Tue, Jul 8, 2025
[Archive]
Volume 20, Issue 12 (December 2022)
IJRM 2022, 20(12): 999-1006 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bahrami Z, Daeifarshbaf N, Amjadi F, Aflatoonian R. The effects of hormonal changes on sperm DNA integrity in oligoasthenoteratospermia individuals: A case-control study. IJRM 2022; 20 (12) :999-1006
URL: http://ijrm.ir/article-1-2160-en.html
URL: http://ijrm.ir/article-1-2160-en.html
1- Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran. Laleh IVF Clinic, Laleh Hospital, Tehran, Iran.
2- Department of Anatomy, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. Infertility Center, Imam Khomeini Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Anatomy, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Endocrinology and Female Infertility at Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran. Laleh IVF Clinic, Laleh Hospital, Tehran, Iran. ,R.aflatoonian@royaninstitute.org
2- Department of Anatomy, School of Medicine, Iran University of Medical Sciences, Tehran, Iran. Infertility Center, Imam Khomeini Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Anatomy, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Endocrinology and Female Infertility at Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran. Laleh IVF Clinic, Laleh Hospital, Tehran, Iran. ,
Full-Text [PDF 824 kb]
(1062 Downloads)
| Abstract (HTML) (1407 Views)

Full-Text: (262 Views)
1. Introduction
Infertility affects approximately 15% of couples attempting pregnancy. Also, the male factor is responsible for about half of these cases (1). It is well-established that most of the male infertility problems associated with qualitative and quantitative defects of spermatogenesis, lead to sexual and fertility dysfunction (1, 2). Abnormalities of the seminal parameters of oligoasthenoteratospermia (OAT) are important causes and the main contributory reasons for male infertility (3). Apart from the variety of known factors for OAT such as age, systemic diseases, varicocele, infection, cryptorchidism, testicular trauma, obstructions, immunological factors, endocrine disorders, and idiopathic factors, one of the critical underlying etiology is hormonal imbalance (4-6).
There are growing concerns that disturbance in hormone levels results in spermatogenesis dysfunction and male infertility. Hormone deficiency in germ cell microenvironments has been indicated as the key reason for immature sperm increase which could adversely affect sperm survival and function and may lead to the authors answering to reviewer's comments and correcting some of them, which was acceptable (7). Single and double DNA strand breaks are positively related to sub-haploid and sperm late apoptosis (8). Given together, it is concluded that high sperm DNA fragmentation index(DFI) levels could reduce fertility capacity by affecting the quality and quantity of the spermatogenesis process (9).
The process of spermatogenesis is thoroughly dependent on downstream hormones secreted in response to gonadotropin-releasing hormone in the hypothalamus-pituitary-testis axis (10). The normal hormonal function of the testis is modulated by pituitary secretion of follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), thyroid-stimulating hormone (TSH), testosterone, and anti-Mullerian hormone (AMH) (11, 12). FSH affects Sertoli cells to produce AMH or serves a direct fundamental role in spermatogenesis through stimulation of cell proliferation, differentiation, and control of surrounding cells apoptosis (10, 13). However, LH acts on Leydig cells to promote testosterone secretion and affects spermatogenesis via testosterone/androgen receptors (14). Although many studies have been carried out on the beneficial effects of these hormones on spermatogenesis, there are many controversial findings in different studies (15-18). Therefore, in the current study, we investigated the effects of known hormones on sperm DFI to draw a more reliable conclusion.
In addition to the most studied hormones, it is reported that PRL and thyroid hormone disorders affect sexual dysfunction and impair the female reproductive function, but their effects on the male reproductive system need to be studied in detail (19, 20). However, there is evidence that the cooperation of PRL and gonadotropins along the hypothalamus-pituitary-testis axis and the effects of thyroid hormones on fetal Sertoli cell maturation and Leydig cell differentiation are crucial for normal spermatogenesis (21, 22).
However, much research is still needed in this area. Hence, the novelty of our study was to investigate the effects of PRL and TSH, along with other hormones including FSH, LH, testosterone, and AMH, on sperm DNA integrity of normal men compared with OATs to design a clinical algorithm for the comprehensive study of male factor infertilities.
There are growing concerns that disturbance in hormone levels results in spermatogenesis dysfunction and male infertility. Hormone deficiency in germ cell microenvironments has been indicated as the key reason for immature sperm increase which could adversely affect sperm survival and function and may lead to the authors answering to reviewer's comments and correcting some of them, which was acceptable (7). Single and double DNA strand breaks are positively related to sub-haploid and sperm late apoptosis (8). Given together, it is concluded that high sperm DNA fragmentation index(DFI) levels could reduce fertility capacity by affecting the quality and quantity of the spermatogenesis process (9).
The process of spermatogenesis is thoroughly dependent on downstream hormones secreted in response to gonadotropin-releasing hormone in the hypothalamus-pituitary-testis axis (10). The normal hormonal function of the testis is modulated by pituitary secretion of follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), thyroid-stimulating hormone (TSH), testosterone, and anti-Mullerian hormone (AMH) (11, 12). FSH affects Sertoli cells to produce AMH or serves a direct fundamental role in spermatogenesis through stimulation of cell proliferation, differentiation, and control of surrounding cells apoptosis (10, 13). However, LH acts on Leydig cells to promote testosterone secretion and affects spermatogenesis via testosterone/androgen receptors (14). Although many studies have been carried out on the beneficial effects of these hormones on spermatogenesis, there are many controversial findings in different studies (15-18). Therefore, in the current study, we investigated the effects of known hormones on sperm DFI to draw a more reliable conclusion.
In addition to the most studied hormones, it is reported that PRL and thyroid hormone disorders affect sexual dysfunction and impair the female reproductive function, but their effects on the male reproductive system need to be studied in detail (19, 20). However, there is evidence that the cooperation of PRL and gonadotropins along the hypothalamus-pituitary-testis axis and the effects of thyroid hormones on fetal Sertoli cell maturation and Leydig cell differentiation are crucial for normal spermatogenesis (21, 22).
However, much research is still needed in this area. Hence, the novelty of our study was to investigate the effects of PRL and TSH, along with other hormones including FSH, LH, testosterone, and AMH, on sperm DNA integrity of normal men compared with OATs to design a clinical algorithm for the comprehensive study of male factor infertilities.
2. Materials and Methods
2.1. Participants
In this case-control study, samples of semen and blood were collected from 60 men (25-45 yr) referred to the infertility clinic for evaluation or treatment of infertility (Infertility Clinic in Laleh hospital, Tehran, Iran), between May 2018 and February 2019. The included men had no history of chronic illness, chemotherapy, radiotherapy, varicocele, and abnormal testicular size. None had used antioxidant or hormonal therapy during the previous 3 months. Since exogenous agents like smoke are important influencing factors on seminal parameters, heavy smokers were excluded from the analysis. 2 groups were included in this study according to semen profiles: an OAT group (n = 30) and a normal control group (n = 30). The control group included men with normal sperm parameters. After semen analysis for the final classification of individuals, DFI checking terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL assay) and hormonal analysis (enzyme-linked immunoassay test) were performed in both groups. Therefore, participants were classified into 4 groups, normospermia with DFI < 19.25, normospermia with DFI > 19.25, OAT with DFI < 19.25, and OAT with DFI > 19.25 (23).
2.2. Analysis of seminal parameters
Samples were collected by masturbation without condom or lubricant cream, after at least 2 days of sexual abstinence and were allowed to liquefy for 30 min at 37°C. After liquefaction, semen parameters (concentration, total motility, and morphology) analyses were conducted according to the World Health Organization 2010 and Kruger’s criteria (24). 3 independent replicates were processed and evaluated separately for each semen sample. Sperm morphology was analyzed with a Diff-Quick kit (BRED Life Science Technology Inc., China) and classified by the Kruger classification. The individual was considered OAT by the following criteria: sperm count below 15 million/ml, motility under 40%, and morphology below 4%.
2.3. DFI analysis
An In-Situ Cell Death Detection kit (Roche Diagnostics GmbH) was used to carry out the TUNEL assay and analysis of terminal deoxynucleotide end labeling in sperm-containing DFI. After the preparation of samples based on the manufacturer’s protocol, fluorescence signals of the spermatozoa were examined by flow cytometric analysis. For this purpose, about 10,000 events were evaluated with an excitation wavelength of 488 nm on the FACS Calibur flow cytometer (Becton and Dickinson Co.). The signals related to TUNEL‐positive cells (green fluorescence) were measured by a 530 ± 30 nm bandpass filter. The positive and negative controls were prepared following the manufacturer’s instruction, by incubating with 50 µg/ml DNase I and omitting terminal deoxytransferase (TdT), respectively (25).
2.4. Laboratory analysis
Blood analysis were performed (Serology laboratory of Infertility Clinic in Laleh hospital, Tehran, Iran). The hormone parameters were analyzed in peripheral blood samples collected in the morning from 7:30-9:00 AM, after fasting overnight. The samples were centrifuged for 10 min at 3000 rpm and the serum was immediately frozen at -20°C temperature. All experiments were performed in triplicate. Then, FSH, LH, AMH, testosterone, PRL, and TSH hormones were measured by an enzymatic immunoassay (ELISA, Vidas, France, Marcy). The normal adult ranges for our laboratory are FSH 2-10 IU/L, LH 1-8 IU/L, AMH 1-6 ng/mL, testosterone 2-8 ng/dL, PRL 2.5-17 ng/mL, and TSH 0.4-4 mIU/L.
2.5. Ethical considerations
All procedures were as per the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments (Code: IR.IUMS.REC.1401.462). Informed consent were obtained before the sample collection from all participants. This study was approved by the Ethics Committee.
2.6. Statistical analysis
Statistical Package for the Social Sciences software (SPSS, version 23; IBM Corp) was used to carry out statistical analysis. One-way ANOVA followed by Tukey's test was performed to compare quantitative variables between groups. All data were presented as mean ± SEM and the p-value < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Sperm parameters and DFI
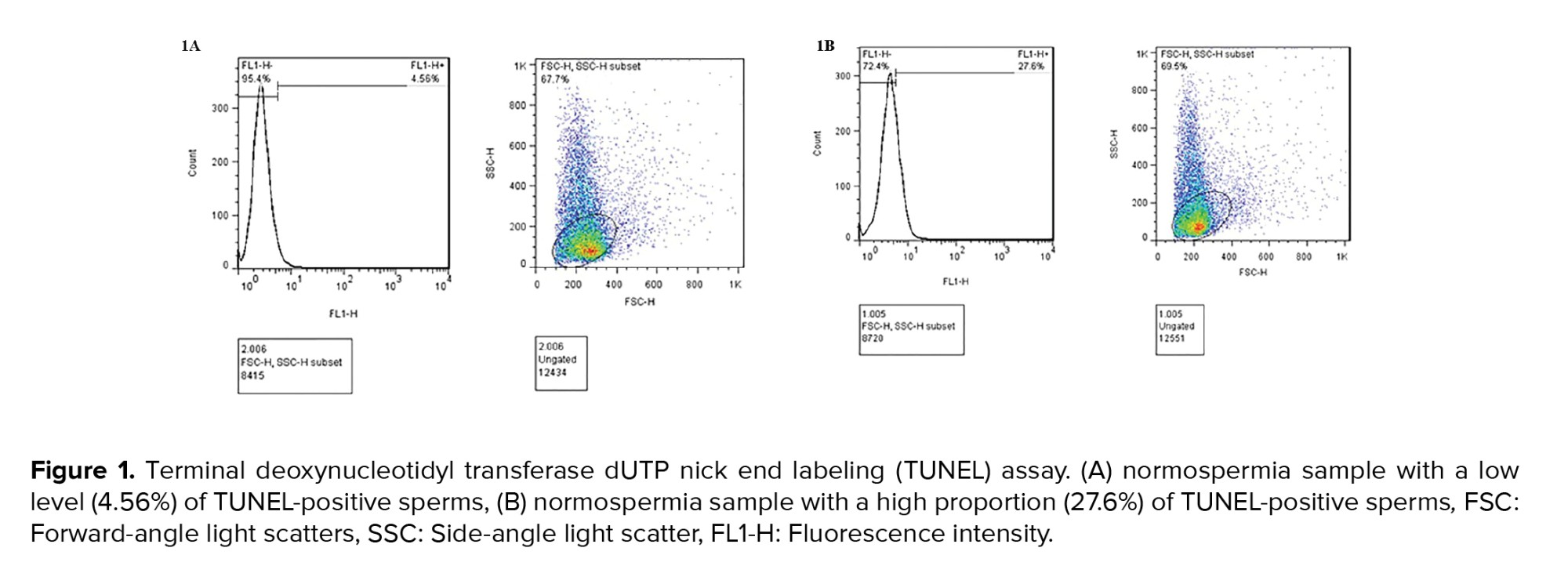
The results of semen analysis and the TUNEL test are demonstrated in table I. Semen volume, sperm concentration, motility, and morphology were analyzed according to the World Health Organization (2010) and Kruger’s criteria. All semen characteristics were significantly different between the control (normospermia) and case (OAT) groups (Table I). Individuals in groups 2 and 4 had statistically significant higher in the percent of DFI compared to the groups 1 and 3 (p = 0.001). Figure 1 shows the result of the TUNEL test. The DFI of sperm was acceptable according to the TUNEL assay with a cutoff point of less than 19.25% (Figure 1A). Specimens with more than 19.25% TUNEL-positive sperms were considered high DFI (Figure 1B).
2.2. Analysis of seminal parameters
Samples were collected by masturbation without condom or lubricant cream, after at least 2 days of sexual abstinence and were allowed to liquefy for 30 min at 37°C. After liquefaction, semen parameters (concentration, total motility, and morphology) analyses were conducted according to the World Health Organization 2010 and Kruger’s criteria (24). 3 independent replicates were processed and evaluated separately for each semen sample. Sperm morphology was analyzed with a Diff-Quick kit (BRED Life Science Technology Inc., China) and classified by the Kruger classification. The individual was considered OAT by the following criteria: sperm count below 15 million/ml, motility under 40%, and morphology below 4%.
2.3. DFI analysis
An In-Situ Cell Death Detection kit (Roche Diagnostics GmbH) was used to carry out the TUNEL assay and analysis of terminal deoxynucleotide end labeling in sperm-containing DFI. After the preparation of samples based on the manufacturer’s protocol, fluorescence signals of the spermatozoa were examined by flow cytometric analysis. For this purpose, about 10,000 events were evaluated with an excitation wavelength of 488 nm on the FACS Calibur flow cytometer (Becton and Dickinson Co.). The signals related to TUNEL‐positive cells (green fluorescence) were measured by a 530 ± 30 nm bandpass filter. The positive and negative controls were prepared following the manufacturer’s instruction, by incubating with 50 µg/ml DNase I and omitting terminal deoxytransferase (TdT), respectively (25).
2.4. Laboratory analysis
Blood analysis were performed (Serology laboratory of Infertility Clinic in Laleh hospital, Tehran, Iran). The hormone parameters were analyzed in peripheral blood samples collected in the morning from 7:30-9:00 AM, after fasting overnight. The samples were centrifuged for 10 min at 3000 rpm and the serum was immediately frozen at -20°C temperature. All experiments were performed in triplicate. Then, FSH, LH, AMH, testosterone, PRL, and TSH hormones were measured by an enzymatic immunoassay (ELISA, Vidas, France, Marcy). The normal adult ranges for our laboratory are FSH 2-10 IU/L, LH 1-8 IU/L, AMH 1-6 ng/mL, testosterone 2-8 ng/dL, PRL 2.5-17 ng/mL, and TSH 0.4-4 mIU/L.
2.5. Ethical considerations
All procedures were as per the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later amendments (Code: IR.IUMS.REC.1401.462). Informed consent were obtained before the sample collection from all participants. This study was approved by the Ethics Committee.
2.6. Statistical analysis
Statistical Package for the Social Sciences software (SPSS, version 23; IBM Corp) was used to carry out statistical analysis. One-way ANOVA followed by Tukey's test was performed to compare quantitative variables between groups. All data were presented as mean ± SEM and the p-value < 0.05 was considered to indicate a statistically significant difference.
3. Results
3.1. Sperm parameters and DFI
The results of semen analysis and the TUNEL test are demonstrated in table I. Semen volume, sperm concentration, motility, and morphology were analyzed according to the World Health Organization (2010) and Kruger’s criteria. All semen characteristics were significantly different between the control (normospermia) and case (OAT) groups (Table I). Individuals in groups 2 and 4 had statistically significant higher in the percent of DFI compared to the groups 1 and 3 (p = 0.001). Figure 1 shows the result of the TUNEL test. The DFI of sperm was acceptable according to the TUNEL assay with a cutoff point of less than 19.25% (Figure 1A). Specimens with more than 19.25% TUNEL-positive sperms were considered high DFI (Figure 1B).
3.2. Hormonal levels and DFI
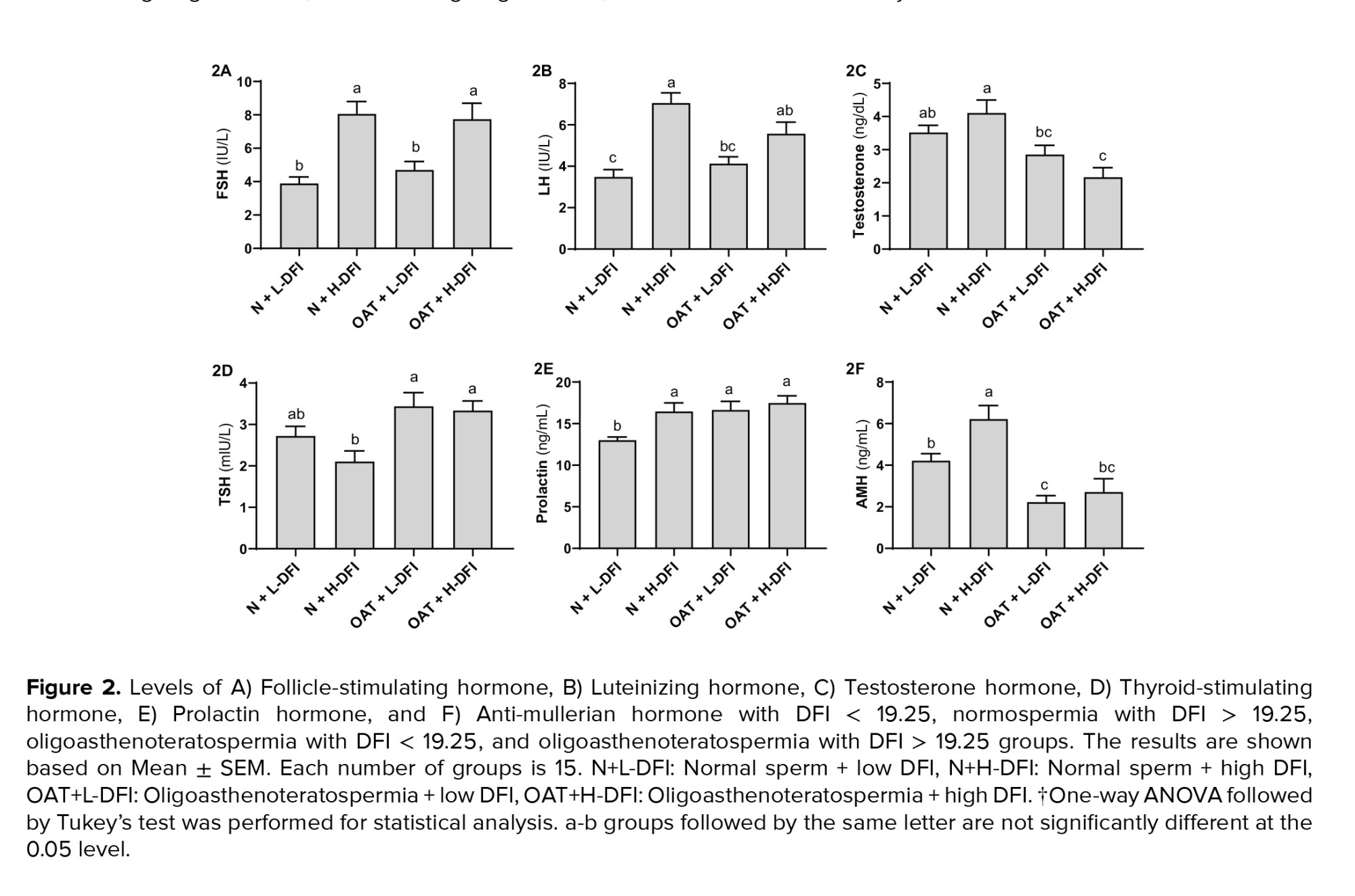
Figure 2 shows the mean concentration of hormones in 4 groups. As depicted in figure 2A, FSH levels in normospermia men with low DFI (control group) decreased significantly when compared to the normospermia and OAT groups with high DFI (p < 0.01). Moreover, FSH concentrations in the OAT group with high DFI were noticeably higher than the OAT group with low DFI (p < 0.01).
LH levels was significantly higher in OAT and normospermia groups with high DFI compared to the control group (p < 0.01, Figure 2B). The level of testosterone (Figure 2C) in normospermia groups reduced significantly in comparison to the OATs with high DFI levels (p < 0.01). Inversely, as shown in figure 2D, the average range of TSH in both OAT groups were significantly higher than normospermia with high DFI (p < 0.01). Although there was a relationship between the last 2 hormones and the sperm concentration, this correlation was positive for testosterone and negative for TSH. As shown in figure 2E, PRL levels in the control group with normal spermogram and low DFI value were remarkably lower than all other 3 experimental groups (p < 0.01). Based on our results, PRL level correlates significantly (p ≤ 0.01) with low sperm concentration and high DFI value simultaneously. AMH levels was remarkably higher in the normospermia group with high levels of DFI compared to the control group (Figure 2F). Additionally, AMH levels were significantly lower in the blood samples of individuals with OAT regardless of sperm DFI level in comparison to the normospermia groups. According to our analysis, increased and decreased levels of AMH had a significant relationship with DFI value and sperm concentration (p ≤ 0.01).



4. Discussion
Our result showed that these hormones have comprehensive effects on male fertility potential and could affect sperm DNA integrity along with semen parameters. OAT, as the most challenging condition in infertility clinics, is associated with the abnormal spermatogenic process, which could be caused by hormonal imbalance (26). Current study on OATs revealed that FSH and LH levels could be indicative of DFI levels, while testosterone and TSH are associated with sperm concentration.
To the best of our knowledge, the present study was the first to examine the effects of PRL and AMH levels on sperm DFI in OAT individuals. Gonadotropin and testosterone concentrations play significant roles in the maintenance of normal spermatogenesis. Furthermore, any alteration in their serum levels could cause abnormal semen profile, sperm DFI, and finally fertility problems (27). Accordingly, our results demonstrated that FSH and LH concentrations were elevated in the groups with increased DFI values.
In the current study, the average concentration of gonadotropins increased significantly by increasing the DFI value, but all levels were in the normal range. Based on our findings, FSH levels were significant determinants of the DFI value which had previously been proven (28). On the other hand, several studies demonstrated the positive effects of FSH pretreatment in the improvement of sperm DNA integrity, but the results are controversial in OATs (29-31). Since OAT is sometimes associated with higher serum levels of gonadotropins, the advantages of FSH treatment on sperm DFI will be promising when endogenous FSH serum level is below 8 IU/L (26, 32). Taken together, it seems that FSH administration for our individuals with high DFI could not be beneficial due to their FSH levels around 8 IU/L.
Pretreatment of these individuals with gonadotropin might alter the hormonal balance, elevated FSH to upper than the normal range, and deteriorate their clinical condition. Therefore, it lightens the possibility that in the case of increased sperm DFI with higher concentrations of serum FSH (> 8 IU/L), alternative antioxidant supplementation could be preferred to suppress the apoptosis and preserve fertility potential. Lu evaluated the relationship between reproductive hormones and sperm DFI in 1010 subfertile men. They observed positive correlations between FSH and LH levels and sperm DFI but no relationship was found between testosterone levels and DFI value which supports our results (28). The present study indicated a relationship between testosterone level and seminal parameters. Our findings are partly consistent with those obtained by Trussell, who observed that in men with unexplained infertility, low testosterone level was associated with abnormal sperm morphology and caused lower live birth rates. Contrary to our results, there was no significant correlation between testosterone level and other parameters of semen such as sperm concentration, motility, and semen volume (33). The variation in the results of these studies could be due to the different target groups (OATs vs. unexplained male patients). Besides FSH, LH, and testosterone, the current study has addressed the effect of TSH, PRL, and AMH on DFI and semen profile. According to our results, TSH showed a significant correlation with seminal parameters in a negative way.
A study showed that men with abnormal semen parameters had higher total T3, T4, and lower TSH levels compared to those with normal semen profiles (34). Nikoobakht investigated the effect of hypothyroidism on semen profile and found that a high level of TSH, in hypothyroidism, adversely affects sperm count, motility, and morphology (35). Unlike previous findings and our results, an investigation indicated that high levels of TSH only is associated with reduced sperm morphology but no other parameters (34).
It has been demonstrated that the excess of PRL, like TSH, could reversibly affect male reproduction. Timely diagnosis of hyperprolactinemia in males can prevent irreversible infertility and unnecessary invasive procedures for achieving pregnancies (36). Untreated hyperprolactinemia could affect spermatogenesis and reduce sperm motility and counts (37). Keskin also evaluated the effects of hyperprolactinemia on semen parameters. However, there was no significant association between the PRL level and seminal parameters, but they suggest that PRL concentrations should be analyzed in moderate and severe oligozoospermias with a sperm concentration < 10 million/ml (38). Our findings are of interest, considering the effect of PRL on sperm DFI. According to our results, there is a positive correlation between PRL levels and DFI value, however, further studies are needed, especially in the hyperprolactinemia individuals after complete treatment. The last hormone evaluated in the current research is peptide growth and differentiation factors called AMH which plays an important role in spermatogenesis. AMH is produced by Sertoli cells which may reflect its critical effects in normal testicular function and spermatogenesis (39). In the support of this fact, the role of AMH in the normal spermatogenic process through a positive relationship with semen parameters has been confirmed previously (40). However, perverse to our results, Appasamy had observed no significant correlation between AMH and sperm DFI value (41). To obtain reliable findings and eliminate the inconsistency of results, it is necessary to further examine the functional mechanism of AMH in maintenance of sperm DNA integrity in male infertilities.
5. Conclusion
In summary, the present study suggests that the fluctuations in the serum levels of mentioned hormones could adversely affect semen profile and sperm DNA integrity which lead to severe fertility problems such as OAT. Accordingly, our findings demonstrated that FSH and LH levels could be indicative of DFI levels, while testosterone and TSH are associated with sperm concentration. Based on the current results, there were also positive correlations between PRL and AMH levels with sperm DFI value. However, the possible effect of these hormones in male factor infertility, on the fertilization rate and embryo quality, needs to be evaluated.
Acknowledgments
This study was supported by the research fund of Iran University of Medical Sciences, Tehran, Iran (grant number: 13580).
Conflict of Interest
The authors declare that they have no competing interest.
To the best of our knowledge, the present study was the first to examine the effects of PRL and AMH levels on sperm DFI in OAT individuals. Gonadotropin and testosterone concentrations play significant roles in the maintenance of normal spermatogenesis. Furthermore, any alteration in their serum levels could cause abnormal semen profile, sperm DFI, and finally fertility problems (27). Accordingly, our results demonstrated that FSH and LH concentrations were elevated in the groups with increased DFI values.
In the current study, the average concentration of gonadotropins increased significantly by increasing the DFI value, but all levels were in the normal range. Based on our findings, FSH levels were significant determinants of the DFI value which had previously been proven (28). On the other hand, several studies demonstrated the positive effects of FSH pretreatment in the improvement of sperm DNA integrity, but the results are controversial in OATs (29-31). Since OAT is sometimes associated with higher serum levels of gonadotropins, the advantages of FSH treatment on sperm DFI will be promising when endogenous FSH serum level is below 8 IU/L (26, 32). Taken together, it seems that FSH administration for our individuals with high DFI could not be beneficial due to their FSH levels around 8 IU/L.
Pretreatment of these individuals with gonadotropin might alter the hormonal balance, elevated FSH to upper than the normal range, and deteriorate their clinical condition. Therefore, it lightens the possibility that in the case of increased sperm DFI with higher concentrations of serum FSH (> 8 IU/L), alternative antioxidant supplementation could be preferred to suppress the apoptosis and preserve fertility potential. Lu evaluated the relationship between reproductive hormones and sperm DFI in 1010 subfertile men. They observed positive correlations between FSH and LH levels and sperm DFI but no relationship was found between testosterone levels and DFI value which supports our results (28). The present study indicated a relationship between testosterone level and seminal parameters. Our findings are partly consistent with those obtained by Trussell, who observed that in men with unexplained infertility, low testosterone level was associated with abnormal sperm morphology and caused lower live birth rates. Contrary to our results, there was no significant correlation between testosterone level and other parameters of semen such as sperm concentration, motility, and semen volume (33). The variation in the results of these studies could be due to the different target groups (OATs vs. unexplained male patients). Besides FSH, LH, and testosterone, the current study has addressed the effect of TSH, PRL, and AMH on DFI and semen profile. According to our results, TSH showed a significant correlation with seminal parameters in a negative way.
A study showed that men with abnormal semen parameters had higher total T3, T4, and lower TSH levels compared to those with normal semen profiles (34). Nikoobakht investigated the effect of hypothyroidism on semen profile and found that a high level of TSH, in hypothyroidism, adversely affects sperm count, motility, and morphology (35). Unlike previous findings and our results, an investigation indicated that high levels of TSH only is associated with reduced sperm morphology but no other parameters (34).
It has been demonstrated that the excess of PRL, like TSH, could reversibly affect male reproduction. Timely diagnosis of hyperprolactinemia in males can prevent irreversible infertility and unnecessary invasive procedures for achieving pregnancies (36). Untreated hyperprolactinemia could affect spermatogenesis and reduce sperm motility and counts (37). Keskin also evaluated the effects of hyperprolactinemia on semen parameters. However, there was no significant association between the PRL level and seminal parameters, but they suggest that PRL concentrations should be analyzed in moderate and severe oligozoospermias with a sperm concentration < 10 million/ml (38). Our findings are of interest, considering the effect of PRL on sperm DFI. According to our results, there is a positive correlation between PRL levels and DFI value, however, further studies are needed, especially in the hyperprolactinemia individuals after complete treatment. The last hormone evaluated in the current research is peptide growth and differentiation factors called AMH which plays an important role in spermatogenesis. AMH is produced by Sertoli cells which may reflect its critical effects in normal testicular function and spermatogenesis (39). In the support of this fact, the role of AMH in the normal spermatogenic process through a positive relationship with semen parameters has been confirmed previously (40). However, perverse to our results, Appasamy had observed no significant correlation between AMH and sperm DFI value (41). To obtain reliable findings and eliminate the inconsistency of results, it is necessary to further examine the functional mechanism of AMH in maintenance of sperm DNA integrity in male infertilities.
5. Conclusion
In summary, the present study suggests that the fluctuations in the serum levels of mentioned hormones could adversely affect semen profile and sperm DNA integrity which lead to severe fertility problems such as OAT. Accordingly, our findings demonstrated that FSH and LH levels could be indicative of DFI levels, while testosterone and TSH are associated with sperm concentration. Based on the current results, there were also positive correlations between PRL and AMH levels with sperm DFI value. However, the possible effect of these hormones in male factor infertility, on the fertilization rate and embryo quality, needs to be evaluated.
Acknowledgments
This study was supported by the research fund of Iran University of Medical Sciences, Tehran, Iran (grant number: 13580).
Conflict of Interest
The authors declare that they have no competing interest.
Type of Study: Original Article |
Subject:
Reproductive Andrology
References
1. 1. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: A review of literature. J Hum Reprod Sci 2015; 8: 191-196.
2. 2. McLachlan RI. Approach to the patient with oligozoospermia. J Clin Endocrinol Metab 2013; 98: 873-880.
3. 3. Azad N, Nazarian H, Ghaffari Novin M, Masteri Farahani R, Piryaei A, Heidari MH, et al. Oligoasthenoteratozoospermic (OAT) men display altered phospholipase C ζ (PLCζ) localization and a lower percentage of sperm cells expressing PLCζ and post-acrosomal sheath WW domain-binding protein (PAWP). Bosn J Basic Med Sci 2018; 18: 178-184.
4. 4. Jimoh AAG, Olawui TS, Omotoso GO, Oyewopo AO, Dare JK. Semen parameters and hormone profile of men investigated for infertility at Midland Fertility Centre, Ilorin, Nigeria. J Basic Appl Sci 2012; 8: 110-113.
5. 5. Moslemi Mehni N, Ketabchi AA, Hosseini E. Combination effect of pentoxifylline and L-carnitine on idiopathic oligoasthenoteratozoospermia. Iran J Reprod Med 2014; 12: 817-824.
6. 6. Wosnitzer M, Goldstein M, Hardy MP. Review of azoospermia. Spermatogenesis 2014; 4: e28218.
7. 7. Darbandi M, Darbandi S, Agarwal A, Sengupta P, Durairajanayagam D, Henkel R, et al. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol 2018; 16: 87.
8. 8. Ribas-Maynou J, Benet J. Single and double strand sperm DNA damage: Different reproductive effects on male fertility. Genes 2019; 10: 105.
9. 9. Qiu Y, Yang H, Li Ch, Xu Ch. Progress in research on sperm DNA fragmentation. Med Sci Monit 2020; 26: e918746.
10. 10. Oduwole OO, Peltoketo H, Huhtaniemi IT. Role of follicle-stimulating hormone in spermatogenesis. Front Endocrinol 2018; 9: 763.
11. 11. McLachlan RI, Wreford NG, Robertson DM, de Kretser DM. Hormonal control of spermatogenesis. Trends Endocrinol Metab 1995; 6: 95-101.
12. 12. Xu H-Y, Zhang H-X, Xiao Zh, Qiao J, Li R. Regulation of anti-müllerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J Androl 2019; 21: 109-114.
13. 13. Grinspon RP, Rey RA. Anti-müllerian hormone and sertoli cell function in paediatric male hypogonadism. Horm Res Paediatr 2010; 73: 81-92.
14. 14. Ramaswamy S, Weinbauer GF. Endocrine control of spermatogenesis: Role of FSH and LH/testosterone. Spermatogenesis 2014; 4: e996025.
15. 15. Wdowiak A, Raczkiewicz D, Stasiak M, Bojar IJNL. Levels of FSH, LH and testosterone, and sperm DNA fragmentation. Neuro Endocrinol Lett 2014; 35: 73-79.
16. 16. Verdi A, Nasr-Esfahani MH, Forouzanfar M, Tavalaee M. The effect of recombinant human follicle-stimulating hormone on sperm quality, chromatin status and clinical outcomes of infertile oligozoospermic men candidate for intracytoplasmic sperm injection: A randomized clinical trial. Int J Fertil Steril 2021; 15: 1-7.
17. 17. Ghasemian F, Mirroshandel SA, Zahiri Z. Impact of hormonal changes on the semen quality and assisted reproductive outcomes in infertile men. J Appl Biomed 2017; 15: 227-232.
18. 18. Simoni M, Santi D, Negri L, Hoffmann I, Muratori M, Baldi E, et al. Treatment with human, recombinant FSH improves sperm DNA fragmentation in idiopathic infertile men depending on the FSH receptor polymorphism p. N680S: A pharmacogenetic study. Hum Reprod 2016; 31: 1960-1969.
19. 19. Bates JN, Kohn TP, Pastuszak AW. Effect of thyroid hormone derangements on sexual function in men and women. Sex Med Rev 2020; 8: 217-230.
20. 20. Wajner SM, Wagner MS, Maia AL. Clinical implications of altered thyroid status in male testicular function. Arq Bras Endocrinol Metabol 2009; 53: 976-982.
21. 21. Castañeda Cortés DC, Langlois VS, Fernandino JI. Crossover of the hypothalamic pituitary-adrenal/ interrenal, -thyroid, and -gonadal axes in testicular development. Front Endocrinol 2014; 5: 139.
22. 22. Singh P, Singh M, Cugati G, Singh AK. Hyperprolactinemia: An often missed cause of male infertility. J Hum Reprod Sci 2011; 4: 102-103.
23. 23. Gardner DK, Weissman A, Howles CM, Shoham Z. Textbook of assisted reproductive techniques. 5th Ed. USA, Florida: CRC Press; 2018.
24. 24. World Health Organization. Who laboratory manual for the examination and processing of human semen. 5th Ed. Switzerland: World Health Organization Press; 2010.
25. 25. Zandieh Z, Ashrafi M, Aflatoonian K, Aflatoonian RJA. Human sperm DNA damage has an effect on immunological interaction between spermatozoa and fallopian tube. Andrology 2019; 7: 228-234.
26. 26. Khourdaji I, Lee H, Smith RP. Frontiers in hormone therapy for male infertility. Transl Androl Urol 2018; 7 (Suppl.): S353-S366.
27. 27. Wdowiak A, Raczkiewicz D, Stasiak M, Bojar I. Levels of FSH, LH and testosterone, and sperm DNA fragmentation. Neuro Endocrinol Lett 2014; 35: 73-79.
28. 28. Lu J-Ch, Jing J, Chen L, Ge Y-F, Feng R-X, Liang Y-J, et al. Analysis of human sperm DNA fragmentation index (DFI) related factors: A report of 1010 subfertile men in China. Reprod Biol Endocrinol 2018; 16: 23.
29. 29. Colacurci N, Monti MG, Fornaro F, Izzo G, Izzo P, Trotta C, et al. Recombinant human FSH reduces sperm DNA fragmentation in men with idiopathic oligoasthenoteratozoospermia. J Androl 2012; 33: 588-593.
30. 30. Muratori M, Baldi E. Effects of FSH on sperm DNA fragmentation: Review of clinical studies and possible mechanisms of action. Front Endocrinol 2018; 9: 734.
31. 31. Colacurci N, de Leo V, Ruvolo G, Piomboni P, Caprio F, Pivonello R, et al. Recombinant FSH improves sperm DNA damage in male infertility: A phase II clinical trial. Front Endocrinol 2018; 9: 383.
32. 32. Wei T-Ch, Huang WJ, Lin ATL, Chen K-K. The role of hormones on semen parameters in patients with idiopathic or varicocele-related oligoasthenoteratozoospermia (OAT) syndrome. J Chin Med Assoc 2013; 76: 624-628.
33. 33. Trussell JC, Coward RM, Santoro N, Stetter Ch, Kunselman A, Diamond MP, et al. Association between testosterone, semen parameters, and live birth in men with unexplained infertility in an intrauterine insemination population. Fertil Steril 2019; 111: 1129-1134.
34. 34. Sharma MK, Parchwani D, Maheria P, Upadhyah A. Relationship between thyroid profile and semen quality. Natl J Commun Med 2012; 3: 20-24.
35. 35. Nikoobakht MR, Aloosh M, Nikoobakht N, Mehrsay A, Biniaz F, Karjalian MA. The role of hypothyroidism in male infertility and erectile dysfunction. Urol J 2012; 9: 405-409.
36. 36. Ralph O, Shroff N. Prolactinoma: A rare, important and treatable cause of male infertility. J Clin Urol 2019; 14: 524-526.
37. 37. Dabbous Z, Atkin SL. Hyperprolactinaemia in male infertility: Clinical case scenarios. Arab J Urol 2018; 16: 44-52.
38. 38. Keskin MZ, Budak S, Gubari S, Celik O, Yalbuzdağ O, Ilbey Y. The effect of hyperprolactinemia on semen parameters. J Clin Anal Med 2016; 7: 757-759.
39. 39. Xu H-Y, Zhang H-X, Xiao Z, Qiao J, Li R. Regulation of anti-Müllerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J Androl 2019; 21: 109-114.
40. 40. Peng L-P, Shao Y, Wang C-C, Zou Z-C, Shen T, Chen L, et al. [Correlation of serum anti-müllerian hormone with semen parameters]. Zhonghua Nan Ke Xue 2017; 23: 531-535. (in Chinese)
41. 41. Appasamy M, Muttukrishna S, Pizzey A, Ozturk O, Groome N, Serhal P, et al. Relationship between male reproductive hormones, sperm DNA damage and markers of oxidative stress in infertility. Reprod Biomed Online 2007; 14: 159-165.
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








