Wed, Jan 28, 2026
[Archive]
Volume 21, Issue 1 (January 2023)
IJRM 2023, 21(1): 17-32 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yunaini L, Pujianto D A. Various gene modification techniques to discover molecular targets for non-hormonal male contraceptives: A review. IJRM 2023; 21 (1) :17-32
URL: http://ijrm.ir/article-1-2404-en.html
URL: http://ijrm.ir/article-1-2404-en.html
1- Doctoral Program of Biomedical Sciences, Faculty of Medicine, Universitas Indonesia, Jakarta Pusat, Indonesia. Department of Medicine Biology, Faculty of Medicine, Universitas Indonesia, Jakarta Pusat, Indonesia. , luluk.yunaini@ui.ac.id
2- Departement of Medicine Biology Faculty of Medicine, Universitas Indonesia,
2- Departement of Medicine Biology Faculty of Medicine, Universitas Indonesia,
Full-Text [PDF 2040 kb]
(1074 Downloads)
| Abstract (HTML) (1643 Views)
1. Introduction
Until now, women dominated contraceptive methods, and men were under-represented (1, 2). Despite varying contraceptive methods, unplanned pregnancy rates remained high (3-5). Male contraceptives include condoms and vasectomy. Contrary to popular belief, vasectomy is irreversible and less reliable than female contraception (4).
Hormonal male contraceptives work by interrupting the negative feedback loop of hypothalamus-pituitary-gonad axis. This method of contraception should be revisited due to several problems and negative effects. Nonhormonal male contraceptives have been created to avoid male hormonal contraceptive side effects (6). One of the candidates for nonhormonal male contraceptives is the epididymal molecule which is suspected to play a role in sperm maturation (7-10). The development of nonhormonal male contraceptives by targeting proteins or genes involved in male fertility is promising for controlling male fertility. A consistent strategy for investigating gene function is necessary to identify candidate genes for use as biomarkers or contraceptive targets. The best way to determine gene function is by genetic modification (GM) techniques in animal model. Common GM approaches include transgenic, knockdown, and knockout/knock-in/gene-trapped (11, 12).
More than 400 genes that play a role in male fertility have been analyzed using animal models (11). This number is less because thousands of genes play a role in the regulation of the complex process in male fertility. GM techniques are developing rapidly. This was followed by an increasingly fast and easy way to make animal models which resulted in more findings of genes that play a role in male fertility as candidates nonhormonal male contraceptives. Although there are many candidate molecular targets for nonhormonal male contraceptives, the development and progress of nonhormonal male contraceptives are slow. The scope of the problem in this article is GM techniques for producing animal models, various genes that play a role in male fertility as nonhormonal male contraceptive candidates, and the development of nonhormonal male contraceptives with reported molecular targets.
2. Material and Methods
The research for relevant articles to this review was acquired from search engines particularly Google Scholar, PubMed, Elsevier, and Scopus distributed between January 2003-December 2021. The keywords used were contraceptive, nonhormonal, GM techniques, animal model, and molecule candidate of male contraceptive. The articles were observed and specified for the current review. This study concentrates on discovering molecule candidates of nonhormonal male contraception from various GM techniques. We categorize 13 articles about the generation of making animal models for infertility by using transgenic, knockout, or Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 (CRISPR/Cas9) techniques. A total of 27 articles in which researchers examined genetically modified to produce sterile or decreased fertility in males were found. Genes that affect infertility in males but do not affect hormones were used as candidates for nonhormonal male contraceptives. Furthermore, a search for candidate nonhormonal male contraceptive molecules was conducted and 26 articles were collected.
3. Results
3.1. Animal model using GM
More than thousand genes are involved in spermatogenesis. As spermatogenesis is complex and there are multiple causes of aberrant spermatogenesis, using experimental animal models with genetic alteration techniques is particularly advantageous. Mice are the most often used experimental animals because they have a short reproductive cycle, genetics similar to humans, and mouse embryos are easily genetically modified (12). Using GM techniques to create experimental animals allows genes from one individual to be transferred to another or between species.
3.1.1. Transgenic
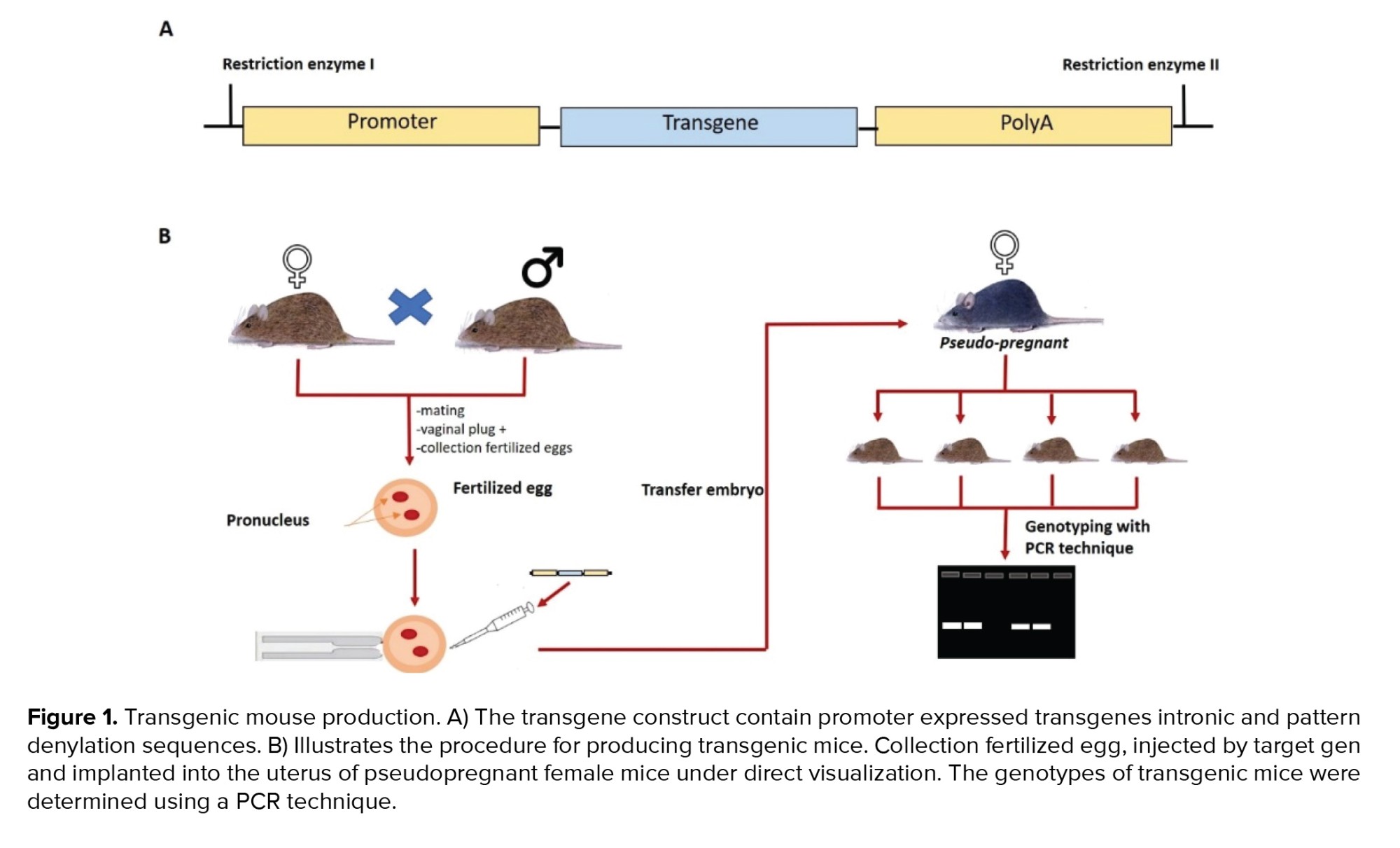
Transgenics are genetic alterations generated through recombinant DNA insertion that can be passed down from generation to generation. Endogenous or exogenous genes or DNA fragments are introduced to boost expression (11). Initially, transgenic individuals were generated through nuclear transfer and microinjection into the pronucleus. Microinjection has persisted because it is more stable than nuclear transfer (12). Figure 1 showed the process of creating transgenic animals.
The microinjection transgenic technique has a number of advantages and disadvantages. The advantages are that almost all cells from these transgenic offspring contain transgenes, production is relatively quick, and the effects of gene overexpression can be analyzed and studied to determine the gene's utility. While disadvantages are integrated randomly in a small percentage of cases, there is a risk of DNA entering critical loci, resulting in genetic mutations, nonphysiological phenotypic effects. Transgenes can enter gene loci silencing (13, 14).
3.1.2. Knockout
The term "knockout" refers to animals whose target genes have been deleted by deleting critical exons to prevent transcription. One strategy for deactivating a gene is to delete its critical exon and replace it with an antibiotic-resistant gene, such as the neomycin gene (neor). After electroporation of the constructed gene into embryonic stem cells (ESCs), the cells are grown in a medium containing the antibiotic neomycin. As a result, the transgenic ESCs will survive in a neomycin-treated medium. Additionally, ESCs containing recombinant DNA will be injected into the blastocyst to generate chimeras. Then, chimeric organisms are mated with wild-type organisms to generate heterozygous offspring. This heterozygous offspring will be crossed with another heterozygous offspring to generate homozygous mutant/knockout offspring (12). Figure 2 depicts a production knockout animal model.
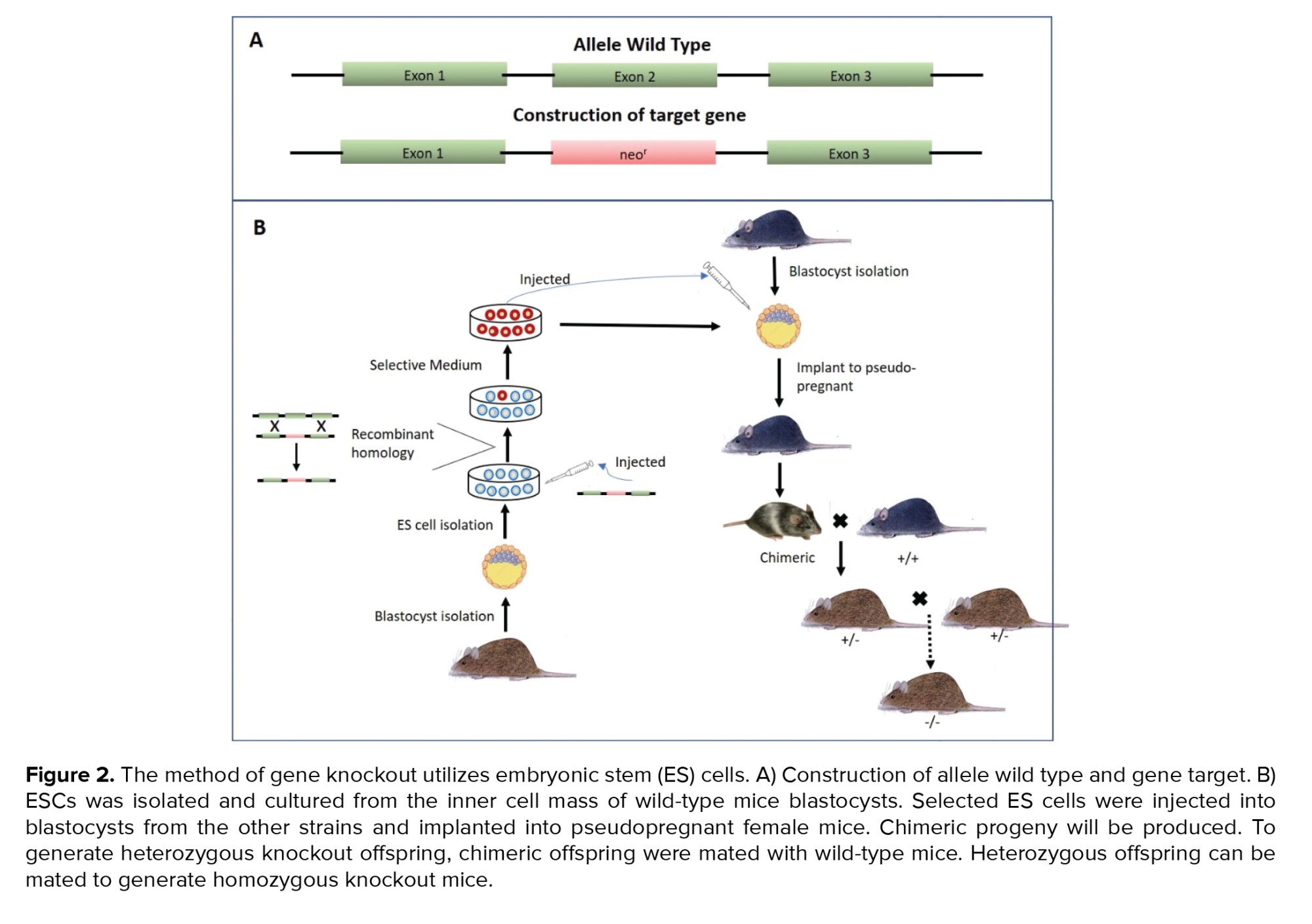
Despite knockout widespread use, this technique is still time-consuming and expensive than transgenic techniques. Knockout can result lethal embryonic or neonatal death caused by target gene is expressed at early embryonic or many tissues that are important for support in neonatal. So they cannot be evaluated postnatally or in adulthood. Another disadvantage of standard knockout is the difficulty distinguishing direct effects on cells or tissues from secondary effects on other organs caused by indirect actions. As a result, it is critical to keep in mind that the observed phenotype may result from both direct and indirect actions of gene products (12, 15). The solution to these problems is to develop knockout techniques such as conditional knockout and knockout inducer. Conditional knockout occurs in specific tissues and organs using the cloning vector pAW8-yEGFP (Cre)-locus of X over (lox) system. The Cre-Lox system comprises 2 components: the Cre recombinase and the recognition site loxP (16).
2 mutant animal strains must be used to generate mutants using the Cre-loxPsystem. The Cre strain was expressed in a specific tissue or organ in the first animal. The second animal is a strain of animal that has a loxP recognition site flanking the target gene that will be excised/cut in a unidirectional orientation. Additionally, the offspring of these 2 animal strains were mated to ensure that target genes were knocked out only in specific tissues/organs. This occurs because Cre is expressed in a limited number of tissues/organs. The Cre protein recognizes the loxP recognition area between the target gene, resulting in the target gene's excision and truncation (17). Procedure conditional knockout can be seen in figure 3.
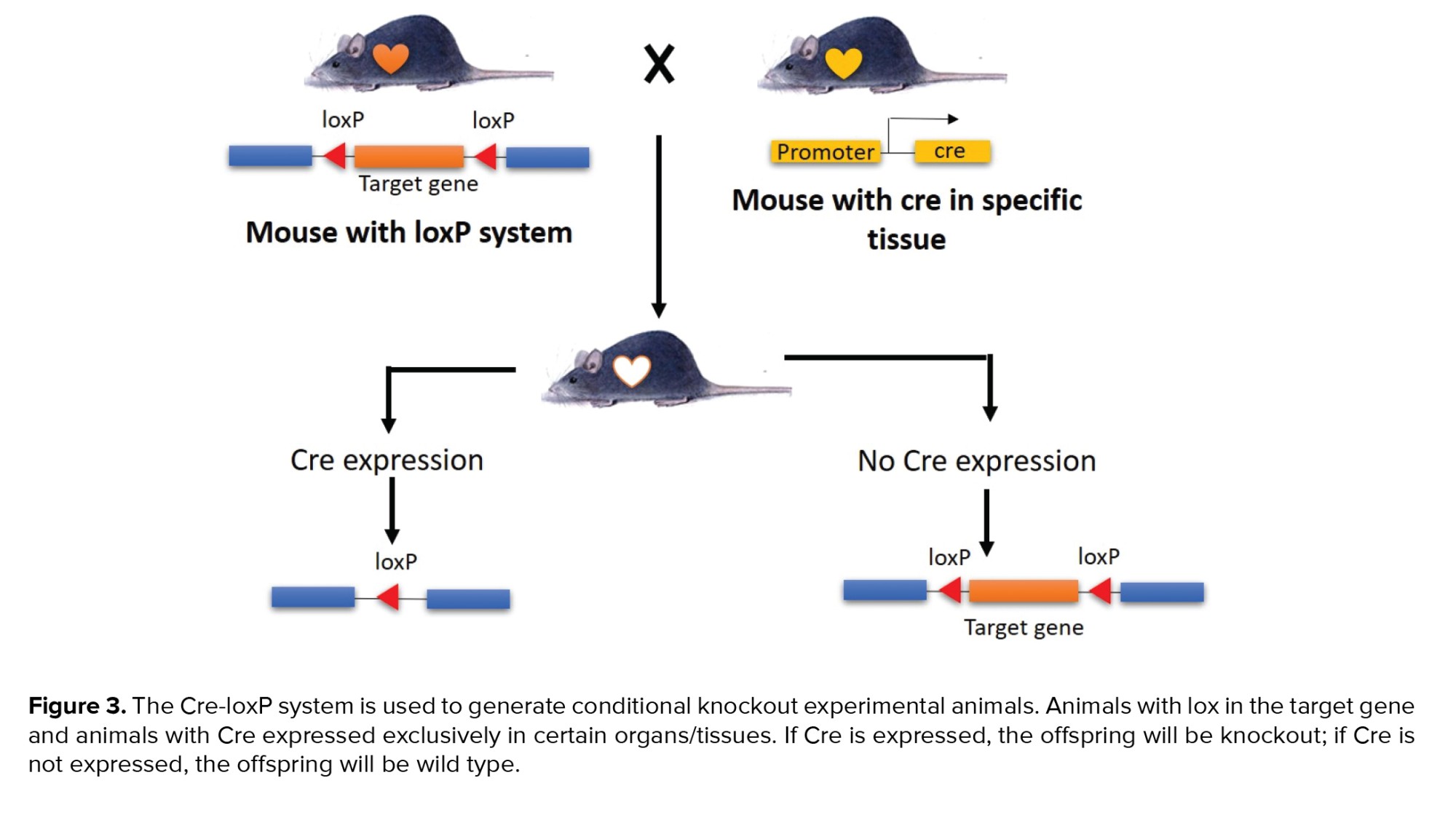
The advantages of conditional knockout are flexibility of the Cre-loxP system, can assess the role of target genes at various developmental stages, and enables analysis of the target genes role at a variety of sites. The disadvantage of this method is that it is difficult to identify a promoter that will specifically direct Cre expression to the desired tissue (16, 17).
Knockout induction (Inducible Cre-loxP system) technique enables precise targeting of Cre activation at specific times and in specific cells. Cell-specific regulatory elements (promoters and enhancers) regulate the inducible Cre system. Tamoxifen (tam) and tetracycline (tet) are 2 frequently used exogenous inducers. The tam-induced Cre-loxP system operates on the principle that when Tam is not present, Cre-Estrogen Receptor (CreER) interacts with heat shock protein 90 and is localized in the cytoplasm. If present in the cytoplasm, Tam inhibits the interaction between heat shock protein 90 and CreER. CreER is translocated to the nucleus. CreER recognizes the loxP site in the nucleus and inactivates target gene in specific tissue. Inducible cre-loxP system using tet is triggered by Dox. This induction is available in 2 modes: Cre (Dox) Tet-on and Cre (Dox) Tet-off. In the Tet-on system, the presence of Dox will induce Cre expression and make deletion of the target gene, whereas in the Tet-off system the presence of Dox inhibit Cre expression and make the target gene expression (17).
The advantage of induced knockout is that it enables precise control of the target gene's expression time, allowing us to avoid the presence of lethal embryos in genes involved in embryology, such as those involved in the cardiovascular system (18). Additionally, this technique can investigate lineage cell tracing. The inducer can substantially affect the phenotype, which is a limitation of the inducible Cre model. For instance, regardless of the target gene's deletion, an induced Cre model with tam would significantly affect estrogen-responsive organs such as bone, uterus, breast, and liver (19).
3.1.3. Clustered regularly interspaced short palindromic repeats (CRISPR)
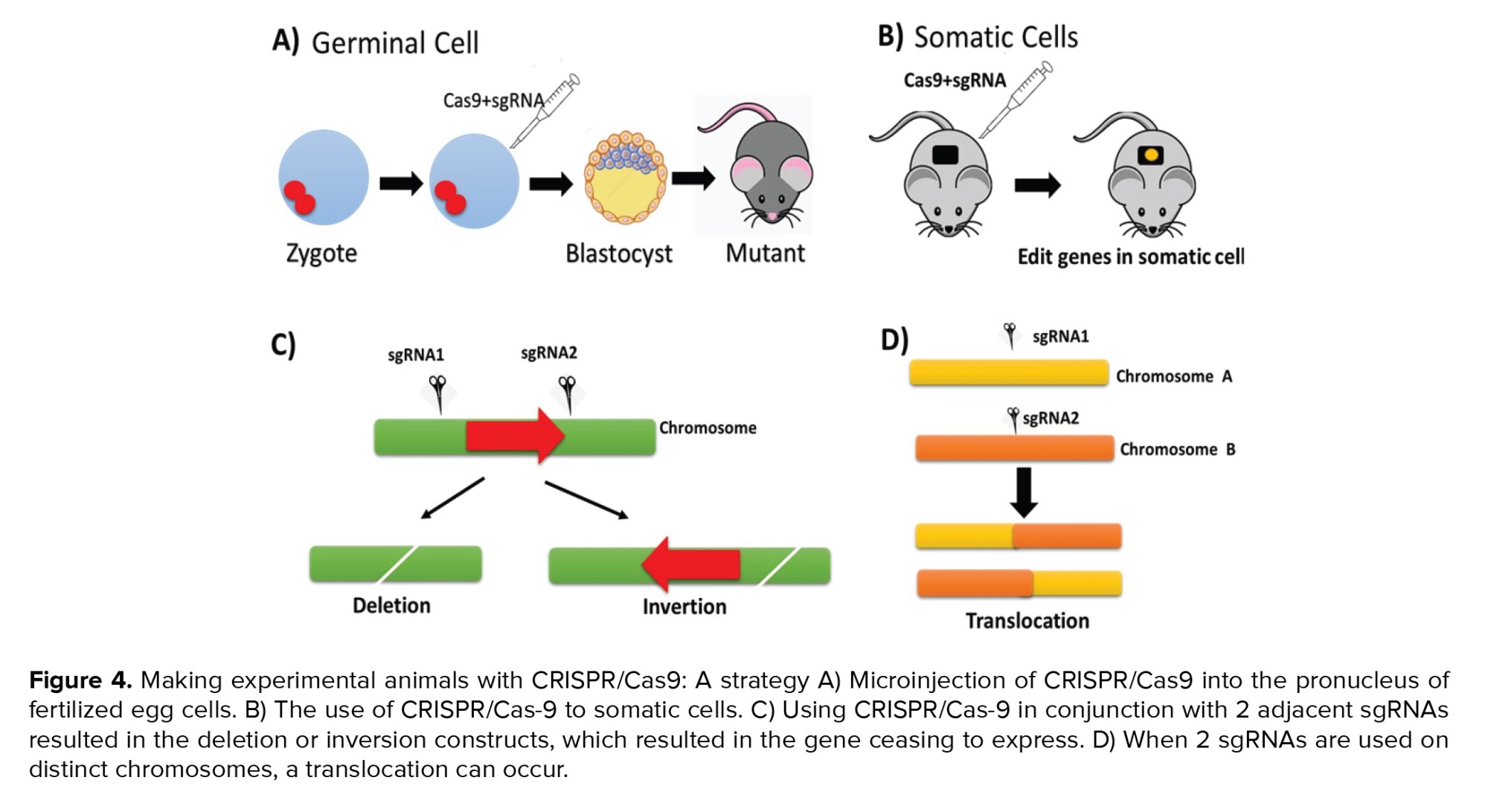
CRISPR is a type of DNA segment found in prokaryotes (bacteria and archaea) consisting of sequences or sequences of nucleotides with short repetitions. The CRISPR gene functions as an immune system in these prokaryotes, protecting them from infection, conjugation, and transformation caused by foreign genetic material (14). CRISPR-Cas9 can be used to edit genes depending on the system's capability. In contrast to transgenic techniques, which perform genetic engineering on a genome randomly, the CRISPR-Cas9 system performs targeted and precise genetic engineering (Figure 4) (20).
The primary benefit of this technology is its ability to automate complex gene targeting vector designs and ESCs manipulation, thereby reducing modeling time and costs. Additionally, CRISPR/Cas9 is not restricted to a rodent's particular strain or genetic background, as ESCs is not required (21). CRISPR-based gene editing makes knockout easier.
One disadvantage of the CRISPR/Cas9 system is that it is inefficient at inserting large DNA sequences (22). To overcome this limitation, it was reported in 2017 that the addition of a CRISPR single stranded DNA (ssDNA) insert (Easi-CRISPR) could be used. These donor sequences can range in length from 500 nt to 2 kb and have been shown to provide highly efficient targeted insertion (23).
The advantage of Easi-CRISPR is that long donor ssDNA sequences do not randomly integrate into the genome, as is the case with short donor dsDNA sequences. Additionally, this donor serves as a superior template for homology repair compared to conventional CRISPR sequences. While Easi-CRISPR appears to be a significant advancement over basic CRISPR technology, ensuring the quality and precision of extremely long ssDNA sequences. The resulting RNA will be reverse-transcribed using reverse transcriptase to generate this sequence. Unlike DNA-dependent polymerases, this enzyme is prone to errors and cannot correct them. This results in sequences that may contain mutations (23).
3.2. Male infertility genes as a nonhormonal contraceptive candidate
The testes and epididymis are 2 organs that are involved in the process of male infertility. The testes are involved in spermatogenesis, while the epididymis is involved in sperm maturation. As a result, numerous studies have examined male infertility at the genomic, transcriptomic, and proteomic levels in these 2 organs. Along with organ analysis, molecular analysis at the cellular level, specifically spermatozoa, was performed. Obtaining a contraceptive target molecule is one of the objectives of the molecular study of male infertility.
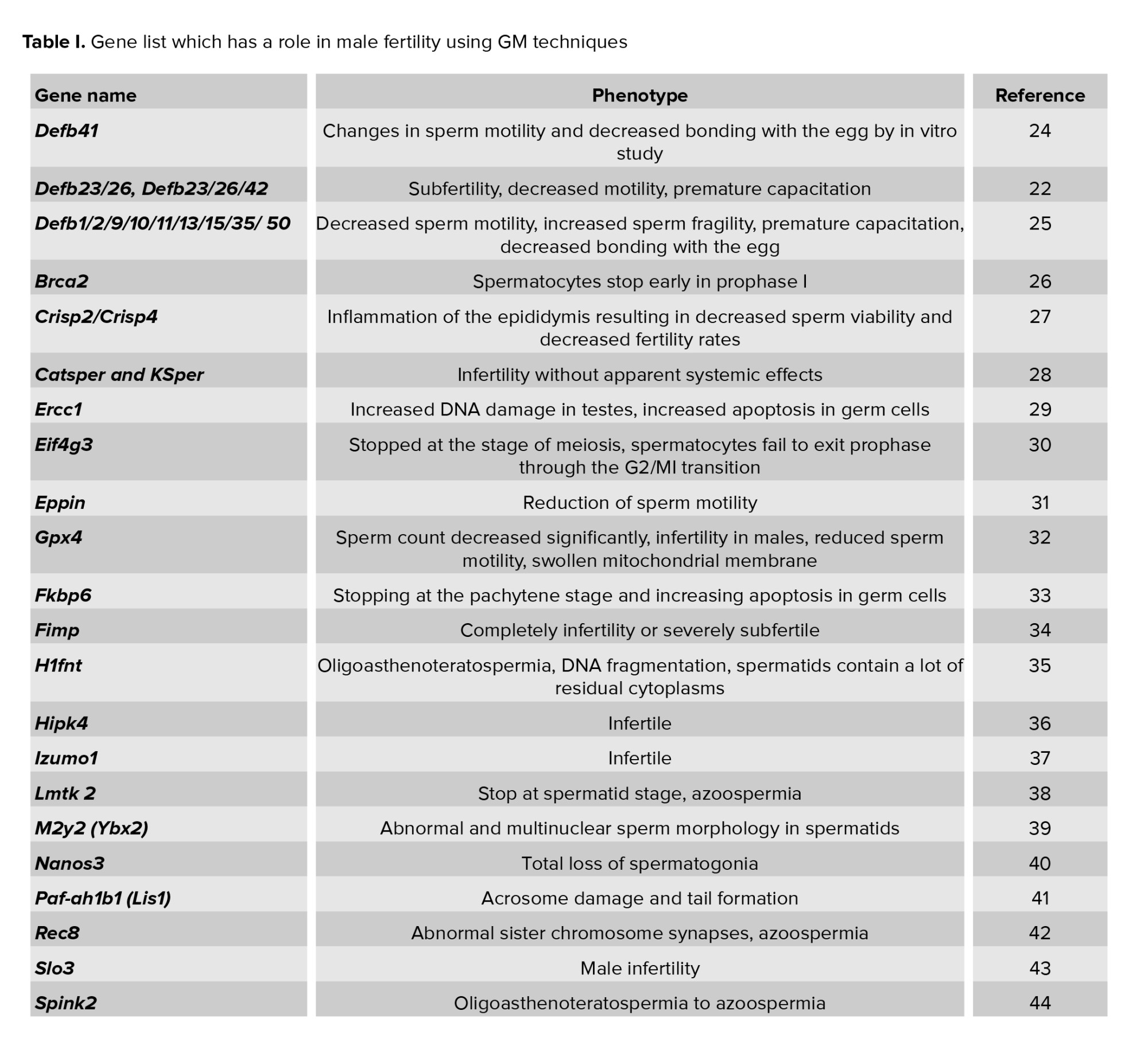
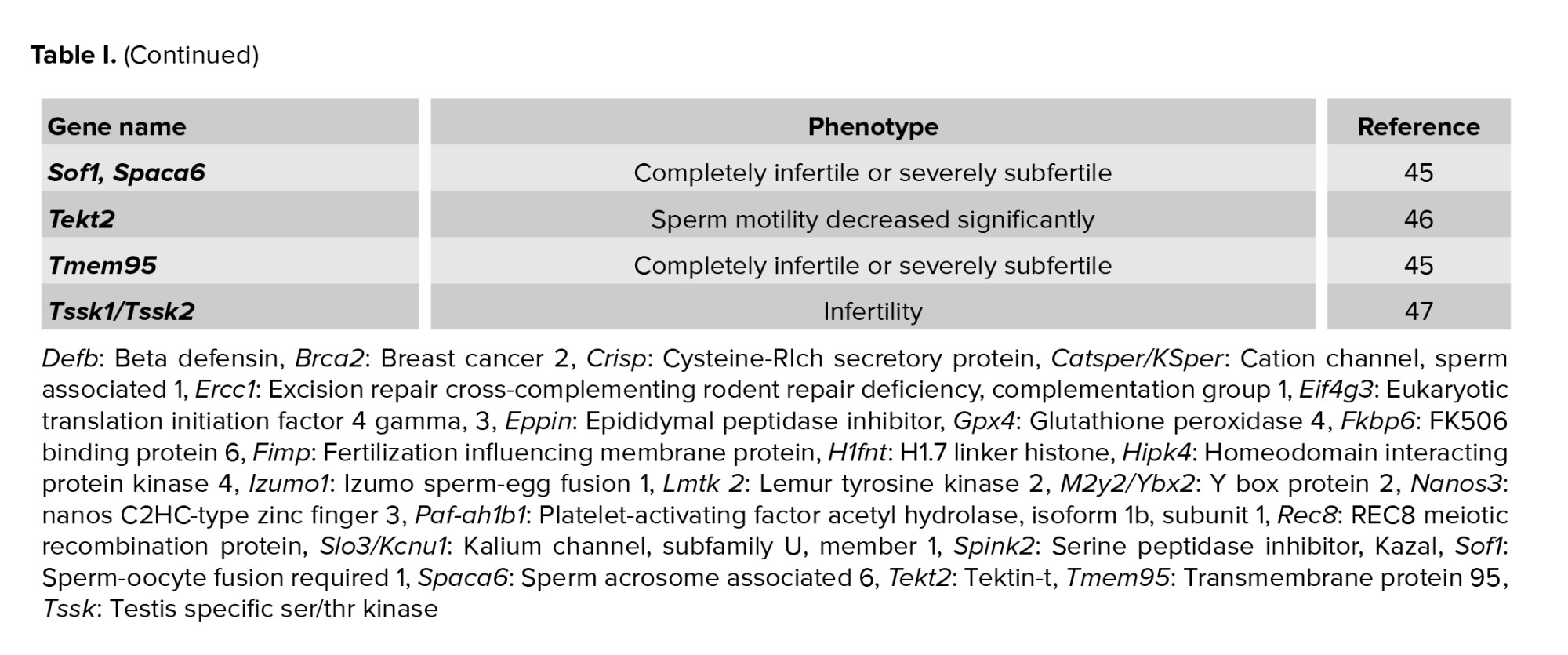
The number of genes and proteins thought to play a role in male fertility must be established by understanding their role and function in fertility. GM techniques, particularly knockout, are one method for analyzing the function and role of genes. Fertility analysis must be performed in vivo due to the complexity of the reproductive process. As a result, it is essential to analyze the function and role of male fertility genes. In table I, we list the genes involved in fertility that have been characterized using knockout animal models.
Spermatozoa become fertile during their transit through the epididymis. Microarray analysis identified over 17,000 genes expressed in the epididymis, but only a few are expressed in the epididymis to a limited extent. To investigate the function of highly expressed genes in the epididymis in vivo, experimental animals in the form of mice were created that were deficient in 9 genes found to be highly expressed in the head and corpus epididymis (Pate1, Pate2, Pate3, Clpsl2, Epp13, Rnase13, Gm1110, Glb1l2, and Glb1l3). The CRISPR/Cas9 system was used to generate knockout mice. The epididymis histology and sperm morphology of all knockout lines were identical to those of control males. Females of wild-type mated with knockout males produced the same number of offspring as control males. Thus, 9 genes that were abundantly expressed in the head and corpus epididymis were discovered to be secreted for sperm function and male fertility. The generation of knockout mice using CRISPR/Cas9 accelerates screening genes expressed in the epididymis for potential reproductive functions (48).
3.3. Development of nonhormonal male contraceptives
Nonhormonal male contraceptives have progressed to the preclinical stage by targeting sperm production or function proteins. The number of target molecules with significant potential as nonhormonal male contraceptives increases year after year. Controlling sperm production or function takes a variety of forms, and each form must be evaluated to ensure that it is both safe and effective as a contraceptive. According to animal models, the target may be effective if specificity is increased to limit off-target effects.
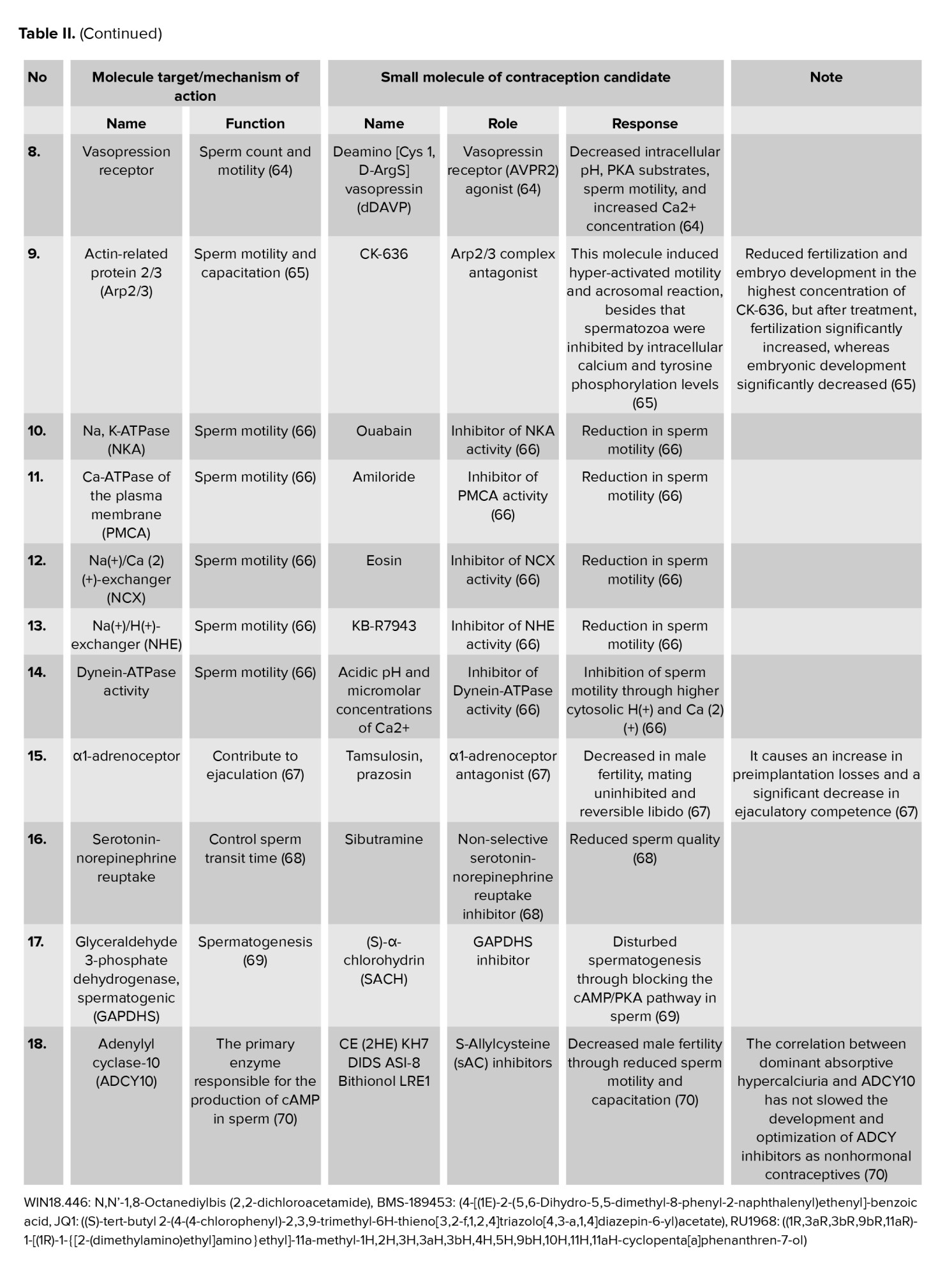
The first candidate molecule for nonhormonal male contraceptives is retinoic acid. It was discovered that male sterility could be caused by vitamin A deficiency (49). Table II showed details of the development of nonhormonal male contraception.
_00-175_Page_09.jpg)
Research on genetic variation of nonhormonal contraceptive target molecules with male fertility has been carried out. Several genes showed associations between gene variations of target molecule and male infertility. ADCY10 is the only soluble adenylate cyclase in the ADCY1-10 family of proteins. Recent whole-exome sequencing of the 2 infertile men revealed that their asthenozoospermia condition, which affects progressive sperm motility, was caused by a homozygous variant upstream of the ADCY10 nucleotide- binding site, which results in premature sperm termination (71). These individuals live normally but are infertile and have a risk of developing calcium oxalate kidney stones.
The testis-specific serine/threonine kinases (TSSK) family consists of 5 members: TSSK1, TSSK2, TSSK3, TSSK4 (also referred to as TSSK5), and TSSK6. Several of its members are critical for spermatogenesis and sperm function. The production of a stable and enzymatically active recombinant human TSSK2 protein represents a significant step forward to eradicate TSSK in humans (72). Furthermore, when men with azoospermia or severe oligozoospermia were compared to fertile controls, single nucleotide polymorphisms in the TSSK2 gene were associated with idiopathic male infertility (73). TSSK2 essay screening revealed a potent TSSK2 inhibitor. This demonstrates the great potential for targeting TSSK with small molecule inhibitors (53, 74).
4. Discussion
Proteomic analyses have been performed on the male reproductive system and the distribution of gene expression in various tissues. Numerous genes were found to be overexpressed in the male reproductive system due to this study's findings. Numerous reports have implicated genes in spermatogenesis and sperm maturation (2). These findings pave the way for the development of candidate target molecules for nonhormonal male contraceptives. For nonhormonal contraception, at least 3 molecular targets are involved in spermatogenesis, sperm maturation, and sperm penetration into oocytes. As a result, numerous analyses of proteins involved in these 3 processes or molecules with high-expression levels in spermatozoa, testes, and epididymis have been conducted.
The high expression in target organs or cells for nonhormonal male contraceptives is frequently accompanied by an ambiguous function and role in male reproduction. Thus, it is critical to employ GM techniques in experimental animals. When developing experimental animals, it is necessary to consider specific genetic mechanisms that disrupt gene function, alter gene activity, or increase gene copy number. If a phenotype is caused by a complete loss of function (zero mutation), the gene can be silenced using gene targeting (gene knockout). Gene knockout can model homozygous recessive traits (inactivation of both copies of the gene by mutation) and haploinsufficient traits (inactivation of one copy of the gene). Transgenic techniques can be used when the phenotype is caused by an increase or new activity of a gene product or an increase in the number of gene copies. If a mutation results in a partial loss of function, it is possible to introduce specific harmful mutations into endogenous genes using gene targeting (knock-in). Additionally, it should be noted that phenotypic changes are not always caused by a loss of gene function in its entirety (null mutation). However, because the null mutation provides the most information about the gene's normal function, it can be used to deduce the molecular mechanism underlying the phenotype (75).
The considerations outlined above can guide the selection of experimental animals using the GM technique. The most recent and most advanced GM technique is CRISPR/Cas-9 for gene editing. This technique can precisely knockout or knock in the target gene. This technique requires less time than other methods, which enables rapid analysis of the function and role of a gene.
Male fertility research is generally associated with genes expressed during the early embryonic and puberty stages. Genes expressed in early embryos are involved in gonad formation and the development of the reproductive tract. Genes are involved in the process of spermatogenesis and sperm maturation during puberty. Numerous genes expressed early in the embryo are also expressed in specific tissues/organs during puberty. The use of gene editing, specifically CRISPR/Cas-9, is optimal for the study of male fertility because the time required to construct the target gene vector is shorter, more precise, and can be combined with the Cre-loxp system (21).
Furthermore, numerous related genes, such as the Crisp and Defb relatives, play a significant role in male infertility. Previous studies have shown that knocking out just one member of a relative gene produces a phenotype identical to the wild type. This is because related genes perform similar functions, and it is suspected that if only one member is deleted, the cell compensates. As a result, it is necessary to eliminate more than one member (22, 27). Thus, combining CRISPR/Cas-9 with the Cre-loxP system accelerates the process and ensures that knockout occurs only in the target tissue or organ. Candidate molecular targets for nonhormonal male contraception will be more precise, specific, and effective using this technique.
Men, according to surveys, want highly effective and irreversible contraception. However, male contraceptives, particularly nonhormonal contraceptives, have developed at a glacial pace until now. The development of GM techniques for analyzing the function and role of genes in the male reproductive system-aided significantly in developing male contraceptive target molecules. Until now, nonhormonal male contraception has been developed in the preclinical stage using experimental animals. The results of experiments on animals frequently differ from one another. This is partly due to the background variations of the experimental animals used, particularly those using ES cells, which have a different background than the blastocyst used, resulting in tillers with a wide range of background variations. These contrasting backgrounds can be minimized by crossing up to several offspring and using the resulting offspring.
It should be noted that when using an experimental animal technique without ESCs, different strains can also produce distinct mutation effects. As a result, it is necessary to analyze the gene in relation to the animal's background. Utilizing the CRISPR/Cas-9 technique makes it possible to minimize background variation in the animals used, as the CRISPR/Cas-9 technique does not require ES cells. As a result, it is necessary to analyze the gene in relation to the animal's background.
The analysis of gene function and its role in the male reproductive system cannot be applied directly to humans. This is because some genes' function and biological function in mice/experimental animals may differ from those in other species. As a result, preclinical testing is frequently conducted on higher-level animals before human testing. SNP analysis and gene expression analysis of candidate contraceptive targets in infertile men can also be used to ascertain the gene-gene association with male infertility. Human ortholog gene analysis is typically performed on gene family members to determine whether the target gene in experimental animals shares a high degree of homology with the human gene.
Once the target molecule is obtained, screening is required to determine which drug molecule can be used as an inhibitor or anti-molecule target antibody. Frequently, when a target molecule has multiple isoforms, it is necessary to analyze the specific bonds and high efficacy of the isoforms. Additionally, if the target molecule is expressed in multiple organs, it will be considered. The first step should be to determine the presence or absence of target molecule isoforms expressed specifically in male reproductive organs such as the testes or epididymis. Moreover, small molecules are synthesized that inhibit the activity of the specific target molecule. The candidate drug molecule must exhibit ideal contraceptive properties, apart from inhibiting the target molecule.
5. Conclusion
A good contraceptive must meet a number of criteria, including being safe, reliable, effective, affordable, easy to use, without serious side effects, readily available, and reversible. It will take a long time to develop the ideal nonhormonal male contraceptive. However, with the rapid advancement of research in the field of reproduction, particularly male reproduction, and the continued innovation of GM techniques especially with CRISPR-Cas9 discovery as an editing gene. The rapid advancement of GM techniques may enable the identification of more precise and specific molecular targets for nonhormonal male contraceptives. So, we are optimistic that one day nonhormonal male contraceptives will be developed and it will be effective and beneficial to the wider community.
Acknowledgments
Thank to the Education Fund Management Institute (LPDP) for supporting the study. Many thanks to Prof Delvac Oceandy, M.D., Ph.D. for the excellent discussion about genetically modified.
Conflict of Interest
There are no conflict of interest.
Full-Text: (277 Views)
1. Introduction
Until now, women dominated contraceptive methods, and men were under-represented (1, 2). Despite varying contraceptive methods, unplanned pregnancy rates remained high (3-5). Male contraceptives include condoms and vasectomy. Contrary to popular belief, vasectomy is irreversible and less reliable than female contraception (4).
Hormonal male contraceptives work by interrupting the negative feedback loop of hypothalamus-pituitary-gonad axis. This method of contraception should be revisited due to several problems and negative effects. Nonhormonal male contraceptives have been created to avoid male hormonal contraceptive side effects (6). One of the candidates for nonhormonal male contraceptives is the epididymal molecule which is suspected to play a role in sperm maturation (7-10). The development of nonhormonal male contraceptives by targeting proteins or genes involved in male fertility is promising for controlling male fertility. A consistent strategy for investigating gene function is necessary to identify candidate genes for use as biomarkers or contraceptive targets. The best way to determine gene function is by genetic modification (GM) techniques in animal model. Common GM approaches include transgenic, knockdown, and knockout/knock-in/gene-trapped (11, 12).
More than 400 genes that play a role in male fertility have been analyzed using animal models (11). This number is less because thousands of genes play a role in the regulation of the complex process in male fertility. GM techniques are developing rapidly. This was followed by an increasingly fast and easy way to make animal models which resulted in more findings of genes that play a role in male fertility as candidates nonhormonal male contraceptives. Although there are many candidate molecular targets for nonhormonal male contraceptives, the development and progress of nonhormonal male contraceptives are slow. The scope of the problem in this article is GM techniques for producing animal models, various genes that play a role in male fertility as nonhormonal male contraceptive candidates, and the development of nonhormonal male contraceptives with reported molecular targets.
2. Material and Methods
The research for relevant articles to this review was acquired from search engines particularly Google Scholar, PubMed, Elsevier, and Scopus distributed between January 2003-December 2021. The keywords used were contraceptive, nonhormonal, GM techniques, animal model, and molecule candidate of male contraceptive. The articles were observed and specified for the current review. This study concentrates on discovering molecule candidates of nonhormonal male contraception from various GM techniques. We categorize 13 articles about the generation of making animal models for infertility by using transgenic, knockout, or Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 (CRISPR/Cas9) techniques. A total of 27 articles in which researchers examined genetically modified to produce sterile or decreased fertility in males were found. Genes that affect infertility in males but do not affect hormones were used as candidates for nonhormonal male contraceptives. Furthermore, a search for candidate nonhormonal male contraceptive molecules was conducted and 26 articles were collected.
3. Results
3.1. Animal model using GM
More than thousand genes are involved in spermatogenesis. As spermatogenesis is complex and there are multiple causes of aberrant spermatogenesis, using experimental animal models with genetic alteration techniques is particularly advantageous. Mice are the most often used experimental animals because they have a short reproductive cycle, genetics similar to humans, and mouse embryos are easily genetically modified (12). Using GM techniques to create experimental animals allows genes from one individual to be transferred to another or between species.
3.1.1. Transgenic
Transgenics are genetic alterations generated through recombinant DNA insertion that can be passed down from generation to generation. Endogenous or exogenous genes or DNA fragments are introduced to boost expression (11). Initially, transgenic individuals were generated through nuclear transfer and microinjection into the pronucleus. Microinjection has persisted because it is more stable than nuclear transfer (12). Figure 1 showed the process of creating transgenic animals.
The microinjection transgenic technique has a number of advantages and disadvantages. The advantages are that almost all cells from these transgenic offspring contain transgenes, production is relatively quick, and the effects of gene overexpression can be analyzed and studied to determine the gene's utility. While disadvantages are integrated randomly in a small percentage of cases, there is a risk of DNA entering critical loci, resulting in genetic mutations, nonphysiological phenotypic effects. Transgenes can enter gene loci silencing (13, 14).

3.1.2. Knockout
The term "knockout" refers to animals whose target genes have been deleted by deleting critical exons to prevent transcription. One strategy for deactivating a gene is to delete its critical exon and replace it with an antibiotic-resistant gene, such as the neomycin gene (neor). After electroporation of the constructed gene into embryonic stem cells (ESCs), the cells are grown in a medium containing the antibiotic neomycin. As a result, the transgenic ESCs will survive in a neomycin-treated medium. Additionally, ESCs containing recombinant DNA will be injected into the blastocyst to generate chimeras. Then, chimeric organisms are mated with wild-type organisms to generate heterozygous offspring. This heterozygous offspring will be crossed with another heterozygous offspring to generate homozygous mutant/knockout offspring (12). Figure 2 depicts a production knockout animal model.

Despite knockout widespread use, this technique is still time-consuming and expensive than transgenic techniques. Knockout can result lethal embryonic or neonatal death caused by target gene is expressed at early embryonic or many tissues that are important for support in neonatal. So they cannot be evaluated postnatally or in adulthood. Another disadvantage of standard knockout is the difficulty distinguishing direct effects on cells or tissues from secondary effects on other organs caused by indirect actions. As a result, it is critical to keep in mind that the observed phenotype may result from both direct and indirect actions of gene products (12, 15). The solution to these problems is to develop knockout techniques such as conditional knockout and knockout inducer. Conditional knockout occurs in specific tissues and organs using the cloning vector pAW8-yEGFP (Cre)-locus of X over (lox) system. The Cre-Lox system comprises 2 components: the Cre recombinase and the recognition site loxP (16).
2 mutant animal strains must be used to generate mutants using the Cre-loxPsystem. The Cre strain was expressed in a specific tissue or organ in the first animal. The second animal is a strain of animal that has a loxP recognition site flanking the target gene that will be excised/cut in a unidirectional orientation. Additionally, the offspring of these 2 animal strains were mated to ensure that target genes were knocked out only in specific tissues/organs. This occurs because Cre is expressed in a limited number of tissues/organs. The Cre protein recognizes the loxP recognition area between the target gene, resulting in the target gene's excision and truncation (17). Procedure conditional knockout can be seen in figure 3.

The advantages of conditional knockout are flexibility of the Cre-loxP system, can assess the role of target genes at various developmental stages, and enables analysis of the target genes role at a variety of sites. The disadvantage of this method is that it is difficult to identify a promoter that will specifically direct Cre expression to the desired tissue (16, 17).
Knockout induction (Inducible Cre-loxP system) technique enables precise targeting of Cre activation at specific times and in specific cells. Cell-specific regulatory elements (promoters and enhancers) regulate the inducible Cre system. Tamoxifen (tam) and tetracycline (tet) are 2 frequently used exogenous inducers. The tam-induced Cre-loxP system operates on the principle that when Tam is not present, Cre-Estrogen Receptor (CreER) interacts with heat shock protein 90 and is localized in the cytoplasm. If present in the cytoplasm, Tam inhibits the interaction between heat shock protein 90 and CreER. CreER is translocated to the nucleus. CreER recognizes the loxP site in the nucleus and inactivates target gene in specific tissue. Inducible cre-loxP system using tet is triggered by Dox. This induction is available in 2 modes: Cre (Dox) Tet-on and Cre (Dox) Tet-off. In the Tet-on system, the presence of Dox will induce Cre expression and make deletion of the target gene, whereas in the Tet-off system the presence of Dox inhibit Cre expression and make the target gene expression (17).
The advantage of induced knockout is that it enables precise control of the target gene's expression time, allowing us to avoid the presence of lethal embryos in genes involved in embryology, such as those involved in the cardiovascular system (18). Additionally, this technique can investigate lineage cell tracing. The inducer can substantially affect the phenotype, which is a limitation of the inducible Cre model. For instance, regardless of the target gene's deletion, an induced Cre model with tam would significantly affect estrogen-responsive organs such as bone, uterus, breast, and liver (19).
3.1.3. Clustered regularly interspaced short palindromic repeats (CRISPR)
CRISPR is a type of DNA segment found in prokaryotes (bacteria and archaea) consisting of sequences or sequences of nucleotides with short repetitions. The CRISPR gene functions as an immune system in these prokaryotes, protecting them from infection, conjugation, and transformation caused by foreign genetic material (14). CRISPR-Cas9 can be used to edit genes depending on the system's capability. In contrast to transgenic techniques, which perform genetic engineering on a genome randomly, the CRISPR-Cas9 system performs targeted and precise genetic engineering (Figure 4) (20).
The primary benefit of this technology is its ability to automate complex gene targeting vector designs and ESCs manipulation, thereby reducing modeling time and costs. Additionally, CRISPR/Cas9 is not restricted to a rodent's particular strain or genetic background, as ESCs is not required (21). CRISPR-based gene editing makes knockout easier.
One disadvantage of the CRISPR/Cas9 system is that it is inefficient at inserting large DNA sequences (22). To overcome this limitation, it was reported in 2017 that the addition of a CRISPR single stranded DNA (ssDNA) insert (Easi-CRISPR) could be used. These donor sequences can range in length from 500 nt to 2 kb and have been shown to provide highly efficient targeted insertion (23).

The advantage of Easi-CRISPR is that long donor ssDNA sequences do not randomly integrate into the genome, as is the case with short donor dsDNA sequences. Additionally, this donor serves as a superior template for homology repair compared to conventional CRISPR sequences. While Easi-CRISPR appears to be a significant advancement over basic CRISPR technology, ensuring the quality and precision of extremely long ssDNA sequences. The resulting RNA will be reverse-transcribed using reverse transcriptase to generate this sequence. Unlike DNA-dependent polymerases, this enzyme is prone to errors and cannot correct them. This results in sequences that may contain mutations (23).
3.2. Male infertility genes as a nonhormonal contraceptive candidate
The testes and epididymis are 2 organs that are involved in the process of male infertility. The testes are involved in spermatogenesis, while the epididymis is involved in sperm maturation. As a result, numerous studies have examined male infertility at the genomic, transcriptomic, and proteomic levels in these 2 organs. Along with organ analysis, molecular analysis at the cellular level, specifically spermatozoa, was performed. Obtaining a contraceptive target molecule is one of the objectives of the molecular study of male infertility.
The number of genes and proteins thought to play a role in male fertility must be established by understanding their role and function in fertility. GM techniques, particularly knockout, are one method for analyzing the function and role of genes. Fertility analysis must be performed in vivo due to the complexity of the reproductive process. As a result, it is essential to analyze the function and role of male fertility genes. In table I, we list the genes involved in fertility that have been characterized using knockout animal models.


Spermatozoa become fertile during their transit through the epididymis. Microarray analysis identified over 17,000 genes expressed in the epididymis, but only a few are expressed in the epididymis to a limited extent. To investigate the function of highly expressed genes in the epididymis in vivo, experimental animals in the form of mice were created that were deficient in 9 genes found to be highly expressed in the head and corpus epididymis (Pate1, Pate2, Pate3, Clpsl2, Epp13, Rnase13, Gm1110, Glb1l2, and Glb1l3). The CRISPR/Cas9 system was used to generate knockout mice. The epididymis histology and sperm morphology of all knockout lines were identical to those of control males. Females of wild-type mated with knockout males produced the same number of offspring as control males. Thus, 9 genes that were abundantly expressed in the head and corpus epididymis were discovered to be secreted for sperm function and male fertility. The generation of knockout mice using CRISPR/Cas9 accelerates screening genes expressed in the epididymis for potential reproductive functions (48).
3.3. Development of nonhormonal male contraceptives
Nonhormonal male contraceptives have progressed to the preclinical stage by targeting sperm production or function proteins. The number of target molecules with significant potential as nonhormonal male contraceptives increases year after year. Controlling sperm production or function takes a variety of forms, and each form must be evaluated to ensure that it is both safe and effective as a contraceptive. According to animal models, the target may be effective if specificity is increased to limit off-target effects.
The first candidate molecule for nonhormonal male contraceptives is retinoic acid. It was discovered that male sterility could be caused by vitamin A deficiency (49). Table II showed details of the development of nonhormonal male contraception.
_00-175_Page_09.jpg)

Research on genetic variation of nonhormonal contraceptive target molecules with male fertility has been carried out. Several genes showed associations between gene variations of target molecule and male infertility. ADCY10 is the only soluble adenylate cyclase in the ADCY1-10 family of proteins. Recent whole-exome sequencing of the 2 infertile men revealed that their asthenozoospermia condition, which affects progressive sperm motility, was caused by a homozygous variant upstream of the ADCY10 nucleotide- binding site, which results in premature sperm termination (71). These individuals live normally but are infertile and have a risk of developing calcium oxalate kidney stones.
The testis-specific serine/threonine kinases (TSSK) family consists of 5 members: TSSK1, TSSK2, TSSK3, TSSK4 (also referred to as TSSK5), and TSSK6. Several of its members are critical for spermatogenesis and sperm function. The production of a stable and enzymatically active recombinant human TSSK2 protein represents a significant step forward to eradicate TSSK in humans (72). Furthermore, when men with azoospermia or severe oligozoospermia were compared to fertile controls, single nucleotide polymorphisms in the TSSK2 gene were associated with idiopathic male infertility (73). TSSK2 essay screening revealed a potent TSSK2 inhibitor. This demonstrates the great potential for targeting TSSK with small molecule inhibitors (53, 74).
4. Discussion
Proteomic analyses have been performed on the male reproductive system and the distribution of gene expression in various tissues. Numerous genes were found to be overexpressed in the male reproductive system due to this study's findings. Numerous reports have implicated genes in spermatogenesis and sperm maturation (2). These findings pave the way for the development of candidate target molecules for nonhormonal male contraceptives. For nonhormonal contraception, at least 3 molecular targets are involved in spermatogenesis, sperm maturation, and sperm penetration into oocytes. As a result, numerous analyses of proteins involved in these 3 processes or molecules with high-expression levels in spermatozoa, testes, and epididymis have been conducted.
The high expression in target organs or cells for nonhormonal male contraceptives is frequently accompanied by an ambiguous function and role in male reproduction. Thus, it is critical to employ GM techniques in experimental animals. When developing experimental animals, it is necessary to consider specific genetic mechanisms that disrupt gene function, alter gene activity, or increase gene copy number. If a phenotype is caused by a complete loss of function (zero mutation), the gene can be silenced using gene targeting (gene knockout). Gene knockout can model homozygous recessive traits (inactivation of both copies of the gene by mutation) and haploinsufficient traits (inactivation of one copy of the gene). Transgenic techniques can be used when the phenotype is caused by an increase or new activity of a gene product or an increase in the number of gene copies. If a mutation results in a partial loss of function, it is possible to introduce specific harmful mutations into endogenous genes using gene targeting (knock-in). Additionally, it should be noted that phenotypic changes are not always caused by a loss of gene function in its entirety (null mutation). However, because the null mutation provides the most information about the gene's normal function, it can be used to deduce the molecular mechanism underlying the phenotype (75).
The considerations outlined above can guide the selection of experimental animals using the GM technique. The most recent and most advanced GM technique is CRISPR/Cas-9 for gene editing. This technique can precisely knockout or knock in the target gene. This technique requires less time than other methods, which enables rapid analysis of the function and role of a gene.
Male fertility research is generally associated with genes expressed during the early embryonic and puberty stages. Genes expressed in early embryos are involved in gonad formation and the development of the reproductive tract. Genes are involved in the process of spermatogenesis and sperm maturation during puberty. Numerous genes expressed early in the embryo are also expressed in specific tissues/organs during puberty. The use of gene editing, specifically CRISPR/Cas-9, is optimal for the study of male fertility because the time required to construct the target gene vector is shorter, more precise, and can be combined with the Cre-loxp system (21).
Furthermore, numerous related genes, such as the Crisp and Defb relatives, play a significant role in male infertility. Previous studies have shown that knocking out just one member of a relative gene produces a phenotype identical to the wild type. This is because related genes perform similar functions, and it is suspected that if only one member is deleted, the cell compensates. As a result, it is necessary to eliminate more than one member (22, 27). Thus, combining CRISPR/Cas-9 with the Cre-loxP system accelerates the process and ensures that knockout occurs only in the target tissue or organ. Candidate molecular targets for nonhormonal male contraception will be more precise, specific, and effective using this technique.
Men, according to surveys, want highly effective and irreversible contraception. However, male contraceptives, particularly nonhormonal contraceptives, have developed at a glacial pace until now. The development of GM techniques for analyzing the function and role of genes in the male reproductive system-aided significantly in developing male contraceptive target molecules. Until now, nonhormonal male contraception has been developed in the preclinical stage using experimental animals. The results of experiments on animals frequently differ from one another. This is partly due to the background variations of the experimental animals used, particularly those using ES cells, which have a different background than the blastocyst used, resulting in tillers with a wide range of background variations. These contrasting backgrounds can be minimized by crossing up to several offspring and using the resulting offspring.
It should be noted that when using an experimental animal technique without ESCs, different strains can also produce distinct mutation effects. As a result, it is necessary to analyze the gene in relation to the animal's background. Utilizing the CRISPR/Cas-9 technique makes it possible to minimize background variation in the animals used, as the CRISPR/Cas-9 technique does not require ES cells. As a result, it is necessary to analyze the gene in relation to the animal's background.
The analysis of gene function and its role in the male reproductive system cannot be applied directly to humans. This is because some genes' function and biological function in mice/experimental animals may differ from those in other species. As a result, preclinical testing is frequently conducted on higher-level animals before human testing. SNP analysis and gene expression analysis of candidate contraceptive targets in infertile men can also be used to ascertain the gene-gene association with male infertility. Human ortholog gene analysis is typically performed on gene family members to determine whether the target gene in experimental animals shares a high degree of homology with the human gene.
Once the target molecule is obtained, screening is required to determine which drug molecule can be used as an inhibitor or anti-molecule target antibody. Frequently, when a target molecule has multiple isoforms, it is necessary to analyze the specific bonds and high efficacy of the isoforms. Additionally, if the target molecule is expressed in multiple organs, it will be considered. The first step should be to determine the presence or absence of target molecule isoforms expressed specifically in male reproductive organs such as the testes or epididymis. Moreover, small molecules are synthesized that inhibit the activity of the specific target molecule. The candidate drug molecule must exhibit ideal contraceptive properties, apart from inhibiting the target molecule.
5. Conclusion
A good contraceptive must meet a number of criteria, including being safe, reliable, effective, affordable, easy to use, without serious side effects, readily available, and reversible. It will take a long time to develop the ideal nonhormonal male contraceptive. However, with the rapid advancement of research in the field of reproduction, particularly male reproduction, and the continued innovation of GM techniques especially with CRISPR-Cas9 discovery as an editing gene. The rapid advancement of GM techniques may enable the identification of more precise and specific molecular targets for nonhormonal male contraceptives. So, we are optimistic that one day nonhormonal male contraceptives will be developed and it will be effective and beneficial to the wider community.
Acknowledgments
Thank to the Education Fund Management Institute (LPDP) for supporting the study. Many thanks to Prof Delvac Oceandy, M.D., Ph.D. for the excellent discussion about genetically modified.
Conflict of Interest
There are no conflict of interest.
Type of Study: Review Article |
Subject:
Reproductive Andrology
References
1. Naz RK, Rowan Sh. Female contraception: Present and future perspectives. Curr Womens Health Rev 2009; 5: 167-175. [DOI:10.2174/157340409789007259]
2. Arifuzzaman S, Rahman MS, Pang M-G. Research update and opportunity of non-hormonal male contraception : Histone demethylase KDM5B-based targeting. Pharmacol Res 2019; 141: 1-20. [DOI:10.1016/j.phrs.2018.12.003] [PMID]
3. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med 2016; 374: 843-852. [DOI:10.1056/NEJMsa1506575] [PMID] [PMCID]
4. Sundaram A, Vaughan B, Kost K, Bankole A, Finer L, Singh S, et al. Contraceptive failure in the United States : Estimates from the 2006-2010. Perspect Sex Reprod Health 2017; 49: 7-16. [DOI:10.1363/psrh.12017] [PMID] [PMCID]
5. Eisenstein M. Stopping sperm as the sperm. Nature 2020; 588: S170-S171. [DOI:10.1038/d41586-020-03534-4] [PMID]
6. Long JE, Lee MS, Blithe DL. Update on novel hormonal and nonhormonal male contraceptive development. J Clin Endocrinol Metab 2021; 106: e2381-e2392. [DOI:10.1210/clinem/dgab034] [PMID] [PMCID]
7. Pujianto DA, Loanda E, Sari P, Midoen YH, Soeharso P. Sperm-associated antigen 11A is expressed exclusively in the principal cells of the mouse caput epididymis in an androgen-dependent manner. Reprod Biol Endocrinol 2013; 11: 59. [DOI:10.1186/1477-7827-11-59] [PMID] [PMCID]
8. Pujianto AD, Muliawati D, Dara M, Parisudha A, Hardiyanto L. Mouse defensin beta 20 (Defb20) is expressed specifically in the caput region of the epididymis and regulated by androgen and testicular factors. Reprod Biol 2020; 20: 536-540. [DOI:10.1016/j.repbio.2020.09.003] [PMID]
9. Browne JA, Leir SH, Yin S, Harris A. Transcriptional networks in the human epididymis. Andrology 2019; 7: 741-747. [DOI:10.1111/andr.12629] [PMID] [PMCID]
10. Pujianto DA, Permatasari S. Mouse CD52 is predominantly expressed in the cauda epididymis, regulated by androgen and lumicrine factors. J Hum Reprod Sci 2021; 14: 350-355. [DOI:10.4103/jhrs.jhrs_29_21] [PMID] [PMCID]
11. Jamsai D, O'Bryan MK. Mouse models in male fertility research. Asian J Androl 2011; 13: 139-151. [DOI:10.1038/aja.2010.101] [PMID] [PMCID]
12. Tamowski S, Aston KI, Carrell DT. The use of transgenic mouse models in the study of male infertility. Syst Biol Reprod Med 2010; 56: 260-273. [DOI:10.3109/19396368.2010.485244] [PMID]
13. Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, et al. Europe PMC funders group origins and functional impact of copy number variation in the human genome. Nature 2010; 464: 704-712. [DOI:10.1038/nature08516] [PMID] [PMCID]
14. Lampreht Tratar U, Horvat S, Cemazar M. Transgenic mouse models in cancer research. Front Oncol 2018; 8: 268. [DOI:10.3389/fonc.2018.00268] [PMID] [PMCID]
15. Takehashi M, Kanatsu-Shinohara M, Shinohara T. Generation of genetically modified animals using spermatogonial stem cells. Dev Growth Differ 2010; 52: 303-310. [DOI:10.1111/j.1440-169X.2009.01167.x] [PMID]
16. Horii T, Morita S, Kimura M, Terawaki N, Shibutani M, Hatada I. Efficient generation of conditional knockout mice via sequential introduction of lox sites. Sci Rep 2017; 7: 7891. [DOI:10.1038/s41598-017-08496-8] [PMID] [PMCID]
17. Kim H, Kim M, Im S-K, Fang S. Mouse Cre-LoxP system : General principles to determine tissue-specific roles of target genes. Lab Anim Res 2018; 34: 147-159. [DOI:10.5625/lar.2018.34.4.147] [PMID] [PMCID]
18. Andersson KB, Winer LH, Mork HK, Molkentin JD, Jaisser F. Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res 2010; 19: 715-725. [DOI:10.1007/s11248-009-9342-4] [PMID]
19. Ye R, Wang QA, Tao C, Vishvanath L, Shao M, Mcdonald JG, et al. Impact of tamoxifen on adipocyte lineage tracing : Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metab 2015; 4: 771-778. [DOI:10.1016/j.molmet.2015.08.004] [PMID] [PMCID]
20. Cong L, Zhang F. Genome engineering using CRISPR-Cas9 system. Methods Mol Biol 2015; 1239: 197-217. [DOI:10.1007/978-1-4939-1862-1_10] [PMID]
21. Mou H, Kennedy Z, Anderson DG, Yin H, Xue W. Precision cancer mouse models through genome editing with CRISPR-Cas9. Genome Med 2015; 7: 53. [DOI:10.1186/s13073-015-0178-7] [PMID] [PMCID]
22. Zhang Ch, Zhou Y, Xie Sh, Yin Q, Tang Ch, Ni Z, et al. CRISPR/Cas9-mediated genome editing reveals the synergistic effects of β-defensin family members on sperm maturation in rat epididymis. FASEB J 2018; 32: 1354-1363. [DOI:10.1096/fj.201700936R] [PMID]
23. Quadros RM, Miura H, Harms DW, Akatsuka H, Sato T, Aida T. Easi-CRISPR: A robust method for one-step generation of mice carrying conditional and insertion alleles using long ssDNA donors and CRISPR ribonucleoproteins. Genome Biol 2017; 18: 92. [DOI:10.1186/s13059-017-1220-4] [PMID] [PMCID]
24. Sipila P, Bjorkgren I. Segment-specific regulation of epididymal gene expression. Reproduction 2016; 152: R91-R99. [DOI:10.1530/REP-15-0533] [PMID]
25. Zhou YS, Webb Sh, Lettice L, Tardif S, Kilanowski F, Tyrrell C, et al. Partial deletion of chromosome 8 b-defensin cluster confers sperm dysfunction and infertility in male mice. PLOS Genet 2013; 9: e1003826. [DOI:10.1371/journal.pgen.1003826] [PMID] [PMCID]
26. Sharan ShK, Pyle A, Coppola V, Babus J, Swaminathan S, Benedict J, et al. BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development 2004; 131: 131-142. [DOI:10.1242/dev.00888] [PMID]
27. Carvajal G, Brukman NG, Weigel Muñoz M, Battistone MA, Guazzone VA, Ikawa M, et al. Impaired male fertility and abnormal epididymal epithelium differentiation in mice lacking CRISP1 and CRISP4. Sci Rep 2018; 8: 17531. [DOI:10.1038/s41598-018-35719-3] [PMID] [PMCID]
28. Carlson AE, Burnett LA, del Camino D, Quill TA, Hille B, Chong JA, et al. Pharmacological targeting of native CatSper channels reveals a required role in maintenance of sperm hyperactivation. PLoS One 2009; 4: e6844. [DOI:10.1371/journal.pone.0006844] [PMID] [PMCID]
29. Hsia K-H, Millar MR, King S, Selfridge J, Redhead NJ, Melton DW, et al. DNA repair gene Ercc1 is essential for normal spermatogenesis and oogenesis and for functional integrity of germ cell DNA in the mouse. Development 2003; 130: 369-378. [DOI:10.1242/dev.00221] [PMID]
30. Sun F, Palmer K, Handel MA. Mutation of Eif4g3, encoding a eukaryotic translation initiation factor, causes male infertility and meiotic arrest of mouse spermatocytes. Development 2010; 137: 1699-1707. [DOI:10.1242/dev.043125] [PMID] [PMCID]
31. O'Rand MG, Hamil KG, Adevai T, Zelinski M. Inhibition of sperm motility in male macaques with EP055, a potential non-hormonal male contraceptive. PLoS One 2018; 13: e0195953. [DOI:10.1371/journal.pone.0195953] [PMID] [PMCID]
32. Imai H, Hakkaku N, Iwamoto R, Suzuki J, Suzuki T, Tajima Y, et al. Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J Biol Chem 2009; 284: 32522-32532. [DOI:10.1074/jbc.M109.016139] [PMID] [PMCID]
33. Crackower MA, Kolas NK, Noguchi J, Sarao R, Kaneko H, Kobayashi E, et al. Essential role of Fkbp6 in male fertility and homologous chromosome pairing in meiosis. Science 2003; 300: 1291-1295. [DOI:10.1126/science.1083022] [PMID] [PMCID]
34. Fujihara Y, Lu Y, Noda T, Oji A, Larasati T, Kojima-Kita K, et al. Spermatozoa lacking fertilization influencing membrane protein (FIMP) fail to fuse with oocytes in mice. Proc Natl Acad Sci U S A 2020; 117: 9393-9400. [DOI:10.1073/pnas.1917060117] [PMID] [PMCID]
35. Martianov I, Brancorsini S, Catena R, Gansmuller A, Kotaja N, Parvinen M, et al. Polar nuclear localization of H1T2, a histone H1 variant, required for spermatid elongation and DNA condensation during spermiogenesis. Proc Natl Acad Sci U S A 2005; 102: 2808-2813. [DOI:10.1073/pnas.0406060102] [PMID] [PMCID]
36. Crapster JA, Rack PG, Hellmann ZJ, Le AD, Adams ChM, Leib RD, et al. HIPK4 is essential for murine spermiogenesis. Elife 2020; 9: e50209. [DOI:10.7554/eLife.50209] [PMID] [PMCID]
37. Aydin H, Sultana A, Li Sh, Thavalingam A, Lee JE. Molecular architecture of the human sperm IZUMO1 and egg JUNO fertilization complex. Nature 2016; 534: 562-565. [DOI:10.1038/nature18595] [PMID] [PMCID]
38. Kawa S, Ito Ch, Toyama Y, Maekawa M, Tezuka T, Nakamura T, et al. Azoospermia in mice with targeted disruption of the Brek/Lmtk2 (brain-enriched kinase/lemur tyrosine kinase 2) gene. Proc Natl Acad Sci U S A 2006; 103: 19344-19349. [DOI:10.1073/pnas.0603603103] [PMID] [PMCID]
39. Yang J, Medvedev S, Yu J, Tang LC, Agno JE, Matzuk MM, et al. Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc Natl Acad Sci U S A 2005; 102: 5755-5760. [DOI:10.1073/pnas.0408718102] [PMID] [PMCID]
40. Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, et al. Conserved role of nanos proteins in germ cell development. Science 2003; 301: 1239-1241. [DOI:10.1126/science.1085222] [PMID]
41. Nayernia K, Vauti F, Meinhardt A, Cadenas C, Schweyer S, Meyer BI, et al. Inactivation of a testis-specific Lis1 transcript in mice prevents spermatid differentiation and causes male infertility. J Biol Chem 2003; 278: 48377-48385. [DOI:10.1074/jbc.M309583200] [PMID]
42. Xu H, Beasley MD, Warren WD, Van Der Horst GTJ, Mckay MJ. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 2005; 8: 949-961. [DOI:10.1016/j.devcel.2005.03.018] [PMID]
43. Zeng X-H, Navarro B, Xia X-M, Clapham DE, Lingle ChJ. Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. J Gen Physiol 2013; 142: 305-313. [DOI:10.1085/jgp.201311011] [PMID] [PMCID]
44. Kherraf Z-E, Christou‐Kent M, Karaouzene T, Amiri‐Yekta A, Martinez G, Vargas AS, et al. SPINK 2 deficiency causes infertility by inducing sperm defects in heterozygotes and azoospermia in homozygotes. EMBO Mol Med 2017; 9: 1132-1149. [DOI:10.15252/emmm.201607461] [PMID] [PMCID]
45. Noda T, Lu Y, Fujihara Y, Oura S, Koyano T, Kobayashi S, et al. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm-oocyte fusion in mice. Proc Natl Acad Sci U S A 2020; 117: 11493-11502. [DOI:10.1073/pnas.1922650117] [PMID] [PMCID]
46. Tanaka H, Iguchi N, Toyama Y, Kitamura K, Takahashi T, Kaseda K, et al. Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol 2004; 24: 7958-7964. [DOI:10.1128/MCB.24.18.7958-7964.2004] [PMID] [PMCID]
47. Xu B, Hao Zh, Jha KN, Zhang Zh, Urekar C, Digilio L, et al. Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsuf fi ciency. Dev Biol 2008; 319: 211-222. [DOI:10.1016/j.ydbio.2008.03.047] [PMID] [PMCID]
48. Noda T, Sakurai N, Nozawa K, Kobayashi S, Devlin DJ, Matzuk MM, et al. Nine genes abundantly expressed in the epididymis are not essential for male fecundity in mice. Andrology 2019; 7: 644-653. [DOI:10.1111/andr.12621] [PMID] [PMCID]
49. Chung SSW, Wang X, Wolgemuth DJ. Prolonged oral administration of a pan-retinoic acid receptor antagonist inhibits spermatogenesis in mice with a rapid recovery and changes in the expression of influx and efflux transporters. Endocrinology 2016; 157: 1601-1612. [DOI:10.1210/en.2015-1675] [PMID] [PMCID]
50. Busada JT, Geyer CB. The role of retinoic acid (RA) in spermatogonial differentiation. Biol Reprod 2016; 94: 10. [DOI:10.1095/biolreprod.115.135145] [PMID] [PMCID]
51. Chen Y, Zhu J-Y, Hong KH, Mikles DC, Georg GI, Goldstein AS, et al. Structural basis of ALDH1A2 inhibition by irreversible and reversible small molecule inhibitors. ACS Chem Biol 2018; 13: 582-590. [DOI:10.1021/acschembio.7b00685] [PMID] [PMCID]
52. Al Noman MDA, Kyzer JL, Chung SSW, Wolgemuth DJ, Georg GI. Retinoic acid receptor antagonists for male contraception: Current status. Biol Reprod 2020; 103: 390-399. [DOI:10.1093/biolre/ioaa122] [PMID] [PMCID]
53. Hawkinson JE, Sinville R, Mudaliar D, Shetty J, Ward T, Herr JC, et al. Potent pyrimidine and pyrrolopyrimidine inhibitors of testis-specific serine/threonine kinase 2 (TSSK2). Chem Med Chem 2017; 12: 1857-1865. [DOI:10.1002/cmdc.201700503] [PMID] [PMCID]
54. Srivastav A, Changkija B, Sharan K, Nagar GK. Influence of antifertility agents dutasteride and nifedipine on Catsper gene level in epididymis during seperm maturation in BALB/c mice. Reproduction 2018; 155: 347-359. [DOI:10.1530/REP-17-0664] [PMID]
55. Matzuk MM, McKeown MR, Filippakopoulos P, Li Q, Ma L, Agno JE, et al. Small-molecule inhibition of BRDT for male contraception. Cell 2012; 150: 673-684. [DOI:10.1016/j.cell.2012.06.045] [PMID] [PMCID]
56. Ayoub AM, Hawk LML, Herzig RJ, Jiang J, Wisniewsk AJ, Gee CT, et al. BET bromodomain inhibitors with one-step synthesis discovered from virtual screen. J Med Chem 2017; 6: 4805-4817. [DOI:10.1021/acs.jmedchem.6b01336] [PMID] [PMCID]
57. Chávez JC, Ferreira JJ, Butler A, De La Vega Beltrán JL, Treviño CL, Darszon A, et al. SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J Biol Chem 2014; 289: 32266-32275. [DOI:10.1074/jbc.M114.607556] [PMID] [PMCID]
58. Rennhack A, Schiffer C, Brenker C, Fridman D, Nitao ET, Cheng YM, et al. A novel cross-species inhibitor to study the function of CatSper Ca2+ channels in sperm. Br J Pharmacol 2018; 175: 3144-3161. [DOI:10.1111/bph.14355] [PMID] [PMCID]
59. O'Rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, et al. Reversible immunocontraception in male monkeys immunized with eppin. Science 2004; 306: 1189-1190. [DOI:10.1126/science.1099743] [PMID]
60. O'Rand M, Silva EJR, Hamil KG. Non-hormonal male contraception: A review and development of an Eppin based contraceptive. Pharmacol Ther 2017; 157: 105-111. [DOI:10.1016/j.pharmthera.2015.11.004] [PMID] [PMCID]
61. D'Francisco F, Merlo M, Vercellini R, Blanco P, Barbeito C, Gobello C. Effect of the indenopyridine RTI-4587-073 (l) on feline testicle. Anim Reprod Sci 2019; 205: 10-17. [DOI:10.1016/j.anireprosci.2019.03.014] [PMID]
62. Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod 2008; 78: 1127-1138. [DOI:10.1095/biolreprod.106.057810] [PMID]
63. Mok K-W, Mruk D, Lie PPY, Lui W-Y, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction 2011; 141: 571-580. [DOI:10.1530/REP-10-0464] [PMID] [PMCID]
64. Kwon W, Park Y, Kim Y, You Y, Kim IC, Pang M. Vasopressin effectively suppresses male fertility. PLoS One 2013; 8: e54192. [DOI:10.1371/journal.pone.0054192] [PMID] [PMCID]
65. Lee JS, Kwon WS, Rahman MS, Yoon SJ, Park YJ, Pang MG. Actin-related protein 2/3 complex-based actin polymerization is critical for male fertility. Andrology 2015; 3: 937-946. [DOI:10.1111/andr.12076] [PMID]
66. Peralta-Aris RD, Vivenes CY, Camejo MI, Pinero S, Proverbio T, Martinez E, et al. ATPases, ion exchangers and human sperm motility. Reproduction 2015; 149: 475-484. [DOI:10.1530/REP-14-0471] [PMID]
67. Sanbe A, Tanaka Y, Fujiwara Y, Tsumura H, Yamauchi J, Cotecchia S, et al. Alpha1-adrenoceptors are required for normal male sexual function. Br J Pharmacol 2007; 152: 332-340. [DOI:10.1038/sj.bjp.0707366] [PMID] [PMCID]
68. Borges CS, Missassi G, Pacini ESA, Kiguti LRA, Sanabria M, Pupo S, et al. Slimmer or fertile? Pharmacological mechanisms involved in reduced sperm quality and fertility in rats exposed to the anorexigen sibutramine. Plos Med 2013; 8: e66091. [DOI:10.1371/journal.pone.0066091] [PMID] [PMCID]
69. Zhang H, Yu H, Wang X, Zheng W, Yang B, Pi J, et al. (S)-α-chlorohydrin inhibits protein tyrosine phosphorylation through blocking cyclic AMP-protein kinase a pathway in spermatozoa. PLoS One 2012; 7: e43004. [DOI:10.1371/journal.pone.0043004] [PMID] [PMCID]
70. Balbach M, Fushimi M, Huggins DJ, Steegborn C, Meinke PT, Levin LR, et al. Optimization of lead compounds into on-demand, nonhormonal contraceptives: Leveraging a public-private drug discovery institute collaboration. Biol Reprod 2020; 103: 176-182. [DOI:10.1093/biolre/ioaa052] [PMID] [PMCID]
71. Akbari A, Pipitone GB, Anvar Z, Jaafarinia M, Ferrari M, Carrera P, et al. ADCY10 frameshift variant leading to severe recessive asthenozoospermia and segregating with absorptive hypercalciuria. Hum Reprod 2019; 34: 1155-1164. [DOI:10.1093/humrep/dez048] [PMID]
72. Shettya J, Sinvilleb R, Shumilinc IA, Minorc W, Zhanga J, Hawkinson JE, et al. Recombinant production of enzymatically active male contraceptive drug target hTSSK2-localization of the TSKS domain phosphorylated by TSSK2. Protein Expr Purif 2016; 121: 88-96. [DOI:10.1016/j.pep.2016.01.009] [PMID] [PMCID]
73. Zhang H, Su D, Yang Y, Zhang W, Liu Y, Bai G, et al. Some single-nucleotide polymorphisms of the TSSK2 gene may be associated with human spermatogenesis impairment. J Androl 2010; 31: 388-392. [DOI:10.2164/jandrol.109.008466] [PMID]
74. Salicioni AM, Gervasi MG, Sosnik J, Tourzani DA, Nayyab S, Caraballo DA, et al. Testis-specific serine kinase protein family in male fertility and as targets for non-hormonal male contraception. Biol Reprod 2020; 103: 264-274. [DOI:10.1093/biolre/ioaa064] [PMID] [PMCID]
75. Conlon RA. Chapter 5 transgenic and gene targeted models of dementia. In: Dam PPDD, Van D. Animal models of dementia neuromethods. Germani: Springer Science; 2011. [DOI:10.1007/978-1-60761-898-0_5]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








