the case and control groups are shown in table I.
The results of the Chi-square test showed that 73.3% (33 people) of the case group had an irregular pattern of menstruation, while no participants in the control group had an irregular pattern of menstruation (p ≤ 0.001). Correlations between betatrophin concentration and clinical, metabolic, and inflammatory parameters of the participants are shown in table II.
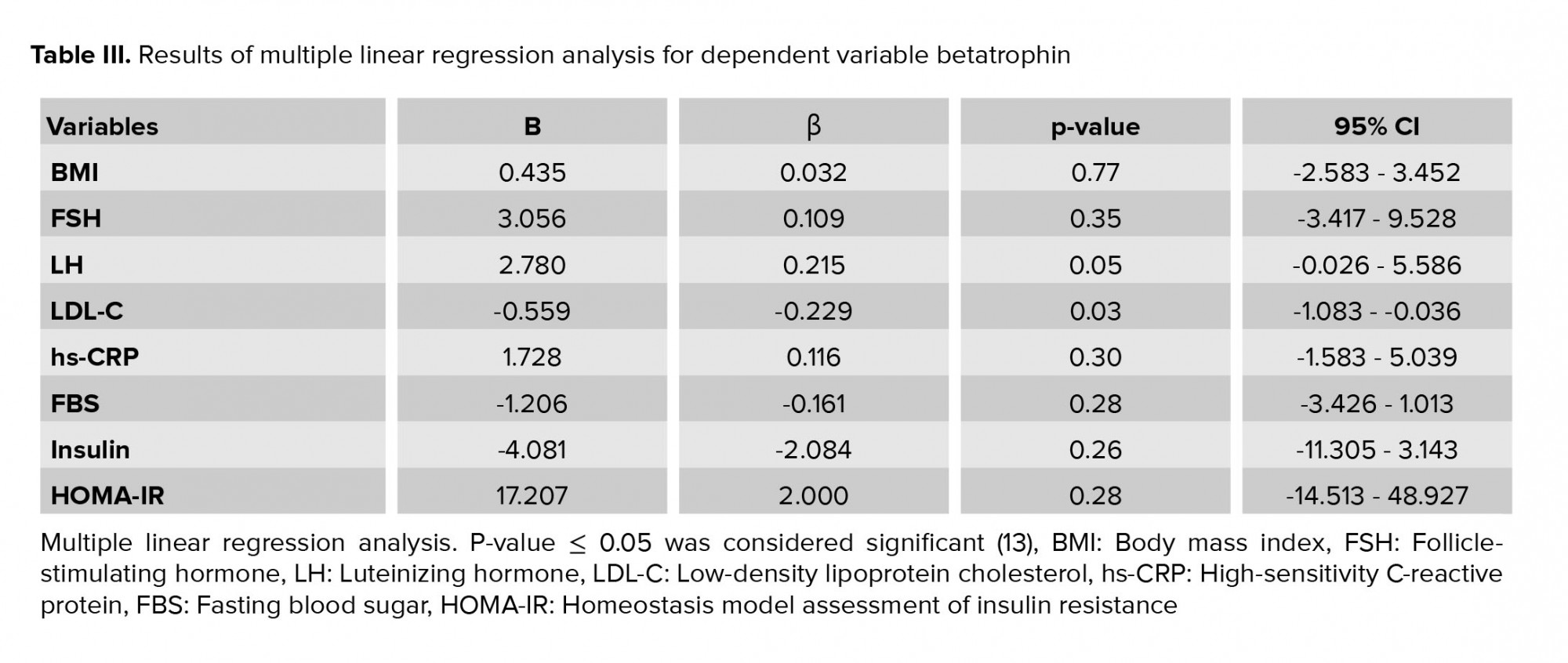
Based on multiple linear regression analyses, the effects of BMI, FSH, LH, LDL-C, hs-CRP, FBS, insulin, and HOMA-IR on betatrophin were not significant (Table III). The results of the independent t test showed no significant difference between groups in folliculogenesis (p = 0.57). The results of the Chi-square test showed that gestational rates were 20.0% and 31.7% in the case and control groups, respectively; this difference was not statistically significant (p = 0.21). Based on the logistic regression model, betatrophin and HOMA-IR did not predict pregnancy.


4. Discussion
Our results showed that the level of betatrophin in women with PCOS was significantly higher than in the control group. In the present study assessing the association of metabolic parameters such as the lipid profiles with betatrophin levels, it is found that amongst lipid profiles only lower LDL-C levels were associated with betatrophin levels in women with PCOS. In addition, the results of the multiple linear regression analysis did not show any effect of any of the inflammatory or metabolic parameters on the betatrophin level.
Betatrophin is a peptide that is produced in the liver as well as in brown and white adipose tissue and it regulates the function of pancreatic beta cells and fat production (5). The results of studies on betatrophin vary. Some studies have shown an increase in betatrophin levels in women with PCOS (3, 14, 15). However, the results of one study showed that betatrophin levels were lower in women with PCOS than in the control group. They also found a significant negative correlation between betatrophin levels, and insulin, BMI and HOMA-IR. The results of their study showed that the only factor affecting beta-dopamine reduction was PCOS (2). Another study found that betatrophin concentration was lower in women with PCOS than in the control group. Their results showed that there was a positive correlation between betatrophin concentration and LDL-C in women with PCOS whose betatrophin concentrations were higher than the cut-off (464.5 ng/l) (6). The results of these studies are not in line with our study.
The results of a study on the association of betatrophin levels with PCOS showed a correlation between PCOS and fasting insulin levels as well as a significant correlation between the levels of betatrophin and fasting insulin levels and HOMA-IR in women with PCOS (14). A negative correlation between serum betatrophin levels and BMI, fasting insulin, and HOMA-IR was demonstrated in another study (15). The present study did not show such correlations. This difference in results may be due to differences in laboratory kits used, ages ethnicities, or sample size.
In our study, women with PCOS had a higher mean BMI than the control group; but, no significant correlation between betatrophin and BMI was found. The results of a recent study done on obese populations was not in line with this study (16). Also some contradictory results regarding the association of betatrophin levels and obesity have been reported (17, 18). The results of a cross-sectional study showed that there was a significant positive correlation between betatrophin levels and BMI in women with PCOS (3), while the results of a case-control study showed the opposite (14). Another study aiming to investigate blood levels of betatrophin in obese children with non-alcoholic fatty liver disease reported no statistically significant difference between the control and experimental groups (19).
This study showed significantly higher levels of fasting insulin in women with PCOS comparing to the control group. This indicates that women with PCOS are insulin resistant. About 80% of women with PCOS have insulin resistance. Betatrophin is produced as a result of insulin resistance (14). The findings of some studies vary regarding the association of betatrophin levels with diabetes (17, 18, 20). In our study, we did not find a significant correlation between the levels of betatrophin and fasting insulin levels. One study showed a significant negative correlation between betatrophin levels and fasting insulin levels in women with PCOS (21), while another showed the opposite (14). The differences in race and lifestyle may have influenced the results of these studies.
Betatrophin has been linked to lipid metabolism changes. But studies’ findings are conflicting. Betatrophin affects the lipid profile by regulating the secretion of very low-density lipoproteins from the liver and inhibiting lipoprotein lipase activity (22). Chinese researchers have reported that there is a positive correlation between betatrophin and TG (23). In another study, the results showed a negative correlation between circulating betatrophin levels and TG, but a positive correlation with high-density lipoprotein cholesterol (18). Furthermore, another study found no association between betatrophin levels and improved fertility outcomes in participants (3).
The results of the present study showed a significant negative correlation between the level of betatrophin and LDL-C in women with PCOS; therefore, it can be concluded that as the level of betatrophin increases, the level of LDL-C decreases. The results of this study were not in line with a cross-sectional study on PCOS women which showed a significant positive correlation between betatrophin and LDL-C levels (3). PCOS is a disease associated with mild chronic inflammation. However, in this study, there was no significant relationship found between betatrophin and inflammatory parameters such as hs-CRP.
One of the limitations of the present study was the small sample size and the attrition in the control group. Also, in the evaluation of insulin resistance, a non-invasive HOM-IR method that was less sensitive than rigorous testing of the euglycemic clamp was used. This may explain the lack of association found between betatrophin levels and insulin resistance in this study. Moreover, the subjects were not evaluated for betatrophin sequence variations.
5. Conclusion
According to the results, betatrophin levels were higher in infertile women with PCOS than in women with other causes of infertility. Given the findings, it can be concluded that there may be an association between increased betatrophin and increased incidence of PCOS. Further studies with a larger sample size are needed to investigate the relationship between betatrophin and insulin resistance and lipid metabolism, and its effects on infertility treatment outcomes.
Acknowledgments
Financial support for the work was provided by the Tehran University of Medical Sciences. We thank the participants and respected staff of the infertility clinic of Imam Khomeini Hospital in Tehran for their cooperation during data collection.
Conflict of Interest
The authors declare that they have no conflict of interest.
Full-Text: (620 Views)
1. Introduction
Polycystic ovary syndrome (PCOS) is a common hormonal health problem that occurs in 5-10% of women of reproductive age (1). Ovulatory dysfunction and hyperandrogenism are two features of PCOS (2). It is hypothesized that the syndrome can be due to several metabolic disorders including low-grade chronic inflammation, resistance to insulin, metabolic syndrome, obesity, hyperlipidemia, glucose intolerance, hypertension, and/or an elevation of the risk of Type 2 diabetes (3).
Two of the most significant and highly prevalent parameters among women with PCOS are insulin resistance and metabolic disorders (4). As a consequence of resistance to insulin, betatrophin levels are expected to vary in the serum as well as in other biological compartments (5). Betatrophin is a peptide hormone derived from liver and adipose tissue (3). This protein regulates glucose homeostasis and lipid metabolism (6). An association between circulating betatrophin levels, Type 1 diabetes, and PCOS has been reported (5); however, the pathogenesis effects of betatrophin on the secretion of insulin and homeostasis of glucose are not fully known (2).
Recent clinical studies have confirmed a positive association between circulating betatrophin levels and serum lipid levels in participants with impaired glucose tolerance, participants with Type 2 diabetes, and healthy individuals. Furthermore, “it has been suggested that betatrophin may be associated with circulating low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, and triglycerides (TG), probably via different mechanisms” (7). A study was conducted to find the relationship between betatrophin levels and anthropometric, hormonal, and metabolic parameters in women with PCOS with a body mass index (BMI) < 25 kg/m2 who were glucose tolerant. The researchers studied 50 of such women using the Rotterdam criteria, and 60 healthy controls who were age and BMI matched and did not present any feature of clinical or biochemical hyperandrogenism. The study confirmed that serum betatrophin levels were significantly higher in glucose tolerant women with PCOS who had a BMI < 25 kg/m2 than in the control group (8).
The results of another study with the aim of investigating betatrophin serum levels in participants with newly-diagnosed PCOS and their influence demonstrated a positive correlation between the serum betatrophin levels in women with PCOS and their fasting blood glucose, TG, BMI, and waist-to-hip ratio (p = 0.01). However, serum betatrophin levels were uncorrelated with the fasting insulin and homeostasis model assessment of insulin resistance (HOMA-IR) (p = 0.05). Multiple stepwise regression analysis showed that the TG level was an independent influence of the serum betatrophin level. However, correlations mentioned above were not found in the control group (9). Many studies have examined the association between betatrophin and metabolic and inflammatory parameters and how they are linked to metabolic diseases (2, 3, 10).
In this study, we aimed to investigate the betatrophin level and its association with metabolic and inflammatory parameters in infertile women with and without PCOS during the intrauterine insemination (IUI) cycle.
2. Materials and Methods
This case-control study was conducted in the infertility clinic of Imam Khomeini Hospital in Tehran, Iran from September 2017 to November 2018.
45 infertile women with a diagnosis of PCOS and 45 infertile women with regular menstruation and with other causes of infertility (idiopathic or male factor) chosen by convenience sampling were included in the study. The participants entered the course of IUI. PCOS diagnosis was determined according to the Rotterdam PCOS Consensus criteria that require at least the presence of two of the following criteria: 1) ovulation dysfunction (anovulation or sporadic ovulation); 2) hyperandrogenism (clinical or biochemical); and 3) polycystic ovaries present on ultrasound (15 follicles 2-10 mm in diameter in either ovary) (11). The other inclusion criteria were: age of 20-40 yr; BMI < 35 kg/m2; at least one tubal patency; and normal levels of thyroid stimulating hormone and prolactin. Women with any underlying disease (e.g., diabetes, hypertension, cardiac or hepatorenal disease, chronic inflammatory disease, etc.), women who had had an infection in the previous two weeks, women taking oral contraceptive pills, antiandrogens or other hormonal drugs in the last month, and women who use recreational drugs, smoke or drink alcohol were excluded.
Participants were interviewed to obtain age and reproductive history, including gravidity, parity, abortion, ectopic pregnancy, menstruation pattern, infertility duration, and stimulation duration. The height and weight of each participant were measured using a digital bathroom scale (Omron Company, Japan) and a SECA stadiometer (SECA Instruments Ltd, Germany). The measurements were recorded to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated by dividing weight (in kilograms) by height squared (in meters) while the participants were weighted barefooted and in light clothing. Fasting brachial venous blood samples were taken to measure the levels of betatrophin, fasting blood sugar (FBS), insulin, luteinizing hormone (LH), follicle-stimulating hormone (FSH), LDL-C, estradiol, and high-sensitivity C-reactive protein (hs-CRP). The participants were on the 3rd day of their menstrual cycle at the time of the blood test. The collected blood was centrifuged at 4000 × g for 10 min right after the test and stored at -80°C until the analysis. To find the amounts of LH, FSH, hs-CRP, FBS, and estradiol, automated chemiluminescence immunoassay systems were used (BT 1500 Biotecnica instruments SPA, Italy). The Friedewald formula (12) and the enzyme-linked immunosorbent assay (ELISA) technique were used for the calculation of LDL-C and hs-CRP, respectively. The intra-assay coefficient of about 5% and inter-assay coefficient of about 10% were determined for each factor. To evaluate the HOMA index, fasting insulin and glucose were used. Insulin resistance was calculated by glucose (mmol/L) multiplied by fasting insulin (mU/L)/22.5 (HOMA-IR formula). The chemiluminescence immunometric assay and a commercial kit (Immulite 2000 Analyzer; CPC, USA) were used to measure fasting insulin. To measure fasting glucose, a glucose oxidase assay (Tosoh Corp., Tosoh, Japan) was used.
ELISA kits (EIAab Science, Wuhan, China; Catalogue No. E11644h) were used to assess fasting serum betatrophin levels following the manufacturer’s instructions. The tests were performed in duplicate, and samples with more than 5% coefficient of variation were excluded.
Every participant in the case or control group was stimulated to ovulate with two clomiphene citrate tablets (Ovumid 50, Iran Hormone Pharmaceutical Co, Tehran, Iran) starting from the 3rd day of their menstrual cycle. On the 8th day of the cycle, 150 units of human menopausal gonadotropin (karma-HMG 75 u, Pharmatech GmbH, Germany) were administered. Following that, serial transvaginal sonography (Philips ultrasound, affinity 70, Japan) was performed until the dominant follicle reached a diameter of 18-20 mm. Subsequently, 10,000 units of human chorionic gonadotropin (karma-HCG 5000 u, Pharmatech GmbH, Germany) were prescribed and 36 hr later, participants were subjected to IUI. Two wk later, the level of beta-human chorionic gonadotropin was checked (MPR-404, ELISAMicroplate Reader, Roedermark, Germany) and, if positive, an ultrasound was performed to evaluate the pregnancy and observe the fetal heart rate.
2.1. Ethical considerations
The study was approved by the Ethics Committee of the Tehran University of Medical Sciences (Code: IR.TUMS.VCR.REC.1396.2259). Written informed consent was obtained from all participants before the initiation of the study.
2.2. Statistical analysis
SPSS (version 25, Armonk, NY, IBM Corp.), Statistical Package for the Social Sciences, were used to analyse the data. The Shapiro-Wilk test was used to test the distribution of the data. Data are presented as mean ± SD or median (interquartile range). An independent t test and a Chi-square test were used to assess between-group differences for continuous variables and categorical variables, respectively. In order to compare group medians for data with non-normal distributions the Mann-Whitney U test was used. Spearman’s Rank Correlation Coefficient was used to analyse Bivariate relations between betatrophin levels and covariates. The determinants of betatrophin (independent variables: BMI, FSH, LH, LDL-C, hs-CRP, FBS, insulin, and HOMA-IR) were evaluated separately using multiple linear regression. Furthermore, the effect of betatrophin and HOMA-IR on pregnancy was investigated using logistic regression.
3. Results
There were 45 participants in each group. Four participants from the control group withdrew from the study before its completion; they did not return after administration of clomiphene citrate for treatment. Age, reproductive history, FSH, LH, LDL-C, hs-CRP, FBS, and HOMA-IR were similar between the groups. As expected, women with PCOS had a higher mean BMI than the control group. Clinical and hormonal details of the participants in the case and control groups are shown in table I.
The results of the Chi-square test showed that 73.3% (33 people) of the case group had an irregular pattern of menstruation, while no participants in the control group had an irregular pattern of menstruation (p ≤ 0.001). Correlations between betatrophin concentration and clinical, metabolic, and inflammatory parameters of the participants are shown in table II.
Based on multiple linear regression analyses, the effects of BMI, FSH, LH, LDL-C, hs-CRP, FBS, insulin, and HOMA-IR on betatrophin were not significant (Table III). The results of the independent t test showed no significant difference between groups in folliculogenesis (p = 0.57). The results of the Chi-square test showed that gestational rates were 20.0% and 31.7% in the case and control groups, respectively; this difference was not statistically significant (p = 0.21). Based on the logistic regression model, betatrophin and HOMA-IR did not predict pregnancy.



4. Discussion
Our results showed that the level of betatrophin in women with PCOS was significantly higher than in the control group. In the present study assessing the association of metabolic parameters such as the lipid profiles with betatrophin levels, it is found that amongst lipid profiles only lower LDL-C levels were associated with betatrophin levels in women with PCOS. In addition, the results of the multiple linear regression analysis did not show any effect of any of the inflammatory or metabolic parameters on the betatrophin level.
Betatrophin is a peptide that is produced in the liver as well as in brown and white adipose tissue and it regulates the function of pancreatic beta cells and fat production (5). The results of studies on betatrophin vary. Some studies have shown an increase in betatrophin levels in women with PCOS (3, 14, 15). However, the results of one study showed that betatrophin levels were lower in women with PCOS than in the control group. They also found a significant negative correlation between betatrophin levels, and insulin, BMI and HOMA-IR. The results of their study showed that the only factor affecting beta-dopamine reduction was PCOS (2). Another study found that betatrophin concentration was lower in women with PCOS than in the control group. Their results showed that there was a positive correlation between betatrophin concentration and LDL-C in women with PCOS whose betatrophin concentrations were higher than the cut-off (464.5 ng/l) (6). The results of these studies are not in line with our study.
The results of a study on the association of betatrophin levels with PCOS showed a correlation between PCOS and fasting insulin levels as well as a significant correlation between the levels of betatrophin and fasting insulin levels and HOMA-IR in women with PCOS (14). A negative correlation between serum betatrophin levels and BMI, fasting insulin, and HOMA-IR was demonstrated in another study (15). The present study did not show such correlations. This difference in results may be due to differences in laboratory kits used, ages ethnicities, or sample size.
In our study, women with PCOS had a higher mean BMI than the control group; but, no significant correlation between betatrophin and BMI was found. The results of a recent study done on obese populations was not in line with this study (16). Also some contradictory results regarding the association of betatrophin levels and obesity have been reported (17, 18). The results of a cross-sectional study showed that there was a significant positive correlation between betatrophin levels and BMI in women with PCOS (3), while the results of a case-control study showed the opposite (14). Another study aiming to investigate blood levels of betatrophin in obese children with non-alcoholic fatty liver disease reported no statistically significant difference between the control and experimental groups (19).
This study showed significantly higher levels of fasting insulin in women with PCOS comparing to the control group. This indicates that women with PCOS are insulin resistant. About 80% of women with PCOS have insulin resistance. Betatrophin is produced as a result of insulin resistance (14). The findings of some studies vary regarding the association of betatrophin levels with diabetes (17, 18, 20). In our study, we did not find a significant correlation between the levels of betatrophin and fasting insulin levels. One study showed a significant negative correlation between betatrophin levels and fasting insulin levels in women with PCOS (21), while another showed the opposite (14). The differences in race and lifestyle may have influenced the results of these studies.
Betatrophin has been linked to lipid metabolism changes. But studies’ findings are conflicting. Betatrophin affects the lipid profile by regulating the secretion of very low-density lipoproteins from the liver and inhibiting lipoprotein lipase activity (22). Chinese researchers have reported that there is a positive correlation between betatrophin and TG (23). In another study, the results showed a negative correlation between circulating betatrophin levels and TG, but a positive correlation with high-density lipoprotein cholesterol (18). Furthermore, another study found no association between betatrophin levels and improved fertility outcomes in participants (3).
The results of the present study showed a significant negative correlation between the level of betatrophin and LDL-C in women with PCOS; therefore, it can be concluded that as the level of betatrophin increases, the level of LDL-C decreases. The results of this study were not in line with a cross-sectional study on PCOS women which showed a significant positive correlation between betatrophin and LDL-C levels (3). PCOS is a disease associated with mild chronic inflammation. However, in this study, there was no significant relationship found between betatrophin and inflammatory parameters such as hs-CRP.
One of the limitations of the present study was the small sample size and the attrition in the control group. Also, in the evaluation of insulin resistance, a non-invasive HOM-IR method that was less sensitive than rigorous testing of the euglycemic clamp was used. This may explain the lack of association found between betatrophin levels and insulin resistance in this study. Moreover, the subjects were not evaluated for betatrophin sequence variations.
5. Conclusion
According to the results, betatrophin levels were higher in infertile women with PCOS than in women with other causes of infertility. Given the findings, it can be concluded that there may be an association between increased betatrophin and increased incidence of PCOS. Further studies with a larger sample size are needed to investigate the relationship between betatrophin and insulin resistance and lipid metabolism, and its effects on infertility treatment outcomes.
Acknowledgments
Financial support for the work was provided by the Tehran University of Medical Sciences. We thank the participants and respected staff of the infertility clinic of Imam Khomeini Hospital in Tehran for their cooperation during data collection.
Conflict of Interest
The authors declare that they have no conflict of interest.
Type of Study: Original Article |
Subject:
Reproductive Endocrinology
References
1. Naghi Jafarabadi M, Mosavi Vahed SH, Afiat M, Ebrahimi Z, Shiva ZS, Haghollahi F. Letrozole plus 2 different gonadotropins regimens for intrauterine insemination in polycystic ovary patients: A randomized clinical trial. Int J Women's Health Reprod Sci 2018; 6: 420-424. [DOI:10.15296/ijwhr.2018.70]
2. Erbag G, Eroglu M, Turkon H, Sen H, Binnetoglu E, Aylanc N, et al. Relationship between betatrophin levels and metabolic parameters in patients with polycystic ovary syndrome. Cell Mol Biol 2016; 62: 20-24.
3. Calan M, Yilmaz O, Kume T, Kocabas GU, Senses PY, Senses YM, et al. Elevated circulating levels of betatrophin are associated with polycystic ovary syndrome. Endocrine 2016; 53: 271-279. [DOI:10.1007/s12020-016-0875-z] [PMID]
4. Wang H, Du L, Wu T, Yang G, Hu W, Wang H, et al. Circulating betatrophin is associated with insulin resistance in humans: Cross-sectional and interventional studies in vivo and in vitro. Oncotarget 2017; 8: 96604-96614. [DOI:10.18632/oncotarget.21852] [PMID] [PMCID]
5. Ersahin AA, Acet M, Ersahin SS, Acet T, Yardim M, Kenanoglu O, et al. Follicular fluid cerebellin and betatrophin regulate the metabolic functions of growing follicles in polycystic ovary syndrome. Clin Exp Reprod Med 2017; 44: 33-39. [DOI:10.5653/cerm.2017.44.1.33] [PMID] [PMCID]
6. Haydardedeoglu FE, Bagir GS, Haydardedeoglu B, Bozkirli E, Bakiner O, Metin K, et al. Serum betatrophin levels are reduced in patients with full-blown polycystic ovary syndrome. Gynecol Endocrinol 2019; 35: 224-227. [DOI:10.1080/09513590.2018.1519791] [PMID]
7. Takebayashi K, Hara K, Terasawa T, Naruse R, Suetsugu M, Tsuchiya T, et al. Serum betatrophin levels and clinical features in patients with poorly controlled type 2 diabetes. J Clin Med Res 2017; 9: 782-787. [DOI:10.14740/jocmr3114w] [PMID] [PMCID]
8. Erol O, Özel MK, Ellidağ HY, Toptaş T, Derbent AU, Yılmaz N. Assessment of circulating betatrophin concentrations in lean glucose-tolerant women with polycystic ovary syndrome. J Obstet Gynaecol 2017; 37: 633-638. [DOI:10.1080/01443615.2017.1286464] [PMID]
9. Junjun HE, Honglin HU, Fang DAI. [Serum levels of betatrophin in patients with newly-diagnosed polycystic ovary syndrome and its influential factors]. Anhui Med J 2018; 2: 166-170. (in Chinese)
10. Hassan AB, Salih ShF, Hassan II, Saadi FS, Abdulah DM, Ahmed IH, et al. Circulating betatrophin in relation to metabolic, inflammatory parameters, and oxidative stress in patients with type 2 diabetes mellitus. Diabetes Metab Syndr 2019; 13: 458-463. [DOI:10.1016/j.dsx.2018.11.016] [PMID]
11. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19: 41-47. [DOI:10.1093/humrep/deh098] [PMID]
12. Knopfholz J, Disserol CCD, Pierin AJ, Schirr FL, Streisky L, Takito LL, et al. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterol 2014; 2014: 261878. [DOI:10.1155/2014/261878] [PMID] [PMCID]
13. McLeod SA. (2019, May 20). What a p-value tells you about statistical significance. Simply Psychology. Available at: www.simplypsychology.org/p-value.html.
14. Qu Q, Zhao D, Zhang F, Bao H, Yang Q. Serum betatrophin levels are increased and associated with insulin resistance in patients with polycystic ovary syndrome. J Int Med Res 2017; 45: 193-202. [DOI:10.1177/0300060516680441] [PMID] [PMCID]
15. Song Sh, Wang J, Guo Ch, Jiang T. [Elevated serum levels of betatrophin in patients with polycystic ovary syndrome and the influential factors]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2016; 41: 969-974. (in Chinese)
16. Abu-Farha M, Sriraman D, Cherian P, AlKhairi I, Elkum N, Behbehani K, et al. Circulating ANGPTL8/betatrophin is increased in obesity and reduced after exercise training. PloS One 2016; 11: e0147367. [DOI:10.1371/journal.pone.0147367] [PMID] [PMCID]
17. Fu Zh, Berhane F, Fite A, Seyoum B, Abou-Samra AB, Zhang R. Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci Rep 2014; 4: 5013. [DOI:10.1038/srep05013] [PMID] [PMCID]
18. Gómez-Ambrosi J, Pascual E, Catalán V, Rodríguez A, Ramírez B, Silva C, et al. Circulating betatrophin concentrations are decreased in human obesity and type 2 diabetes. J Clin Endocrinol Metab 2014; 99: E2004-E2009. [DOI:10.1210/jc.2014-1568] [PMID]
19. Battal F, Türkön H, Aylanç N, Aylanç H, Yıldırım Ş, Kaymaz N, et al. Investigation of blood betatrophin levels in obese children with non-alcoholic fatty liver disease. Pediatr Gastroenterol Hepatol Nutr 2018; 21: 111-117. [DOI:10.5223/pghn.2018.21.2.111] [PMID] [PMCID]
20. Abu-Farha M, Abubaker J, Al-Khairi I, Cherian P, Noronha F, Hu FB, et al. Higher plasma betatrophin/ANGPTL8 level in type 2 diabetes subjects does not correlate with blood glucose or insulin resistance. Sci Rep 2015; 5: 10949. [DOI:10.1038/srep10949] [PMID] [PMCID]
21. Duan Y, Liu D, Qu H, Wei H, Luo Y, Feng Zh, et al. Decreased circulating levels of betatrophin in Chinese women with polycystic ovary syndrome. Int J Clin Exp Med 2017; 10: 5196-5202.
22. Ghasemi H, Tavilani H, Khodadadi I, Saidijam M, Karimi J. Circulating betatrophin levels are associated with the lipid profile in type 2 diabetes. Chonnam Med J 2015; 51: 115-119. [DOI:10.4068/cmj.2015.51.3.115] [PMID] [PMCID]
23. Gao T, Jin K, Chen P, Jin H, Yang L, Xie X, et al. Circulating betatrophin correlates with triglycerides and postprandial glucose among different glucose tolerance statuses: A case-control study. PloS One 2015; 10: e0133640. [DOI:10.1371/journal.pone.0133640] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





