Tue, Jul 15, 2025
[Archive]
Volume 20, Issue 1 (January 2022)
IJRM 2022, 20(1): 21-28 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jamal A, Naemi M, Eslamian L, Marsoosi V, Moghfeghi M, Nurzadeh M, et al . The association between fetal renal artery indices in late pregnancy and birth weight in gestational diabetes mellitus: A cohort study. IJRM 2022; 20 (1) :21-28
URL: http://ijrm.ir/article-1-2198-en.html
URL: http://ijrm.ir/article-1-2198-en.html
Ashraf Jamal1 

 , Mahsa Naemi1
, Mahsa Naemi1 

 , Laleh Eslamian1
, Laleh Eslamian1 

 , Vajiheh Marsoosi1
, Vajiheh Marsoosi1 

 , Maryam Moghfeghi2
, Maryam Moghfeghi2 

 , Maryam Nurzadeh1
, Maryam Nurzadeh1 

 , Taraneh Geran3
, Taraneh Geran3 

 , Marjan Ghaemi4
, Marjan Ghaemi4 

 , Leila Zanbagh *5
, Leila Zanbagh *5 




 , Mahsa Naemi1
, Mahsa Naemi1 

 , Laleh Eslamian1
, Laleh Eslamian1 

 , Vajiheh Marsoosi1
, Vajiheh Marsoosi1 

 , Maryam Moghfeghi2
, Maryam Moghfeghi2 

 , Maryam Nurzadeh1
, Maryam Nurzadeh1 

 , Taraneh Geran3
, Taraneh Geran3 

 , Marjan Ghaemi4
, Marjan Ghaemi4 

 , Leila Zanbagh *5
, Leila Zanbagh *5 


1- Fetomaternal Department, Shariati Hospital Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute, Tehran, Iran.
3- Department of Obstetrics and Gynecology, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran.
4- Vali-e-Asr Reproductive Health Research Center, Tehran University of Medical Sciences, Tehran, Iran.
5- Fetomaternal Department, Shariati Hospital Tehran University of Medical Sciences, Tehran, Iran. ,GHAEMIMARJAN59@GMAIL.COM
2- Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute, Tehran, Iran.
3- Department of Obstetrics and Gynecology, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran.
4- Vali-e-Asr Reproductive Health Research Center, Tehran University of Medical Sciences, Tehran, Iran.
5- Fetomaternal Department, Shariati Hospital Tehran University of Medical Sciences, Tehran, Iran. ,
Keywords: Fetus, Gestational diabetes mellitus, Infant, Middle cerebral artery, Renal artery, Doppler ultrasound, Umbilical artery.
Full-Text [PDF 271 kb]
(1415 Downloads)
| Abstract (HTML) (1814 Views)
Full-Text: (590 Views)
1. Introduction
Gestational diabetes mellitus (GDM) is one of the most common endocrine diseases and it affects around 7-10% of all pregnancies worldwide (1). Prevalence rates vary due to the local screening practices and diagnostic criteria as well as population characteristics such as body mass index, maternal age, history of type II diabetes, and ethnicity (2). GDM is associated with adverse maternal and neonatal outcomes (3). Doppler vascular ultrasound is a non-invasive method that can evaluate vascular impedance distal to the area.
The placenta transports oxygen and nutrients to the fetus. For evaluation of the fetus blood supply, hemodynamic parameters such as the systolic/diastolic ratio (S/D), the pulsatility index (PI), and the resistance index (RI) play an important role (4). The kidney is one of the main organs of the fetus that is sensitive to hypoxia (5). The resistance of the renal artery may increase in intrauterine growth restriction (IUGR). Therefore, evaluation of the renal artery indices, such as the PI, RI and S/D, provide valuable information on renal vessels. Few studies have focused on fetal renal arteries in women with GDM and there is no consensus on the validity of their parameters (6).
Therefore, the current study sought to compare in late pregnancy, in women with vs. without GDM, the correlations of hemodynamic indices (S/D, PI, and RI) of the umbilical artery, middle cerebral artery, and renal artery and fetal growth with the newborn birth weight. The findings could then be used to determine whether fetal hemodynamic indices can assist clinicians in estimating newborn birth weight and possibly facilitate early detection of hypoxia in fetuses.
2. Materials and Methods
2.1. Participants
This cohort study was performed in Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran from January to December 2020. Two hundred forty-six pregnant women in their late pregnancy period who were referred for ultrasound were divided into two groups. GDM group: the women with GDM, diagnosed by blood test at 24-28 weeks of gestational age, treated with insulin or managed with diet; and the control group: women without GDM. The inclusion criteria were women aged 18-40 yr with singleton viable pregnancy and gestational age from 37 to 40 wk. In the GDM group, women newly identified with GDM according to the 2018 diagnostic criteria of the American Diabetes Association were recruited. GDM was diagnosed by a two-hour oral glucose tolerance test at 24-28 wk of gestational age. A diagnosis was made when any of the following was met or exceeded: fasting glucose level of 92 mg/dl, one-hour level of 180 mg/dl, and/or two-hour level of 153 mg/dl (7). Exclusion criteria in both groups were: multiple pregnancies, pre-gestational diabetes mellitus, preeclampsia (pregnancy-induced hypertension), the use of alcohol/cigarettes, IUGR, or other well-known conditions affecting fetal blood flow. Participants underwent physical examinations and were investigated for fasting blood glucose level, two-hour postprandial blood glucose level, and HbA1C at 37 wk. Participants with a fasting glucose level below 92 mg/dl, an HbA1C below 6, and a 2hPP below 120 mg/dl were defined as having well-controlled GDM. The age, gestational age, mode of delivery, birth weight, Apgar score, umbilical arterial blood pH, neonatal blood glucose level, and neonatal intensive care unit admission were evaluated in both groups.
2.2. Ultrasound assessment
Transabdominal 2D ultrasound was performed for all participants to assess biometry and amniotic fluid index with the exclusion of congenital fetal malformations. Ultrasound assessments were performed by one perinatologist using the Affiniti 70 ultrasound machine (Philips, the Netherlands) and a C6-2 convex probe with a frequency of 2-6 MHz. All recordings were obtained weekly from 37 wk of gestation until delivery, and the last records were evaluated in the analysis. All Doppler measurements were done in the time of lack of fetal movements and breathing (8).
Fetal biometrics included biparietal diameter, femur length, abdominal circumference, head circumference, and fetal weight, were calculated according to Hadlock’s formula (9).
The color flow pattern was selected to measure the RI, PI, and S/D of the umbilical, middle cerebral, and renal arteries. This measurement was performed for the umbilical artery 5 cm apart prom placenta, in the way that the angle between blood flow and the ultrasound beam below 20o.
For MCA Doppler, the ultrasound probe was located toward the brain basement membrane to see a pair of alisphenoids in the distance of the anterior and middle cranial fossa to reveal the Willis circle (10). The sampling volume (2 mm) was located in the proximal of the third portion of the mentioned artery, just after its origin Willis circle (11).
The evaluation of the fetal renal artery, were done in the coronal plane near to the renal hilum, and the angle between blood flow and the ultrasound beam below 20o. The Doppler indices mean of two renal arteries was applied in the final analysis. For the final analysis, the mean of five serial Doppler velocities were used (10).
2.3. Sample size calculation
To obtain a correlation coefficient of at least 0.25 between birth weight and fetal renal artery Doppler hemodynamic indices, with an error of the first type equal to 0.05 and a power of 80%, the required sample size for each group was estimated to be 123.
2.4. Ethical considerations
The study protocol was approved by the Institutional Review Board of Tehran University of Medical Sciences, Teheran, Iran (Code: IR.TUMS.MEDICINE.REC.1399.758). Written informed consent was obtained from all participants before the study. This study was conducted according to the principles of the Helsinki Declaration.
2.5. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 19.0 (IBM, USA). Continuous data were compared between the two groups with the independent t test (if normally distributed) or Mann-Whitney U test (if non-normally distributed). The categorical variables were compared with the Chi-square test. The Pearson or Spearman's rho correlation analysis was carried out to assess the linear relationship between fetal growth and Doppler hemodynamic indices with neonatal birth weight. A two-tailed p < 0.05 was considered significant.
3. Results
Table I shows the comparison of maternal, fetal, and obstetric characteristics of the GDM and control groups. Fetal growth indices and estimated fetal weight were not significantly different between the two groups. The number of days between the last ultrasound and delivery was four in the GDM group and three in the control group, with a range of zero to seven days and without any significant difference between the groups (p = 0.34).
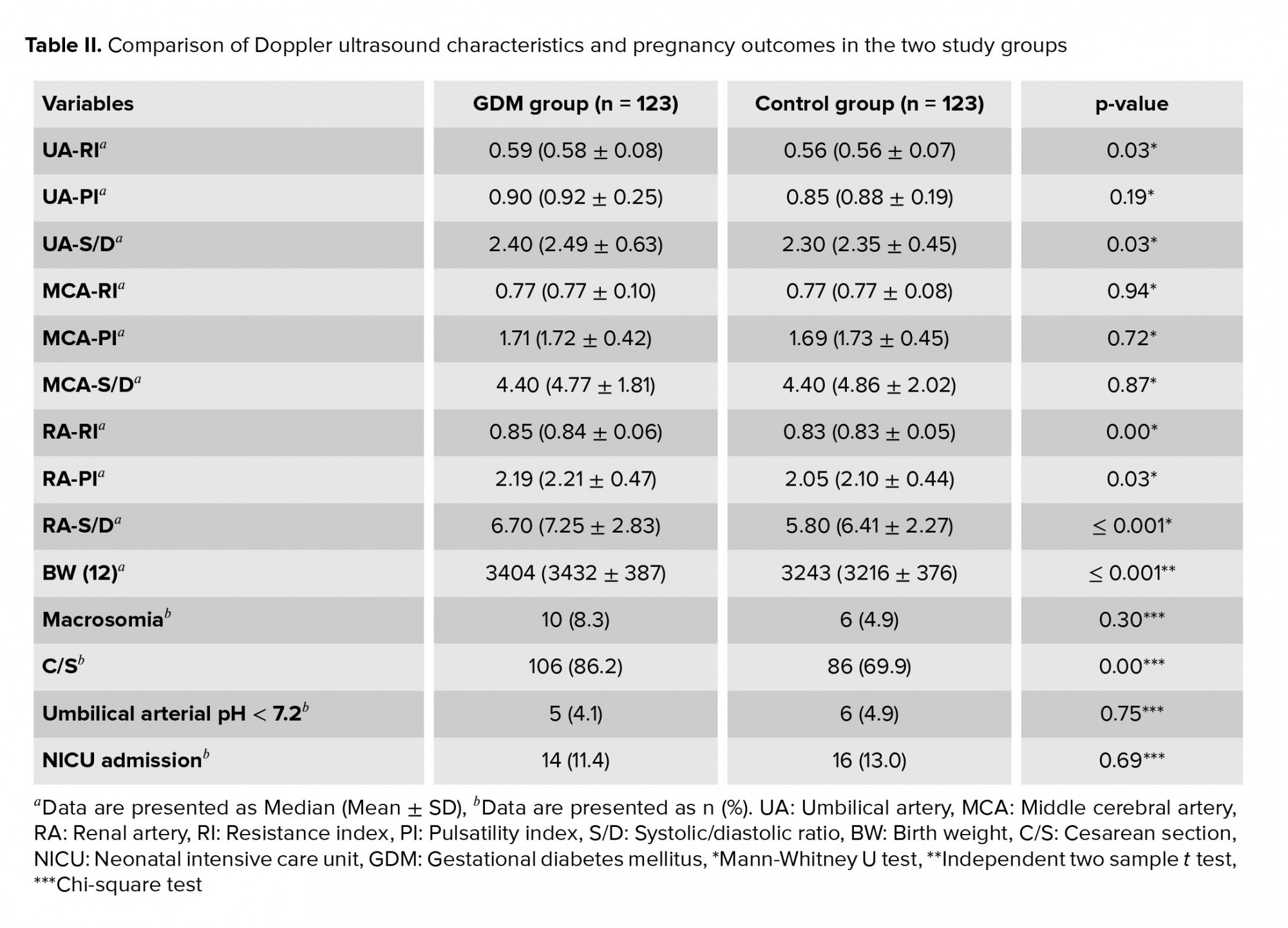
As seen in table II, the GDM group had a higher median umbilical artery RI, umbilical artery S/D, renal artery RI, renal artery PI and renal artery S/D than the control group.
The mean neonatal birth weight in the GDM group was 3404 gr vs. 3243 gr in the control group (p < 0.01). Ten (8.3%) newborns in the GDM group and 6 (4.9%) in the control group had a birth weight ≥ 4000 gr; this difference was not significant.
Only one neonate of each group had an Apgar score below seven at five min after birth. There was no significant difference between the two groups in terms of umbilical arterial blood pH below 7.2 or neonatal intensive care unit admission. The cesarean section rate was 86.2% in the GDM group vs. 69.9% in the control group (p < 0.01).
In both groups, there was a significant linear correlation between the fetal growth indices and birth weight (p < 0.01) (Table III). The relation between the Doppler indices of the renal and umbilical arteries with neonatal weight is listed in table III.
52 (41.5%) cases in the GDM group were treated with insulin and 70 (58.5%) were only managing their GM through their diet. None of the cases had metformin or other oral drugs administered. Given the defined criteria for measuring how GM is controlled, 69 (56.0%) cases were well controlled. The proportion of well-controlled cases was significantly different according to their treatment. Twenty-one (41.2%) cases in the insulin subgroup vs. 48 (66.7%) in the diet subgroup were well controlled (p = 0.01). The Doppler indices in the two diabetic subgroups (well controlled vs. poorly controlled) were not significantly different.
The newborn blood sugar testing revealed five cases (4.1%) of hypoglycemia. Two (3.9%) newborns in the insulin subgroup and three (4.2%) in the diet subgroup were hypoglycemic. Two (2.9%) newborns in the well-controlled GDM subgroup and 3 (5.6%) in the poorly-controlled GDM subgroup were hypoglycemic; this difference was not significant (p = 0.65). None of the newborns in the control group were hypoglycemic.


4. Discussion
In our study, impaired Doppler indices in the GDM group were associated with lower birth weight, and these symptoms were first observed in renal artery indices. Fetal weight is not a good predictor of being small for gestational age (SGA) or IUGR in GDM, and biometric factors are not valid either. Hypoxia and SGA or IUGR may occur in GDM mothers, even in a fetus with a weight above the 10th percentile for their gestational age. Gestational diabetes is associated with some fetal and maternal complications, such as preeclampsia fetal demise or malformation. Indeed, between 20-40% of the fetus being macrosomic in GDM. Increased glycemic indices in GDM can lead to fetal growth abnormalities especially in late pregnancy (13).
Ultrasonography is a non-invasive tool with reproducibility that is cost-effective. It is the optimum method to screen the fetal wellbeing. The umbilical, middle cerebral artery as well as the renal artery is the main arteries of the circulatory system of the fetus. Their indices may be a good indicator for fetal growth is the predictors' fetal distress, IUGR, and other poor fetal or maternal outcomes.
The renal artery in face with hypoxia and ischemia redistribute blood flow. Therefore, to ensure an adequate blood supply to the main organs such as the brain and liver, it's S/D, PI, and RI increases. We did not expect to see such redistribution in healthy fetuses. In this study, no significant correlation was observed between renal artery indices and birth weight in the control group, which is similar to the results of another previous study (10). As we excluded women with well-known conditions affecting fetal blood flow such as IUGR and hypoxemia in both groups, we did not expect any disturbance in renal artery Doppler indices in the control group. In the GDM group, lower birth weight was assumed to be related to an early hypoxic state in the fetus. A significant correlation was found between blood oxygen deficit and increased renal artery PI (14). A limitation of our study was that we did not directly assess fetal hypoxia, as doing so requires invasive testing.
We found that the hemodynamic indices of the fetal renal artery in term pregnancy (37-40 wk) were inversely correlated with neonatal birth weight in the GDM group. This is contrary to another study that found a statistically significant positive correlation between birth weight and renal artery RI, PI, and S/D in women with GDM (all p < 0.01). This may be because the criteria used for diagnosing diabetes in pregnancy were different.
With increasing gestational age, placental blood is supplemented, the blood volume of the umbilical artery rises, and vascular resistance reduces. In the GDM group, there was a significant negative correlation between newborn birth weight and indices of the umbilical artery (S/D and PI), but we did not detect a significant correlation with RI. This may be because PI is often a more sensitive item compared to RI. A significant correlation was found between birth weight and umbilical artery PI, but not with the values of the uterine artery PI, which is consistent with the current study (15). In another study on pregnant women with type 1 diabetes, macrosomic fetuses had a significant decline in umbilical artery PI compared with normal-weight fetuses. Their findings demonstrated a negative correlation between umbilical PI and neonatal birth weight (16). A further study also showed that there can be a negative correlation between umbilical PI and fetal weight which is in line with the current study (17). And a recent study of 226 women with GDM revealed that the umbilical artery hemodynamic indices in late pregnancy had a negative correlation with neonatal birth weight, but there was no such correlation with fetal growth indices (18).
If blood demand increases, the fetus may be at risk for hypoxia. In such cases, blood flow is altered to spare the brain, which leads to an increase in MCA blood supply, providing 80% of cerebral hemisphere perfusion (19). However, we did not find any differences in the middle cerebral artery indices between the groups as we excluded cases of IUGR from our study.
5. Conclusion
Our study showed that in mothers with GDM, the hemodynamic indices of the fetal renal artery in term pregnancies (37-40 wk) were inversely correlated with neonatal birth weight. Based on our findings, we propose that the use of Doppler hemodynamic indices of the renal artery in late pregnancy can be a helpful parameter in GDM mothers to detect hypoxic fetuses who are at risk of SGA and IUGR even when normal weight (above the 10th percentile for their gestational age). These renal artery Doppler indices can help diagnose hypoxia in these fetuses even earlier than the umbilical artery Doppler indices.
Acknowledgments
This study is a thesis of L.Z for fellowship degree. The authors would like to thank the Clinical Research Development Unit of Shariati Hospital, Tehran, Iran. This study was supported financially by Tehran University of Medical Sciences, Tehran, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Gestational diabetes mellitus (GDM) is one of the most common endocrine diseases and it affects around 7-10% of all pregnancies worldwide (1). Prevalence rates vary due to the local screening practices and diagnostic criteria as well as population characteristics such as body mass index, maternal age, history of type II diabetes, and ethnicity (2). GDM is associated with adverse maternal and neonatal outcomes (3). Doppler vascular ultrasound is a non-invasive method that can evaluate vascular impedance distal to the area.
The placenta transports oxygen and nutrients to the fetus. For evaluation of the fetus blood supply, hemodynamic parameters such as the systolic/diastolic ratio (S/D), the pulsatility index (PI), and the resistance index (RI) play an important role (4). The kidney is one of the main organs of the fetus that is sensitive to hypoxia (5). The resistance of the renal artery may increase in intrauterine growth restriction (IUGR). Therefore, evaluation of the renal artery indices, such as the PI, RI and S/D, provide valuable information on renal vessels. Few studies have focused on fetal renal arteries in women with GDM and there is no consensus on the validity of their parameters (6).
Therefore, the current study sought to compare in late pregnancy, in women with vs. without GDM, the correlations of hemodynamic indices (S/D, PI, and RI) of the umbilical artery, middle cerebral artery, and renal artery and fetal growth with the newborn birth weight. The findings could then be used to determine whether fetal hemodynamic indices can assist clinicians in estimating newborn birth weight and possibly facilitate early detection of hypoxia in fetuses.
2. Materials and Methods
2.1. Participants
This cohort study was performed in Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran from January to December 2020. Two hundred forty-six pregnant women in their late pregnancy period who were referred for ultrasound were divided into two groups. GDM group: the women with GDM, diagnosed by blood test at 24-28 weeks of gestational age, treated with insulin or managed with diet; and the control group: women without GDM. The inclusion criteria were women aged 18-40 yr with singleton viable pregnancy and gestational age from 37 to 40 wk. In the GDM group, women newly identified with GDM according to the 2018 diagnostic criteria of the American Diabetes Association were recruited. GDM was diagnosed by a two-hour oral glucose tolerance test at 24-28 wk of gestational age. A diagnosis was made when any of the following was met or exceeded: fasting glucose level of 92 mg/dl, one-hour level of 180 mg/dl, and/or two-hour level of 153 mg/dl (7). Exclusion criteria in both groups were: multiple pregnancies, pre-gestational diabetes mellitus, preeclampsia (pregnancy-induced hypertension), the use of alcohol/cigarettes, IUGR, or other well-known conditions affecting fetal blood flow. Participants underwent physical examinations and were investigated for fasting blood glucose level, two-hour postprandial blood glucose level, and HbA1C at 37 wk. Participants with a fasting glucose level below 92 mg/dl, an HbA1C below 6, and a 2hPP below 120 mg/dl were defined as having well-controlled GDM. The age, gestational age, mode of delivery, birth weight, Apgar score, umbilical arterial blood pH, neonatal blood glucose level, and neonatal intensive care unit admission were evaluated in both groups.
2.2. Ultrasound assessment
Transabdominal 2D ultrasound was performed for all participants to assess biometry and amniotic fluid index with the exclusion of congenital fetal malformations. Ultrasound assessments were performed by one perinatologist using the Affiniti 70 ultrasound machine (Philips, the Netherlands) and a C6-2 convex probe with a frequency of 2-6 MHz. All recordings were obtained weekly from 37 wk of gestation until delivery, and the last records were evaluated in the analysis. All Doppler measurements were done in the time of lack of fetal movements and breathing (8).
Fetal biometrics included biparietal diameter, femur length, abdominal circumference, head circumference, and fetal weight, were calculated according to Hadlock’s formula (9).
The color flow pattern was selected to measure the RI, PI, and S/D of the umbilical, middle cerebral, and renal arteries. This measurement was performed for the umbilical artery 5 cm apart prom placenta, in the way that the angle between blood flow and the ultrasound beam below 20o.
For MCA Doppler, the ultrasound probe was located toward the brain basement membrane to see a pair of alisphenoids in the distance of the anterior and middle cranial fossa to reveal the Willis circle (10). The sampling volume (2 mm) was located in the proximal of the third portion of the mentioned artery, just after its origin Willis circle (11).
The evaluation of the fetal renal artery, were done in the coronal plane near to the renal hilum, and the angle between blood flow and the ultrasound beam below 20o. The Doppler indices mean of two renal arteries was applied in the final analysis. For the final analysis, the mean of five serial Doppler velocities were used (10).
2.3. Sample size calculation
To obtain a correlation coefficient of at least 0.25 between birth weight and fetal renal artery Doppler hemodynamic indices, with an error of the first type equal to 0.05 and a power of 80%, the required sample size for each group was estimated to be 123.
2.4. Ethical considerations
The study protocol was approved by the Institutional Review Board of Tehran University of Medical Sciences, Teheran, Iran (Code: IR.TUMS.MEDICINE.REC.1399.758). Written informed consent was obtained from all participants before the study. This study was conducted according to the principles of the Helsinki Declaration.
2.5. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 19.0 (IBM, USA). Continuous data were compared between the two groups with the independent t test (if normally distributed) or Mann-Whitney U test (if non-normally distributed). The categorical variables were compared with the Chi-square test. The Pearson or Spearman's rho correlation analysis was carried out to assess the linear relationship between fetal growth and Doppler hemodynamic indices with neonatal birth weight. A two-tailed p < 0.05 was considered significant.
3. Results
Table I shows the comparison of maternal, fetal, and obstetric characteristics of the GDM and control groups. Fetal growth indices and estimated fetal weight were not significantly different between the two groups. The number of days between the last ultrasound and delivery was four in the GDM group and three in the control group, with a range of zero to seven days and without any significant difference between the groups (p = 0.34).
As seen in table II, the GDM group had a higher median umbilical artery RI, umbilical artery S/D, renal artery RI, renal artery PI and renal artery S/D than the control group.
The mean neonatal birth weight in the GDM group was 3404 gr vs. 3243 gr in the control group (p < 0.01). Ten (8.3%) newborns in the GDM group and 6 (4.9%) in the control group had a birth weight ≥ 4000 gr; this difference was not significant.
Only one neonate of each group had an Apgar score below seven at five min after birth. There was no significant difference between the two groups in terms of umbilical arterial blood pH below 7.2 or neonatal intensive care unit admission. The cesarean section rate was 86.2% in the GDM group vs. 69.9% in the control group (p < 0.01).
In both groups, there was a significant linear correlation between the fetal growth indices and birth weight (p < 0.01) (Table III). The relation between the Doppler indices of the renal and umbilical arteries with neonatal weight is listed in table III.
52 (41.5%) cases in the GDM group were treated with insulin and 70 (58.5%) were only managing their GM through their diet. None of the cases had metformin or other oral drugs administered. Given the defined criteria for measuring how GM is controlled, 69 (56.0%) cases were well controlled. The proportion of well-controlled cases was significantly different according to their treatment. Twenty-one (41.2%) cases in the insulin subgroup vs. 48 (66.7%) in the diet subgroup were well controlled (p = 0.01). The Doppler indices in the two diabetic subgroups (well controlled vs. poorly controlled) were not significantly different.
The newborn blood sugar testing revealed five cases (4.1%) of hypoglycemia. Two (3.9%) newborns in the insulin subgroup and three (4.2%) in the diet subgroup were hypoglycemic. Two (2.9%) newborns in the well-controlled GDM subgroup and 3 (5.6%) in the poorly-controlled GDM subgroup were hypoglycemic; this difference was not significant (p = 0.65). None of the newborns in the control group were hypoglycemic.



4. Discussion
In our study, impaired Doppler indices in the GDM group were associated with lower birth weight, and these symptoms were first observed in renal artery indices. Fetal weight is not a good predictor of being small for gestational age (SGA) or IUGR in GDM, and biometric factors are not valid either. Hypoxia and SGA or IUGR may occur in GDM mothers, even in a fetus with a weight above the 10th percentile for their gestational age. Gestational diabetes is associated with some fetal and maternal complications, such as preeclampsia fetal demise or malformation. Indeed, between 20-40% of the fetus being macrosomic in GDM. Increased glycemic indices in GDM can lead to fetal growth abnormalities especially in late pregnancy (13).
Ultrasonography is a non-invasive tool with reproducibility that is cost-effective. It is the optimum method to screen the fetal wellbeing. The umbilical, middle cerebral artery as well as the renal artery is the main arteries of the circulatory system of the fetus. Their indices may be a good indicator for fetal growth is the predictors' fetal distress, IUGR, and other poor fetal or maternal outcomes.
The renal artery in face with hypoxia and ischemia redistribute blood flow. Therefore, to ensure an adequate blood supply to the main organs such as the brain and liver, it's S/D, PI, and RI increases. We did not expect to see such redistribution in healthy fetuses. In this study, no significant correlation was observed between renal artery indices and birth weight in the control group, which is similar to the results of another previous study (10). As we excluded women with well-known conditions affecting fetal blood flow such as IUGR and hypoxemia in both groups, we did not expect any disturbance in renal artery Doppler indices in the control group. In the GDM group, lower birth weight was assumed to be related to an early hypoxic state in the fetus. A significant correlation was found between blood oxygen deficit and increased renal artery PI (14). A limitation of our study was that we did not directly assess fetal hypoxia, as doing so requires invasive testing.
We found that the hemodynamic indices of the fetal renal artery in term pregnancy (37-40 wk) were inversely correlated with neonatal birth weight in the GDM group. This is contrary to another study that found a statistically significant positive correlation between birth weight and renal artery RI, PI, and S/D in women with GDM (all p < 0.01). This may be because the criteria used for diagnosing diabetes in pregnancy were different.
With increasing gestational age, placental blood is supplemented, the blood volume of the umbilical artery rises, and vascular resistance reduces. In the GDM group, there was a significant negative correlation between newborn birth weight and indices of the umbilical artery (S/D and PI), but we did not detect a significant correlation with RI. This may be because PI is often a more sensitive item compared to RI. A significant correlation was found between birth weight and umbilical artery PI, but not with the values of the uterine artery PI, which is consistent with the current study (15). In another study on pregnant women with type 1 diabetes, macrosomic fetuses had a significant decline in umbilical artery PI compared with normal-weight fetuses. Their findings demonstrated a negative correlation between umbilical PI and neonatal birth weight (16). A further study also showed that there can be a negative correlation between umbilical PI and fetal weight which is in line with the current study (17). And a recent study of 226 women with GDM revealed that the umbilical artery hemodynamic indices in late pregnancy had a negative correlation with neonatal birth weight, but there was no such correlation with fetal growth indices (18).
If blood demand increases, the fetus may be at risk for hypoxia. In such cases, blood flow is altered to spare the brain, which leads to an increase in MCA blood supply, providing 80% of cerebral hemisphere perfusion (19). However, we did not find any differences in the middle cerebral artery indices between the groups as we excluded cases of IUGR from our study.
5. Conclusion
Our study showed that in mothers with GDM, the hemodynamic indices of the fetal renal artery in term pregnancies (37-40 wk) were inversely correlated with neonatal birth weight. Based on our findings, we propose that the use of Doppler hemodynamic indices of the renal artery in late pregnancy can be a helpful parameter in GDM mothers to detect hypoxic fetuses who are at risk of SGA and IUGR even when normal weight (above the 10th percentile for their gestational age). These renal artery Doppler indices can help diagnose hypoxia in these fetuses even earlier than the umbilical artery Doppler indices.
Acknowledgments
This study is a thesis of L.Z for fellowship degree. The authors would like to thank the Clinical Research Development Unit of Shariati Hospital, Tehran, Iran. This study was supported financially by Tehran University of Medical Sciences, Tehran, Iran.
Conflict of Interest
The authors declare that there is no conflict of interest.
Type of Study: Original Article |
Subject:
Pregnancy Health
References
1. Ferrara A. Increasing prevalence of gestational diabetes mellitus: A public health perspective. Diabetes Care 2007; 30 (Suppl.): S141-S146. [DOI:10.2337/dc07-s206] [PMID]
2. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr Diab Rep 2016; 16: 7. [DOI:10.1007/s11892-015-0699-x] [PMID] [PMCID]
3. Farrar D, Simmonds M, Bryant M, Sheldon TA, Tuffnell D, Golder S, et al. Hyperglycaemia and risk of adverse perinatal outcomes: Systematic review and meta-analysis. BMJ 2016; 354: i4694. [DOI:10.1136/bmj.i4694] [PMID] [PMCID]
4. Fu J, Olofsson P. Relations between fetal brain-sparing circulation, oxytocin challenge test, mode of delivery and fetal outcome in growth-restricted term fetuses. Acta Obstet Gynecol Scand 2011; 90: 227-230. [DOI:10.1111/j.1600-0412.2010.01042.x] [PMID]
5. Suranyi A, Kozinszky Z, Molnar A, Nyari T, Bito T, Pal A. Placental three-dimensional power doppler indices in mid-pregnancy and late pregnancy complicated by gestational diabetes mellitus. Prenat Diagn 2013; 33: 952-958. [DOI:10.1002/pd.4172] [PMID]
6. James DK, Parker MJ, Smoleniec JS. Comprehensive fetal assessment with three ultrasonographic characteristics. Am J Obstet Gynecol 1992; 166: 1486-1495. [DOI:10.1016/0002-9378(92)91624-J]
7. American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 2018; 41 (Suppl.): S13-S27. [DOI:10.2337/dc18-S002] [PMID]
8. Benzer N, Pekin AT, Yilmaz SA, Kerimoglu OS, Dogan NU, Celik C. Predictive value of second and third trimester fetal renal artery doppler indices in idiopathic oligohydramnios and polyhydramnios in low-risk pregnancies: A longitudinal study. J Obstet Gynaecol Res 2015; 41: 523-528. [DOI:10.1111/jog.12601] [PMID]
9. Fatone AM, Moadel AB, Foley FW, Fleming M, Jandorf L. Urban voices: The quality-of-life experience among women of color with breast cancer. Palliat Support Care 2007; 5: 115-125. [DOI:10.1017/S1478951507070186] [PMID]
10. Liu F, Liu Y, Lai YP, Gu XN, Liu DM, Yang M. Fetal hemodynamics and fetal growth indices by ultrasound in late pregnancy and birth weight in gestational diabetes mellitus. Chin Med J 2016; 129: 2109-2114. [DOI:10.4103/0366-6999.189057] [PMID] [PMCID]
11. Norton ME, Scoutt LM, Feldstein VA. Callen's ultrasonography in obstetrics and gynecology. Amsterdam, Netherlands: Elsevier; 2016.
12. Petersmann A, Nauck M, Muller-Wieland D, Kerner W, Muller UA, Landgraf R, et al. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 2018; 126: 406-410. [DOI:10.1055/a-0584-6223] [PMID]
13. Gluckman PD. The role of pituitary hormones, growth factors and insulin in the regulation of fetal growth. Oxf Rev Reprod Biol 1986; 8: 1-60.
14. Vyas S, Nicolaides KH, Campbell S. Renal artery flow-velocity waveforms in normal and hypoxemic fetuses. Am J Obstet Gynecol 1989; 161: 168-172. [DOI:10.1016/0002-9378(89)90257-3]
15. Quintero-Prado R, Bugatto F, Sanchez-Martin P, Fajardo-Exposito MA, Torrejon R, Bartha JL. The influence of placental perfusion on birthweight in women with gestational diabetes. J Matern Fetal Neonatal Med 2016; 29: 32-35. [DOI:10.3109/14767058.2014.985201] [PMID]
16. Maruotti GM, Rizzo G, Sirico A, Sarno L, Cirigliano L, Arduini D, et al. Are there any relationships between umbilical artery pulsatility index and macrosomia in fetuses of type I diabetic mothers? J Matern Fetal Neonatal Med 2014; 27: 1776-1781. [DOI:10.3109/14767058.2013.879706] [PMID]
17. Verburg BO, Jaddoe VW, Wladimiroff JW, Hofman A, Witteman JC, Steegers EA. Fetal hemodynamic adaptive changes related to intrauterine growth: The generation R study. Circulation 2008; 117: 649-659. [DOI:10.1161/CIRCULATIONAHA.107.709717] [PMID]
18. Li J, Chen YP, Dong YP, Yu CH, Lu YP, Xiao XM, et al. The impact of umbilical blood flow regulation on fetal development differs in diabetic and non-diabetic pregnancy. Kidney Blood Press Res 2014; 39: 369-377. [DOI:10.1159/000355815] [PMID]
19. Leung WC, Lam H, Lee CP, Lao TT. Doppler study of the umbilical and fetal middle cerebral arteries in women with gestational diabetes mellitus. Ultrasound Obstet Gynecol 2004; 24: 534-537. [DOI:10.1002/uog.1730] [PMID]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





