Sat, Feb 21, 2026
[Archive]
Volume 20, Issue 6 (June 2022)
IJRM 2022, 20(6): 501-510 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghadirkhomi E, Angaji S A, Khosravi M, Mashayekhi M R. Association study of novel single nucleotide polymorphisms of androgen receptor and estrogen receptor-α genes with male infertility in North West of Iran: a case- control study. IJRM 2022; 20 (6) :501-510
URL: http://ijrm.ir/article-1-2184-en.html
URL: http://ijrm.ir/article-1-2184-en.html
1- Department of Genetics, Faculty of Biological Science, North Tehran Branch, Islamic Azad University, Tehran, Iran.
2- Department of Cell and Molecular Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran. ,vo83ge@yahoo.com
3- Department of Biology, Faculty of Biological Sciences, North Tehran Branch, Islamic Azad University, Tehran, Iran.
4- Department of Genetics, Faculty of Biological Science, Tabriz Branch, Islamic Azad University, Tabriz, Iran.
2- Department of Cell and Molecular Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran. ,
3- Department of Biology, Faculty of Biological Sciences, North Tehran Branch, Islamic Azad University, Tehran, Iran.
4- Department of Genetics, Faculty of Biological Science, Tabriz Branch, Islamic Azad University, Tabriz, Iran.
Full-Text [PDF 287 kb]
(1284 Downloads)
| Abstract (HTML) (1945 Views)
Full-Text: (435 Views)
1. Introduction
Infertility is a universally common issue defined as a failure to achieve pregnancy after a year of unprotected intercourse (1). According to the World Health Organization report, around 60-80 million couples in reproductive age suffer from infertility worldwide (2).
Although a complex network of environmental factors and genetic aberrations play a crucial role in the etiology of male infertility, the most possible cause of impaired spermatogenesis is unknown (3). In the case of spermatogenesis, androgen receptor (AR) mediates the androgens signaling in the testis. It has been pinpointed that disruption of the AR-signaling pathway results in a disturbance of maintenance of spermatogonial numbers, blood-testis barrier integrity, completion of meiosis, adhesion of spermatids, and spermiation (4, 5). Due to the fundamental effects of AR in spermatogenesis, various gene polymorphisms of AR concerning male infertility were studied. Several meta-analyses have reported the association between AR gene CAG repeat length polymorphism and male infertility (6-9). Despite these conclusions, some population-based primary studies did not find any significant associations between AR gene CAG as well as GGN repeat length polymorphisms and infertility among their male population (8, 10, 11).
However, the current local survey in southwest Iran has revealed that GGN repeat length polymorphisms are one important polymorphism leading to male infertility among Iranian population (12). These findings have led to the hypothesis that other polymorphisms in the AR gene should be examined which might be considered a risk factor for male infertility. Another hormonal receptor that was studied is estrogen receptor alpha (ER-α). Female hormones and their receptor gene polymorphisms, in particular estrogen receptors, have been challenged in elucidating their role in male infertility (13). It should be borne in mind that similar to AR, ERs also contribute to several steps of spermatogenesis such as modulating sperm metabolism, reduced testicular size, and severe oligospermia. Besides, gene expression of ER significantly reduces in Sertoli cell-only tests (14). Evidence has added new significant findings in the association between ER gene polymorphism especially p and x alleles and increased male infertility (10, 15).
Most of the previous studies have focused on some limited ER gene polymorphisms and additional information is needed on the association between ER polymorphisms and male infertility. Considering the high infertility rate among men, especially Iranian, understanding the main genetic factors, in particular sex hormone-related contributors, might shed light on the cause and mechanisms of male infertility which can be used in therapeutic strategies. We are aware of the lack of data on various hormonal gene polymorphisms and the risk of male infertility. Therefore, it seems that other observational studies are needed to elucidate novel gene polymorphism of AR and ER-α and their causative role in therisk of infertility among men.
The present case-control study, therefore, examined the association of different polymorphisms of AR and ER-α gene with male infertility among the Iranian population.
2. Materials and Methods
2.1. Participants
This population-based, case-control study was carried out in 120 infertile men aged 25-45 yr presenting at the infertility treatment center of Valiasr hospital, Tabriz, Iran from March 2018 to July 2019. All cases were of idiopathic azoospermia or severe oligospermia (sperm count < 5 × 106/ml) and their azoospermia was diagnosed and confirmed by a specialist. Participants with any identified causes were excluded from the study. Hence, all infertile cases were assessed for physical examination and all necessary hormonal and genetic tests including karyotyping, micro-deletion of the Y chromosome, and cystic fibrosis transmembrane conductance regulator mutations. The controls were 25-45 yr old and included 120 healthy men who had normal semen tests and at least one child at the time of the study. To determine the quality and quantity of sperm samples, semen analysis was done twice for all participants using microscopic examination methods according to the World Health Organization standard values (16).
2.2. Extraction of peripheral blood DNA
Blood samples were collected in an Ethylenediaminetetraacetic acid‐containing vacutainer tubes (Greiner Bio-One, Germany) from 240 randomly chosen individuals. Samples were stored at -80ºC and the genomic DNA was extracted from all participants’ blood samples using rapid extract polymerase chain reaction (PCR) kit (PCR bio Systems Company, UK) according to the manufacturer’s protocol. The concentration and quality of extracted DNA were measured at 260 nm and 280 nm (A260/280) using a Nano Drop 2000 spectrophotometer (Ds-11 spectrophotometer, Denovix company, USA).
2.3. Genotyping
The allele-specific PCR or amplification refractory mutation system method was applied to identify the genetic polymorphisms. Genotype analysis for ER-α gene was done according to tetra-primer amplification refractory mutation system PCR method. PCR primer sets were designed and optimized using primer 3 version 4.1.0 and primer 1 software. The quality and specificity of primers were analyzed by Oligo Analyzer software and primer blast on the NCBI website. To amplify the out target genes, a ready-to-use mastermix was used with a volume of 13 μL (UK. PCR Biosystems. Ltd.). The PCR reactions were performed with a final volume of 25 μL. PCR products were analyzed by 2% gel electrophoresis using novel juice stain (Cat.No.LD001-1000, GeneDireX, Taiwan). Samples were re-genotyped to confirm the results.
2.4. AR single nucleotide polymorphisms (SNPs)
The ARA>T (rs137852568) was amplified by using common reverse primer 5`-ATC TGAAAGGGGGCATGAGC- 3`, wild type forward primer 5`-GTTCACTTTTGACCTGCTAAT AA-3`, and mutant type forward primer 5`-GTT CAC TTT TGA CCT GCT AAAT-3`. The cycling condition for wild-type amplification was 95oC for 3 min for the first cycle, 95oC for 30 sec, 57oC for 30 sec, 72oC for 1 min for 35 cycles, and the final extensional time of 7 min at 72oC. All cycling conditions for mutant and wild types were the same. The primers used to amplify AR A>C (rs137852599) were as follows: common forward primer 5`-AGT CTC TCTTCCTTCCCA ATA G-3`, wild type reverse primer 5`-TCAGGCTGGTTGTTGTCG T-3`, and mutant type reverse primer 5`-TCAGTCTGGTTGTTGTCG G-3`. The cycling condition for all primers was 95oC for 3 min for the first cycle, 95oC for 30 sec, 55oC for 30 sec, 72oC for 30 sec for 30 cycles, and the final extension in 72oC for 5 min. Primers for AR G>A (rs137852563) were as follows: common reverse primer 5`-GGG CAG AAA AGC ACC AGA CA-3`, wild type forward primer 5`-GCT TGT ACA CGT GGT CAA GTT G-3`, and mutant forward primer 5`-GCT TGT ACA CGT GGT CAA GTT A-3`. PCR conditions for both wild and mutant types were 95oC for 3 min at the first cycle, 95oC for 45 sec, 58oC for 30 sec, and 72oC for 1 min for 30 cycles. The final extension was at 72oC for 5 min. Sizes of PCR products were 185, 224, and 132 base pairs (bp) for rs137852568, rs137852599, and rs137852563 polymorphisms, respectively.
2.5. ER-α SNPs
To amplify the ER-α A>G (rs796065354), 4 primers were used as follows: forward inner primer 5`-CTG ACCAAACGCTCTAAG AA-3`, reverse inner primer 5`-ACAAAGCCAGGCTGT TCC-3`, forward outer primer 5`-GCAGGGATACGAAAAGAC C-3`, and reverse outer primer 5`-CTTACC TGGCACCCTCTT C-3`. The cycling condition for all primers wasin this manner: for the first cycle, 95oC for 5 min, then 95oC for 40 sec, 55oC for 35 sec, 72oC for 45 sec, for 29 cycles, and final extension at 72oC for 7 min for all types of primers. Other polymorphism of ER-α C>T, (rs104893956), was amplified using forward inner primer 5`-CCAGGCAAACTTCAGATAATC- 3`, reverse inner primer 5`-TTCTTACACCCTGGCGTC A-3`, forward outer primer 5`-ACA CAA TTT CCC CTC AAG G- 3`, and reverse outer primer 5`-TCTCTTAGGATCTGCTCATAG G-3`with the cycling condition 95oC for 5 min for the first cycle, 95oC for 30 sec, 55oC for 30 sec, 72oC for 45 sec for 29 cycles, and the final extension time of 7 min at 72oC. The cycling conditions for all primer types were the same. The rs104893956 polymorphism PCR products were 443, 252, and 230 bp for forwarding outer and reverse outer, forward inner and reverse outer, and reverse inner and forward outer, respectively. The PCR products of rs104893956 polymorphism were as follows: 345, 213, and 169 bp for outer forward and outer reverse, inner forward and outer reverse, and outer forward and inner reverse, respectively.
2.6. Ethical considerations
This study was conducted following the ethical principles, the national norms, relevant guidelines, and regulations for conducting Medical Research in Iran. Written informed consent was obtained from all eligible subjects. The project was approved by the Science and Research Branch of Tehran’s Islamic Azad University Ethical Committee, Tehran, Iran (Code: IR.IAU.SRB.REC.1398.001).
2.7. Statistical analysis
The frequency of genotypes in the case and control groups were evaluated for Hardy-Weinberg equilibrium (HWE) and Chi-square was calculated. The association of the SNPs of AR and ESR with male infertility was assessed by using conditional logistic regression. Statistical analyses were carried out using SPSS version 22 (IBM, Armonk, NY). P-values ˂ 0.05 were considered significant.
3. Results
Concentration of extracted DNAs was in the range of 77-247 ng/μL. Obtained OD 260/280 was in the range of 1.78-1.89.
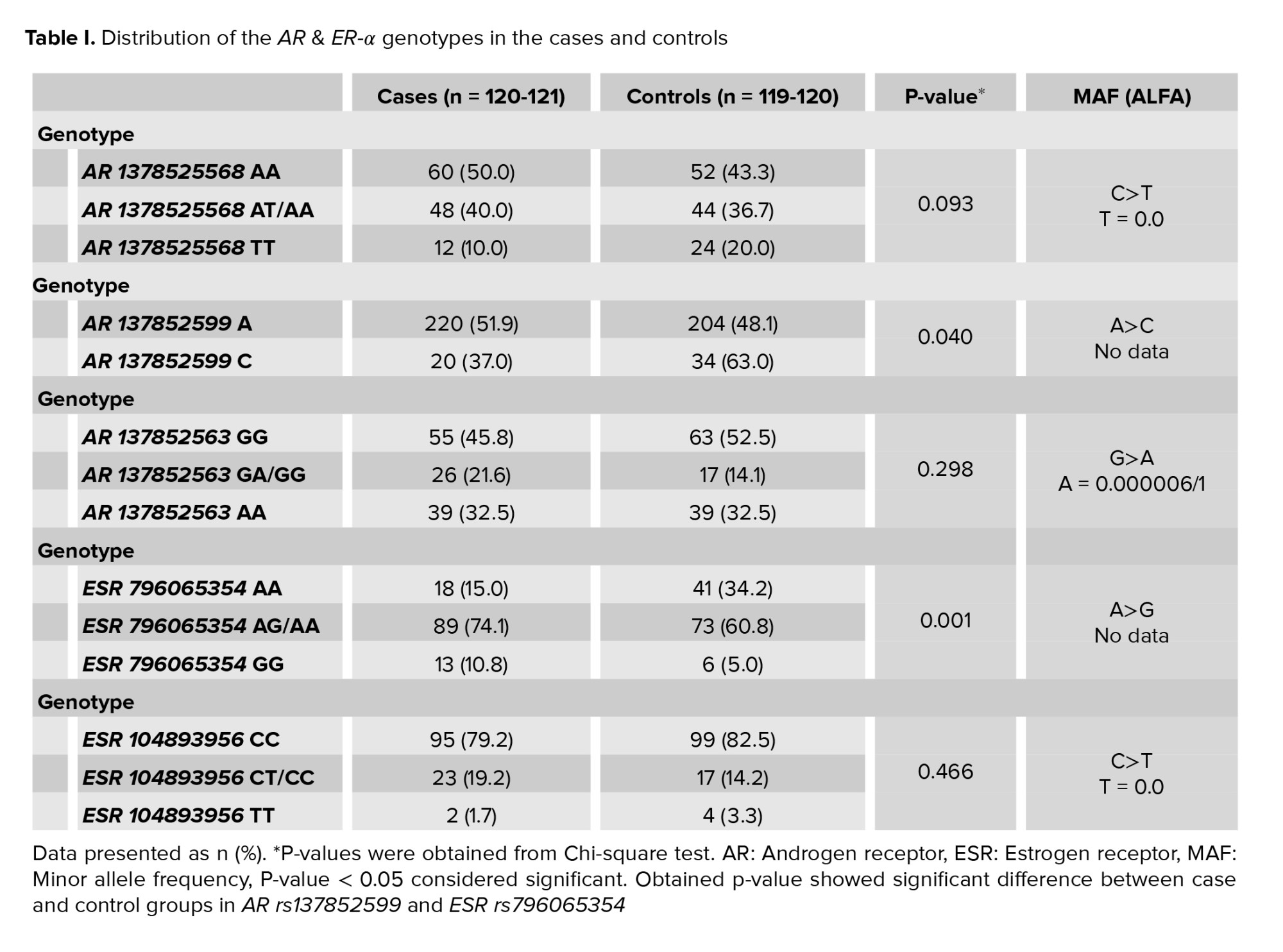
Table I shows the genotypic frequencies of AR and ER-α variants in fertile and infertile men. A total of 3 variants for the AR gene and 2 variants for the ER-α gene were identified. There were no significant differences between the 2 groups in terms of AR rs1378525568 and rs137852563 variants and ER-α gene rs104893956, however, there was a significant difference between cases and controls in terms of ER-α rs796065354 (p = 0.001) and AR rs137852599 (p = 0.040).
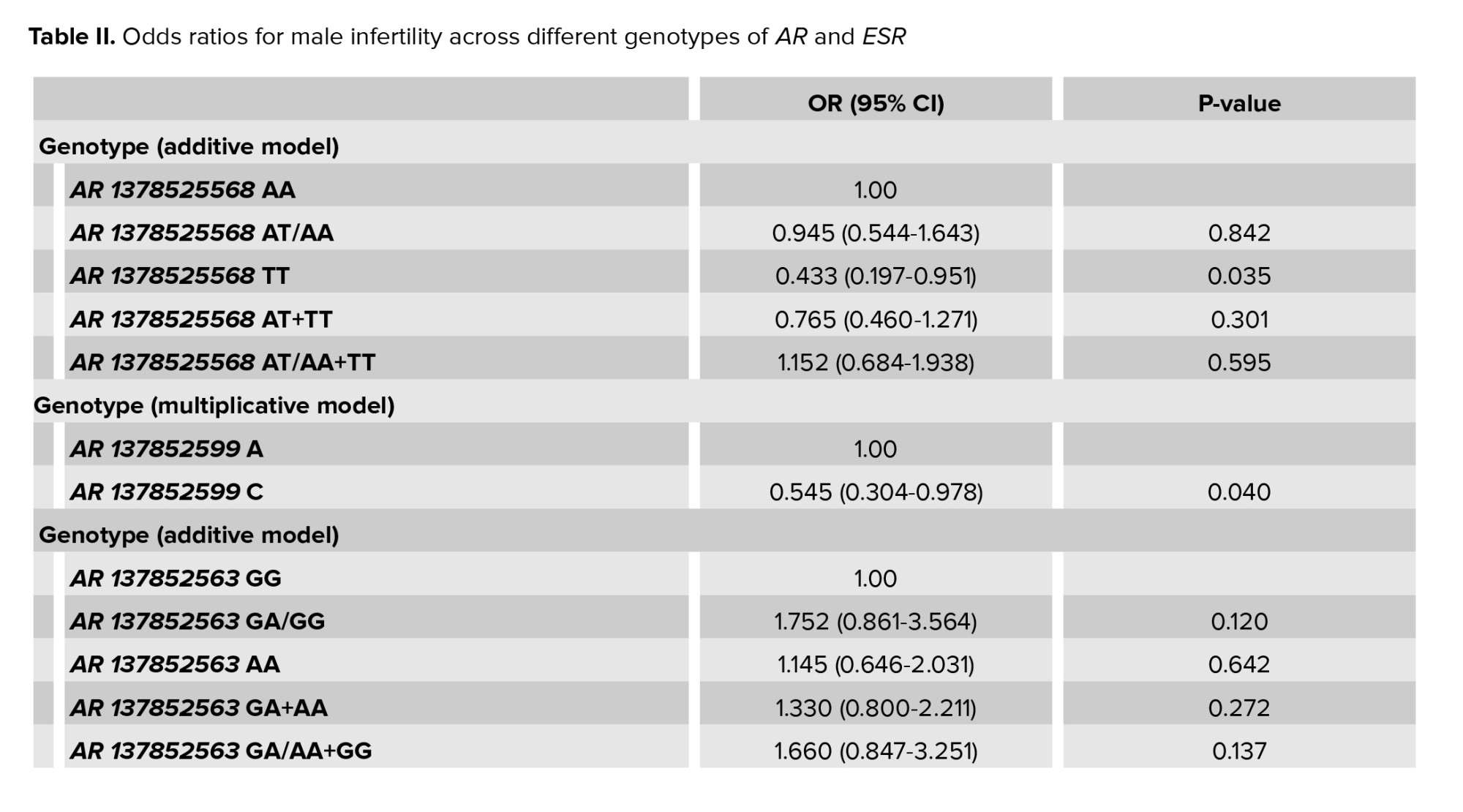
The associations of AR and ER-α polymorphisms with male infertility are shown in table II. We observed a significant association between ARrs1378525568 TT genotype and odds of idiopathic azoospermiaor severe oligospermia compared with normal homozygote (p: 0.035, OR: 0.433, CI: 0.197-0.951). The HWE examination test revealed that this equilibrium for AR rs1378525568 (A>T) has been disrupted in healthy controls not in cases (Chi2 = 6.0449, and 0.2721, respectively) and the additive pattern was considered. To examine whether HWE in a group is disrupted or not, calculated Chi-square was compared to statistics table Chi-square. If the calculated Chi-square was bigger than the statistics table Chi-square (3.8), the H0 hypothesis will be rejected which means HWE is disrupted in that group, and otherwise H1 hypothesis will be rejected that means the group is in HWE. In terms of AR rs137852599, there was a significant association between the C allele (recessive allele) and odds of male infertility compared with the dominant allele (OR: 0.545, CI: 0.304-0.978). Men with this polymorphism were at a 45% decreased risk of infertility. The examination of HWE for AR rs137852599 (A>C) indicated that both controls and cases were in HWE (Chi2 = 1.384, and 1.9438, respectively). Therefore, statistical analysis was performed based on the multiplicative pattern. There were no significant associations between AR rs137852563 genotypes and infertility risk among men compared to normal homozygote. For rs137852563 (G>A), the HWE has been disrupted in 2 study groups (Chi2 = 58.6723, and 37.474 for controls and cases, respectively), therefore additive model was used for statistical analysis.
The results showed a significant association between ER-α rs796065354 genotypes (AG/AA, GG, GA+GG, and GA/GG+A) and the risk of idiopathic azoospermiaor severe oligospermia compared to normal homozygote. For ER-αrs796065354 (A>G); none of the cases or controls were in HWE (Chi2 =28.3334, and 13.0515, respectively) that showed the additive pattern. Heterozygote over dominance was also observed in this SNP. In terms of ER-α rs104893956, there was no significant relationship between any genotypes of CT/CC, TT, CT+TT, and CT/TT+CC and male infertility compared with normal homozygote. The HWE examination test for ER-α rs104893956 (C>T) revealed that this equilibrium has been disrupted in healthy controls not in cases (Chi2 = 6.9657, and 0.1936, respectively) and the additive pattern was considered.

4. Discussion
Due to the fundamental effects of genetic contributors on the etiology of infertility, examining the SNPs is of high priority. Among the genes involving the fertilization process, in particular, in men, hormone-related genes and their receptors due to multifunctional properties in male physiology and spermatogenesis are strongly targeted in various infertility studies. AR as one of the interesting receptors has been studied frequently and its role in spermatogenesis, the survival of germ cells, and their maturation is well-defined (5, 17).
The results of this population-based, case-control study suggested that AR rs1378525568 TT genotype and AR rs137852599 C allele have a protective effect on fertility and may reduce idiopathic azoospermia or severe oligospermia risk. We also observed an association between ER-α rs796065354 genotypes (AG/AA, GG, GA+GG, and GA/GG+A) and odds of infertility among men. However, they failed to find any significant association between other polymorphisms of AR and ER-α genes with the risk of infertility among our study population. To the best of our knowledge, this study is among the first case-control studies examining the association between specific polymorphisms of AR and ER-α genes with the risk of male infertility among the Iranian population.
Most meta-analysis studies have concluded that the increase in CAG repeat lengthin ARis associated with male infertility (6-9). However, the findings of up-to-date meta-analysis which were classified by region and sub-types of male infertility were inconsistent. It seems that Caucasian populations are more susceptible to this polymorphism due to AR dysfunctions (7-9). We found for the first time, an inverse association between AR rs1378525568 TT genotype and AR rs137852599 C allele with male infertility. Most previous studies have focused on CAG repeat length polymorphism, which their results were in contrast to our findings (12, 18, 19). Besides, some studies failed to find any significant relation between AR polymorphisms and odds of various sub-types of male infertility (20). However, similar to other reports, we failed to find a significant association between other alleles of AR rs1378525568 as well as AR rs137852599 and AR rs137852563 variants and fertilization ability of men. It should be mentioned that previous data on AR polymorphisms have been limited to the CAG repeat length polymorphism, so previous findings differ from ours which has studied novel polymorphisms. Moreover, some basic differences concerning environmental factors, study population, and sample size might contribute to the conflicting findings between studies. Overall, it is suggested that further studies are required to investigate the relationship between different types of AR polymorphism and male reproductive function.
Despite estrogens and their receptors (ERs; including ER-α, ER-β, and ER-γ) conventionally regarded as female hormones, there is a wide range of investigation showing their profound effects on the male reproductive system (3). In the case of infertility, the exact physiological role of estrogens in spermatogenesis is not clearly understood. However, based on recent studies, they are regarded as a “survival factor”. According to this concept, the absence of ER-α results in reducing the epididymis sperm content, sperm motility, and fertilizing ability (21, 22). It seems that ER-α confers a stronger estrogen effect. We found, as a first case-control study, ER-α rs796065354 genotypes (AG/AA, GG, GA+GG and GA/GG+A) might increase the odds of male infertility. Similar to AR, ER-α gene polymorphisms investigated in previous observational studies were different from the polymorphisms we studied. However, it has been reported that the (TA)n repeat polymorphism may negatively influence spermatogenesis (22). Some studies demonstrated that XbaI and PvuII polymorphisms of the ER-α gene are associated with the risk of male infertility (15, 23). ER-α gene polymorphism might affect sperm quality in men. ER-α 397T/T and ER-α 351A/A genotypes were associated with lower sperm motility in men with oligozoospermia (24). Semen variables including sperm count, motility, velocity, and morphology as well as sperm acrosin activity were significantly higher among infertile oligoasthenoteratozoospermia men who had pp and xx genotype (15). Nevertheless, we found no significant relation between ER-α rs104893956 genotype and idiopathic azoospermia or severe oligospermia among our study population. It should be brought in mind that our studied polymorphisms were completely different from other studies; therefore, it may lead to some inconsistency. Also, some controversial findings might be a reflection of the difference in study design and population. Similar to AR, it is suggested to conduct more observational studies to elucidate the association between different types of ER-α genotypes with male infertility.
The exact mechanisms through which AR and ER-α polymorphisms influence male infertility risk are complex and not well-established. Given the role of AR and ER-α in spermatogenesis and male infertility, one might assume that binding of the AR- androgen complex to HSPs and the subsequent transfer of the complex to the nucleolus modify the transcription of various genes involved in spermatogenesis (5). Such classic genomic action was considered to ER-α where translocation of ER-α-estrogen complex to the nucleolus, and its interaction with DNA-binding elements in the genome, alters the expression of steroid-responsive genes (25).
Being the first report on novel polymorphism of AR and ER-αassociation with odds of male reproductive function as well as age-matched design that led to control the age-dependent conflictions are the strength of the present study. However, some limitations should be considered in the interpretation of our findings. Control genotypes should be in HWE, as long as the population they are selected from is random and large in size. A significant result showing that controls are not in HWE could arise because of 1) random chance: One of every 20 markers tested will give a p-value < 0.05 by chance; 2) incorrect genotyping; and 3) heterogeneous population. Provided the controls are in HWE, the cases may then be tested. If theSNP has a true genetic effect that is not controlled by a multiplicative model, the cases will not be in HWE. Although the test has little power to detect small departures from HWE (26).
Environmental factors, smoking, high-risk jobs, residence in high-risk areas, etc. are some confounders that were not included in this study. Therefore, the generalizability of the findings should be done cautiously.
5. Conclusion
In conclusion, we found pieces of evidence of an association between novel polymorphisms of AR and ER-α and male infertility among the Iranian population. Based on the results, AR rs1378525568 TT genotype and AR rs137852599 C allele may have a protective effect on male fertility. In contrast, there was a significant association between predisposition polymorphism ESR-α rs796065354 and male infertility that may be considered as a biomarker for male infertility. However, further studies with different ethnic populations and larger samples are needed to validate the findings.
Acknowledgements
The authors are thankful to Dr. Danaei for her help in sampling and medical diagnosis, and to Ms. Beikzadeh for data analysis. This research received no specific grant from any funding agency in the public, commercial, or not-profit sectors.
Conflict of Interest
The authors declare that they have no competing interests.
Infertility is a universally common issue defined as a failure to achieve pregnancy after a year of unprotected intercourse (1). According to the World Health Organization report, around 60-80 million couples in reproductive age suffer from infertility worldwide (2).
Although a complex network of environmental factors and genetic aberrations play a crucial role in the etiology of male infertility, the most possible cause of impaired spermatogenesis is unknown (3). In the case of spermatogenesis, androgen receptor (AR) mediates the androgens signaling in the testis. It has been pinpointed that disruption of the AR-signaling pathway results in a disturbance of maintenance of spermatogonial numbers, blood-testis barrier integrity, completion of meiosis, adhesion of spermatids, and spermiation (4, 5). Due to the fundamental effects of AR in spermatogenesis, various gene polymorphisms of AR concerning male infertility were studied. Several meta-analyses have reported the association between AR gene CAG repeat length polymorphism and male infertility (6-9). Despite these conclusions, some population-based primary studies did not find any significant associations between AR gene CAG as well as GGN repeat length polymorphisms and infertility among their male population (8, 10, 11).
However, the current local survey in southwest Iran has revealed that GGN repeat length polymorphisms are one important polymorphism leading to male infertility among Iranian population (12). These findings have led to the hypothesis that other polymorphisms in the AR gene should be examined which might be considered a risk factor for male infertility. Another hormonal receptor that was studied is estrogen receptor alpha (ER-α). Female hormones and their receptor gene polymorphisms, in particular estrogen receptors, have been challenged in elucidating their role in male infertility (13). It should be borne in mind that similar to AR, ERs also contribute to several steps of spermatogenesis such as modulating sperm metabolism, reduced testicular size, and severe oligospermia. Besides, gene expression of ER significantly reduces in Sertoli cell-only tests (14). Evidence has added new significant findings in the association between ER gene polymorphism especially p and x alleles and increased male infertility (10, 15).
Most of the previous studies have focused on some limited ER gene polymorphisms and additional information is needed on the association between ER polymorphisms and male infertility. Considering the high infertility rate among men, especially Iranian, understanding the main genetic factors, in particular sex hormone-related contributors, might shed light on the cause and mechanisms of male infertility which can be used in therapeutic strategies. We are aware of the lack of data on various hormonal gene polymorphisms and the risk of male infertility. Therefore, it seems that other observational studies are needed to elucidate novel gene polymorphism of AR and ER-α and their causative role in therisk of infertility among men.
The present case-control study, therefore, examined the association of different polymorphisms of AR and ER-α gene with male infertility among the Iranian population.
2. Materials and Methods
2.1. Participants
This population-based, case-control study was carried out in 120 infertile men aged 25-45 yr presenting at the infertility treatment center of Valiasr hospital, Tabriz, Iran from March 2018 to July 2019. All cases were of idiopathic azoospermia or severe oligospermia (sperm count < 5 × 106/ml) and their azoospermia was diagnosed and confirmed by a specialist. Participants with any identified causes were excluded from the study. Hence, all infertile cases were assessed for physical examination and all necessary hormonal and genetic tests including karyotyping, micro-deletion of the Y chromosome, and cystic fibrosis transmembrane conductance regulator mutations. The controls were 25-45 yr old and included 120 healthy men who had normal semen tests and at least one child at the time of the study. To determine the quality and quantity of sperm samples, semen analysis was done twice for all participants using microscopic examination methods according to the World Health Organization standard values (16).
2.2. Extraction of peripheral blood DNA
Blood samples were collected in an Ethylenediaminetetraacetic acid‐containing vacutainer tubes (Greiner Bio-One, Germany) from 240 randomly chosen individuals. Samples were stored at -80ºC and the genomic DNA was extracted from all participants’ blood samples using rapid extract polymerase chain reaction (PCR) kit (PCR bio Systems Company, UK) according to the manufacturer’s protocol. The concentration and quality of extracted DNA were measured at 260 nm and 280 nm (A260/280) using a Nano Drop 2000 spectrophotometer (Ds-11 spectrophotometer, Denovix company, USA).
2.3. Genotyping
The allele-specific PCR or amplification refractory mutation system method was applied to identify the genetic polymorphisms. Genotype analysis for ER-α gene was done according to tetra-primer amplification refractory mutation system PCR method. PCR primer sets were designed and optimized using primer 3 version 4.1.0 and primer 1 software. The quality and specificity of primers were analyzed by Oligo Analyzer software and primer blast on the NCBI website. To amplify the out target genes, a ready-to-use mastermix was used with a volume of 13 μL (UK. PCR Biosystems. Ltd.). The PCR reactions were performed with a final volume of 25 μL. PCR products were analyzed by 2% gel electrophoresis using novel juice stain (Cat.No.LD001-1000, GeneDireX, Taiwan). Samples were re-genotyped to confirm the results.
2.4. AR single nucleotide polymorphisms (SNPs)
The ARA>T (rs137852568) was amplified by using common reverse primer 5`-ATC TGAAAGGGGGCATGAGC- 3`, wild type forward primer 5`-GTTCACTTTTGACCTGCTAAT AA-3`, and mutant type forward primer 5`-GTT CAC TTT TGA CCT GCT AAAT-3`. The cycling condition for wild-type amplification was 95oC for 3 min for the first cycle, 95oC for 30 sec, 57oC for 30 sec, 72oC for 1 min for 35 cycles, and the final extensional time of 7 min at 72oC. All cycling conditions for mutant and wild types were the same. The primers used to amplify AR A>C (rs137852599) were as follows: common forward primer 5`-AGT CTC TCTTCCTTCCCA ATA G-3`, wild type reverse primer 5`-TCAGGCTGGTTGTTGTCG T-3`, and mutant type reverse primer 5`-TCAGTCTGGTTGTTGTCG G-3`. The cycling condition for all primers was 95oC for 3 min for the first cycle, 95oC for 30 sec, 55oC for 30 sec, 72oC for 30 sec for 30 cycles, and the final extension in 72oC for 5 min. Primers for AR G>A (rs137852563) were as follows: common reverse primer 5`-GGG CAG AAA AGC ACC AGA CA-3`, wild type forward primer 5`-GCT TGT ACA CGT GGT CAA GTT G-3`, and mutant forward primer 5`-GCT TGT ACA CGT GGT CAA GTT A-3`. PCR conditions for both wild and mutant types were 95oC for 3 min at the first cycle, 95oC for 45 sec, 58oC for 30 sec, and 72oC for 1 min for 30 cycles. The final extension was at 72oC for 5 min. Sizes of PCR products were 185, 224, and 132 base pairs (bp) for rs137852568, rs137852599, and rs137852563 polymorphisms, respectively.
2.5. ER-α SNPs
To amplify the ER-α A>G (rs796065354), 4 primers were used as follows: forward inner primer 5`-CTG ACCAAACGCTCTAAG AA-3`, reverse inner primer 5`-ACAAAGCCAGGCTGT TCC-3`, forward outer primer 5`-GCAGGGATACGAAAAGAC C-3`, and reverse outer primer 5`-CTTACC TGGCACCCTCTT C-3`. The cycling condition for all primers wasin this manner: for the first cycle, 95oC for 5 min, then 95oC for 40 sec, 55oC for 35 sec, 72oC for 45 sec, for 29 cycles, and final extension at 72oC for 7 min for all types of primers. Other polymorphism of ER-α C>T, (rs104893956), was amplified using forward inner primer 5`-CCAGGCAAACTTCAGATAATC- 3`, reverse inner primer 5`-TTCTTACACCCTGGCGTC A-3`, forward outer primer 5`-ACA CAA TTT CCC CTC AAG G- 3`, and reverse outer primer 5`-TCTCTTAGGATCTGCTCATAG G-3`with the cycling condition 95oC for 5 min for the first cycle, 95oC for 30 sec, 55oC for 30 sec, 72oC for 45 sec for 29 cycles, and the final extension time of 7 min at 72oC. The cycling conditions for all primer types were the same. The rs104893956 polymorphism PCR products were 443, 252, and 230 bp for forwarding outer and reverse outer, forward inner and reverse outer, and reverse inner and forward outer, respectively. The PCR products of rs104893956 polymorphism were as follows: 345, 213, and 169 bp for outer forward and outer reverse, inner forward and outer reverse, and outer forward and inner reverse, respectively.
2.6. Ethical considerations
This study was conducted following the ethical principles, the national norms, relevant guidelines, and regulations for conducting Medical Research in Iran. Written informed consent was obtained from all eligible subjects. The project was approved by the Science and Research Branch of Tehran’s Islamic Azad University Ethical Committee, Tehran, Iran (Code: IR.IAU.SRB.REC.1398.001).
2.7. Statistical analysis
The frequency of genotypes in the case and control groups were evaluated for Hardy-Weinberg equilibrium (HWE) and Chi-square was calculated. The association of the SNPs of AR and ESR with male infertility was assessed by using conditional logistic regression. Statistical analyses were carried out using SPSS version 22 (IBM, Armonk, NY). P-values ˂ 0.05 were considered significant.
3. Results
Concentration of extracted DNAs was in the range of 77-247 ng/μL. Obtained OD 260/280 was in the range of 1.78-1.89.
Table I shows the genotypic frequencies of AR and ER-α variants in fertile and infertile men. A total of 3 variants for the AR gene and 2 variants for the ER-α gene were identified. There were no significant differences between the 2 groups in terms of AR rs1378525568 and rs137852563 variants and ER-α gene rs104893956, however, there was a significant difference between cases and controls in terms of ER-α rs796065354 (p = 0.001) and AR rs137852599 (p = 0.040).
The associations of AR and ER-α polymorphisms with male infertility are shown in table II. We observed a significant association between ARrs1378525568 TT genotype and odds of idiopathic azoospermiaor severe oligospermia compared with normal homozygote (p: 0.035, OR: 0.433, CI: 0.197-0.951). The HWE examination test revealed that this equilibrium for AR rs1378525568 (A>T) has been disrupted in healthy controls not in cases (Chi2 = 6.0449, and 0.2721, respectively) and the additive pattern was considered. To examine whether HWE in a group is disrupted or not, calculated Chi-square was compared to statistics table Chi-square. If the calculated Chi-square was bigger than the statistics table Chi-square (3.8), the H0 hypothesis will be rejected which means HWE is disrupted in that group, and otherwise H1 hypothesis will be rejected that means the group is in HWE. In terms of AR rs137852599, there was a significant association between the C allele (recessive allele) and odds of male infertility compared with the dominant allele (OR: 0.545, CI: 0.304-0.978). Men with this polymorphism were at a 45% decreased risk of infertility. The examination of HWE for AR rs137852599 (A>C) indicated that both controls and cases were in HWE (Chi2 = 1.384, and 1.9438, respectively). Therefore, statistical analysis was performed based on the multiplicative pattern. There were no significant associations between AR rs137852563 genotypes and infertility risk among men compared to normal homozygote. For rs137852563 (G>A), the HWE has been disrupted in 2 study groups (Chi2 = 58.6723, and 37.474 for controls and cases, respectively), therefore additive model was used for statistical analysis.
The results showed a significant association between ER-α rs796065354 genotypes (AG/AA, GG, GA+GG, and GA/GG+A) and the risk of idiopathic azoospermiaor severe oligospermia compared to normal homozygote. For ER-αrs796065354 (A>G); none of the cases or controls were in HWE (Chi2 =28.3334, and 13.0515, respectively) that showed the additive pattern. Heterozygote over dominance was also observed in this SNP. In terms of ER-α rs104893956, there was no significant relationship between any genotypes of CT/CC, TT, CT+TT, and CT/TT+CC and male infertility compared with normal homozygote. The HWE examination test for ER-α rs104893956 (C>T) revealed that this equilibrium has been disrupted in healthy controls not in cases (Chi2 = 6.9657, and 0.1936, respectively) and the additive pattern was considered.



4. Discussion
Due to the fundamental effects of genetic contributors on the etiology of infertility, examining the SNPs is of high priority. Among the genes involving the fertilization process, in particular, in men, hormone-related genes and their receptors due to multifunctional properties in male physiology and spermatogenesis are strongly targeted in various infertility studies. AR as one of the interesting receptors has been studied frequently and its role in spermatogenesis, the survival of germ cells, and their maturation is well-defined (5, 17).
The results of this population-based, case-control study suggested that AR rs1378525568 TT genotype and AR rs137852599 C allele have a protective effect on fertility and may reduce idiopathic azoospermia or severe oligospermia risk. We also observed an association between ER-α rs796065354 genotypes (AG/AA, GG, GA+GG, and GA/GG+A) and odds of infertility among men. However, they failed to find any significant association between other polymorphisms of AR and ER-α genes with the risk of infertility among our study population. To the best of our knowledge, this study is among the first case-control studies examining the association between specific polymorphisms of AR and ER-α genes with the risk of male infertility among the Iranian population.
Most meta-analysis studies have concluded that the increase in CAG repeat lengthin ARis associated with male infertility (6-9). However, the findings of up-to-date meta-analysis which were classified by region and sub-types of male infertility were inconsistent. It seems that Caucasian populations are more susceptible to this polymorphism due to AR dysfunctions (7-9). We found for the first time, an inverse association between AR rs1378525568 TT genotype and AR rs137852599 C allele with male infertility. Most previous studies have focused on CAG repeat length polymorphism, which their results were in contrast to our findings (12, 18, 19). Besides, some studies failed to find any significant relation between AR polymorphisms and odds of various sub-types of male infertility (20). However, similar to other reports, we failed to find a significant association between other alleles of AR rs1378525568 as well as AR rs137852599 and AR rs137852563 variants and fertilization ability of men. It should be mentioned that previous data on AR polymorphisms have been limited to the CAG repeat length polymorphism, so previous findings differ from ours which has studied novel polymorphisms. Moreover, some basic differences concerning environmental factors, study population, and sample size might contribute to the conflicting findings between studies. Overall, it is suggested that further studies are required to investigate the relationship between different types of AR polymorphism and male reproductive function.
Despite estrogens and their receptors (ERs; including ER-α, ER-β, and ER-γ) conventionally regarded as female hormones, there is a wide range of investigation showing their profound effects on the male reproductive system (3). In the case of infertility, the exact physiological role of estrogens in spermatogenesis is not clearly understood. However, based on recent studies, they are regarded as a “survival factor”. According to this concept, the absence of ER-α results in reducing the epididymis sperm content, sperm motility, and fertilizing ability (21, 22). It seems that ER-α confers a stronger estrogen effect. We found, as a first case-control study, ER-α rs796065354 genotypes (AG/AA, GG, GA+GG and GA/GG+A) might increase the odds of male infertility. Similar to AR, ER-α gene polymorphisms investigated in previous observational studies were different from the polymorphisms we studied. However, it has been reported that the (TA)n repeat polymorphism may negatively influence spermatogenesis (22). Some studies demonstrated that XbaI and PvuII polymorphisms of the ER-α gene are associated with the risk of male infertility (15, 23). ER-α gene polymorphism might affect sperm quality in men. ER-α 397T/T and ER-α 351A/A genotypes were associated with lower sperm motility in men with oligozoospermia (24). Semen variables including sperm count, motility, velocity, and morphology as well as sperm acrosin activity were significantly higher among infertile oligoasthenoteratozoospermia men who had pp and xx genotype (15). Nevertheless, we found no significant relation between ER-α rs104893956 genotype and idiopathic azoospermia or severe oligospermia among our study population. It should be brought in mind that our studied polymorphisms were completely different from other studies; therefore, it may lead to some inconsistency. Also, some controversial findings might be a reflection of the difference in study design and population. Similar to AR, it is suggested to conduct more observational studies to elucidate the association between different types of ER-α genotypes with male infertility.
The exact mechanisms through which AR and ER-α polymorphisms influence male infertility risk are complex and not well-established. Given the role of AR and ER-α in spermatogenesis and male infertility, one might assume that binding of the AR- androgen complex to HSPs and the subsequent transfer of the complex to the nucleolus modify the transcription of various genes involved in spermatogenesis (5). Such classic genomic action was considered to ER-α where translocation of ER-α-estrogen complex to the nucleolus, and its interaction with DNA-binding elements in the genome, alters the expression of steroid-responsive genes (25).
Being the first report on novel polymorphism of AR and ER-αassociation with odds of male reproductive function as well as age-matched design that led to control the age-dependent conflictions are the strength of the present study. However, some limitations should be considered in the interpretation of our findings. Control genotypes should be in HWE, as long as the population they are selected from is random and large in size. A significant result showing that controls are not in HWE could arise because of 1) random chance: One of every 20 markers tested will give a p-value < 0.05 by chance; 2) incorrect genotyping; and 3) heterogeneous population. Provided the controls are in HWE, the cases may then be tested. If the
Environmental factors, smoking, high-risk jobs, residence in high-risk areas, etc. are some confounders that were not included in this study. Therefore, the generalizability of the findings should be done cautiously.
5. Conclusion
In conclusion, we found pieces of evidence of an association between novel polymorphisms of AR and ER-α and male infertility among the Iranian population. Based on the results, AR rs1378525568 TT genotype and AR rs137852599 C allele may have a protective effect on male fertility. In contrast, there was a significant association between predisposition polymorphism ESR-α rs796065354 and male infertility that may be considered as a biomarker for male infertility. However, further studies with different ethnic populations and larger samples are needed to validate the findings.
Acknowledgements
The authors are thankful to Dr. Danaei for her help in sampling and medical diagnosis, and to Ms. Beikzadeh for data analysis. This research received no specific grant from any funding agency in the public, commercial, or not-profit sectors.
Conflict of Interest
The authors declare that they have no competing interests.
Type of Study: Original Article |
Subject:
Reproductive Urology
References
1. Parsanezhad ME, Namavar Jahromi B, Zare N, Keramati P, Khalili A, Parsa-Nezhad M. Epidemiology and etiology of infertility in Iran: Systematic review and meta-analysis. J Womens Health, Issues Care, 2013; 2: 6. [DOI:10.4172/2325-9795.1000121]
2. Akhondi MM, Ranjbar F, Shirzad M, Behjati Ardakani Z, Kamali K, Mohammad K. Practical difficulties in estimating the prevalence of primary infertility in Iran. Int J Fertil Steril 2019; 13: 113-117.
3. Sen S, Dixit A, Thakur Ch, Gokral J, Hinduja I, Zaveri K, et al. Association of progesterone receptor gene polymorphism with male infertility and clinical outcome of ICSI. J Assist Reprodu Genet 2013; 30: 1133-1139. [DOI:10.1007/s10815-013-0074-2] [PMID] [PMCID]
4. O'Hara L, Smith LB. Androgen receptor roles in spermatogenesis and infertility. Best Pract Res Clin Endocrinol Metab 2015; 29: 595-605. [DOI:10.1016/j.beem.2015.04.006] [PMID]
5. O'Hara L, Smith LB. The genetics of androgen receptor signalling in male fertility. In: Voght PH. Genetics of human infertility. New Yok: Karger Publishers; 2017: 86-100. [DOI:10.1159/000477280]
6. Davis-Dao CA, Tuazon ED, Sokol RZ, Cortessis VK. Male infertility and variation in CAG repeat length in the androgen receptor gene: A meta-analysis. J Clin Endocrinol Metab 2007; 92: 4319-4326. [DOI:10.1210/jc.2007-1110] [PMID]
7. Xiao F, Lan A, Lin Zh, Song J, Zhang Y, Li J, et al. Impact of CAG repeat length in the androgen receptor gene on male infertility: A meta-analysis. Reprod Biomed Online 2016; 33: 39-49. [DOI:10.1016/j.rbmo.2016.03.012] [PMID]
8. Pan B, Li R, Chen Y, Tang Q, Wu W, Chen L, et al. Genetic association between androgen receptor gene CAG repeat length polymorphism and male infertility: A meta-analysis. Medicine 2016; 95: e2878. [DOI:10.1097/MD.0000000000002878] [PMID] [PMCID]
9. Mobasseri N, Babaei F, Karimian M, Nikzad H. Androgen receptor (AR)-CAG trinucleotide repeat length and idiopathic male infertility: A case-control trial and a meta-analysis. EXCLI J 2018; 17: 1167-1179.
10. Cerván-Martín M, Castilla JA, Palomino-Morales RJ, Carmona FD. Genetic landscape of nonobstructive azoospermia and new perspectives for the clinic. J Clin Med 2020; 9: 300. [DOI:10.3390/jcm9020300] [PMID] [PMCID]
11. Rivera-Diaz PA, Patricia Ortiz C, Ricardo Delgado D. The crucial role of estrogen/androgen hormones and their receptors in male infertility risk. Central Asian J Med Pharm Sci Innovat 2021; 1: 35-43.
12. Moghadam M, Khatami SR, Galehdari H. Association of androgen receptor GGN repeat length polymorphism and male infertility in Khuzestan, Iran. Iran J Reprod Med 2015; 13: 305-310.
13. Nemati H, Sadeghi M, Nazeri M, Mohammadi M. Evaluation of the association between polymorphisms of PRM1 and PRM2 and the risk of male infertility: A systematic review, meta-analysis, and meta-regression. Sci Rep 2020; 10: 17228. [DOI:10.1038/s41598-020-74233-3] [PMID] [PMCID]
14. Andrade DL, Viana MC, Esteves SC. Differential diagnosis of azoospermia in men with infertility. J Clin Med 2021; 10: 3144. [DOI:10.3390/jcm10143144] [PMID] [PMCID]
15. Zalata A, Abdalla HA, El‐Bayoumy Y, Mostafa T. Oestrogen receptor alpha gene polymorphisms relationship with semen variables in infertile men. Andrologia 2014; 46: 618-624. [DOI:10.1111/and.12123] [PMID]
16. Tournaye H, Krausz C, Oates RD. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol 2017; 5: 544-553.
https://doi.org/10.1016/S2213-8587(16)30043-2 [DOI:10.1016/S2213-8587(16)30040-7]
17. Mongan NP, Tadokoro-Cuccaro R, Bunch T, Hughes IA. Androgen insensitivity syndrome. Best Pract Res Clin Endocrinol Metab 2015; 29: 569-580. [DOI:10.1016/j.beem.2015.04.005] [PMID]
18. Ferlin A, Bartoloni L, Rizzo G, Roverato A, Garolla A, Foresta C. Androgen receptor gene CAG and GGC repeat lengths in idiopathic male infertility. Mol Hum Reprod 2004; 10: 417-421. [DOI:10.1093/molehr/gah054] [PMID]
19. Giagulli VA, Carbone MD, De Pergola G, Guastamacchia E, Resta F, Licchelli B, et al. Could androgen receptor gene CAG tract polymorphism affect spermatogenesis in men with idiopathic infertility? J Assist Reprod Genet 2014; 31: 689-697. [DOI:10.1007/s10815-014-0221-4] [PMID] [PMCID]
20. Demirkan S, Sayın DB, Gündüz Ö. CAG polymorphism in the androgen receptor gene in women may be associated with nodulocystic acne. Postepy Dermatol Alergol 2019; 36: 173-176. [DOI:10.5114/ada.2019.84592] [PMID] [PMCID]
21. Guido C, Perrotta I, Panza S, Middea E, Avena P, Santoro M, et al. Human sperm physiology: Estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) influence sperm metabolism and may be involved in the pathophysiology of varicocele‐associated male infertility. J Cell Physiol 2011; 226: 3403-3412. [DOI:10.1002/jcp.22703] [PMID]
22. Fernández R, Delgado-Zayas E, Ramírez K, Cortés-Cortés J, Gómez-Gil E, Esteva E, et al. Analysis of four polymorphisms located at the promoter of the estrogen receptor Alpha ESR1 gene in a population with gender incongruence. Sex Med 2020; 8: 490-500. [DOI:10.1016/j.esxm.2020.04.002] [PMID] [PMCID]
23. Liaqat S, Hasnain Sh, Muzammil S, Hayat S. Polymorphism analysis in estrogen receptors alpha and beta genes and their association with infertile population in Pakistan. EXCLIJ 2015; 14: 1085.
24. Lazaros LA, Xita NV, Kaponis AI, Zikopoulos KA, Plachouras NI, Georgiou IA. Estrogen receptor α and β polymorphisms are associated with semen quality. J Androl 2010; 31: 291-298. [DOI:10.2164/jandrol.109.007542] [PMID]
25. Dutta S, Sengupta P, Muhamad S. Male reproductive hormones and semen quality. Asian Pac J Reprod 2019; 8: 189-194. [DOI:10.4103/2305-0500.268132]
26. Abramovs N, Brass A, Tassabehji M. Hardy-weinberg equilibrium in the large scale genomic sequencing era. Front Genet 2020; 11: 210. [DOI:10.3389/fgene.2020.00210] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








