Sun, Feb 1, 2026
[Archive]
Volume 20, Issue 9 (September 2022)
IJRM 2022, 20(9): 779-786 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Polim A A, Handayani N, Nurputra D K, Lubis A M, Sirait B, Jacobus D, et al . Birth of spinal muscular atrophy unaffected baby from genetically at-risk parents following a pre-implantation genetic screening: A case report. IJRM 2022; 20 (9) :779-786
URL: http://ijrm.ir/article-1-2194-en.html
URL: http://ijrm.ir/article-1-2194-en.html
Arie Adrainus Polim *1 

 , Nining Handayani2
, Nining Handayani2 

 , Dian Kesumapramudya Nurputra3
, Dian Kesumapramudya Nurputra3 

 , Anggia Melanie Lubis4
, Anggia Melanie Lubis4 

 , Batara Sirait5
, Batara Sirait5 

 , Dennis Jacobus6
, Dennis Jacobus6 

 , Arief Boediono7
, Arief Boediono7 

 , Ivan Sini2
, Ivan Sini2 




 , Nining Handayani2
, Nining Handayani2 

 , Dian Kesumapramudya Nurputra3
, Dian Kesumapramudya Nurputra3 

 , Anggia Melanie Lubis4
, Anggia Melanie Lubis4 

 , Batara Sirait5
, Batara Sirait5 

 , Dennis Jacobus6
, Dennis Jacobus6 

 , Arief Boediono7
, Arief Boediono7 

 , Ivan Sini2
, Ivan Sini2 


1- Morula IVF Jakarta Clinic, Jakarta, Indonesia. IRSI Research and Training Centre, Jakarta, Indonesia. Department of Obstetrics and Gynecology, School of Medicine and Health Sciences, Atmajaya Catholic University of Indonesia, Jakarta, Indonesia. , polim.arie@irsi-bunda.org
2- Morula IVF Jakarta Clinic, Jakarta, Indonesia. IRSI Research and Training Centre, Jakarta, Indonesia.
3- Department of Child Health, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, D.I.Y Jogjakarta, Indonesia. Graduate Program of Clinical Medicine Science, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, D.I.Y Jogjakarta, Indonesia.
4- Morula IVF Jakarta Clinic, Jakarta, Indonesia.
5- Morula IVF Jakarta Clinic, Jakarta, Indonesia. IRSI Research and Training Centre, Jakarta, Indonesia. Department of Obstetrics and Gynaecology, Faculty of Medicine Universitas Kristen Indonesia, Jakarta, Indonesia.
6- Diagnos Genomics, Jakarta, Indonesia.
7- Morula IVF Jakarta Clinic, Jakarta, Indonesia. IRSI Research and Training Centre, Jakarta, Indonesia. Department of Anatomy, Physiology and Pharmacology, IPB University, Bogor, Indonesia.
2- Morula IVF Jakarta Clinic, Jakarta, Indonesia. IRSI Research and Training Centre, Jakarta, Indonesia.
3- Department of Child Health, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, D.I.Y Jogjakarta, Indonesia. Graduate Program of Clinical Medicine Science, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, D.I.Y Jogjakarta, Indonesia.
4- Morula IVF Jakarta Clinic, Jakarta, Indonesia.
5- Morula IVF Jakarta Clinic, Jakarta, Indonesia. IRSI Research and Training Centre, Jakarta, Indonesia. Department of Obstetrics and Gynaecology, Faculty of Medicine Universitas Kristen Indonesia, Jakarta, Indonesia.
6- Diagnos Genomics, Jakarta, Indonesia.
7- Morula IVF Jakarta Clinic, Jakarta, Indonesia. IRSI Research and Training Centre, Jakarta, Indonesia. Department of Anatomy, Physiology and Pharmacology, IPB University, Bogor, Indonesia.
Full-Text [PDF 1174 kb]
(1250 Downloads)
| Abstract (HTML) (1874 Views)
1. Introduction
Spinal muscular atrophy (SMA) is a monogenic neuromuscular disorder that is estimated to occur in 1 per 10.000 live births with 1 in 50 people being the genetic carrier of the disease worldwide. SMA is a homozygous autosomal recessive disorder caused by the inactivation of the survival alpha motor neuron-1 (SMN1) gene caused by the gene’s deletion or point mutation. In 95% of the cases, SMA-affected people present homozygous deletion of SMN-1 exon 7, while approximately 5% carry deleterious variants or point mutations of the gene.
The genetic alteration ultimately leads to a diminished expression of the survival motor neuron protein that is crucial in maintaining healthy functional motor neuron cells within the spinal cord. Inadequate expression of SMN protein compromises the survival and ability of the spinal motor neuron cells to transmit neural signals. Consequently, symptoms of SMA would include proximal muscle weakness, muscular atrophy, or hypotonia, which manifest mostly in the lower extremities (1).
SMN-1 and survival alpha motor neuron-2 (SMN2) genes share a considerably similar structure. The diploid nature of the human genome implies that a normal individual carries 2 copies of both the SMN1 and SMN2 genes. SMN2 gene also produces the SMN protein, but during its transcription, a nucleotide substitution at the 5’ end of exon 7 generates unstable and subsequently degraded protein transcripts. Therefore, the SMN2 gene contributes to only a limited production of functional SMN proteins. While the inactivation of the SMN1 gene is the main cause of inherited SMA, the gene copy number of SMN2 could determine the severity of the disease in which a higher SMN2 gene copy number in affected people has been associated with less severe SMA symptoms (1).
Implementation of pre-implantation genetic testing for monogenic diseases (PGT-M) through in vitro fertilization (IVF) has become a promising conception strategy for couples who are at risk of passing on an abnormal gene to their child. Following procedures of IVF such as ovarian stimulation, ovum pick-up, insemination, and embryo culture, the screening of embryos for genetic disorders can be performed to permit the transfer of genetically unaffected embryos. According to a survey study conducted at 137 IVF clinics in the United States, 82% of these clinics have offered pre-implantation genetic diagnosis for single-gene disorders (2). Furthermore, the European Society Human Reproduction and Endocrinology PGT consortium data collection XVI-XVIII reported that, in 2015, approximately 2.661 cycles of IVF-PGT-M were conducted, in which 50% were utilized to detect autosomal dominant diseases while 26% were performed to detect autosomal recessive diseases including SMA (3).
Hereby, we report the first successful birth of a healthy baby from parents who are both carriers of SMA following a combination of IVF and PGT-M program at our IVF Clinic Center.
2. Case presentation
2.1. Couple history
The couple visited Morula IVF Jakarta Clinic, Jakarta, Indonesia in October 2019 to seek an IVF expert opinion regarding PGT-M for SMA. The wife was 26 while the husband was 32 yr. Both had a normal karyotype (46XX and 46XY, respectively). Additionally, both spouses were related as cousins. A passing of a cousin was because of SMA was recorded in the husband’s family history. Previously, the couple had their 1st female baby who was diagnosed with SMA at the age of 7 months and passed away at the age of 15 months due to hypotonia and respiratory distress. SMA screening analysis of the affected 1st-born exhibited the deletion of the SMN-1 gene and the presence of 2 SMN-2 gene copies. To avoid having another child who is affected with SMA, the couple decided to consider IVF-PGT-M.
2.2. IVF and embryo biopsy
A complete hormonal basal profile measurements of the wife presented normal values (follicle-stimulating hormone; 6.71 mIU/mL, estradiol: 22.94 pg/mL, progesterone: 0.10 ng/mL, luteinizing hormone: 4.5 mIU/mL, and anti-Mullerian hormone: 2.73 ng/mL). The detected antral follicle count was 15. The wife was then subjected to a gonadotropin-releasing hormone antagonist protocol. The initial dose of gonadotropin (Gonal F, Merck Serono, Switzerland) was 225 IU which was not altered for 9 days with a total dose of 2025 IU. Daily priming injections of 0.25 mg Cetrotide (Merck KGaA, Darmstadt, Germany) started on day 6 of the menstrual cycle. Maturation trigger injection of 0.3 cc Buserelin (Suprefact, Sanofi-Aventis, Germany) was given 36 hrs before the ovum pick-up procedure. Buserelin was administered to prevent ovarian hyperstimulation syndrome caused by the high level of estradiol on trigger day (3115 pg/mL).
A total of 18 mature oocytes were retrieved and each was injected with a single spermatozoon from the husband through intracytoplasmic morphologically selected sperm injection. Thirteen oocytes were successfully fertilized and were subsequently cultured until day 5 after the injection procedure. On day 4, all developed embryos were subjected to a series of 3 4-μm diameter laser pulses (OCTAX Laser Shoot-TM) to facilitate trophectoderm (TE) herniation. On day 5, 3 top-quality blastocysts were then biopsied for the PGT-M process. During the biopsy process, protruted TE cells were lasered at the junction by a maximum of 3 pulses followed by aspiration into the biopsy pipette (Blastomere aspiration pipette; COOK Ireland, Ltd). Approximately 2-4 TE cells were eventually collected in a 0.2 mL micro-centrifuge tubes subsequent to washing in sterile phosphate-buffered saline. The embryos were then vitrified and stored in liquid nitrogen.
2.3. PGT-A and PGT-M
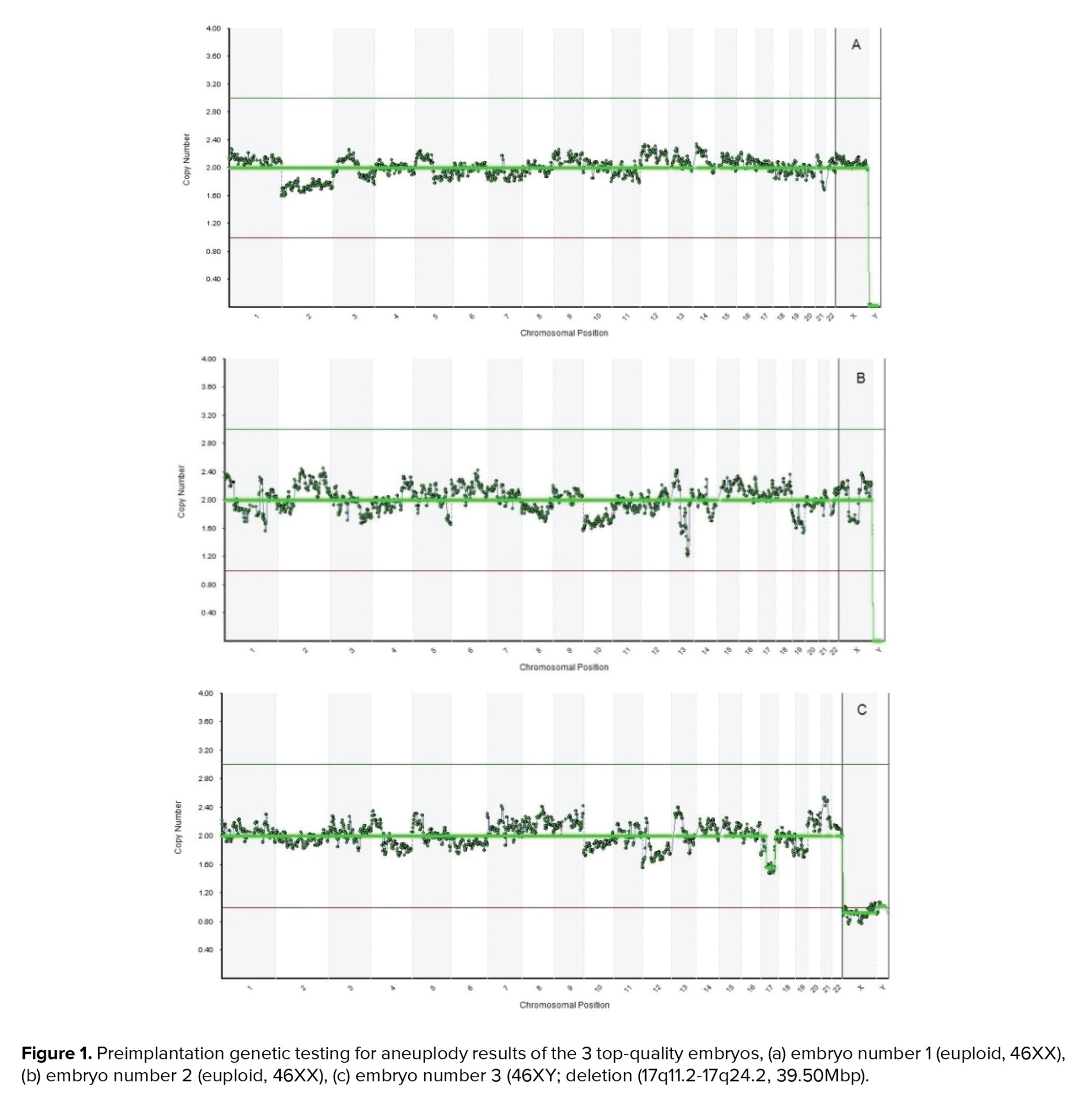
Polymerase chain reaction (PCR)-based method of whole-genome amplification (WGA) was conducted on the DNA extracted from the biopsied cells by utilizing SurePlex DNA Amplification system (Illumina, San Diego CA). Next-generation sequencing procedure using a Veriseq PGS-MiSeq kit (Illumina, USA) was utilized for the ploidy screening. Data was interpreted using the BlueFuse Multi Software V4.5 (32178) (Illumina, USA). Eventually, 2 embryos were found to be euploid (both of which were 46XX) while the remaining one was euploid with mild mosaicism (46XY; deletion (17q11.2-17q24.2, 39.50Mbp) (Figure 1).
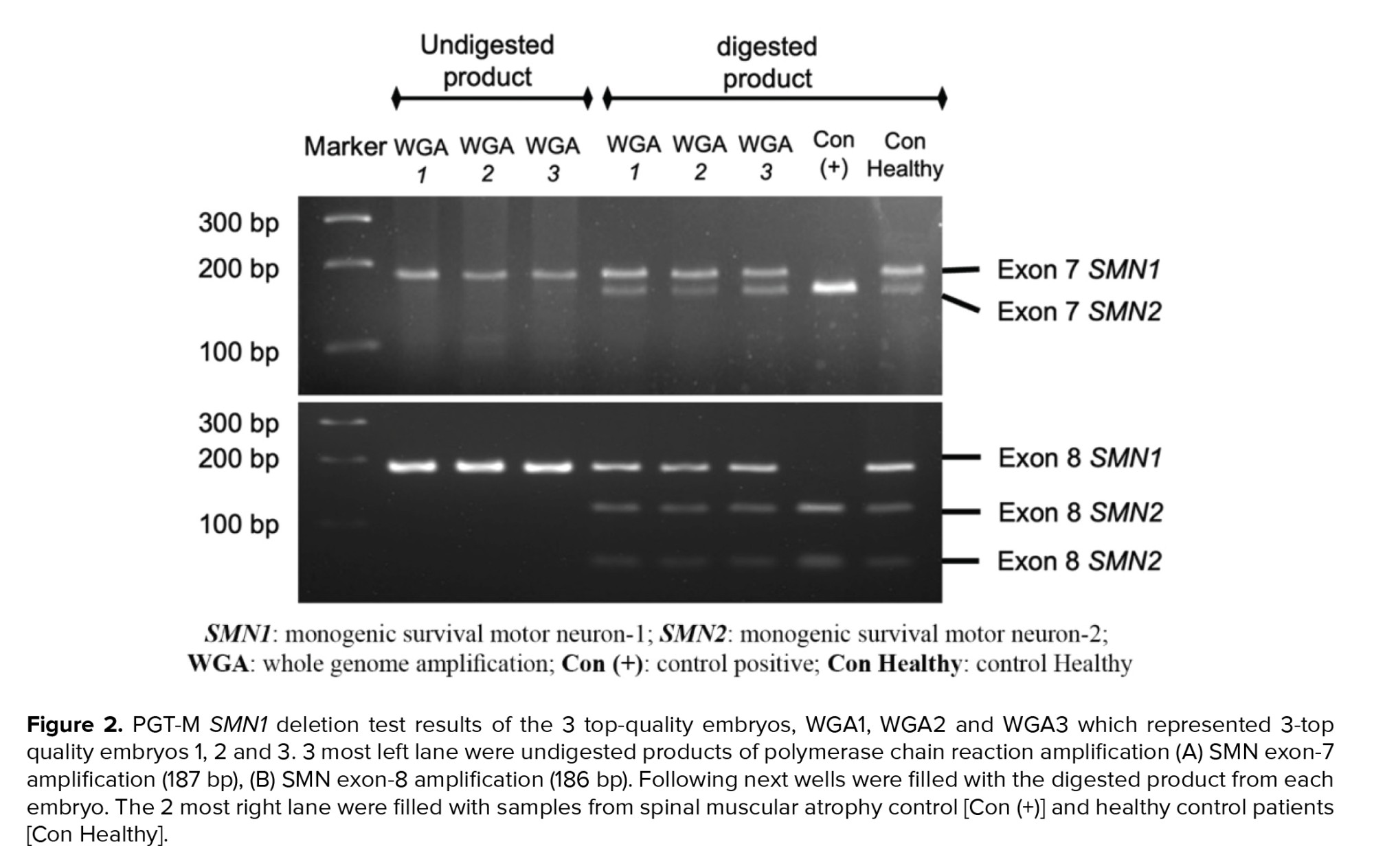
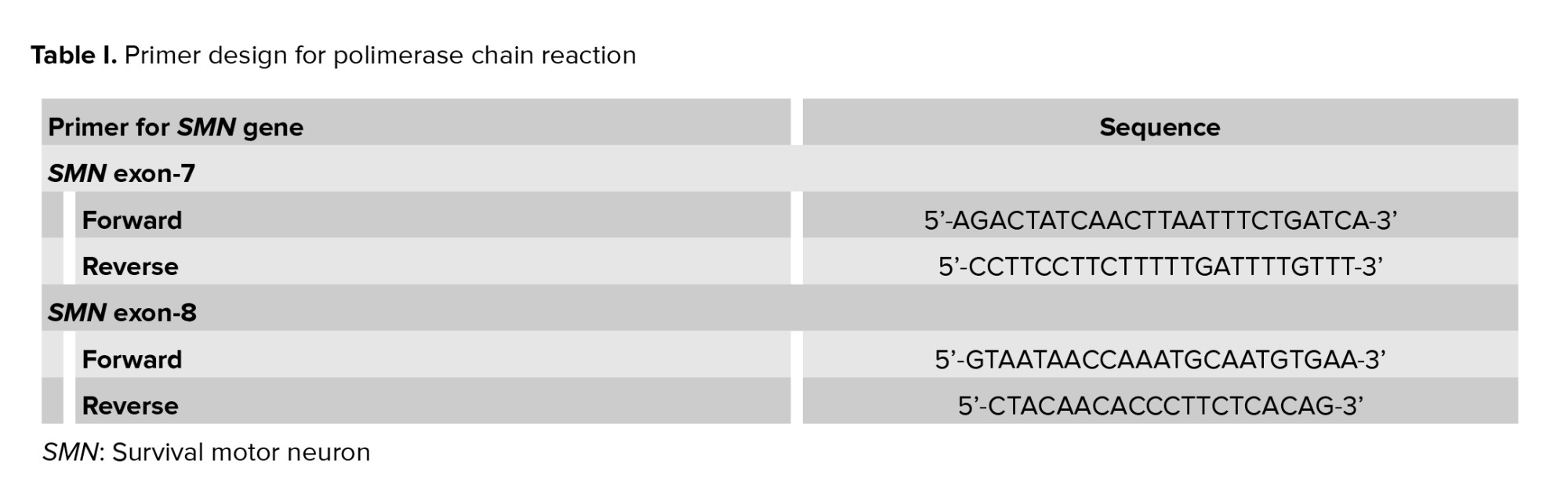
PGT-M for SMA was subsequently performed at the Genetics Laboratory of Universitas Gadjah Mada, Faculty of Medicine, Public Health and Nursing. WGA DNA samples of the embryos were tested for SMN1 and SMN2 exon 7-8 deletion using the PCR-restriction fragment length polymorphism (RFLP) method. Previously described primer sequences were designed to amplify the allele-specific SMN-1 and SMN-2 genes (Table I) (4, 5). The resulting PCR products were subsequently digested with restriction enzymes DraI and DdeI to isolate the DNA sequence of interest: exon 7 and exon 8, respectively. The digested DNA was then gel-electrophorized in 4% agarose and 3% agarose for exon 7 and exon 8, respectively. The DNA bands were then identified under ultra-violet light. The expected bands for SMN exon 7 and exon 8 after PCR were 187 bp and 186 bp. After enzymatic digestion, the SMN exon 7 band in the healthy embrio was expected to be cleaved into 2 bands, while exon 8 was cleaved into 3 bands, as shown in figure 2. SMA positive and negative control samples were provided for comparison and quality control of the PCR and digestion process. Here, SMN1 deletion was confirmed when the digestion only resulted in 1 band of SMN exon 7 and 2 bands of SMN exon 8.
The results indicated that all the embryos possessed the full-length functional sequence of SMN1 and SMN2 exon 7-8, implying that the embryos were not affected by the SMN1-deletion type SMA.
2.4. Frozen embryo transfer
After genetic counseling and discussion, the couple eventually opted to undergo a frozen embryo transfer of one of the euploid embryos (number 1) through a natural cycle. The embryo survived the thawing process and was successfully transferred into the mother’s uterus without any difficulties. Luteal support using 200 mg Utrogestan (Bezins-Belgium) was vaginally administered every 12 hr starting on the day of the embryo transfer until the beta human chorionic gonadotropin test day. Chemically tested beta human chorionic gonadotropin level was 6904.00 mIU/mL suggesting successful implantation of the embryo. Fetal growth was frequently monitored. There were no complications during the pregnancy and a healthy female baby was born at 37 wk and 6 days. Overall clinical presentation of the born baby was favorable. The weight, body length and head circumference at birth were 3020 gr, 49 cm, and 34 cm, respectively, with an Apgar score of 9/10. The baby had normal active movements, normal muscle tones, and suckling reflex. Genetic analysis of the newborn baby was carried out 21 days after birth to identify SMN1 and SMN2 exon 7-8 deletion. The PCR - RFLP results showed presence of the SMN1 exon 7-8 and SMN2 exon 7-8 which implied that the baby was not affected with the congenital SMA.
2.5. Ethical considerations
A signed informed consent was obtained from the couple for the publication of this study. The case protocol was approved by the Faculty of Medicine, University of Indonesia Ethics Committee, Jakarta, Indonesia (Code: ND-120/UN2.F1/ETIK/PPM.00.02/2021).
3. Discussion
This study presented the usefulness of the PGT-A-PGT-M service in a private IVF clinic as a screening strategy to prevent conceiving a baby who would inherit SMA disorder from parents with SMA carrier status (heterozygous deletion of SMN1). The IVF-PGT-M procedures allow early detection of the disease at the pre-implantation blastocysts stage by utilizing minimal biopsy of the TE cells. Although IVF is complex and costly, the couple was driven to go through with the program to avoid the mental anguish of overcoming possible pregnancy terminations and having another child who would suffer from the same life-threatening conditions. Geneticists and IVF specialists also took an important role during the counselling in which clear and comprehensive information concerning the possible risks and advantages of the program was given to the couple. An increasing trend of genetically at-risk couples considering PGT-M in their attempt to conceive through IVF is observed in several studies (6, 7). According to a survey study, the supply of clear information on the accuracy of PGT-M served as one of the most important factors that determined the couples’ decision to go through with the IVF/PGT-M procedure (6).
The relatively high carrier frequency of SMA has demanded the development of a reliable molecular diagnostic technique, particularly in Indonesia, to accommodate the specific needs of couples who are at risk of passing on the genetic condition to their child. Multiple genetic testing modalities for SMA have long been developed such as sequencing, quantitative PCR (qPCR), PCR- RLFP. Each of these methods, however, has its limitations and advantages (8). Notably, one of the main challenges of performing PGT-M is the limited amount of genetic sample volume for the analysis. Multiple displacement amplification was therefore suggested as a promising technique for the amplification of single or few cell samples (7).
Detection of SMN1 deletion combined with several marker-based linkage analyses that have a high predictive value for heterozygosity have also been demonstrated (9). In this case, we utilized PCR to amplify exon 7-8 of SMN followed by RFLP employing DraI and DdeI enzymes to identify the SMN1 gene exon 7-8 deletion. This method has a limitation in which the copy number of SMN1 and SMN2 genes could not be examined; thus, the SMA carrier status of the baby could not be confirmed. Additional diagnostic procedures such as quantitative PCR are essential to obtain comprehensive results. Identification of SMN1 gene exon 7 and the gene copy number could be achieved by combining PCR-RFLP with multiplex ligation-dependent probe amplification method (5, 10). However, to date, several studies have reported the effectiveness of a modified nested PCR-RFLP in depicting the copy number of the SMN1 gene.
Despite limitation, the advantages of using PCR-RFLP method for PGT-M are quite apparent, such as: 1) inter-laboratory reproducibility, 2) low cost, 3) utilization of simple standard equipment, 4) straightforward qualitative analysis without the need for a quantification procedure to determine the positive status, 5) yield of robust results while utilizing a small quantity of DNA derived from an embryo biopsy sample. Additionally, given that 95% of people who are clinically diagnosed with SMA have the undetectable exon 7 and 8 of SMN1, this method permits an efficient early diagnosis for SMA (10).
Although most embryos biopsy for PGT-M were executed at the cleavage stage (3), we believe that performing the biopsy on the blastocyst stage is more convenient, especially for developing countries such as Indonesia. Considering the great expense of IVF treatments along with the additional costs of PGT-A and PGT-M, conducting a genetic test at the cleavage-stage is less appropriate as the development of the embryos up to the blastocyst stage would not be guaranteed; patients could end up with blastocysts that are not suitable for transfer thus the outcomes of prior genetic test at the cleavage stage would be futile.
4. Conclusion
The success of our 1st IVF-PGT-M case has proven the effectiveness of the pre-implantation molecular diagnosis of SMA. Although other diagnostic tools are needed to confirm the copy number of SMN genes, detection of SMN1 gene deletion is feasible through the RFLP-PCR method.
Acknowledgements
The authors thank Aditya Adinata M.D., Ulfa Khaerani M.D., Anggraeni M.D. and Aulia Shafira M.D. from the Medical Faculty of UGM for their technical assistance in handling the PGT-M SMN1-deletion test. We also thank Arief Setiawan, M.Sc. for his assistance in providing PGT-A pictures.
Conflict of Interest
The authors declared that there is no conflict of interest.
Full-Text: (450 Views)
1. Introduction
Spinal muscular atrophy (SMA) is a monogenic neuromuscular disorder that is estimated to occur in 1 per 10.000 live births with 1 in 50 people being the genetic carrier of the disease worldwide. SMA is a homozygous autosomal recessive disorder caused by the inactivation of the survival alpha motor neuron-1 (SMN1) gene caused by the gene’s deletion or point mutation. In 95% of the cases, SMA-affected people present homozygous deletion of SMN-1 exon 7, while approximately 5% carry deleterious variants or point mutations of the gene.
The genetic alteration ultimately leads to a diminished expression of the survival motor neuron protein that is crucial in maintaining healthy functional motor neuron cells within the spinal cord. Inadequate expression of SMN protein compromises the survival and ability of the spinal motor neuron cells to transmit neural signals. Consequently, symptoms of SMA would include proximal muscle weakness, muscular atrophy, or hypotonia, which manifest mostly in the lower extremities (1).
SMN-1 and survival alpha motor neuron-2 (SMN2) genes share a considerably similar structure. The diploid nature of the human genome implies that a normal individual carries 2 copies of both the SMN1 and SMN2 genes. SMN2 gene also produces the SMN protein, but during its transcription, a nucleotide substitution at the 5’ end of exon 7 generates unstable and subsequently degraded protein transcripts. Therefore, the SMN2 gene contributes to only a limited production of functional SMN proteins. While the inactivation of the SMN1 gene is the main cause of inherited SMA, the gene copy number of SMN2 could determine the severity of the disease in which a higher SMN2 gene copy number in affected people has been associated with less severe SMA symptoms (1).
Implementation of pre-implantation genetic testing for monogenic diseases (PGT-M) through in vitro fertilization (IVF) has become a promising conception strategy for couples who are at risk of passing on an abnormal gene to their child. Following procedures of IVF such as ovarian stimulation, ovum pick-up, insemination, and embryo culture, the screening of embryos for genetic disorders can be performed to permit the transfer of genetically unaffected embryos. According to a survey study conducted at 137 IVF clinics in the United States, 82% of these clinics have offered pre-implantation genetic diagnosis for single-gene disorders (2). Furthermore, the European Society Human Reproduction and Endocrinology PGT consortium data collection XVI-XVIII reported that, in 2015, approximately 2.661 cycles of IVF-PGT-M were conducted, in which 50% were utilized to detect autosomal dominant diseases while 26% were performed to detect autosomal recessive diseases including SMA (3).
Hereby, we report the first successful birth of a healthy baby from parents who are both carriers of SMA following a combination of IVF and PGT-M program at our IVF Clinic Center.
2. Case presentation
2.1. Couple history
The couple visited Morula IVF Jakarta Clinic, Jakarta, Indonesia in October 2019 to seek an IVF expert opinion regarding PGT-M for SMA. The wife was 26 while the husband was 32 yr. Both had a normal karyotype (46XX and 46XY, respectively). Additionally, both spouses were related as cousins. A passing of a cousin was because of SMA was recorded in the husband’s family history. Previously, the couple had their 1st female baby who was diagnosed with SMA at the age of 7 months and passed away at the age of 15 months due to hypotonia and respiratory distress. SMA screening analysis of the affected 1st-born exhibited the deletion of the SMN-1 gene and the presence of 2 SMN-2 gene copies. To avoid having another child who is affected with SMA, the couple decided to consider IVF-PGT-M.
2.2. IVF and embryo biopsy
A complete hormonal basal profile measurements of the wife presented normal values (follicle-stimulating hormone; 6.71 mIU/mL, estradiol: 22.94 pg/mL, progesterone: 0.10 ng/mL, luteinizing hormone: 4.5 mIU/mL, and anti-Mullerian hormone: 2.73 ng/mL). The detected antral follicle count was 15. The wife was then subjected to a gonadotropin-releasing hormone antagonist protocol. The initial dose of gonadotropin (Gonal F, Merck Serono, Switzerland) was 225 IU which was not altered for 9 days with a total dose of 2025 IU. Daily priming injections of 0.25 mg Cetrotide (Merck KGaA, Darmstadt, Germany) started on day 6 of the menstrual cycle. Maturation trigger injection of 0.3 cc Buserelin (Suprefact, Sanofi-Aventis, Germany) was given 36 hrs before the ovum pick-up procedure. Buserelin was administered to prevent ovarian hyperstimulation syndrome caused by the high level of estradiol on trigger day (3115 pg/mL).
A total of 18 mature oocytes were retrieved and each was injected with a single spermatozoon from the husband through intracytoplasmic morphologically selected sperm injection. Thirteen oocytes were successfully fertilized and were subsequently cultured until day 5 after the injection procedure. On day 4, all developed embryos were subjected to a series of 3 4-μm diameter laser pulses (OCTAX Laser Shoot-TM) to facilitate trophectoderm (TE) herniation. On day 5, 3 top-quality blastocysts were then biopsied for the PGT-M process. During the biopsy process, protruted TE cells were lasered at the junction by a maximum of 3 pulses followed by aspiration into the biopsy pipette (Blastomere aspiration pipette; COOK Ireland, Ltd). Approximately 2-4 TE cells were eventually collected in a 0.2 mL micro-centrifuge tubes subsequent to washing in sterile phosphate-buffered saline. The embryos were then vitrified and stored in liquid nitrogen.
2.3. PGT-A and PGT-M
Polymerase chain reaction (PCR)-based method of whole-genome amplification (WGA) was conducted on the DNA extracted from the biopsied cells by utilizing SurePlex DNA Amplification system (Illumina, San Diego CA). Next-generation sequencing procedure using a Veriseq PGS-MiSeq kit (Illumina, USA) was utilized for the ploidy screening. Data was interpreted using the BlueFuse Multi Software V4.5 (32178) (Illumina, USA). Eventually, 2 embryos were found to be euploid (both of which were 46XX) while the remaining one was euploid with mild mosaicism (46XY; deletion (17q11.2-17q24.2, 39.50Mbp) (Figure 1).
PGT-M for SMA was subsequently performed at the Genetics Laboratory of Universitas Gadjah Mada, Faculty of Medicine, Public Health and Nursing. WGA DNA samples of the embryos were tested for SMN1 and SMN2 exon 7-8 deletion using the PCR-restriction fragment length polymorphism (RFLP) method. Previously described primer sequences were designed to amplify the allele-specific SMN-1 and SMN-2 genes (Table I) (4, 5). The resulting PCR products were subsequently digested with restriction enzymes DraI and DdeI to isolate the DNA sequence of interest: exon 7 and exon 8, respectively. The digested DNA was then gel-electrophorized in 4% agarose and 3% agarose for exon 7 and exon 8, respectively. The DNA bands were then identified under ultra-violet light. The expected bands for SMN exon 7 and exon 8 after PCR were 187 bp and 186 bp. After enzymatic digestion, the SMN exon 7 band in the healthy embrio was expected to be cleaved into 2 bands, while exon 8 was cleaved into 3 bands, as shown in figure 2. SMA positive and negative control samples were provided for comparison and quality control of the PCR and digestion process. Here, SMN1 deletion was confirmed when the digestion only resulted in 1 band of SMN exon 7 and 2 bands of SMN exon 8.
The results indicated that all the embryos possessed the full-length functional sequence of SMN1 and SMN2 exon 7-8, implying that the embryos were not affected by the SMN1-deletion type SMA.
2.4. Frozen embryo transfer
After genetic counseling and discussion, the couple eventually opted to undergo a frozen embryo transfer of one of the euploid embryos (number 1) through a natural cycle. The embryo survived the thawing process and was successfully transferred into the mother’s uterus without any difficulties. Luteal support using 200 mg Utrogestan (Bezins-Belgium) was vaginally administered every 12 hr starting on the day of the embryo transfer until the beta human chorionic gonadotropin test day. Chemically tested beta human chorionic gonadotropin level was 6904.00 mIU/mL suggesting successful implantation of the embryo. Fetal growth was frequently monitored. There were no complications during the pregnancy and a healthy female baby was born at 37 wk and 6 days. Overall clinical presentation of the born baby was favorable. The weight, body length and head circumference at birth were 3020 gr, 49 cm, and 34 cm, respectively, with an Apgar score of 9/10. The baby had normal active movements, normal muscle tones, and suckling reflex. Genetic analysis of the newborn baby was carried out 21 days after birth to identify SMN1 and SMN2 exon 7-8 deletion. The PCR - RFLP results showed presence of the SMN1 exon 7-8 and SMN2 exon 7-8 which implied that the baby was not affected with the congenital SMA.
2.5. Ethical considerations
A signed informed consent was obtained from the couple for the publication of this study. The case protocol was approved by the Faculty of Medicine, University of Indonesia Ethics Committee, Jakarta, Indonesia (Code: ND-120/UN2.F1/ETIK/PPM.00.02/2021).



3. Discussion
This study presented the usefulness of the PGT-A-PGT-M service in a private IVF clinic as a screening strategy to prevent conceiving a baby who would inherit SMA disorder from parents with SMA carrier status (heterozygous deletion of SMN1). The IVF-PGT-M procedures allow early detection of the disease at the pre-implantation blastocysts stage by utilizing minimal biopsy of the TE cells. Although IVF is complex and costly, the couple was driven to go through with the program to avoid the mental anguish of overcoming possible pregnancy terminations and having another child who would suffer from the same life-threatening conditions. Geneticists and IVF specialists also took an important role during the counselling in which clear and comprehensive information concerning the possible risks and advantages of the program was given to the couple. An increasing trend of genetically at-risk couples considering PGT-M in their attempt to conceive through IVF is observed in several studies (6, 7). According to a survey study, the supply of clear information on the accuracy of PGT-M served as one of the most important factors that determined the couples’ decision to go through with the IVF/PGT-M procedure (6).
The relatively high carrier frequency of SMA has demanded the development of a reliable molecular diagnostic technique, particularly in Indonesia, to accommodate the specific needs of couples who are at risk of passing on the genetic condition to their child. Multiple genetic testing modalities for SMA have long been developed such as sequencing, quantitative PCR (qPCR), PCR- RLFP. Each of these methods, however, has its limitations and advantages (8). Notably, one of the main challenges of performing PGT-M is the limited amount of genetic sample volume for the analysis. Multiple displacement amplification was therefore suggested as a promising technique for the amplification of single or few cell samples (7).
Detection of SMN1 deletion combined with several marker-based linkage analyses that have a high predictive value for heterozygosity have also been demonstrated (9). In this case, we utilized PCR to amplify exon 7-8 of SMN followed by RFLP employing DraI and DdeI enzymes to identify the SMN1 gene exon 7-8 deletion. This method has a limitation in which the copy number of SMN1 and SMN2 genes could not be examined; thus, the SMA carrier status of the baby could not be confirmed. Additional diagnostic procedures such as quantitative PCR are essential to obtain comprehensive results. Identification of SMN1 gene exon 7 and the gene copy number could be achieved by combining PCR-RFLP with multiplex ligation-dependent probe amplification method (5, 10). However, to date, several studies have reported the effectiveness of a modified nested PCR-RFLP in depicting the copy number of the SMN1 gene.
Despite limitation, the advantages of using PCR-RFLP method for PGT-M are quite apparent, such as: 1) inter-laboratory reproducibility, 2) low cost, 3) utilization of simple standard equipment, 4) straightforward qualitative analysis without the need for a quantification procedure to determine the positive status, 5) yield of robust results while utilizing a small quantity of DNA derived from an embryo biopsy sample. Additionally, given that 95% of people who are clinically diagnosed with SMA have the undetectable exon 7 and 8 of SMN1, this method permits an efficient early diagnosis for SMA (10).
Although most embryos biopsy for PGT-M were executed at the cleavage stage (3), we believe that performing the biopsy on the blastocyst stage is more convenient, especially for developing countries such as Indonesia. Considering the great expense of IVF treatments along with the additional costs of PGT-A and PGT-M, conducting a genetic test at the cleavage-stage is less appropriate as the development of the embryos up to the blastocyst stage would not be guaranteed; patients could end up with blastocysts that are not suitable for transfer thus the outcomes of prior genetic test at the cleavage stage would be futile.
4. Conclusion
The success of our 1st IVF-PGT-M case has proven the effectiveness of the pre-implantation molecular diagnosis of SMA. Although other diagnostic tools are needed to confirm the copy number of SMN genes, detection of SMN1 gene deletion is feasible through the RFLP-PCR method.
Acknowledgements
The authors thank Aditya Adinata M.D., Ulfa Khaerani M.D., Anggraeni M.D. and Aulia Shafira M.D. from the Medical Faculty of UGM for their technical assistance in handling the PGT-M SMN1-deletion test. We also thank Arief Setiawan, M.Sc. for his assistance in providing PGT-A pictures.
Conflict of Interest
The authors declared that there is no conflict of interest.
Type of Study: Case Report |
Subject:
Reproductive Genetics
References
1. Wirth B, Karakaya M, Kye MJ, Mendoza-Ferreira N. Twenty-five years of spinal muscular atrophy research: From phenotype to genotype to therapy, and what comes next. Annu Rev Genomics Hum Genet 2020; 21: 231-261. [DOI:10.1146/annurev-genom-102319-103602] [PMID]
2. Stern HJ. Preimplantation genetic diagnosis: Prenatal testing for embryos finally achieving its potential. J Clin Med 2014; 3: 280-309. [DOI:10.3390/jcm3010280] [PMID] [PMCID]
3. Coonen E, van Montfoort A, Carvalho F, Kokkali G, Moutou C, Rubio C, et al. ESHRE PGT consortium data collection XVI-XVIII: Cycles from 2013 to 2015. Hum Reprod Open 2020; 2020: hoaa043. [DOI:10.1093/hropen/hoaa043] [PMID] [PMCID]
4. Niba ETE, Rochmah MA, Harahap NIF, Awano H, Morioka I, Iijima K, et al. Spinal muscular atrophy: New screening system with real-time mCOP-PCR and PCR-RFLP for SMN1 deletion. Kobe J Med Sci 2019; 65: 44-48.
5. Rashnonejad A, Onay H, Atik T, Atan Sahin O, Gokben S, Tekgul H, et al. Molecular genetic analysis of survival motor neuron gene in 460 Turkish cases with suspicious spinal muscular atrophy disease. Iran J Child Neurol 2016; 10: 30-35.
6. Lee I, Alur-Gupta S, Gallop R, Dokras A. Utilization of preimplantation genetic testing for monogenic disorders. Fertil Steril 2020; 114: 854-860. [DOI:10.1016/j.fertnstert.2020.05.045] [PMID]
7. Fu Y, Shen X, Wu H, Chen D, Zhou C. Preimplantation genetic testing for monogenic disease of spinal muscular atrophy by multiple displacement amplification: 11 unaffected livebirths. Int J Med Sci 2019; 16: 1313-1319. [DOI:10.7150/ijms.32319] [PMID] [PMCID]
8. Hamzi K, Bellayou H, Itri M, Nadifi S. PCR-RFLP, sequencing, and quantification in molecular diagnosis of spinal muscular atrophy: Limits and advantages. J Mol Neurosci 2013; 50: 270-274. [DOI:10.1007/s12031-012-9944-9] [PMID]
9. Zhao M, Lian M, Cheah FSH, Tan ASC, Agarwal A, Chong SS. Identification of novel microsatellite markers flanking the SMN1 and SMN2 duplicated region and inclusion into a single-tube tridecaplex panel for haplotype-based preimplantation genetic testing of spinal muscular atrophy. Front Genet 2019; 10: 1105. [DOI:10.3389/fgene.2019.01105] [PMID] [PMCID]
10. Liu Zh, Zhang P, He X, Liu Sh, Tang Sh, Zhang R, et al. New multiplex real-time PCR approach to detect gene mutations for spinal muscular atrophy. BMC Neurol 2016; 16: 141. [DOI:10.1186/s12883-016-0651-y] [PMID] [PMCID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





